Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity
Abstract
1. Introduction
2. Definition of AMPs
3. Properties and Structure of AMPs
4. Comparison between AMPs and Antibiotics
5. Synthesis of AMPs
5.1. Defensins
5.2. Bacteriocin
5.3. Antimicrobial Substrates Produced in a Ribosome-Independent Way
6. Biological Functions of AMPs
6.1. Antiviral Activity
6.2. Antibacterial Activity
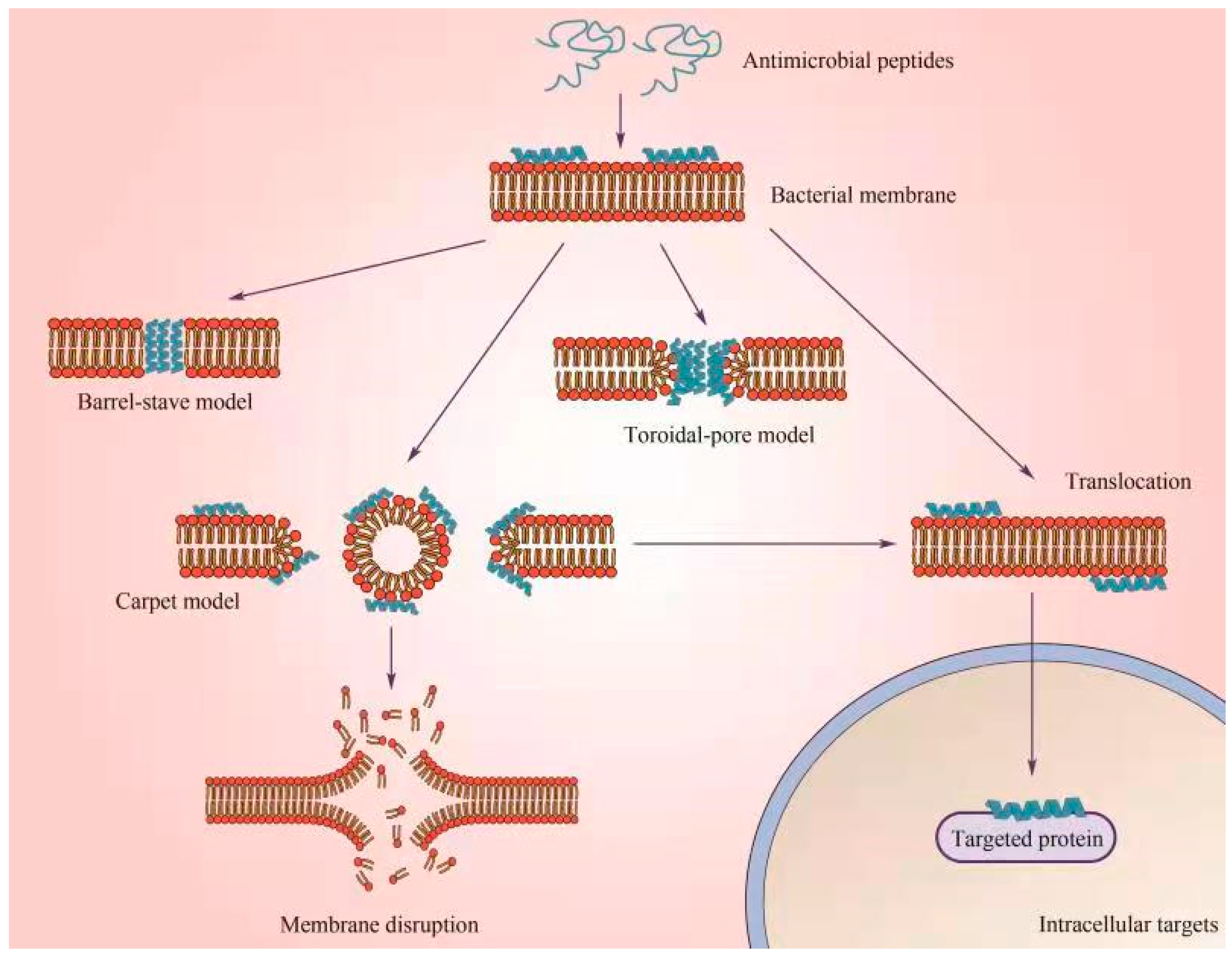
6.2.1. Membrane-Targeting Mechanism
6.2.2. Non-Membrane-Targeting Mechanism
6.3. Antifungal Activity
6.4. Antiparasitic Function
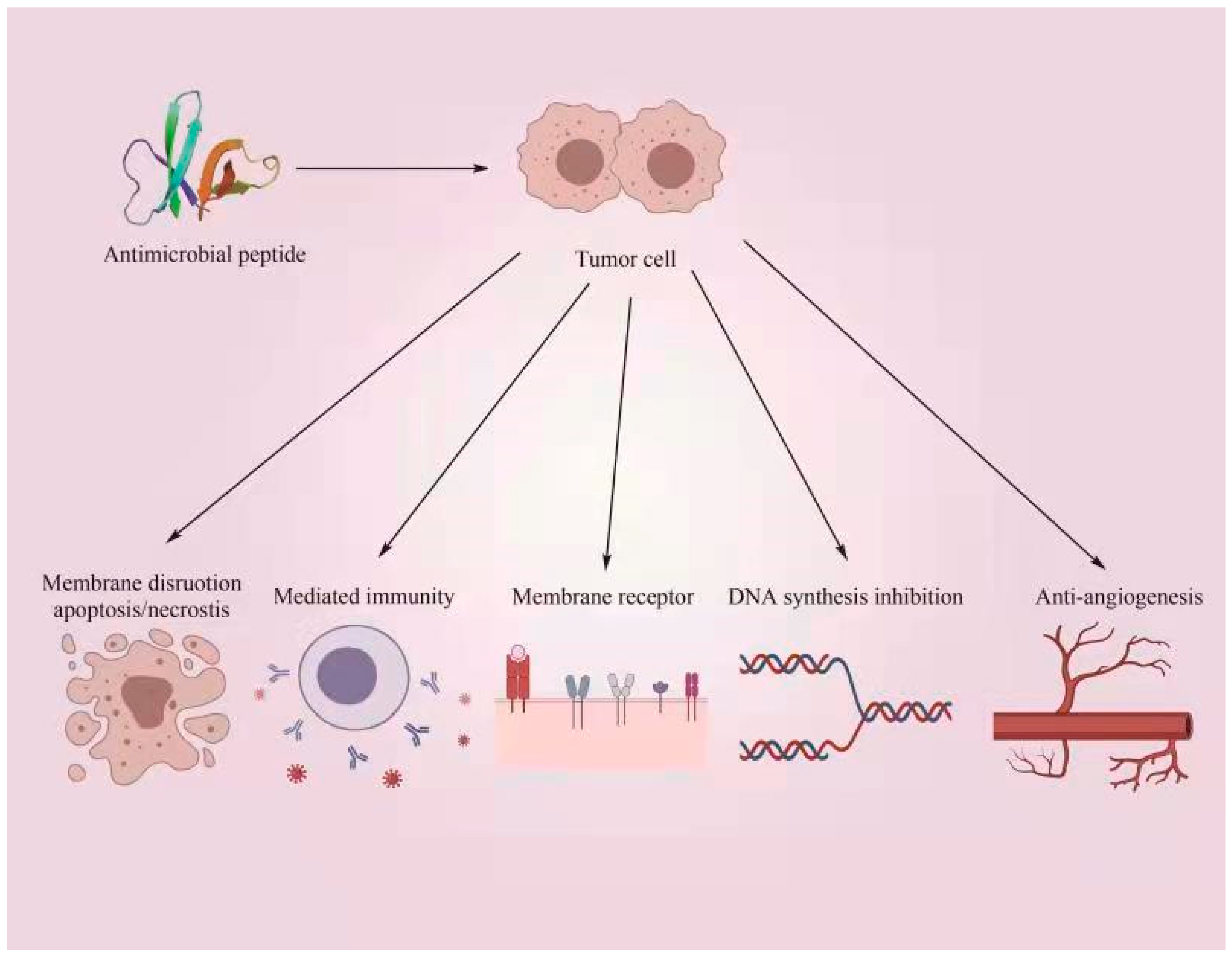
6.5. Antineoplastic Activity
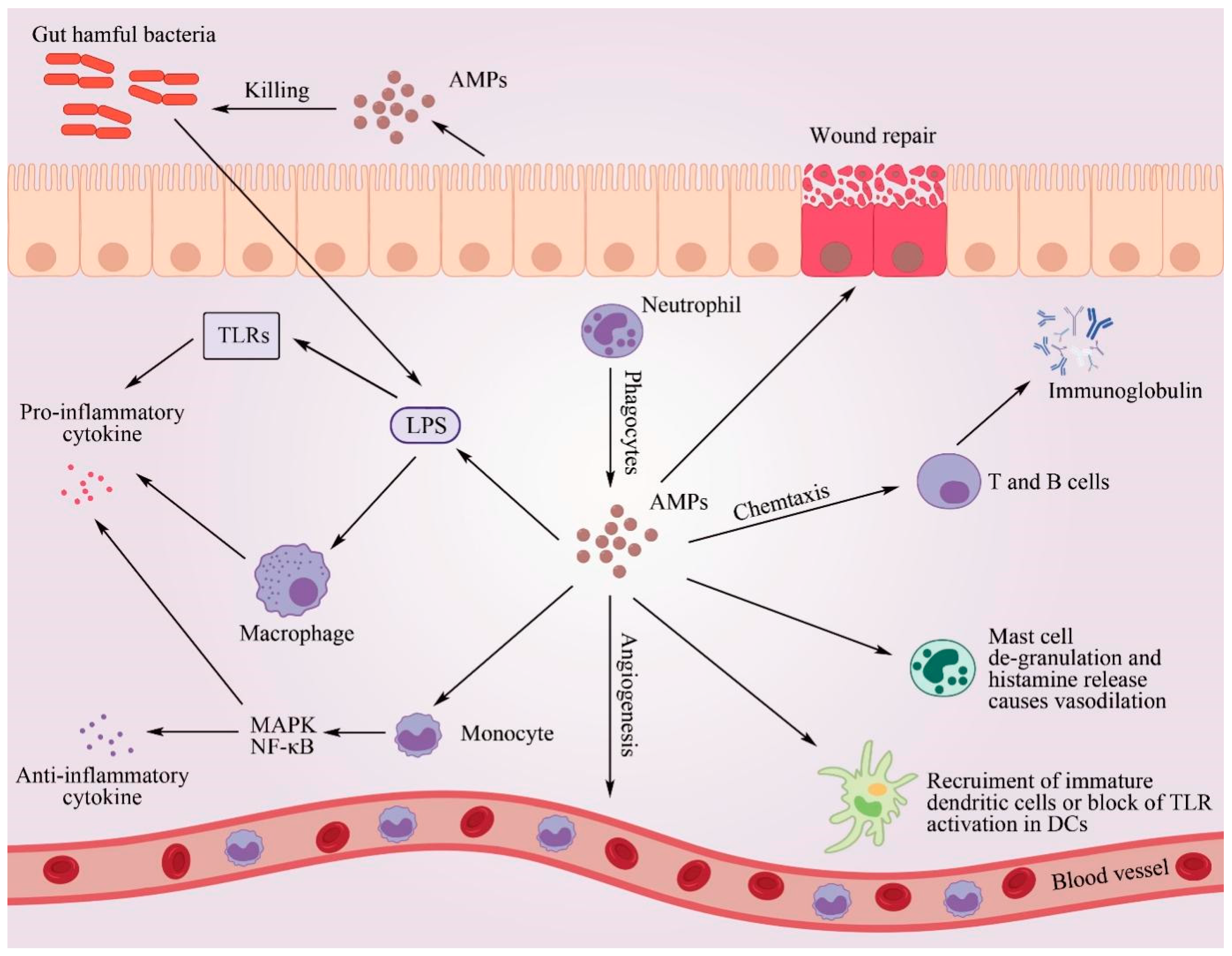
6.6. Regulation of the Host Immune System
6.6.1. Regulation of Pro-Inflammatory and Anti-Inflammatory Immune Responses
6.6.2. Chemotaxis Function
6.6.3. Enhanced Ability of the Host Immune System to Kill Harmful Bacteria
7. Factors Affecting the Biological Functions of AMPs
7.1. Conformation of AMPs
7.2. Electrical Charge Number of AMPs
7.3. Hydrophilic and Hydrophobic Properties of AMPs
8. Biological Expression Systems for AMPs
8.1. Escherichia coli Expression System
8.2. Yeast Expression Systems
8.3. Plant Expression Systems
9. Current Questions regarding the Application of AMPs
9.1. Sources of AMPs
9.2. Toxicity of AMPs to Host Cells
9.3. Stability of AMPs
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.L.; Li, X.J.; Ma, N.; Jin, X.F.; Yuan, X.F.; Qu, C.; Tang, H.M.; Liu, Z.; Zhang, Z. Bacterial quorum sensing molecules promote allergic airway inflammation by activating the retinoic acid response. iScience 2020, 23, 101288. [Google Scholar] [CrossRef]
- Lai, Y.P.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef]
- Li, Y.F. Recombinant production of antimicrobial peptides in Escherichia coli: A review. Protein Expr. Purif. 2011, 80, 260–267. [Google Scholar] [CrossRef]
- Cregg, J.M.; Tolstorukov, I.; Kusari, A.; Sunga, J.; Madden, K.; Chappell, T. Expression in the yeast Pichia pastoris. Methods Enzymol. 2009, 463, 169–189. [Google Scholar]
- Huang, Y.C.; Kong, Q.; Mao, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Mangoni, M.L.; Pasquazzi, C.; Fiocco, D.; Marangi, M.; Miele, R.; Zechini, B.; Borro, M.; Versace, I.; Simmaco, M. Alpha-defensin increase in peripheral blood mononuclear cells from patients with hepatitis C virus chronic infection. J. Viral Hepat. 2006, 13, 821–827. [Google Scholar] [CrossRef]
- Lyu, W.; Curtis, A.R.; Sunkara, L.T.; Zhang, G. Transcriptional regulation of antimicrobial host defense peptides. Curr. Protein Pept. Sci. 2015, 16, 672–679. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Johansson, M.E.; Stabler, R.A.; Dalgakiran, F.; Hansson, G.C.; Wren, B.W.; Luzio, J.P.; Taylor, P.W. Altered innate defenses in the neonatal gastrointestinal tract in response to colonization by neuropathogenic Escherichia coli. Infect. Immun. 2013, 81, 3264–3275. [Google Scholar] [CrossRef]
- De, G.; Chantal, V.G.; Zhu, L.Y.; Oman, T.J.; van der Donk, W.A. NMR structure of the S-linked glycopeptide Sublancin 168. ACS Chem. Biol. 2014, 9, 796–801. [Google Scholar]
- Malmsten, M. Antimicrobial peptides. Upsala J. Med. Sci. 2014, 119, 199–204. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, Q.; Zhang, Q.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Yin, L.M.; Edwards, M.A.; Li, J.; Yip, C.M.; Deber, C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J. Biol. Chem. 2012, 287, 7738–7745. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Gera, L.; Vasil, M.L.; Hodges, R.S. Rational design of α-helical antimicrobial peptides to target Gram-negative pathogens, Acinetobacter baumannii and Pseudomonas aeruginosa: Utilization of charge, ‘specificity determinants,’ total hydrophobicity, hydrophobe type and location as design parameters to improve the therapeutic ratio. Chem. Biol. Drug Des. 2011, 77, 225–240. [Google Scholar]
- Takahashi, D.; Shukla, S.K.; Prakash, O.; Zhang, G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 2010, 92, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-helical antimicrobial peptides. Eur. J. Biochem. 2001, 55, 4–30. [Google Scholar] [CrossRef]
- Shaw, J.E.; Alattia, J.R.; Verity, J.E.; Privé, G.G.; Yip, C.M. Mechanisms of antimicrobial peptides action: Studies of indolicidin assembly at model membrane interfaces by in situ atomic force microscopy. J. Struct. Biol. 2006, 154, 42–58. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Thennarasu, S.; Tan, A.; Gottipati, K.; Sreekumar, S.; Heyl, D.L.; An, F.Y.; Shelburne, C.E. Deletion of all cysteines in tachyplesin I abolishes hemolytic activity and retains antimicrobial activity and lipopolysaccharide selective binging. Biochemistry 2006, 45, 6529–6540. [Google Scholar] [CrossRef]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial peptides: Primeval molecules of future drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef]
- van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.F.; Yang, Q.; Qiao, S.Y. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Siriwardena, T.N.; Stach, M.; Tinguely, R.; Kasraian, S.; Luzzaro, F.; Leib, S.L.; Darbre, T.; Reymond, J.L.; Endimiani, A. In vitro activity of the novel antimicrobial peptide dendrimer G3KL against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 7915–7918. [Google Scholar] [CrossRef]
- Rajamuthiah, R.; Jayamani, E.; Conery, A.L.; Fuchs, B.B.; Kim, W.; Johnston, T.; Vilcinskas, A.; Ausubel, F.M.; Mylonakis, E.A. A defensin from the model beetle Tribolium castaneum acts synergistically with Telavancin and Daptomycin against multidrug resistant Staphylococcus aureus. PLoS ONE 2015, 10, e128576. [Google Scholar] [CrossRef]
- Cuperus, T.; Coorens, M.; van Dijk, A.; Haagsman, H.P. Avian host defense peptides. Dev. Comp. Immunol. 2013, 41, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sunkara, L.T. Avian antimicrobial host defense peptides: From biology to therapeutic applications. Pharmaceuticals 2014, 7, 220–247. [Google Scholar] [CrossRef] [PubMed]
- Semple, F.; Dorin, J.R. Beta-defensins: Multifunctional modulators of infection, inflammation and more? J. Innate Immun. 2012, 4, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Cole, R.I.; Selsted, M.E. Theta-defensins: Cyclic peptides with endless potential. J. Biol. Chem. 2012, 287, 27014–27019. [Google Scholar] [CrossRef] [PubMed]
- Krizsan, A.; Volke, D.; Weinert, S.; Strater, N.; Knappe, D.; Hoffmann, R. Insect-derived proline-rich antimicrobial peptides kill bacteria by inhibiting bacterial protein translation at the 70S ribosome. Angew. Chem. Int. Ed. 2014, 53, 12236–12239. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.J. Antimicrobial peptides from plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Rebuffat, S. Microcins in action: Amazing defence strategies of enterobacteria. Biochem. Soc. Trans. 2012, 40, 1456–1462. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Bierbaum, G.; Sahl, H. Lantibiotics: Mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 2009, 10, 2–18. [Google Scholar] [CrossRef]
- Vondenhoff, G.H.M.; Blanchaert, B.; Geboers, S.; Kazakov, T.; Datsenko, K.A.; Wanner, B.L.; Rozenski, J.; Severinov, K.; Van Aerschot, A. Characterization of peptide chain length and constituency requirements for YejABEF-mediated uptake of Microcin C analogues. J. Bacteriol. 2011, 193, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Bantysh, O.; Serebryakova, M.; Zukher, I.; Kulikovsky, A.; Tsibulskaya, D.; Dubiley, S.; Severinov, K. Enzymatic synthesis and functional characterization of bioactive Microcin C-like compounds with altered peptide sequence and length. J. Bacteriol. 2015, 197, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.; Li, J.; Ross, A.C.; Jensen, S. Paenibacillus polymyxa PKB1 produces variants of polymyxin in B-type antibiotics. Chem. Biol. 2012, 18, 16440–16448. [Google Scholar] [CrossRef]
- Thasana, N.; Prapagdee, B.; Rangkadilok, N.; Sallabhan, R.; Aye, S.L.; Ruchirawat, S.; Loprasert, S. Bacillus subtilis SSE4 produces subtulene A, a new lipopeptides antibiotic possessing as unusual C15 unsaturated beta-amino acid. FEBS Lett. 2010, 584, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Tscheka, C.; Edwards, K.; Heerklotz, H. All-or-none membrane permeabilization by fengycin-type lipopeptides from Bacillus subtilis QST713. Biochim. Biophys. Acta 2011, 1808, 2000–2008. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Ng, T.B.; Zhang, J.; Zhou, M.; Song, F.; Lu, F.; Liu, Y. Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides 2007, 28, 553–559. [Google Scholar] [CrossRef]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional cationic host defense peptides and their clinical applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Malmsten, M. Interactions of antimicrobial peptides with bacterial membranes and membrane components. Curr. Top. Med. Chem. 2016, 16, 16–24. [Google Scholar] [CrossRef]
- Hazrati, E.; Galen, B.; Lu, W.; Wang, W.; Ouyang, Y.; Keller, M.J.; Lehrer, R.J.; Herold, B.C. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 2006, 177, 8658–8666. [Google Scholar] [CrossRef]
- Hsieh, I.N.; Hartshorn, K.L. The role of antimicrobial peptides in influenza virus infection and their potential as antiviral and immunomodulatory therapy. Pharmaceuticals 2016, 9, 53. [Google Scholar] [CrossRef]
- Wiens, M.E.; Wilson, S.S.; Lucero, C.M.; Smith, J.G. Defensins and viral infection: Dispelling common misconceptions. PLoS Pathog. 2014, 10, e1004186. [Google Scholar] [CrossRef]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.C.; Strandberg, E.; Grau-Campistany, A.; Wadhwani, P.; Reichert, J.; Bürck, J.; Rabanal, F.; Auger, M.; Paquin, J.; Ulrich, A.S. Influence of the length and charge on the activity of α-helical amphipathic antimicrobial peptides. Biochemistry 2017, 56, 1680–1695. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Yang, Y.; Lyu, X.; Dong, N.; Shan, A. Antimicrobial activity, improved cell selectivity and mode of action of short PMAP-36-derived peptides against bacteria and Candida. Sci. Rep. 2016, 6, 27258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Ebenhan, T.; Gheysens, O.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M.M. Antimicrobial peptides: Their role as infection-selective tracers for molecular imaging. Biomed. Res. Int. 2014, 2014, 867381. [Google Scholar] [CrossRef]
- Priya, A.; Swetha, T.K.; Pandian, S.K. Antimicrobial peptides as a potent therpeutic regimen to quench biofilm-mediated antimicrobial resistance. In Microbial and Matural Macromolecules; Academic Press: Cambridge, MA, USA, 2021; pp. 531–570. [Google Scholar]
- Lee, T.H.; Hall, K.N.; Aguilar, M.I. Antimicrobial peptide structure and mechanism of action: A focus on the role of membrane structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- de Leeuw, E.; Li, C.; Zeng, P.; Li, C.D.; Buin, M.; Lu, W.Y.; Breukink, E.; Lu, W. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett. 2010, 584, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Varney, K.M.; Bonvin, A.M.J.J.; Pazgier, M.; Malin, J.; Yu, W.; Ateh, E.; Oashi, T.; Lu, W.; Huang, J.D.; Buin, M.; et al. Turning defense into offense: Defensin mimetics as novel antibiotics targeting lipid II. PLoS Pathog. 2013, 9, E1003732. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Bayer, A.S.; Xiong, Y.Q.; Yeaman, M.R. Advances in antimicrobial peptide immunobiology. Pept. Sci. 2006, 84, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.L.; Tian, J.H.; Yang, W.Y.; Wen, S.Y. Bactrocerin-1: A novel inducible antimicrobial peptide from pupae of oriental fruit fly Bactrocera dorsalis Hende. Arch. Insect Biochem. Physiol. 2009, 71, 117–129. [Google Scholar] [CrossRef]
- Vale, N.; Aguiar, L.; Gomes, P. Antimicrobial peptides: A new class of antimalarial drugs? Front. Pharmacol. 2014, 5, 275. [Google Scholar] [CrossRef]
- McGwire, B.S.; Kulkarni, M.M. Interactions of antimicrobial peptides with Leishmania and trypanosomes and their functional role in host parasitism. Exp. Parasitol. 2010, 126, 397–405. [Google Scholar] [CrossRef]
- Neshani, A.; Zare, H.; Akbari Eidgahi, M.R.; Khaledi, A.; Ghazvini, K. Epinecidin-1, a highly potent marine antimicrobial peptide with anticancer and immunomodulatory activities. BMC Pharmacol. Toxicol. 2019, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jiang, W.; Cao, S.; Zhao, P.; Liu, J.; Dong, H.; Guo, Y.; Liu, Q.; Gong, P. In vitro leishmanicidal activity of antimicrobial peptide KDEL against Leishmania tarentolae. Acta Biochim. Biophys. Sin. 2019, 51, 1286–1292. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Castanho, M.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Y.; Yin, Q.; Liang, H.; She, R. Chicken intestine defensins activated murine peripheral blood mononuclear cells through the TLR4-NF-κB pathway. Vet. Immunol. Immunopathol. 2009, 133, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Recombinant antimicrobial peptide microcin J25 alleviates DSS-induced colitis via regulating intestinal barrier function and modifying gut microbiota. Biomed. Pharmacother. 2021, 139, 111127. [CrossRef] [PubMed]
- Silva, O.N.; de la Fuente-Nunez, C.; Haney, E.F.; Fensterseifer, I.C.M.; Ribeiro, S.M.; Porto, W.F.; Brown, P.; Faria-Junior, C.; Rezende, T.M.B.; Moreno, S.E.; et al. An anti-infective synthetic peptide with dual antimicrobial and immunomodulatory activities. Sci. Rep. 2016, 6, 35465. [Google Scholar] [CrossRef]
- van der Does, A.M.; Bogaards, S.J.; Ravensbergen, B.; Beekhuizen, H.; van Dissel, J.T.; Nibbering, P.H. Antimicrobial peptide hLF1-11 directs granulocyte-macrophage colony-stimulating factor-driven monocyte differentiation toward macrophages with enhanced recognition and clearance of pathogens. Antimicrob. Agents Chemother. 2010, 54, 811–816. [Google Scholar] [CrossRef]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.E.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Lippert, D.N.; Hamill, P.; Falsafi, R.; Nijnik, A.; Kindrachuk, J.; Pistolic, J.; Gardy, J.; Miri, P.; Naseer, M. Intracellular receptor for human host defense peptide LL-37 in monocytes. J. Immunol. 2009, 183, 2688–2696. [Google Scholar] [CrossRef]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef]
- Fritz, J.; Brunner, S.; Birnstiel, M.; Buschle, M.; Gabain, A.V.; Mattner, F.; Zauner, W. The artificial antimicrobial peptide KLKLLLLLKLK induces predominantly a TH2-type immune response to co-injected antigens. Vaccine 2004, 22, 3274–3284. [Google Scholar] [CrossRef] [PubMed]
- Nijnik, A.; Madera, L.; Ma, S.; Waldbrook, M.; Elliott, M.R.; Easton, D.M.; Mayer, M.L.; Mullaly, S.C.; Kindrachuk, J.; Jenssen, H.; et al. Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J. Immunol. 2010, 184, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Nijnik, A.; Pistolic, J.; Filewod, N.C.; Hancock, R.E.W. Signaling pathways mediating chemokine induction in keratinocytes by cathelicidin LL-37 and flagellin. J. Innate Immun. 2012, 4, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Hancock, R.E.W. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 2007, 64, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Madera, L.; Afacan, N.; Okumura, K.; Ogawa, H.; Hancock, R.E.W. The innate defense regulator peptides IDR-HH2, IDR-1002, and IDR-1018 modulate human neutrophil functions. J. Leukoc. Biol. 2013, 94, 159–170. [Google Scholar] [CrossRef] [PubMed]
- von Kockritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Borrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Jena, P.; Mehta, R.K.; Pati, R.; Banerjee, B.; Pati, S.V.; Sonawane, A. Cationic antimicrobial peptides and biogenic silver nanoparticles kill mycobacteria without eliciting DNA damage and cytotoxicity in mouse macrophages. Antimicrob. Agents Chemother. 2013, 57, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Falciani, C.; Lozzi, L.; Pollini, S.; Luca, V.; Caenicell, V.; Brunetti, J.; Lelli, B.; Bindl, S.; Scali, S.; Giulio, A.D.; et al. Isomerization of an antimicrobial peptide broadens antimicrobial spectrum to gram-positive bacterial pathogens. PLoS ONE 2012, 7, e46259. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Qiu, S.; Wang, J.; Peng, J.; Zhao, P.; Zhu, R.; Wang, H.; Li, Y.; Wang, K. Antimicrobial activity and stability of the D-amino acid substituted derivatives of antimicrobial peptide polybia-MPI. AMB Express 2016, 6, 122. [Google Scholar] [CrossRef]
- Berthold, N.; Czihal, P.; Fritsche, S.; Sauer, U.; Schiffer, G.; Knappe, D.; Alber, G.; Hoffmann, R. Novel apidaecin 1b analogs with superior serum stabilities for treatment of infections by gram-negative pathogens. Antimicrob. Agents Chemother. 2013, 57, 402–409. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Gong, F.; Li, S.; Chang, H.; Xie, H.; Gao, H.; Tan, Y.; Ji, S. Design of anα-helical antimicrobial peptide with improved cell selective and potent anti-biofilm activity. Sci. Rep. 2016, 6, 27394. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuert, H.; Hancock, R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Khiari, Z.; Pietrasik, Z.; Gaudette, N.J.; Betti, M. Poultry protein isolate prepared using an acid solubilization/precipitation extraction influences the microstructure, the functionality and the consumer acceptability of a processed meat product. Food Struct. 2014, 2, 49–60. [Google Scholar] [CrossRef]
- Gao, J.X.; Zhang, M.H.; Zhang, F.; Wang, Y.; Ouyang, J.; Luo, X.; Yang, H.; Zhang, D.; Chen, Y.; Yu, H.; et al. Design of a Sea snake antimicrobial peptide derivative with therapeutic potential against drug-resistant bacterial infection. ACS Infect. Dis. 2020, 6, 2451–2467. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, A.; Martinez, M.; Noguera, M.E.; Augusto, M.T.; Disalvo, A.; Santos, N.C.; Semorile, L.; Maffia, P.C. Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptide-membrane interactions of three related antimicrobial peptides. Colloids Surf. B Biointerfaces 2016, 1, 528–536. [Google Scholar] [CrossRef]
- Musa, M.; Radman, M.; Krisko, A. Decreasing translation error rate in Escherichia coli increases protein function. BMC Biotechnol. 2016, 19, 16–28. [Google Scholar] [CrossRef]
- Herbel, V.; Schafer, H.; Wink, M. Recombinant production of snakin-2 (an antimicrobial peptide from tomato) in E. coli and analysis of its bioactivity. Molecules 2015, 20, 14889–14901. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Q.; Li, Z.; Zhang, Y.; Zhao, J.; Wang, L. Molecular cloning, expression, purification, and functional characterization of palustrin-2CE, an antimicrobial peptide of Rana chensinensis. Biosci. Biotechnol. Biochem. 2012, 76, 157–162. [Google Scholar] [CrossRef]
- Li, Y. Carrier proteins for fusion expression of antimicrobial peptides in Escherichia coli. Biotechnol. Appl. Biochem. 2009, 54, 1–9. [Google Scholar] [CrossRef]
- Malakhov, M.P.; Mattern, M.R.; Malakhova, O.A.; Drinker, M.; Stephen, D.; Butt, T.R. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genom. 2004, 5, 75–86. [Google Scholar] [CrossRef]
- Soundrarajan, N.; Cho, H.S.; Ahn, B.; Choi, M.; Thong, L.M.; Choi, H.; Cha, S.; Kim, J.; Park, C.; Seo, K.; et al. Green fluorescent protein as a scaffold for high efficiency production of functional bacteriotoxic proteins in Escherichia coli. Sci. Rep. 2016, 6, 20661. [Google Scholar] [CrossRef]
- Mattanovich, D.; Branduardi, P.; Dato, L.; Gasser, B.; Sauer, M.; Porro, D. Recombinant protein production in yeasts. Methods Mol. Biol. 2012, 824, 329–358. [Google Scholar]
- Vogl, T.; Gebbie, L.; Palfreyman, R.W.; Speight, R. Effect of plasmid design and type of integration event on recombinant protein expression in Pichia pastoris. Appl. Environ. Microbiol. 2018, 84, e02712-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, S.; Shen, M.; Chen, F.; Zou, Z.; Ran, X.; Cheng, T.; Su, Y.; Wang, J. High level expression and purification of bioactive human alpha-defensin 5 mature peptide in Pichia pastoris. Appl. Microbiol. Biotechnol. 2009, 84, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wang, Q.; Zhou, Y.; Yin, L.; Zhang, G.; Ma, Y. High-level expression of a ZEN-detoxifying gene by codon ptimization and biobrick in Pichia pastoris. Microbiol. Res. 2016, 193, 48–56. [Google Scholar] [CrossRef]
- Strasser, R.; Altmann, F.; Mach, L.; Glössl, J.; Steinkellner, S. Generation of Arabidopsis thaliana plants with complex N-glycans lacking beta 1,2-linked xylose and core alpha 1,3-linked fucose. FEBS Lett. 2004, 561, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Huether, C.M.; Lienhart, O.; Baur, A.; Stemmer, C.; Gorr, G.; Reski, R.; Decker, E.L. Glyco-engineering of moss lacking plant-specific sugar residues. Plant Biol. 2005, 7, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.; Li, H.; Hu, Z. Expression of tandem repeat cecropin B in chlamydomonas reinhardtii and its antibacterial effect. Prog. Biochem. Biophys. 2012, 39, 344–351. [Google Scholar] [CrossRef]
- Morizane, S.; Yamasaki, K.; Mühleisen, B.; Kotol, P.F.; Murakami, M.; Iwatsuki, Y.; Hata, T.; Gallo, R.L. Cathelicidin antimicrobial peptides LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J. Investig. Dermatol. 2011, 132, 135–143. [Google Scholar] [CrossRef]
- Yang, D.; Biragyn, A.; Hoover, D.M.; Lubkowski, J.; Oppenheim, J.J. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 2007, 22, 181–215. [Google Scholar] [CrossRef]
- Giuliani, A.; Rinaldi, A.C. Beyond natural antimicrobial peptides: Multimeric peptides and other peptidomimetic approaches. Cell. Mol. Life Sci. 2011, 68, 2255–2266. [Google Scholar] [CrossRef]
- Molhoek, E.M.; van Dijk, A.; Veldhuizen, E.J.A.; Haagsman, H.P.; Bikker, F.J. Improved proteolytic stability of chicken cathelicidin-2 derived peptides by D-amino acid substitutions and cyclization. Peptides 2011, 32, 875–880. [Google Scholar] [CrossRef] [PubMed]

| Property | Eukaryotic AMPs | Prokaryotic AMPs | Antibiotics |
|---|---|---|---|
| Biological synthesis | Ribosome | Ribosome, modification | Non-ribosome |
| Spectrum | Selective | Narrow | Broad |
| Host cell immunity | No immunity | No immunity | No immunity |
| Targets | Multiple | Multiple | One or a class |
| Resistance | Not easy | Difficult | Easy |
| Stability | Unstable | Moderately stable | Stable |
| Bioactivity | High | Low | Very high |
| Production cost | Very high | High | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Z.; Yang, P.; Lei, J.; Zhao, J. Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity. Antibiotics 2023, 12, 1037. https://doi.org/10.3390/antibiotics12061037
Lyu Z, Yang P, Lei J, Zhao J. Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity. Antibiotics. 2023; 12(6):1037. https://doi.org/10.3390/antibiotics12061037
Chicago/Turabian StyleLyu, Zhiqian, Pan Yang, Jian Lei, and Jinbiao Zhao. 2023. "Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity" Antibiotics 12, no. 6: 1037. https://doi.org/10.3390/antibiotics12061037
APA StyleLyu, Z., Yang, P., Lei, J., & Zhao, J. (2023). Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity. Antibiotics, 12(6), 1037. https://doi.org/10.3390/antibiotics12061037






