Reduced Prognostic Role of Serum PCT Measurement in Very Frail Older Adults Admitted to the Emergency Department
Abstract
1. Introduction
2. Results
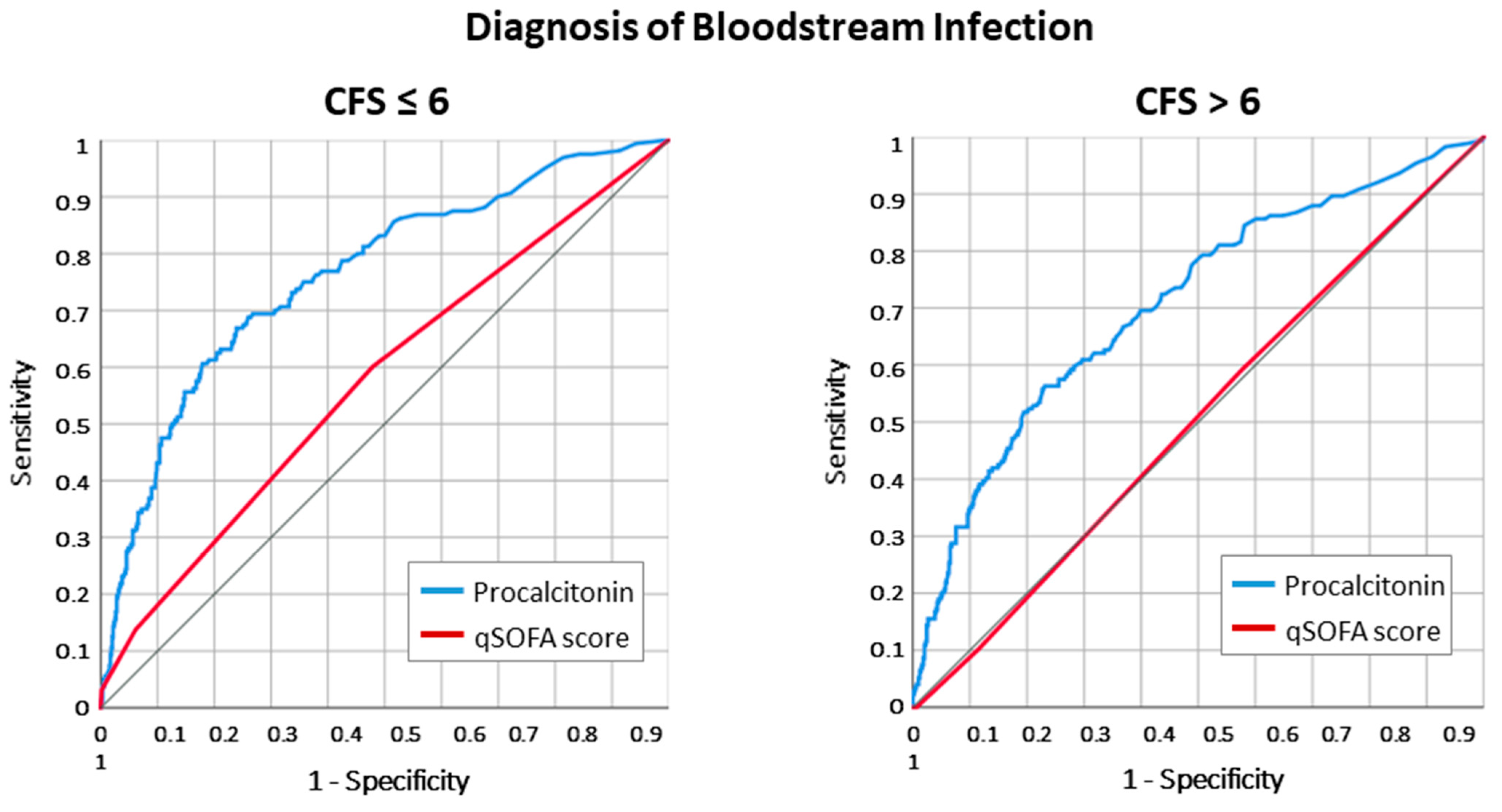
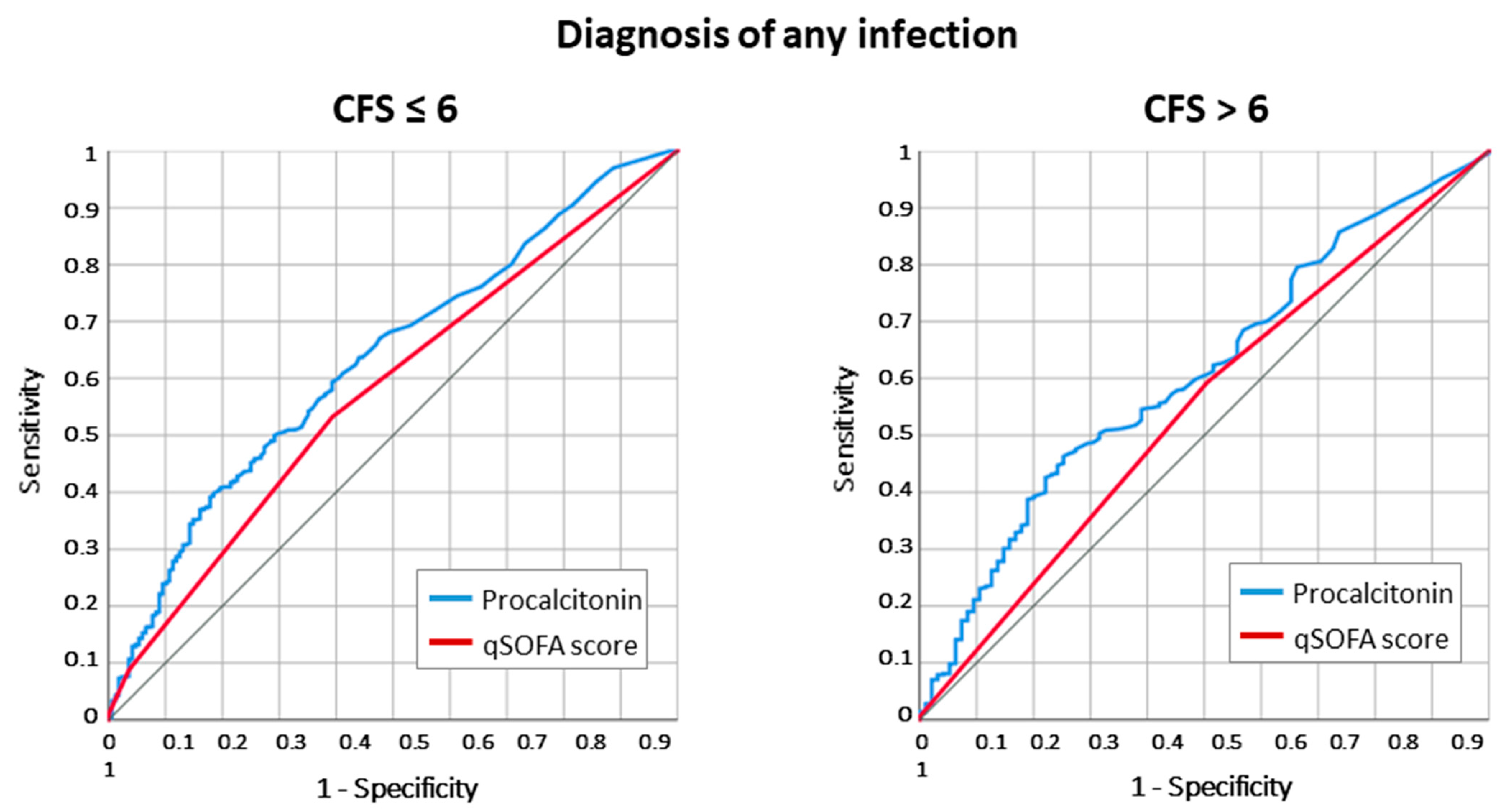
2.1. Infection Diagnosis According to Frailty Status
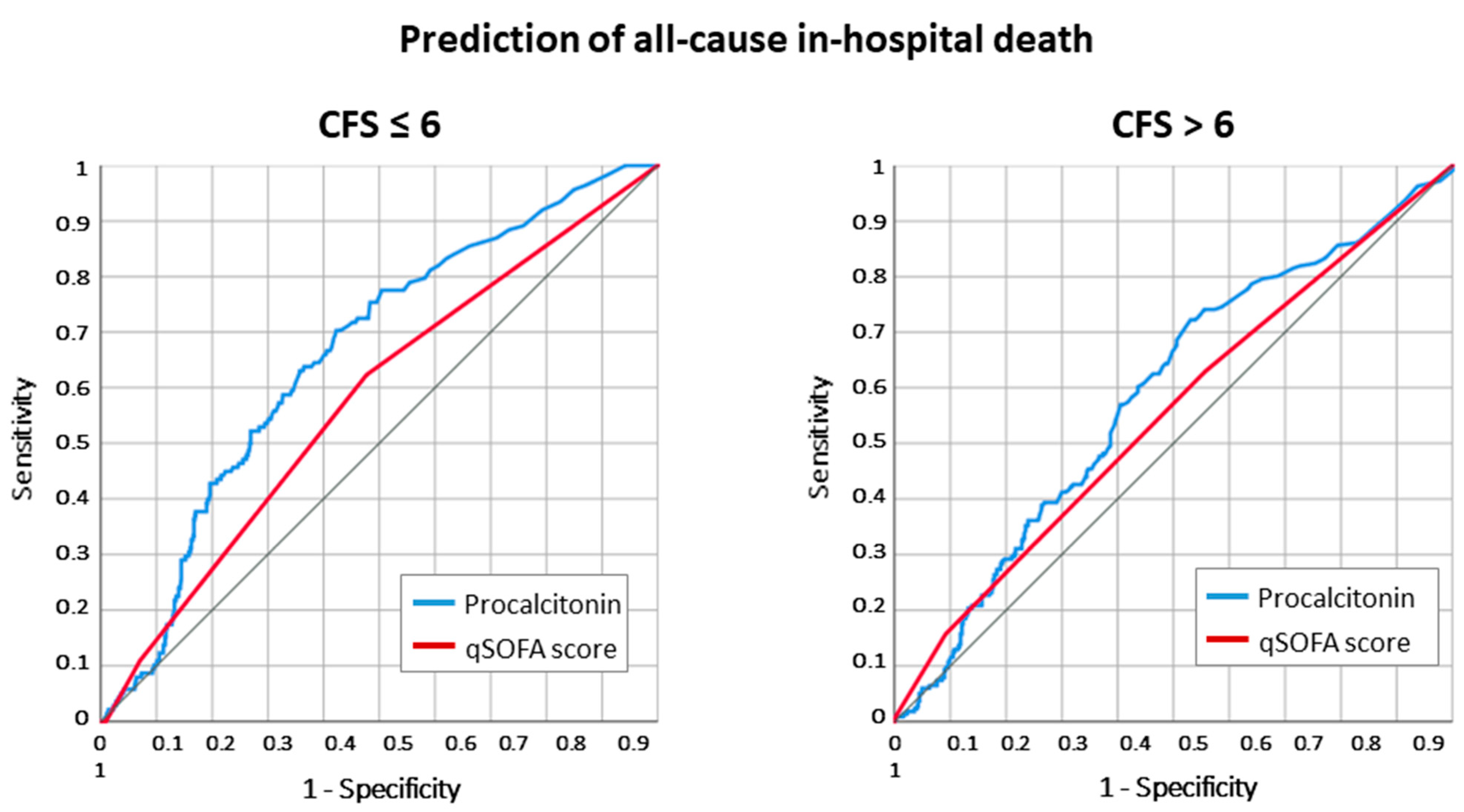
2.2. All-Cause Death According to Frailty Status
2.3. Adjusted Odds for Death and Infection Diagnosis According to Frailty
3. Materials and Methods
3.1. Study Design
3.2. Study Variables
3.3. Outcome Measures
3.4. Statistical Analysis
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pallin, D.J.; Allen, M.B.; Espinola, J.A.; Camargo, C.A.; Bohan, J.S. Population aging and emergency departments: Visits will not increase, lengths-of-stay and hospitalizations will. Health Aff. 2013, 32, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Hoogendijk, E.O.; Cardona-Morrell, M.; Hillman, K. Frailty in emergency departments. Lancet 2016, 387, 434. [Google Scholar] [CrossRef] [PubMed]
- Covino, M.; Petruzziello, C.; Onder, G.; Migneco, A.; Simeoni, B.; Franceschi, F.; Ojetti, V. A 12-year retrospective analysis of differences between elderly and oldest old patients referred to the emergency department of a large tertiary hospital. Maturitas 2019, 120, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Font, M.D.; Thyagarajan, B.; Khanna, A.K. Sepsis and Septic Shock—Basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. N. Am. 2020, 104, 573–585. [Google Scholar] [CrossRef]
- Usman, O.A.; Usman, A.A.; Ward, M.A. Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the Emergency Department. Am. J. Emerg. Med. 2019, 37, 1490–1497. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Zaccone, V.; Falsetti, L.; Nitti, C.; Gentili, T.; Marchetti, A.; Piersantelli, M.N.; Sampaolesi, M.; Riccomi, F.; Raponi, A.; Salvi, A. The Prognostic Role of Procalcitonin in Critically Ill Patients Admitted in a Medical Stepdown Unit: A Retrospective Cohort Study. Sci. Rep. 2020, 10, 4531. [Google Scholar] [CrossRef]
- Covino, M.; Manno, A.; De Matteis, G.; Taddei, E.; Carbone, L.; Piccioni, A.; Simeoni, B.; Fantoni, M.; Franceschi, F.; Murri, R. Prognostic Role of Serum Procalcitonin Measurement in Adult Patients Admitted to the Emergency Department with Fever. Antibiotics 2021, 10, 788. [Google Scholar] [CrossRef]
- Yunus, I.; Fasih, A.; Wang, Y. The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics. PLoS ONE 2018, 13, e0206527. [Google Scholar] [CrossRef]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Tuna Doğrul, R.; Doğan Varan, H.; Kızılarslanoğlu, M.C.; Kılıç, M.K.; Arık, G.; Kara, Ö.; Halil, M.; Cankurtaran, M.; Yavuz, B.B. Relationship Between Frailty and Inflammation. Eur. J. Geriatr. Gerontol. 2019, 1, 17–23. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, Q.; Flaherty, J.H.; Cao, L.; Zhou, J.; Su, L.; Shen, Y.; Dong, B. Comparison of procalcitonin, a potentially new inflammatory biomarker of frailty, to interleukin-6 and C-reactive protein among older Chinese hospitalized patients. Aging Clin. Exp. Res. 2018, 30, 1459–1464. [Google Scholar] [CrossRef]
- Gómez-Rubio, P.; Trapero, I.; Cauli, O.; Buigues, C. Salivary IL-6 Concentration Is Associated with Frailty Syndrome in Older Individuals. Diagnostics 2022, 12, 117. [Google Scholar] [CrossRef]
- Liang, S.Y. Sepsis and Other Infectious Disease Emergencies in the Elderly. Emerg. Med. Clin. N. Am. 2016, 34, 501–522. [Google Scholar] [CrossRef]
- Hofman, M.R.; van den Hanenberg, F.; Sierevelt, I.N.; Tulner, C.R. Elderly patients with an atypical presentation of illness in the emergency department. Neth. J. Med. 2017, 75, 241–246. [Google Scholar] [PubMed]
- Jarrett, P.G.; Rockwood, K.; Carver, D.; Stolee, P.; Cosway, S. Illness presentation in elderly patients. Arch. Intern. Med. 1995, 155, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and Comprehensive Geriatric Assessment. J. Korean Med. Sci. 2020, 35, e16. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Salini, S.; Giovannini, S.; Covino, M.; Barillaro, C.; Acampora, N.; Gravina, E.M.; Loreti, C.; Damiano, F.P.; Franceschi, F.; Russo, A. Frailty Network in an Acute Care Setting: The New Perspective for Frail Older People. Diagnostics 2022, 12, 1228. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Hogan, D.B.; Fox, R.A. The atypical presentation of infection in old age. Age Ageing 1987, 16, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.C. Fever in the elderly. Clin. Infect. Dis. 2000, 31, 148–151. [Google Scholar] [CrossRef]
- Bellmann-Weiler, R.; Weiss, G. Pitfalls in the diagnosis and therapy of infections in elderly patients—A mini-review. Gerontology 2009, 55, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Limpawattana, P.; Phungoen, P.; Mitsungnern, T.; Laosuangkoon, W.; Tansangworn, N. Atypical presentations of older adults at the emergency department and associated factors. Arch. Gerontol. Geriatr. 2016, 62, 97–102. [Google Scholar] [CrossRef]
- Norman, D.C. Clinical Features of Infection in Older Adults. Clin. Geriatr. Med. 2016, 32, 433–441. [Google Scholar] [CrossRef]
- Vrettos, I.; Voukelatou, P.; Panayiotou, S.; Kyvetos, A.; Tsigkri, A.; Makrilakis, K.; Sfikakis, P.P.; Niakas, D. Factors Associated with Mortality in Elderly Hospitalized Patients at Admission. Cureus 2022, 14, e22709. [Google Scholar] [CrossRef]
- Boonmee, P.; Ruangsomboon, O.; Limsuwat, C.; Chakorn, T. Predictors of Mortality in Elderly and Very Elderly Emergency Patients with Sepsis: A Retrospective Study. West J. Emerg. Med. 2020, 21, 210–218. [Google Scholar] [CrossRef]
- Rosa, F.; Covino, M.; Russo, A.; Salini, S.; Forino, R.; Della Polla, D.; Fransvea, P.; Quero, G.; Fiorillo, C.; La Greca, A.; et al. Frailty assessment as independent prognostic factor for patients ≥65 years undergoing urgent cholecystectomy for acute cholecystitis. Dig. Liver Dis. 2023, 55, 505–512. [Google Scholar] [CrossRef]
- Covino, M.; Salini, S.; Russo, A.; De Matteis, G.; Simeoni, B.; Maccauro, G.; Sganga, G.; Landi, F.; Gasbarrini, A.; Franceschi, F. Frailty Assessment in the Emergency Department for Patients ≥80 Years Undergoing Urgent Major Surgical Procedures. J. Am. Med. Dir. Assoc. 2022, 23, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Covino, M.; Russo, A.; Salini, S.; De Matteis, G.; Simeoni, B.; Della Polla, D.; Sandroni, C.; Landi, F.; Gasbarrini, A.; Franceschi, F. Frailty Assessment in the Emergency Department for Risk Stratification of COVID-19 Patients Aged ≥80 Years. J. Am. Med. Dir. Assoc. 2021, 22, 1845–1852.e1. [Google Scholar] [CrossRef] [PubMed]
- Hoeboer, S.H.; van der Geest, P.J.; Nieboer, D.; Groeneveld, A.B.J. The diagnostic accuracy of procalcitonin for bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2015, 21, 474–481. [Google Scholar] [CrossRef]
- Chirouze, C.; Schuhmacher, H.; Rabaud, C.; Rabaud, C.; Gil, H.; Khayat, N.; Estavoyer, J.-M.; May, T.; Hoen, B. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin. Infect. Dis. 2002, 35, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.E.; Fiechtl, J.F.; Brown, M.D.; Ballew, J.J.; Kline, J.A. Procalcitonin test in the diagnosis of bacteremia: A meta-analysis. Ann. Emerg. Med. 2007, 50, 34–41. [Google Scholar] [CrossRef]
- Tang, B.M.P.; Eslick, G.D.; Craig, J.C.; McLean, A.S. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: Systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 210–217. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Hao, J.; Zhang, F.; Liu, G. Value of serum procalcitonin and acute physiology and chronic health evaluation II score on predicting the prognosis of sepsis in elderly patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2023, 35, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Heppner, H.J.; Bertsch, T.; Alber, B.; Esslinger, A.S.; Dragonas, C.; Bauer, J.M.; Sieber, C.C. Procalcitonin: Inflammatory biomarker for assessing the severity of community-acquired pneumonia—A clinical observation in geriatric patients. Gerontology 2010, 56, 385–389. [Google Scholar] [CrossRef]
| All Patients n 1459 | Fit or Moderately Frail (CFS ≤ 6) n 796 | Frail (CFS > 6) n 663 | p Value | |
|---|---|---|---|---|
| Age | 85 (82–89) | 85 (82–89) | 86 (82–90) | 0.014 |
| Sex (Male) | 718 (49.2) | 428 (53.8) | 290 (43.7) | <0.001 |
| ED Presentation | ||||
| Triage | ||||
| -Emergency | 269 (18.4) | 121 (15.2) | 148 (22.3) | |
| -Urgency | 842 (57.7) | 470 (59.0) | 372 (56.1) | 0.001 |
| -Minor urgency | 348 (23.9) | 205 (25.8) | 143 (21.6) | |
| qSOFA ≥ 2 | 136 (9.3%) | 61 (7.7%) | 75 (11.3%) | 0.017 |
| Dyspnea | 540 (37.0) | 285 (35.8) | 255 (38.5) | 0.295 |
| Fever | 706 (48.4) | 413 (51.9) | 293 (44.2) | 0.003 |
| Chest pain | 62 (4.2) | 40 (5.0) | 22 (3.3) | 0.108 |
| Vomit | 136 (9.3) | 90 (11.3) | 46 (6.9) | 0.004 |
| Abdominal pain | 151 (10.3) | 107 (13.4) | 44 (6.6) | <0.001 |
| Confusion | 138 (9.5) | 81 (10.2) | 57 (8.6) | 0.305 |
| Malaise/fatigue | 199 (13.6) | 136 (17.1) | 63 (9.5) | <0.001 |
| Laboratory values | ||||
| Procalcitonin (ng/mL) | 0.34 [0.13–1.61] | 0.29 [0.12–1.53] | 0.40 [0.15–1.92] | 0.005 |
| Procalcitonin > 0.5 ng/mL | 624 (42.8%) | 330 (41.5%) | 294 (44.3%) | 0.267 |
| Hemoglobin (g/dl) | 11.5 [10.1–12.7] | 11.6 [10.2–12.8] | 11.4 [9.9–12.6] | 0.349 |
| WBC (×109/L) | 11.3 [8.5–17.3] | 11.2 [8.4–16.9] | 11.3 [8.7–17.4] | 0.725 |
| Platelets (×109/L) | 223 [169–299] | 211 [157–284] | 239 [184–346] | 0.017 |
| Fibrinogen (mg/dL) | 558 [432–765] | 589 [404–808] | 546 [437–729] | 0.876 |
| Creatinine (mg/dL) | 1.32 [0.86–1.86] | 1.32 [0.91–1.71] | 1.28 [0.84–1.91] | 0.634 |
| Glucose (mg/dL) | 129 [108–168] | 122 [106–168] | 133 [112–178] | 0.054 |
| CRP (mg/L) | 121 [39–214] | 112 [37–232] | 123 [39–182] | 0.527 |
| Comorbidities | ||||
| Charlson Comorbidity Index | 7 (6–9) | 7 (6–9) | 7 (6–9) | 0.005 |
| Hypertension | 598 (41.0) | 350 (44.0) | 248 (37.4) | 0.011 |
| Ischemic heart disease | 330 (22.6) | 184 (23.1) | 146 (22.0) | 0.619 |
| Congestive heart failure | 570 (39.1) | 310 (38.9) | 260 (39.2) | 0.916 |
| Peripheral vascular disease | 531 (36.4) | 265 (33.3) | 266 (40.1) | 0.007 |
| Cerebrovascular disease | 197 (13.5) | 84 (10.6) | 113 (17.0) | <0.001 |
| Dementia | 249 (17.1) | 73 (9.2) | 176 (26.5) | <0.001 |
| COPD | 364 (24.9) | 209 (26.3) | 155 (23.4) | 0.206 |
| Diabetes | 362 (24.8) | 181 (22.7) | 181 (27.3) | 0.045 |
| Liver chronic disease | 34 (2.3) | 20 (2.5) | 14 (2.1) | 0.613 |
| Rheumatologic disease | 28 (1.9) | 19 (2.4) | 9 (1.4) | 0.154 |
| Chronic kidney disease | 538 (36.9) | 291 (36.6) | 247 (37.3) | 0.783 |
| Malignancy | 295 (20.2) | 170 (21.4) | 125 (18.9) | 0.236 |
| Site of infection | ||||
| Any infection | 1196 (82.0%) | 628 (78.9%) | 568 (85.7%) | 0.001 |
| Sepsis | 334 (22.9) | 160 (20.1) | 174 (26.2) | 0.005 |
| Pneumonia | 638 (43.7) | 328 (41.2) | 310 (46.8) | 0.033 |
| UTI | 297 (20.4) | 153 (19.2) | 144 (21.7) | 0.238 |
| Abdominal infection | 186 (12.7) | 117 (14.7) | 69 (10.4) | 0.014 |
| Others | 79 (5.4) | 46 (5.8) | 33 (5.0) | 0.501 |
| Outcomes | ||||
| Length of stay | 11.3 [7.2–17.7] | 10.7 [7.3–17.2] | 11.5 [7.2–18.5] | 0.321 |
| In-hospital death | 354 (24.3%) | 138 (17.3%) | 216 (32.6%) | <0.001 |
| Fit or Moderately Frail (CFS ≤ 6) | Frail (CFS > 6) | |||||
|---|---|---|---|---|---|---|
| Infection (Any) n 628 | Non-Infected n 168 | p | Infection (Any) n 568 | Non-Infected n 95 | p | |
| Age | 85 [83–89] | 85 [82–89] | 0.249 | 86 [83–90] | 85 [82–89] | 0.159 |
| Sex (Male) | 341 (54.3) | 87 (51.8) | 0.562 | 253 (44.5) | 37 (38.9) | 0.309 |
| CFS | 5 [4–6] | 5 [4–6] | 0.526 | 8 [7,8] | 7 [7–8] | 0.011 |
| ED Presentation | ||||||
| Triage | ||||||
| -Emergency | 98 (15.6%) | 23 (13.7) | 130 (22.9%) | 18 (18.9) | ||
| -Urgency | 368 (58.6) | 102 (60.7) | 0.810 | 315 (55.5) | 57 (60.0) | 0.644 |
| -Minor urgency | 162 (25.8) | 43 (25.6) | 123 (21.7) | 20 (21.1) | ||
| QSOFA ≥ 2 | 55 (8.8%) | 6 (3.6) | 0.025 | 66 (11.6) | 9 (9.5) | 0.541 |
| Dyspnea | 234 (37.3) | 51 (30.4) | 0.097 | 235 (41.4) | 20 (21.1) | <0.001 |
| Fever > 38 °C in ED | 351 (55.9) | 62 (36.9) | <0.001 | 268 (47.2) | 25 (26.3) | <0.001 |
| Chest pain | 28 (4.5) | 12 (7.1) | 0.157 | 19 (3.3) | 3 (3.2) | 0.925 |
| Vomiting | 66 (10.5) | 24 (14.3) | 0.170 | 38 (6.7) | 8 (8.4) | 0.539 |
| Abdominal pain | 89 (14.2) | 18 (10.7) | 0.243 | 36 (6.3) | 8 (8.4) | 0.450 |
| Diarrhea | 48 (7.6) | 9 (5.4) | 0.307 | 39 (6.9) | 8 (8.4) | 0.585 |
| Neurological sympt. | 62 (9.9) | 19 (11.3) | 0.584 | 51 (9.0) | 6 (6.3) | 0.391 |
| Malaise/fatigue | 100 (15.9) | 36 (21.4) | 0.092 | 54 (9.5) | 9 (9.5) | 0.992 |
| Laboratory values | ||||||
| PCT (ng/mL) | 0.39 [0.14–2.48] | 0.18 [0.10–0.56] | <0.001 | 0.46 [0.16–2.45] | 0.28 [0.10–0.58] | 0.001 |
| PCT > 0.5 ng/mL | 287 (45.6) | 43 (25.6) | <0.001 | 268 (47.2) | 26 (27.4) | <0.001 |
| Hemoglobin (g/dL) | 11.8 [10.2–13.1] | 11.1 [10.2–12.2] | 0.196 | 11.2 [9.8–12.6] | 11.8 [10.4–12.6] | 0.559 |
| WBC (×109/L) | 11.3 [8.5–17.3] | 11.2 [9.0–13.9] | 0.888 | 12.3 [8.8–18.5] | 11.3 [8.6–17.4] | 0.812 |
| Platelets (×109/L) | 223 [169–299] | 225 [155–292] | 0.417 | 223 [174–351] | 250 [225–343] | 0.202 |
| Fibrinogen (mg/dL) | 591 [423–809] | 588 [374–528] | 0.296 | 548 [437–750] | 533 [444–682] | 0.852 |
| Creatinine (mg/dL) | 1.30 [0.88–1.68] | 1.49 [1.21–4.37] | 0.010 | 1.25 [0.83–1.90] | 1.50 [0.90–2.29] | 0.434 |
| Glucose (mg/dL) | 121 [105–166] | 128 [106–188] | 0.482 | 132 [115–180] | 135 [105–188] | 0.849 |
| CRP (mg/L) | 146 [56–234] | 38 [18–155] | 0.019 | 131 [41–86] | 43 [12–177] | 0.154 |
| Comorbidities | ||||||
| Charlson Index | 7 [6–9] | 7 [6–9] | 0.258 | 7 [6–9] | 8 [6–9] | 0.101 |
| Hypertension | 276 (43.9) | 74 (44.0) | 0.982 | 210 (37.0) | 38 (40.0) | 0.572 |
| IHD | 146 (23.2) | 38 (22.6) | 0.864 | 126 (22.2) | 20 (21.1) | 0.806 |
| CHF | 244 (38.9) | 66 (39.3) | 0.919 | 218 (38.4) | 42 (44.2) | 0.281 |
| PVD | 208 (33.1) | 57 (33.9) | 0.844 | 222 (39.1) | 44 (46.3) | 0.183 |
| Previous stroke | 63 (10.0) | 21 (12.5) | 0.355 | 93 (16.4) | 20 (21.1) | 0.262 |
| Dementia | 59 (9.4) | 14 (8.3) | 0.672 | 144 (25.4) | 32 (33.7) | 0.089 |
| COPD | 180 (28.7) | 27 (17.3) | 0.003 | 144 (25.4) | 11 (11.6) | 0.003 |
| Diabetes | 145 (23.1) | 36 (21.4) | 0.648 | 152 (26.8) | 29 (30.5) | 0.446 |
| Chronic liver disease | 14 (2.2) | 6 (3.6) | 0.324 | 11 (1.9) | 3 (3.2) | 0.443 |
| Rheumatologic | 11 (1.8) | 8 (4.8) | 0.023 | 9 (1.6) | 0 (0.0) | 0.217 |
| CKD | 234 (37.3) | 57 (33.9) | 0.426 | 213 (37.5) | 34 (35.8) | 0.450 |
| Malignancy | 127 (20.2) | 43 (25.6) | 0.131 | 100 (17.6) | 25 (26.3) | 0.045 |
| Outcomes | ||||||
| Length of stay | 11.3 [7.4–17.4] | 9.5 [6.3–15.6] | 0.012 | 11.6 [7.2–18.8] | 11.4 [5.6–17.1] | 0.298 |
| Death | 108 (17.2%) | 30 (17.9%) | 0.841 | 187 (32.9%) | 29 (30.5%) | 0.645 |
| Fit or Moderately Frail (CFS ≤ 6) | Frail (CFS ≥ 6) | |||||
|---|---|---|---|---|---|---|
| Deceased n 138 | Alive n 658 | p Value | Deceased n 216 | Alive n 447 | p Value | |
| Variable | ||||||
| Age | 86 [83–90] | 85 [82–89] | 0.108 | 86 [83–91] | 86 [82–90] | 0.250 |
| Sex (Male) | 71 (51.4) | 357 (54.3) | 0.548 | 102 (47.2) | 188 (42.1) | 0.209 |
| Clinical Frailty Scale | 5 (5–6) | 5 (4–6) | 0.054 | 8 (7–8) | 7 (7–8) | 0.055 |
| ED Presentation | ||||||
| Triage | ||||||
| -Emergency | 33 (23.9%) | 88 (13.4) | 74 (34.3%) | 74 (16.6) | ||
| -Urgency | 77 (55.8) | 393 (59.7) | 0.005 | 108 (50.0) | 264 (59.1) | <0.001 |
| -Minor urgency | 28 (20.3) | 177 (26.9) | 34 (15.7) | 109 (24.4) | ||
| QSOFA ≥ 2 | 15 (10.9%) | 46 (7.0%) | 0.119 | 34 (15.7%) | 41 (9.2%) | 0.012 |
| Dyspnea | 62 (44.9) | 223 (33.9) | 0.014 | 86 (39.8) | 169 (37.8) | 0.619 |
| Fever | 57 (41.3) | 356 (54.1) | 0.006 | 81 (37.5) | 212 (47.4) | 0.016 |
| Chest pain | 6 (4.3) | 34 (5.2) | 0.689 | 4 (1.9) | 18 (4.0) | 0.143 |
| Vomiting | 16 (11.6) | 74 (11.2) | 0.907 | 14 (6.5) | 32 (7.2) | 0.748 |
| Abdominal pain | 12 (8.7) | 95 (14.4) | 0.072 | 9 (4.2) | 35 (7.8) | 0.076 |
| Diarrhea | 15 (10.9) | 42 (6.4) | 0.063 | 10 (4.6) | 37 (8.3) | 0.086 |
| Neurological symptoms | 14 (10.1) | 67 (10.2) | 0.989 | 13 (6.0) | 44 (9.8) | 0.100 |
| Malaise/fatigue | 24 (17.4) | 112 (17.0) | 0.916 | 21 (9.7) | 42 (9.4) | 0.893 |
| Laboratory values | ||||||
| Procalcitonin (ng/mL) | 1.11 [0.27–4.29] | 0.26 [0.12–1.38] | <0.001 | 0.57 [0.22–3.34] | 0.31 [0.14–0.26] | <0.001 |
| Procalcitonin > 0.5 ng/mL | 88 (63.8%) | 242 (36.8%) | <0.001 | 117 (54.2%) | 177 (39.6%) | <0.001 |
| Hemoglobin (g/dL) | 11.5 [10.2– 12.7] | 12.3 [10.2–13.4] | 0.170 | 10.6 [8.3–11.6] | 11.8 [10.2–12.7] | 0.002 |
| WBC (×109/L) | 14.2 [7.7–18.3] | 11.0 [8.4–16.7] | 0.466 | 11.3 [8.4–17.4] | 12.3 [9.2–17.5] | 0.459 |
| Platelets (×109/L) | 225 [179–291] | 208 [155–284] | 0.279 | 220 [169–368] | 248 [189–343] | 0.574 |
| Fibrinogen (mg/dL) | 600 [398–770] | 588 [404–810] | 0.900 | 551 [453–731] | 533 [415–722] | 0.482 |
| Creatinine (mg/dL) | 1.38 [0.8–3.24] | 1.32 [0.92–1.69] | 0.544 | 1.45 [1.0–1.94] | 1.16 [0.78–1.89] | 0.208 |
| Glucose (mg/dL) | 120 [106–148] | 122 [106–170] | 0.676 | 135 [102–173] | 133 [115–182] | 0.628 |
| CRP (mg/L) | 226 [53–258] | 107 [33–218] | 0.107 | 131 [36–188] | 121 [39–184] | 0.736 |
| Clinical History | ||||||
| Charlson Comorbidity Index | 7 (6–9) | 7 (6–8) | 0.551 | 7 (6–9) | 8 (7–8) | 0.023 |
| Hypertension | 51 (37.0) | 299 (45.4) | 0.068 | 68 (31.5) | 180 (40.3) | 0.028 |
| IHD | 38 (27.5) | 146 (22.2) | 0.175 | 54 (25.0) | 92 (20.6) | 0.198 |
| CHF | 63 (45.7) | 247 (37.5) | 0.076 | 103 (47.7) | 157 (35.1) | 0.002 |
| PVD | 52 (37.7) | 213 (32.4) | 0.229 | 86 (39.8) | 180 (40.3) | 0.911 |
| Previous stroke | 18 (13.0) | 66 (10.0) | 0.295 | 31 (14.4) | 82 (18.3) | 0.200 |
| Dementia | 16 (11.6) | 57 (8.7) | 0.278 | 42 (19.4) | 134 (30.0) | 0.004 |
| COPD | 30 (21.7) | 179 (27.2) | 0.185 | 48 (22.2) | 107 (23.9) | 0.625 |
| Diabetes | 32 (23.2) | 149 (22.6) | 0.890 | 61 (28.2) | 120 (26.8) | 0.706 |
| Liver chronic disease | 4 (2.9) | 16 (2.4) | 0.750 | 1 (0.5) | 13 (2.9) | 0.040 |
| Rheumatologic | 3 (2.2) | 16 (2.4) | 0.857 | 0 (0.0) | 9 (2.0) | 0.036 |
| Chronic kidney disease | 56 (40.6) | 235 (35.7) | 0.281 | 76 (35.2) | 171 (38.3) | 0.444 |
| Malignancy | 36 (26.1) | 134 (20.4) | 0.136 | 39 (18.1) | 86 (19.2) | 0.715 |
| Outcomes—Site of Infection | ||||||
| Sepsis | 37 (26.8) | 123 (18.7) | 0.030 | 90 (41.7) | 84 (18.8) | <0.001 |
| Pneumonia | 72 (52.2) | 256 (38.9) | 0.004 | 101 (46.8) | 209 (46.8) | 0.999 |
| UTI | 9 (6.5) | 144 (21.9) | <0.001 | 26 (12.0) | 118 (26.4) | <0.001 |
| Abdominal infection | 8 (5.8) | 109 (16.6) | 0.001 | 14 (6.5) | 55 (12.3) | 0.021 |
| Others | 5 (3.6) | 41 (6.2) | 0.233 | 14 (6.5) | 19 (4.3) | 0.216 |
| Length of stay (days) | 10.3 [4.36–18.00] | 11.01 [7.42–7.19] | 0.043 | 8.74 [3.65–6.29] | 12.6 [8.30–19.59] | <0.001 |
| Prediction of Bloodstream Infection—Non-Frail or Moderately Frail Patients (CFS ≤ 6) | |||
| Variable | Wald Statistic | Odds Ratio [95% CI] | p Value |
| PCT values | 44.413 | 1.06 [1.04–1.08] | <0.001 |
| Age | 1.616 | 0.97 [0.93–1.01] | 0.204 |
| qSOFA ≥ 2 | 10.038 | 2.52 [1.42–4.46] | 0.002 |
| Charlson index | 0.0966 | 1.01 [0.93–1.09] | 0.756 |
| Prediction of bloodstream infection—Very Frail patients (CFS > 6) | |||
| PCT values | 31.694 | 1.05 [1.03–1.07] | <0.001 |
| Age | 4.482 | 0.96 [0.92–0.99] | 0.034 |
| qSOFA ≥ 2 | 1.311 | 0.70 [0.38–1.29] | 0.252 |
| Charlson index | 6.130 | 0.91 [0.84–0.98] | 0.013 |
| Prediction of any infection—Non-frail or moderately frail patients (CFS ≤ 6) | |||
| PCT values | 6.798 | 1.04 [1.01–1.08] | 0.009 |
| Age | 1.719 | 1.03 [0.99–1.07] | 0.190 |
| qSOFA ≥ 2 | 4.653 | 2.59 [1.09–6.14] | 0.031 |
| Charlson index | 0.327 | 0.98 [0.91–1.05] | 0.568 |
| Prediction of any infection—Very Frail patients (CFS > 6) | |||
| PCT values | 2.687 | 1.02 [0.99–1.06] | 0.130 |
| Age | 2.293 | 1.04 [0.99–1.09] | 0.734 |
| qSOFA ≥ 2 | 0.658 | 1.14 [0.54–2.38] | 0.734 |
| Charlson index | 0.327 | 0.97 [0.88–1.05] | 0.417 |
| Prediction of all-cause death—Non-frail or moderately frail patients (CFS ≤ 6) | |||
| Procalcitonin value | 2.599 | 1.01 [1.00–1.02] | 0.047 |
| Age | 0.895 | 1.03 [0.99–1.07] | 0.118 |
| Urgency | 10.332 | 2.28 [1.28–4.06] | 0.006 |
| Emergency | 7.766 | 1.09 [0.68–1.77] | 0.005 |
| Non-Urgency | 0.148 | Reference | 0.701 |
| Charlson index | 0.767 | 1.13 [1.04–1.22] | 0.002 |
| Prediction of all-cause death—Very Frail patients (CFS > 6) | |||
| Procalcitonin value | 0.004 | 1.00 [0.98–1.02] | 0.948 |
| Age | 2.446 | 1.03 [0.99–1.07] | 0.118 |
| Emergency | 23.576 | 2.99 [1.79–4.99] | <0.001 |
| Urgency | 17.640 | 1.26 [0.80–1.98] | <0.001 |
| Non-Urgency | 0.999 | Reference | 0.318 |
| Charlson index | 1.003 | 0.97 [0.90–1.03] | 0.317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Salini, S.; Gava, G.; Merra, G.; Piccioni, A.; De Matteis, G.; Tullo, G.; Novelli, A.; Petrucci, M.; Gasbarrini, A.; et al. Reduced Prognostic Role of Serum PCT Measurement in Very Frail Older Adults Admitted to the Emergency Department. Antibiotics 2023, 12, 1036. https://doi.org/10.3390/antibiotics12061036
Russo A, Salini S, Gava G, Merra G, Piccioni A, De Matteis G, Tullo G, Novelli A, Petrucci M, Gasbarrini A, et al. Reduced Prognostic Role of Serum PCT Measurement in Very Frail Older Adults Admitted to the Emergency Department. Antibiotics. 2023; 12(6):1036. https://doi.org/10.3390/antibiotics12061036
Chicago/Turabian StyleRusso, Andrea, Sara Salini, Giordana Gava, Giuseppe Merra, Andrea Piccioni, Giuseppe De Matteis, Gianluca Tullo, Angela Novelli, Martina Petrucci, Antonio Gasbarrini, and et al. 2023. "Reduced Prognostic Role of Serum PCT Measurement in Very Frail Older Adults Admitted to the Emergency Department" Antibiotics 12, no. 6: 1036. https://doi.org/10.3390/antibiotics12061036
APA StyleRusso, A., Salini, S., Gava, G., Merra, G., Piccioni, A., De Matteis, G., Tullo, G., Novelli, A., Petrucci, M., Gasbarrini, A., Landi, F., Franceschi, F., & Covino, M. (2023). Reduced Prognostic Role of Serum PCT Measurement in Very Frail Older Adults Admitted to the Emergency Department. Antibiotics, 12(6), 1036. https://doi.org/10.3390/antibiotics12061036









