The Genetic Diversity and Antimicrobial Resistance of Pyogenic Pathogens Isolated from Porcine Lymph Nodes
Abstract
1. Introduction
2. Results
2.1. Bacteriological Examination
2.2. Phenotypes and Genotypes of Antimicrobial Resistance
2.2.1. Study for S. aureus
2.2.2. Study for Streptococcus spp.
2.2.3. Study for R. equi
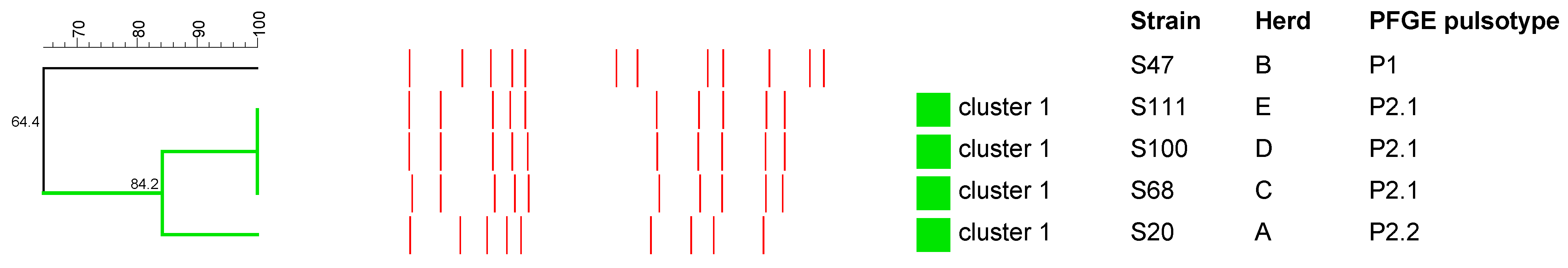
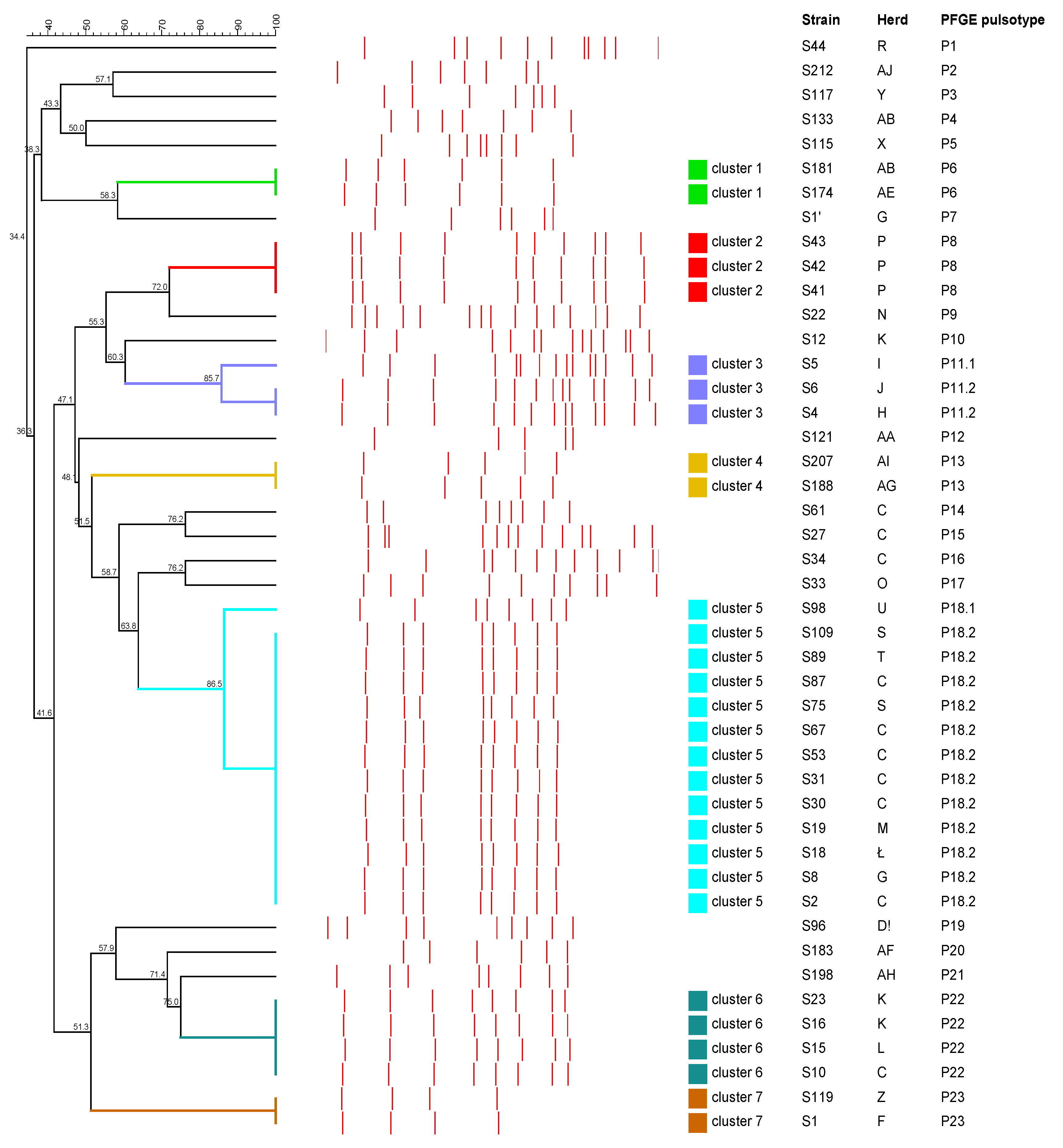
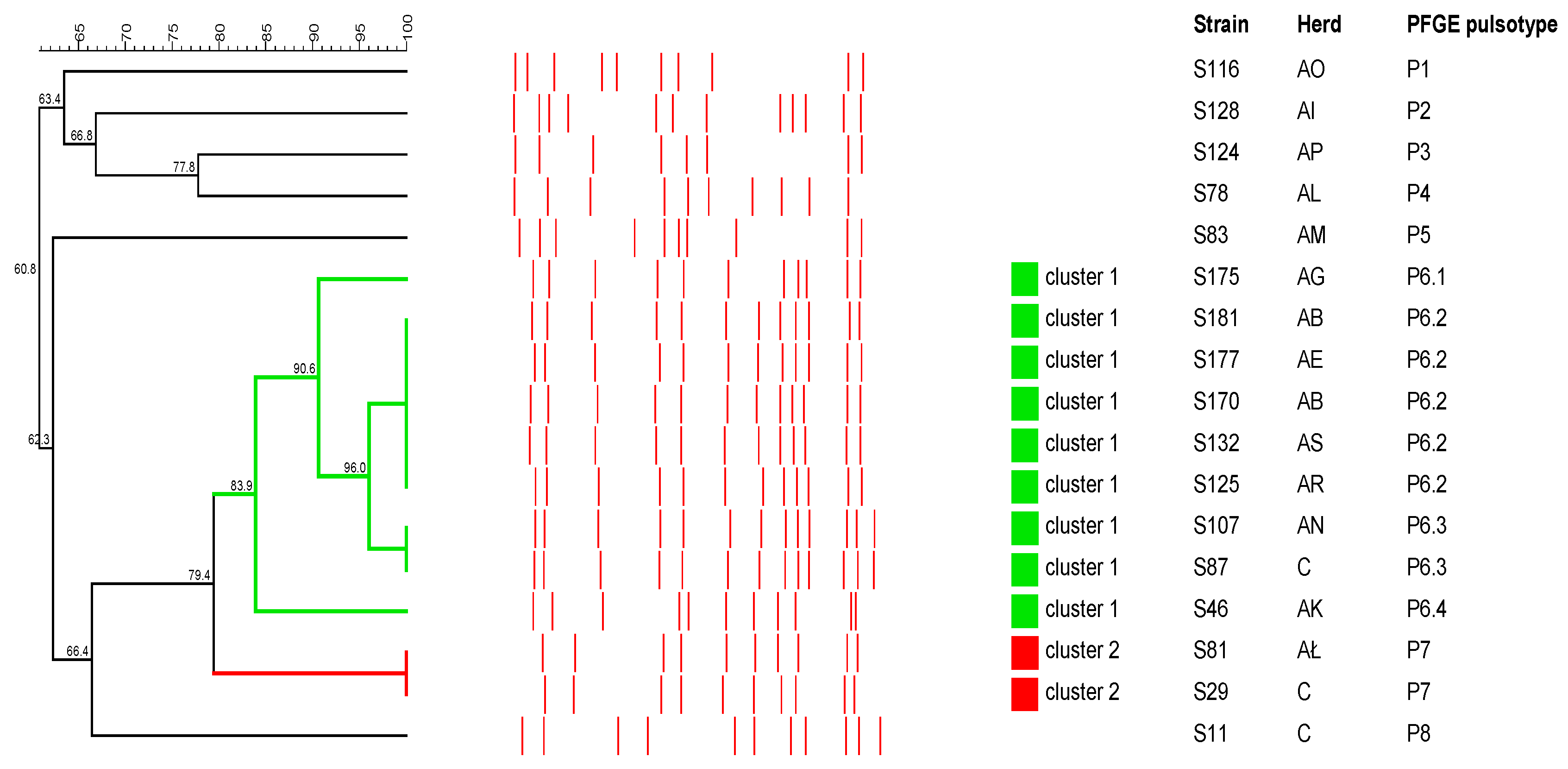
2.3. Pulsed-Field Gel Electrophoresis Analysis
2.3.1. S. aureus
2.3.2. Streptococcus spp.
2.3.3. R. equi
3. Discussion
4. Materials and Methods
4.1. Material Collection
4.2. Bacterial Isolation and Identification
4.3. DNA Extraction
4.4. Antimicrobial Susceptibility Testing
4.5. Detection of Antimicrobial Resistance Genes
4.6. Pulsed-Field Gel Electrophoresis
4.6.1. Analysis of S. aureus Isolates
4.6.2. Analysis of Streptococcus spp. Isolates
4.6.3. Analysis of R. equi Isolates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso-Toset, F.; Gómez-Laguna, J.; Amarilla, S.P.; Vela, A.I.; Carrasco, L.; Fernández-Garayzábal, J.F.; Astorga, R.J.; Luque, I. Multi-Etiological Nature of Tuberculosis-like Lesions in Condemned Pigs at the Slaughterhouse. PLoS ONE 2015, 10, e0139130. [Google Scholar]
- Kaczmarkowska, A.; Didkowska, A.; Brzezińska, S.; Klich, D.; Kwiecień, E.; Dolka, I.; Kociuba, P.; Rzewuska, M.; Augustynowicz-Kopeć, E.; Anusz, K. Could the type and severity of gross lesions in pig lymph nodes play a role in the detection of Mycobacterium avium? PLoS ONE 2022, 17, e0269912. [Google Scholar] [CrossRef]
- Kawata, K.; Minakami, T.; Mori, Y.; Katsumi, M.; Kataoka, Y.; Ezawa, A.; Kikuchi, N.; Takahashi, T. rDNA sequence analyses of Streptococcus dysgalactiae subsp. equisimilis isolates from pigs. Int. J. Syst. Evol. Microbiol. 2003, 53 Pt 6, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Komijn, R.E.; Wisselink, H.J.; Rijsman, V.M.C.; Stockhofe-Zurwieden, N.; Bakker, D.; van Zijderveld, F.G.; Eger, T.; Wagenaar, J.A.; Putirulan, F.F.; Urlings, B.A.P. Granulomatous lesions in lymph nodes of slaughter pigs bacteriologically negative for Mycobacterium avium subsp. avium and positive for Rhodococcus equi. Vet. Microbiol. 2007, 120, 352–357. [Google Scholar] [PubMed]
- Cardoso-Toset, F.; Gómez-Laguna, J.; Gómez-Gascón, L.; Rodríguez-Gómez, I.M.; Galán-Relaño, A.; Carrasco, L.; Tarradas, C.; Vela, A.I.; Luque, I. Histopathological and microbiological study of porcine lymphadenitis: Contributions to diagnosis and control of the disease. Porc. Health Manag. 2020, 6, 36. [Google Scholar] [CrossRef]
- Brandt, C.M.; Spellerberg, B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin. Infect. Dis. 2009, 49, 766–772. [Google Scholar] [CrossRef]
- Oh, S.I.; Kim, J.W.; Kim, J.; So, B.; Kim, B.; Kim, H.Y. Molecular subtyping and antimicrobial susceptibility of Streptococcus dysgalactiae subspecies equisimilis isolates from clinically diseased pigs. J. Vet. Sci. 2020, 21, e57. [Google Scholar] [CrossRef]
- Gottschalk, M. Streptococcosis. In Disease of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K., Stevenson, G.W., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2012; pp. 841–855. [Google Scholar]
- Costa, M.O.; Harding, J.C.S.; Huang, Y.; Nosach, R. Streptococcus equi subsp. zooepidemicus infection of pigs leads to shedding in faeces and a carrier state. Transbound. Emerg. Dis. 2022, 69, e1503–e1509. [Google Scholar]
- Baracco, G.J. Infections Caused by Group C and G Streptococcus (Streptococcus dysgalactiae subsp. equisimilis and Others): Epidemiological and Clinical Aspects. Microbiol. Spectr. 2019, 7. [Google Scholar]
- Kim, M.; Heo, S.T.; Oh, H.; Kim, M.; Jo, J.; Kim, Y.R.; Lee, K.H.; Yoo, J.R. Human zoonotic infectious disease caused by Streptococcus equi subsp. zooepidemicus. Zoonoses Public Health 2022, 69, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Salasia, S.I.; Wibawan, I.W.; Pasaribu, F.H.; Abdulmawjood, A.; Lammler, C. Persistent occurrence of a single Streptococcus equi subsp. zooepidemicus clone in the pig and monkey population in Indonesia. J. Vet. Sci. 2004, 5, 263–265. [Google Scholar] [PubMed]
- Lara, G.H.; Ribeiro, M.G.; Leite, C.Q.; Paes, A.C.; Guazzelli, A.; da Silva, A.V.; Santos, A.C.; Listoni, F.J. Occurrence of Mycobacterium spp. and other pathogens in lymph nodes of slaughtered swine and wild boars (Sus scrofa). Res. Vet. Sci. 2011, 90, 185–188. [Google Scholar] [CrossRef]
- Lin, W.V.; Kruse, R.L.; Yang, K.; Musher, D.M. Diagnosis and management of pulmonary infection due to Rhodococcus equi. Clin. Microbiol. Infect. 2019, 25, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kubota, H.; Madarame, H.; Takase, F.; Takahashi, K.; Sasaki, Y.; Kakuda, T.; Takai, S. Pathogenicity and genomic features of vapN-harboring Rhodococcus equi isolated from human patients. Int. J. Med. Microbiol. 2021, 311, 151519. [Google Scholar] [CrossRef]
- Samutela, M.T.; Kwenda, G.; Simulundu, E.; Nkhoma, P.; Higashi, H.; Frey, A.; Bates, M.; Hang’ombe, B.M. Pigs as a potential source of emerging livestock-associated Staphylococcus aureus in Africa: A systematic review. Int. J. Infect. Dis. 2021, 109, 38–49. [Google Scholar] [CrossRef]
- Martínez, J.; Jaro, P.J.; Aduriz, G.; Gómez, E.A.; Peris, B.; Corpa, J.M. Carcass condemnation causes of growth retarded pigs at slaughter. Vet. J. 2007, 174, 160–164. [Google Scholar] [CrossRef]
- Di Marco, V.; Mazzone, P.; Capucchio, M.T.; Boniotti, M.B.; Aronica, V.; Russo, M.; Fiasconaro, M.; Cifani, N.; Corneli, S.; Biasibetti, E.; et al. Epidemiological significance of the domestic black pig (Sus scrofa) in maintenance of bovine tuberculosis in Sicily. J. Clin. Microbiol. 2012, 50, 1209–1218. [Google Scholar] [CrossRef]
- Martín-Hernando, M.P.; Höfle, U.; Vicente, J.; Ruiz-Fons, F.; Vidal, D.; Barral, M.; Garrido, J.M.; de la Fuente, J.; Gortazar, C. Lesions associated with Mycobacterium tuberculosis complex infection in the European wild boar. Tuberculosis 2007, 87, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Toset, F.; Luque, I.; Morales-Partera, A.; Galán-Relaño, A.; Barrero-Domínguez, B.; Hernández, M.; Gómez-Laguna, J. Survival of Streptococcus suis, Streptococcus dysgalactiae and Trueperella pyogenes in dry-cured Iberian pork shoulders and loins. Food Microbiol. 2017, 61, 66–71. [Google Scholar] [CrossRef]
- Tang, Y.; Larsen, J.; Kjeldgaard, J.; Andersen, P.S.; Skov, R.; Ingmer, H. Methicillin-resistant and -susceptible Staphylococcus aureus from retail meat in Denmark. Int. J. Food Microbiol. 2017, 249, 72–76. [Google Scholar] [CrossRef]
- Tegegne, H.A.; Koláčková, I.; Florianová, M.; Gelbíčová, T.; Madec, J.Y.; Haenni, M.; Karpíšková, R. Detection and molecular characterisation of methicillin-resistant Staphylococcus aureus isolated from raw meat in the retail market. J. Glob. Antimicrob. Resist. 2021, 26, 233–238. [Google Scholar] [CrossRef]
- Arbeit, R.D. Laboratory procedures for the epidemiological analysis of microorganisms. In Manual of Clinical Microbiology, 6th ed.; Murray, P.R., Baron, E.J.O., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society for Microbiology: Washington, DC, USA, 1995; pp. 190–208. [Google Scholar]
- Vela, A.I.; Goyache, J.; Tarradas, C.; Luque, I.; Mateos, A.; Moreno, M.A.; Borge, C.; Perea, J.A.; Domínguez, L.; Fernández-Garayzábal, J.F. Analysis of genetic diversity of Streptococcus suis clinical isolates from pigs in Spain by pulsed-field gel electrophoresis. J. Clin. Microbiol. 2003, 41, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Monger, X.C.; Gilbert, A.A.; Saucier, L.; Vincent, A.T. Antibiotic Resistance: From Pig to Meat. Antibiotics 2021, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
- CA-SFM Comité de L’antibiogramme de la Société Française de Microbiologie. Antibiogram Committee of the French Society of Microbiology Guidelines: Recommandations Vétérinaires 2021. Available online: https://www.sfm-microbiologie.org/wp-content/uploads/2021/12/CASFM_VET2021.pdf (accessed on 14 February 2023). (In French).
- CLSI. Performance Standards for Antimicrobial Disk and Dilutionn Susceptibility Test for Bacteria Isolated from Animals, 4th ed.; CLSI Supplement VET08; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Methods for Antimicrobial Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria Isolated from Animals, 1st ed.; CLSI Supplement VET06; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Zientara, W. Production of live pigs in Poland—Conditions and prospects. Annals PAAAE 2019, 21, 101–110. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug. Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Koh, T.H.; Binte, A.R.N.; Sessions, O.M. Comparative genomic analysis of Streptococcus dysgalactiae subspecies dysgalactiae, an occasional cause of zoonotic infection. Pathology 2020, 52, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Schrieber, L.; Towers, R.; Muscatello, G.; Speare, R. Transmission of Streptococcus dysgalactiae subsp. equisimilis between child and dog in an Aboriginal Australian community. Zoonoses Public Health 2014, 61, 145–148. [Google Scholar]
- Takai, S.; Sawada, N.; Nakayama, Y.; Ishizuka, S.; Nakagawa, R.; Kawashima, G.; Sangkanjanavanich, N.; Sasaki, Y.; Kakuda, T.; Suzuki, Y. Reinvestigation of the virulence of Rhodococcus equi isolates from patients with and without AIDS. Lett. Appl. Microbiol. 2020, 71, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Boland, J.A.; Giguère, S.; Hapeshi, A.; MacArthur, I.; Anastasi, E.; Valero-Rello, A. Rhodococcus equi: The many facets of a pathogenic actinomycete. Vet. Microbiol. 2013, 167, 9–33. [Google Scholar] [CrossRef]
- Deliwala, S.; Beere, T.; Samji, V.; Mcdonald, P.J.; Bachuwa, G. When Zoonotic Organisms Cross Over-Trueperella pyogenes Endocarditis Presenting as a Septic Embolic Stroke. Cureus 2020, 12, e7740. [Google Scholar] [CrossRef]
- Witkowski, L.; Rzewuska, M.; Takai, S.; Kizerwetter-Świda, M.; Kita, J. Molecular epidemiology of Rhodococcus equi in slaughtered swine, cattle and horses in Poland. BMC Microbiol. 2016, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Alves-Barroco, C.; Brito, P.H.; Santos-Sanches, I.; Fernandes, A.R. Phylogenetic analysis and accessory genome diversity reveal insight into the evolutionary history of Streptococcus dysgalactiae. Front. Microbiol. 2022, 13, 952110. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, M.; Kataoka, Y.; Takahashi, T.; Kikuchi, N.; Hiramune, T. Biochemical and serological examination of beta-hemolytic streptococci isolated from slaughtered pigs. J. Vet. Med. Sci. 1998, 60, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Ogura, K.; Miyoshi-Akiyama, T.; Nakamura, M.; Kaya, H.; Okamoto, S. Prevalence and genomic characterization of Group A Streptococcus dysgalactiae subsp. equisimilis isolated from patients with invasive infections in Toyama prefecture, Japan. Microbiol. Immunol. 2020, 64, 113–122. [Google Scholar] [CrossRef]
- Sabat, A.J.; Budimir, A.; Nashev, D.; Sa-Leao, R.; van Dijl, J.M.; Laurent, F.; Grundmann, H.; Friedrich, A.; ESCMID Study Group of Epidemiological Markers (ESGEM). Overview of molecular typing methods for outbreak detection and epidemiological surveillance. Eurosurveillance 2013, 18, 20380. [Google Scholar] [CrossRef]
- Broens, E.M.; Graat, E.A.; Van Der Wolf, P.J.; Van De Giessen, A.W.; De Jong, M.C. Transmission of methicillin-resistant Staphylococcus aureus among pigs during transportation from farm to abattoir. Vet. J. 2011, 189, 302–305. [Google Scholar] [CrossRef]
- Gómez-Sanz, E.; Torres, C.; Lozano, C.; Fernández-Pérez, R.; Aspiroz, C.; Ruiz-Larrea, F.; Zarazaga, M. Detection, Molecular Characterization, and Clonal Diversity of Methicillin-Resistant Staphylococcus aureus CC398 and CC97 in Spanish Slaughter Pigs of Different Age Groups. Foodborne Pathog. Dis. 2010, 7, 1269–1277. [Google Scholar] [CrossRef]
- Molla, B.; Byrne, M.; Abley, M.; Mathews, J.; Jackson, C.R.; Fedorka-Cray, P.; Sreevatsan, S.; Wang, P.; Gebreyes, W.A. Epidemiology and genotypic characteristics of methicillin-resistant Staphylococcus aureus strains of porcine origin. J. Clin. Microbiol. 2012, 50, 3687–3693. [Google Scholar] [CrossRef]
- Lo, Y.P.; Wan, M.T.; Chen, M.M.; Su, H.Y.; Lauderdale, T.L.; Chou, C.C. Molecular characterization and clonal genetic diversity of methicillin-resistant Staphylococcus aureus of pig origin in Taiwan. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Pate, M.; Ocepek, M.; Zdovc, I.; Minato, C.; Ohtsu, Y.; Matsuoka, M.; Honda, Y.; Hashimoto, L.; Sasaki, Y.; Kakuda, T.; et al. Intermediately virulent Rhodococcus equi isolates from pigs in Slovenia: Discovery of new plasmid types and assessment of genetic diversity by pulsed-field gel electrophoresis. Vet. Med. 2009, 54, 111–117. [Google Scholar] [CrossRef]
- Moreno, L.Z.; da Costa, B.L.; Matajira, C.E.; Gomes, V.T.; Mesquita, R.E.; Silva, A.P.; Moreno, A.M. Molecular and antimicrobial susceptibility profiling of Streptococcus dysgalactiae isolated from swine. Diagn. Microbiol. Infect. Dis. 2016, 86, 178–180. [Google Scholar] [CrossRef]
- Renzhammer, R.; Loncaric, I.; Ladstätter, M.; Pinior, B.; Roch, F.F.; Spergser, J.; Ladinig, A.; Unterweger, C. Detection of Various Streptococcus spp. and Their Antimicrobial Resistance Patterns in Clinical Specimens from Austrian Swine Stocks. Antibiotics 2020, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Byun, J.H.; Park, H.; Lee, J.; Lee, H.S.; Yoshida, H.; Shibayama, A.; Fujita, T.; Tsuyuki, Y.; Takahashi, T. Molecular epidemiological features and antibiotic susceptibility patterns of Streptococcus dysgalactiae subsp. equisimilis isolates from Korea and Japan. Ann. Lab. Med. 2018, 38, 212–219. [Google Scholar] [PubMed]
- Savini, V.; Catavitello, C.; Talia, M.; Manna, A.; Pompetti, F.; Di Bonaventura, G.; Di Giuseppe, N.; Febbo, F.; Balbinot, A.; Di Zacomo, S.; et al. β-Lactam failure in treatment of two group G Streptococcus dysgalactiae subsp. equisimilis Pharyngitis patients. J. Clin. Microbiol. 2008, 46, 814–816. [Google Scholar] [CrossRef]
- Smith, M.; Do, T.N.; Gibson, J.S.; Jordan, D.; Cobbold, R.N.; Trott, D.J. Comparison of antimicrobial resistance phenotypes and genotypes in enterotoxigenic Escherichia coli isolated from Australian and Vietnamese pigs. J. Glob. Antimicrob. Resist. 2014, 2, 162–167. [Google Scholar] [CrossRef]
- Dressler, A.E.; Scheibel, R.P.; Wardyn, S.; Harper, A.L.; Hanson, B.M.; Kroeger, J.S.; Diekema, D.J.; Bender, J.B.; Gray, G.C.; Smith, T.C. Prevalence, antibiotic resistance and molecular characterisation of Staphylococcus aureus in pigs at agricultural fairs in the USA. Vet. Rec. 2012, 170, 495. [Google Scholar] [CrossRef]
- Peeters, L.E.J.; Argudín, M.A.; Azadikhah, S.; Butaye, P. Antimicrobial resistance and population structure of Staphylococcus aureus recovered from pigs farms. Vet. Microbiol. 2015, 180, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cisek, A.A.; Rzewuska, M.; Witkowski, L.; Binek, M. Antimicrobial resistance in Rhodococcus equi. Acta Biochim. Pol. 2014, 61, 633–638. [Google Scholar] [CrossRef]
- Berghaus, L.J.; Giguère, S.; Guldbech, K.; Warner, E.; Ugorji, U.; Berghaus, R.D. Comparison of Etest, disk diffusion, and broth macrodilution for in vitro susceptibility testing of Rhodococcus equi. J. Clin. Microbiol. 2015, 53, 314–318. [Google Scholar] [CrossRef]
- Giguère, S.; Berghaus, L.J.; Willingham-Lane, J.M. Antimicrobial Resistance in Rhodococcus equi. Microbiol. Spectr. 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Stoś, K.; Rychlik, E.; Woźniak, A.; Ołtarzewski, M. Red and Processed Meat Consumption in Poland. Foods 2022, 11, 3283. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR Method for Species Identification of Coagulase-Positive Staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Riffon, R.; Sayasith, K.; Khalil, H.; Dubreuil, P.; Drolet, M.; Lagacé, J. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J. Clin. Microbiol. 2001, 39, 2584–2589. [Google Scholar] [CrossRef] [PubMed]
- Alber, J.; El-Sayed, A.; Lämmler, C.; Hassan, A.A.; Weiss, R.; Zschöck, M. Multiplex polymerase chain reaction for identification and differentiation of Streptococcus equi subsp. zooepidemicus and Streptococcus equi subsp. equi. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 455–458. [Google Scholar] [CrossRef]
- Clermont, D.; Chesneau, O.; De Cespédès, G.; Horaud, T. New tetracycline resistance determinants coding for ribosomal protection in streptococci and nucleotide sequence of tet(T) isolated from Streptococcus pyogenes A498. Antimicrob. Agents Chemother. 1997, 41, 112–116. [Google Scholar] [CrossRef]
- Nawaz, M.; Wang, J.; Zhou, A.; Ma, C.; Wu, X.; Moore, J.E.; Millar, B.C.; Xu, J. Characterization and Transfer of Antibiotic Resistance in Lactic Acid Bacteria from Fermented Food Products. Curr. Microbiol. 2011, 62, 1081–1089. [Google Scholar] [CrossRef]
- Gibreel, A.; Tracz, D.M.; Nonaka, L.; Ngo, T.M.; Connell, S.R.; Taylor, D.E. Incidence of Antibiotic Resistance in Campylobacter jejuni Isolated in Alberta, Canada, from 1999 to 2002, with Special Reference to tet (O)-Mediated Tetracycline Resistance. Antimicrob. Agents Chemother. 2004, 48, 3442–3450. [Google Scholar] [CrossRef]
- Pang, Y.; Bosch, T.; Roberts, M.C. Single polymerase chain reaction for the detection of tetracycline-resistant determinants Tet K and Tet L. Mol. Cell. Probes 1994, 8, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Toomey, N.; Bolton, D.; Fanning, S. Characterisation and transferability of antibiotic resistance genes from lactic acid bacteria isolated from Irish pork and beef abattoirs. Res. Microbiol. 2010, 161, 127–135. [Google Scholar] [CrossRef]
- Hennekinne, J.A.; Kerouanton, A.; Brisabois, A.; De Buyser, M.L. Discrimination of Staphylococcus aureus biotypes by pulsed-field gel electrophoresis of DNA macro-restriction fragments. J. Appl. Microbiol. 2003, 94, 321–329. [Google Scholar] [CrossRef]
- Szaluś-Jordanow, O.; Chrobak, D.; Pyrgiel, M.; Lutyńska, A.; Kaba, J.; Czopowicz, M.; Witkowski, L.; Kizerwetter-Świda, M.; Binek, M.; Frymus, T. PFGE and AFLP genotyping of Staphylococcus aureus subsp. anaerobius isolated from goats with Morel’s disease. Arch. Microbiol. 2013, 195, 37–41. [Google Scholar] [PubMed]
- Cohen, N.D.; Smith, K.E.; Ficht, T.A.; Takai, S.; Libal, M.C.; West, B.R.; DelRosario, L.S.; Becu, T.; Leadon, D.P.; Buckley, T.; et al. Epidemiologic study of results of pulsed-field gel electrophoresis of isolates of Rhodococcus equi obtained from horses and horse farms. Am. J. Vet. Res. 2003, 64, 153–161. [Google Scholar] [CrossRef]
- Witkowski, L.; Rzewuska, M.; Cisek, A.A.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Czopowicz, M.; Welz, M.; Kita, J. Prevalence and genetic diversity of Rhodococcus equi in wild boars (Sus scrofa), roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in Poland. BMC Microbiol. 2015, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial straintyping. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
| Samples | Streptococcus dysgalactiae | Streptococcus equi subsp. zooepidemicus | Staphylococcus aureus | Rhodococcus equi |
|---|---|---|---|---|
| L.n. mandibular with lesions (n = 86) | 28 | 0 | 4 | 8 |
| L.n. mandibular without lesions (n = 113) | 14 | 3 | 1 | 9 |
| L.n. mediastinal with lesions (n = 1) | 1 | 0 | 0 | 0 |
| L.n. of the hilum of a liver with lesions (n = 1) | 1 | 0 | 0 | 0 |
| Liver with lesions (n = 1) | 1 | 0 | 0 | 0 |
| L.n. of the first rib with lesions (n = 1) | 0 | 0 | 0 | 0 |
| L.n. retropharyngeal with lesions (n = 1) | 0 | 0 | 0 | 0 |
| L.n. tracheobronchial with lesions (n = 2) | 0 | 0 | 0 | 0 |
| L.n. mesenteric with lesions (n = 1) | 0 | 0 | 0 | 0 |
| Lung with lesions (n = 1) | 0 | 0 | 0 | 0 |
| Total | 45 | 3 | 5 | 17 |
| Antimicrobial Agent a | MIC (μg/mL) | MIC Breakpoints (μg/mL) b | |||
|---|---|---|---|---|---|
| MIC50 | MIC90 | S | R | ||
| Streptococcus spp. | PEN | 0.0006 | 0.016 | ≤0.25 | >16 c |
| AMC | 0.016 | 0.047 | ≤0.25/0.12 | ≥1/0.5 d | |
| CTX | 0.032 | 0.094 | ≤2 | ≥8 d | |
| CIP | 0.5 | 0.75 | ≤0.5 | >2 c | |
| ERY | 0.19 | 0.75 | ≤1 | >4 c | |
| DOX | 0.5 | 24 | ≤4 | >8 c | |
| GEN | 1.5 | 3 | ≤250 | >500 c | |
| SXT | 0.38 | 3 | ≤2/38 | >8/152 c | |
| Rhodococcus equi | PEN | 12 | >32 | ≤0.25 | >0.25 c |
| AMC | 1.5 | 2 | ≤0.25/0.12 | ≥1/0.5 d | |
| CTX | >32 | >32 | ≤2 | ≥8 d | |
| CIP | 0.75 | 1.5 | ≤2 | >2 c | |
| ERY | 0.25 | 0.38 | ≤0.5 | ≥8 e | |
| DOX | 8 | 12 | ≤4 | ≥16 e | |
| GEN | 0.5 | 0.75 | ≤1 | >1 c | |
| SXT | 2 | >32 | ≤2/38 | >8/152 c | |
| Staphylococcus aureus | PEN | 0.032 | 0.125 | ≤0.25 | >0.25 c |
| AMC | 0.125 | 0.25 | ≤0.25/0.12 | ≥1/0.5 d | |
| CTX | 4 | >32 | ≤2 | ≥8 d | |
| CIP | 0.25 | 0.75 | ≤2 | >2 c | |
| ERY | 0.094 | 0.5 | ≤1 | >4 c | |
| DOX | 0.38 | 0.5 | ≤4 | >8 c | |
| GEN | 0.19 | 0.19 | ≤1 | >1 c | |
| SXT | 0.19 | 0.38 | ≤2/38 | >8/152 c | |
| Primer Designation | Primer Sequence (5′-3′) | Target Gene | Annealing Temperature (°C) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| DI_F | GAYACICCIGGICAYRTIGAYTT | Tet a | 53 b | 1100 | [60] |
| DII_R | GCCCARWAIGGRTTIGGIGGIACYTC | ||||
| tetM_F | TTAAATAGTGTTCTTGGAG | tetM | 54 c | 656 | [61] |
| tetM_R | CTAAGATATGGCTCTAACAA | ||||
| tetO_F | GGCGTTTTGTTTATGTGCG | tetO | 50 c | 559 | [62] |
| tetO_R | ATGGACAACCCGACAGAAGC | ||||
| TKI_F | CCTGTTCCCTCTGATAAA | tetK/L | 50 b | 1050 | [63] |
| TL32_R | CAAACTGGGTGAACACAG | ||||
| ermA_F | TCTAAAAAGCATGTAAAAGAA | ermA | 52 c | 645 | [64] |
| ermA_R | CTTCGATAGTTTATTAATATTAGT | ||||
| ermB_F | GAAAAGGTACTCAACCAAATA | ermB | 52 c | 639 | [64] |
| ermB_R | AGTAACGGTACTTAAATTGTTTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarkowska, A.; Kwiecień, E.; Didkowska, A.; Stefańska, I.; Rzewuska, M.; Anusz, K. The Genetic Diversity and Antimicrobial Resistance of Pyogenic Pathogens Isolated from Porcine Lymph Nodes. Antibiotics 2023, 12, 1026. https://doi.org/10.3390/antibiotics12061026
Kaczmarkowska A, Kwiecień E, Didkowska A, Stefańska I, Rzewuska M, Anusz K. The Genetic Diversity and Antimicrobial Resistance of Pyogenic Pathogens Isolated from Porcine Lymph Nodes. Antibiotics. 2023; 12(6):1026. https://doi.org/10.3390/antibiotics12061026
Chicago/Turabian StyleKaczmarkowska, Aleksandra, Ewelina Kwiecień, Anna Didkowska, Ilona Stefańska, Magdalena Rzewuska, and Krzysztof Anusz. 2023. "The Genetic Diversity and Antimicrobial Resistance of Pyogenic Pathogens Isolated from Porcine Lymph Nodes" Antibiotics 12, no. 6: 1026. https://doi.org/10.3390/antibiotics12061026
APA StyleKaczmarkowska, A., Kwiecień, E., Didkowska, A., Stefańska, I., Rzewuska, M., & Anusz, K. (2023). The Genetic Diversity and Antimicrobial Resistance of Pyogenic Pathogens Isolated from Porcine Lymph Nodes. Antibiotics, 12(6), 1026. https://doi.org/10.3390/antibiotics12061026






