Antibiotic Resistance in Selected Emerging Bacterial Foodborne Pathogens—An Issue of Concern?
Abstract
1. Introduction
2. Emergence Phenomenon among Foodborne Pathogens
3. Emerging Pathogens
4. Emerging Foodborne Bacterial Pathogens—Characteristics and Antibiotic Resistance of the Most Important Species
4.1. Aliarcobacter spp.
Antimicrobial Resistance of Aliarcobacter spp.
| Genus/Species | Resistance to | Genes | References |
|---|---|---|---|
| Aliarobacter spp. | tetracycline | tetA, tetO, tetW | [43] |
| quinolones | gnrS, gyrA | [44] | |
| fluorochinolones (especially ciprofloxacin) | gyrA | [45] | |
| beta-lactamases | bla1, bla2 | [42,46] | |
| ampicilin | bla2 | [46] | |
| chloramfenicol | cat3 | ||
| macrolides | macA1, macB2 | [40] | |
| polymyxin | arnB, eptA | ||
| various classes of antibiotics | rlmN | ||
| suspected to be involved in multidrug resistance | hipA | [47] | |
| Aeromonas spp. | streptomycin | aadA1 | [48,49] |
| spectinomycin | aadA2 | ||
| streptothricin | sat1 | ||
| tetracycline | tetA, tetB, tettC, tetD, tetE, tetH, tetG, tetM | [50,51,52] | |
| quinolone | qnrS2, parC, mutation in gyrA | ||
| [53] | |||
| sulphonamide | sul1, sul2 | [54,55] | |
| aminoglycosides | aac (6′)-Ib-cr | ||
| beta-lactam | blaKPC-2, blaP1, blaTEM, blaVEB-1a, blaSHV-12 | ||
| ciprofloxacin | aac(6′)-ib-cr | [55] | |
| trimethoprim | dfrA1, dfrA1/7, dfrA12 | [56] | |
| aminoglycosides | aadA1a, aadA2, aadA7, aacA4, aacA, strA-strB | [57,58,59,60] | |
| Escherichia coli | β-lactams | blaCTX-M-1, blaCTX-M-14, blaTEM-52, blaSHV-12, blaCTX-M, blaTEM, blaSHV (ESBL genes) | [13,61] |
| carbapenems | blaNDM-1, blaNDM-5, blaVIM-1, blaIMP-4, blaOXA-48, blaOXA-181, blaKPC-2 | [62,63,64,65,66,67] | |
| quinolones | gyrA | [68] | |
| aminoglycosides | armA | [69] | |
| fosfomycin | mutations in the glpT and uhpA/T genes, fosA | [70,71] | |
| tetracycline | tet(A), tet(B), tet(C), tet(D), tet(E), tet(G), tet(J), tet(L), tet(Y) | [72] | |
| phenicols | cmlA, floR, cfr | [73] | |
| sulphonamide | sul1, sul2, sul3 | [74] | |
| trimethoprim | dfrA, dfrB | [75] | |
| polymyxin | pmrCAB | [76] | |
| Salmonella spp. | β-lactams | blaTEM, blaCTX-M | [77,78] |
| aminoglycosides | aac(3)-IV, aac(60)-Iaa, aadA1, aadA2 | [79] | |
| sulfonamides | sul, dfrA1, dfrA12 | [80] | |
| tetracyclines | tetA, tetB | [81] | |
| quinolones | oqxAB, qnrA, qnrB, qnrC, qnrD, qnrS, aac(6′)lb-cr | [82] | |
| chloramphenicol | cmlA, catB | ||
| colistin | mcr-1, mcr-3 | [83,84] | |
| Vibrio spp. | aminoglycoside | str | [85] |
| β-lactams | blAOXA, blaPSE, ampC | [86] | |
| tetracycline | tetA, tetE | [87,88] | |
| sulfonamide | sul1, sul2 | ||
| quinolone | qnr | [89] | |
| chloramphenicol | cat, floR | [87] | |
| macrolides | erm, mef, aac, aphA | [90] | |
| Campylobacter spp. | ciprofloxacin | mutation in gyrA gene | [91,92] |
| tetracycline | tetA, tetB, tetC, tetD, tetK, tetM | ||
| erythromycin | ermM | ||
| chloramphenicol | catI, catII | [92] | |
| gentamycin | aac(3)-IIa-(aacC2) | ||
| ampicillin | ampC | ||
| imipenem | imi, vim, kpc | ||
| Cronobacter spp. | colistin | mcr-1, mcr-10, mcr-9.1 | [93,94,95,96] |
| β-lactams | blaTEM, blaOXA, blaSHV, blaCTX-M-1, blaCTX-M-2, blaCTX-M-8, blaCTX-M-9 | [97,98] | |
| fosfomycin | glpT | [98] | |
| cephalothin | blaCSA | [96] | |
| fluoroquinolone | marA, marR, adeF, emrR, emrB | ||
| nitroimidazole | msbA | ||
| macrolide | kpnE, kpnF, kpnH | ||
| aminoglycoside | baeR | ||
| Listeria monocytogenes | fosfomycin | fosX | [99] |
| lincosamides | lin | ||
| quinolones | norB | ||
| tetracyclines | tetA, tetC, tetM, tetS | ||
| vancomycin | nacC, vanR, vanT, vanXY-C | [100,101] | |
| lincomycin | abc-f | [101,102] | |
| trimethoprim | drfE | [101] | |
| macrolide, linesoide and streptogramin B | ermB, ermC | [101,103,104] | |
| β-lactams | blaTEM-116 | [101] | |
| macrolide | mphB | ||
| Staphylococcus aurues | penicillins | blaZ | [105,106] |
| beta-lactams (MRSA) | mecA, mecC | [107] | |
| aminoglycoside | aac(6′)/aph(2″), aph(3′)-IIIa, ant(4′)-Ia | [108,109,110] | |
| macrolides, lincosamides, streptogramin B | ermA, ermB, ermC, ermY | [111,112] | |
| macrolides | msrA, msrB, mphC | [113] | |
| linezolid | cfr, optrA, poxtA | [114] | |
| tetracycline | tetK, tetM | [111] | |
| vancomycin | vanA | [115] | |
| fluoroquinolones | norA | [116] | |
| trimethoprim | dfrA | [116] | |
| Streptococcus suis | tetracyclines | tetM, tetO, tetQ, tetT, tetW, tetK, tetL | [117] |
| macrolides | ermB, ermA, ermTR | [118] | |
| lincosamides | lnu(B), lnu(C) | [117] | |
| aminoglycosides (including kanamycin and neomycin) | aph(3′)-IIIa | [117] | |
| vancomycin | vanG | [119,120] | |
| amphenicols | cfr, cat | [121,122] | |
| Clostridioides difficilie | β-lactams | CDD1, CCD2 | [123] |
| aminoglycosides | aph, aac, ant, npmA | [124] | |
| tetracyclines | tet44, tetM, tetW, tetA, tetB | [125,126,127] | |
| vancomycin | murG, vanS/, vanG | [125,128,129] | |
| metronidazole | glyC, nifJ | [130,131] | |
| fidaxomicin | rpoB | [128,132,133] | |
| rifamycins | rdxA | [134,135] | |
| fluorochinolones | gyrA, gyrB | [136] | |
| chloramfenicol | cat(P), cat(D) | [125] | |
| linezolid | cfrC, rplC | [134] | |
| Helicobacter pylori | metronidazole | rdxA | [137,138] |
| amoxicillin | pbp1A | ||
| fluoroquinolone (especially levofloxacin) | gyrA, gyrB | ||
| clarithromycin | cla |
4.2. Aeromonas spp.
Antimicrobial Resistance of Aeromonas spp.
4.3. Escherichia coli—Various Pathotypes
Antimicrobial Resistance of E. coli
4.4. Salmonella spp.
Antibiotic Resistance of Salmonella spp.
4.5. Vibrio spp.
Antimicrobial Resistance of Vibrio spp.
4.6. Campylobacter spp.
Antimicrobial Resistance of Campylobacter spp.
4.7. Cronobacter spp.
4.8. Helicobacter pylori
Antibiotic Resistance of H. pylori
4.9. Listeria monocytogenes
Antimicrobial Resistance of Listeria monocytogenes
4.10. Staphylococcus aureus
Antimicrobial Resistance of Staphylococcus aureus
4.11. Streptococcus suis
Antibiotic Resistance Streptococcus suis
4.12. Clostridioides difficile
Antimicrobial Resistance of C. difficile
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Food Safety. Key Facts. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 13 December 2022).
- Trastoy, R.; Manso, T.; Fernández-García, L.; Blasco, L.; Ambroa, A.; Pérez Del Molino, M.L.; Bou, G.; García-Contreras, R.; Wood, T.K.; Tomás, M. Mechanisms of Bacterial Tolerance and Persistence in the Gastrointestinal and Respiratory Environments. Clin. Microbiol. Rev. 2018, 31, e00023-18. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Wu, Q.; Zhang, Z.; Liu, J.; Tian, C.; Huang, Z.; Malakar, P.K.; Pan, Y.; Zhao, Y. Meta-Analysis for the Global Prevalence of Foodborne Pathogens Exhibiting Antibiotic Resistance and Biofilm Formation. Front. Microbiol. 2022, 13, 906490. [Google Scholar] [CrossRef]
- Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Skowron, K.; Kijewska, A.; Bernaciak, Z.; Bauza-Kaszewska, J.; Kraszewska, Z.; Gospodarek-Komkowska, E. Comparison of Selected Phenotypic Features of Persistent and Sporadic Strains of Listeria monocytogenes Sampled from Fish Processing Plants. Foods 2022, 11, 1492. [Google Scholar] [CrossRef]
- Tauxe, R.V. Emerging foodborne pathogens. Int. J. Food Microbiol. 2002, 78, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Future Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef]
- Whitehouse, C.A.; Zhao, S.; Tate, H. Antimicrobial Resistance in Campylobacter Species: Mechanisms and Genomic Epidemiology. Adv. Appl. Microbiol. 2018, 103, 1–47. [Google Scholar] [PubMed]
- Nair, D.V.T.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef]
- Eng, S.K.; Pusparajah, P.; Ab Mutalib, N.S.; Leng, S.H.; Chan, K.G.; Learn Han, L. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Erb, A.; Stürmer, T.; Marre, R.; Brenner, H. Prevalence of antibiotic resistance in Escherichia coli: Overview of geographical, temporal, and methodological variations. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 83–90. [Google Scholar] [CrossRef]
- Ito, A.; Taniuchi, A.; May, T.; Kawata, K.; Okabe, S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 2009, 75, 4093–4100. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 14. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of Antibiotic Resistance in Listeria monocytogenes Isolated from Food Products: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Panera-Martínez, S.; Rodríguez-Melcón, C.; Serrano-Galán, V.; Alonso-Calleja, C.; Capita, R. Prevalence, quantification and antibiotic resistance of Listeria monocytogenes in poultry preparations. Food Control 2022, 135, 108608. [Google Scholar] [CrossRef]
- Smith, J.L.; Fratamico, P.M. Emerging and Re-Emerging Foodborne Pathogens. Foodborne Pathog. Dis. 2018, 15, 737–757. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Lianou, A.; Sofos, J.N. Food Safety: Emerging Pathogens. In Encyclopedia of Agriculture and Food Systems, 2nd ed.; Van Alfen, N.K., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 250–272. [Google Scholar]
- Buchanan, R.L. Identifying and controlling emerging foodborne pathogens: Research needs. Emerg. Infect. Dis. 1997, 3, 517–521. [Google Scholar] [CrossRef]
- Behravesh, C.B.; Williams, I.T.; Tauxe, R.V. Emerging foodborne pathogens and problems: Expanding prevention efforts before slaughter or harvest. In Improving Food Safety Through a One Health Approach; Choffnes, E.R., Relman, D.A., Olsen, L.A., Hutton, R., Mack, A., Eds.; National Academies of Sciences, Engineering, and Medicine: National Academies Press: Washington, DC, USA, 2012; pp. 307–331. [Google Scholar]
- Skovgaard, N. New trends in emerging pathogens. Inter. J. Food Microbiol. 2007, 120, 217–224. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Den Besten, H.M.W.; Böhnlein, C.; Gareis, M.; Zwietering, M.H.; Fusco, V. Microbial food safety in the 21st century: Emerging challenges and foodborne pathogenic bacteria. Trends Food Sci. Technol. 2018, 81, 155–158. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Tsiodras, S.; Poulakou, G. Emerging and Re-Emerging Infectious Diseases: Humankind’s Companions and Competitors. Microorganisms 2022, 10, 98. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Current Outbreak List. Available online: https://www.cdc.gov/outbreaks/index.html (accessed on 13 March 2023).
- U.S. Food & Drug Administration (FDA). Investigations of Foodborne Illness Outbreaks. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/investigations-foodborne-illness-outbreaks (accessed on 13 March 2023).
- Yesilmen, S.; Vural, A.; Erkan, M.E.; Yildirim, I.H. Prevalence and antimicrobial susceptibility of Arcobacter species in cow milk, water buffalo milk and fresh village cheese. Int. J. Food Microbiol. 2014, 188, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cataluña, A.; Salas-Massó, N.; Diéguez, A.L.; Balboa, S.; Lema, A.; Romalde, J.L.; Figueras, M.J. Revisiting the Taxonomy of the Genus Arcobacter: Getting Order From the Chaos. Front. Microbiol. 2018, 9, 2077. [Google Scholar] [CrossRef] [PubMed]
- Chuan, J.; Belov, A.; Cloutier, M.; Li, X.; Khan, I.U.H.; Chen, W. Comparative genomics analysis and virulence-related factors in novel Aliarcobacter faecis and Aliarcobacter lanthieri species identified as potential opportunistic pathogens. BMC Genom. 2022, 23, 471. [Google Scholar] [CrossRef] [PubMed]
- Simaluiza, R.J.; Ambuludi, D.R.; Fernández, H. First case of diarrhea due to Aliarcobacter butzleri (formerly Arcobacter butzleri) in Ecuador. Infect. Dis. Now 2021, 51, 564–566. [Google Scholar] [CrossRef]
- Müller, E.; Abdel-Glil, M.Y.; Hotzel, H.; Hänel, I.; Tomaso, H. Aliarcobacter butzleri from Water Poultry: Insights into Antimicrobial Resistance, Virulence and Heavy Metal Resistance. Genes 2020, 11, 1104. [Google Scholar] [CrossRef]
- Hänel, I.; Müller, E.; Santamarina, B.G.; Tomaso, H.; Hotzel, H.; Busch, A. Antimicrobial Susceptibility and Genomic Analysis of Aliarcobacter cibarius and Aliarcobacter thereius, Two Rarely Detected Aliarcobacter Species. Front. Cell. Infect. Microbiol. 2021, 11, 532989. [Google Scholar] [CrossRef]
- Girbau, C.; Guerra, C.; Martínez-Malaxetxebarria, I.; Alonso, R.; Fernández-Astorga, A. Prevalence of ten putative virulence genes in the emerging foodborne pathogen Arcobacter isolated from food products. Food Microbiol. 2015, 52, 146–149. [Google Scholar] [CrossRef]
- Ferreira, S.; Luís, Â.; Oleastro, M.; Pereira, L.; Domingues, F.C. A meta-analytic perspective on Arcobacter spp. antibiotic resistance. J. Glob. Antimicrob. Resist. 2019, 16, 130–139. [Google Scholar] [CrossRef]
- Uljanovas, D.; Gölz, G.; Brückner, V.; Grineviciene, A.; Tamuleviciene, E.; Alter, T.; Malakauskas, M. Prevalence, antimicrobial susceptibility and virulence gene profiles of Arcobacter species isolated from human stool samples, foods of animal origin, ready-to-eat salad mixes and environmental water. Gut Pathog. 2021, 13, 76. [Google Scholar] [CrossRef]
- Drancourt, M. Acute Diarrhea. Infect. Dis. 2017, 1, 335–340.e2. [Google Scholar] [CrossRef]
- Šilha, D.; Pejchalová, M.; Šilhová, L. Susceptibility to 18 drugs and multidrug resistance of Arcobacter isolates from different sources within the Czech Republic. J. Glob. Antimicrob. Res. 2017, 9, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Jehanne, Q.; Bénéjat, L.; Ducournau, A.; Bessède, E.; Lehours, P. Molecular Cut-off Values for Aliarcobacter butzleri Susceptibility Testing. Microbiol. Spectr. 2022, 10, e0100322. [Google Scholar] [CrossRef]
- Kayman, T.; Abay, S.; Hizlisoy, H.; Atabay, H.I.; Diker, K.S.; Aydin, F. Emerging pathogen Arcobacter spp. in acute gastroenteritis: Molecular identification, antibiotic susceptibilities and genotyping of the isolated arcobacters. J. Med. Microbiol. 2012, 61, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Van den Abeele, A.M.; Vogelaers, D.; Vanlaere, E.; Houf, K. Antimicrobial susceptibility testing of Arcobacter butzleri and Arcobacter cryaerophilus strains isolated from Belgian patients. J. Antimicrob. Chemother. 2016, 71, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Di Pinto, A.; Mottola, A.; Mule, G.; Chieffi, D.; Baruzzi, F.; Tantillo, G.; Fusco, V. Genomic Characterization of Arcobacter butzleri Isolated From Shellfish: Novel Insight Into Antibiotic Resistance and Virulence Determinants. Front. Microbiol. 2019, 10, 670. [Google Scholar] [CrossRef]
- Vicente-Martins, S.; Oleastro, M.; Domingues, F.C.; Ferreira, S. Arcobacter spp. at retail food from Portugal: Prevalence, genotyping and antibiotics resistance. Food Control 2018, 85, 107–112. [Google Scholar] [CrossRef]
- Müller, E.; Hotzel, H.; Ahlers, C.; Hänel, I.; Tomaso, H.; Abdel-Glil, M.Y. Genomic Analysis and Antimicrobial Resistance of Aliarcobacter cryaerophilus Strains From German Water Poultry. Front. Microbiol. 2020, 11, 1549. [Google Scholar] [CrossRef]
- Zambri, M.; Cloutier, M.; Adam, Z.; Lapen, D.R.; Wilkes, G.; Sunohara, M.; Topp, E.; Talbot, G.; Khan, I.U.H. Novel Virulence, Antibiotic Resistance and Toxin Gene-Specific PCR-Based Assays for Rapid Pathogenicity Assessment of Arcobacter faecis and Arcobacter lanthieri. BMC Microbiol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Abdelbaqi, K.; Ménard, A.; Prouzet-Mauleon, V.; Bringaud, F.; Lehours, P.; Mégraud, F. Nucleotide Sequence of the GyrA Gene of Arcobacter Species and Characterization of Human Ciprofloxacin-Resistant Clinical Isolates. FEMS Immunol. Med. Microbiol. 2007, 49, 337–345. [Google Scholar] [CrossRef]
- Hausdorf, L.; Neumann, M.; Bergmann, I.; Sobiella, K.; Mundt, K.; Fröhling, A.; Schlüter, O.; Klocke, M. Occurrence and Genetic Diversity of Arcobacter Spp. in a Spinach-Processing Plant and Evaluation of Two Arcobacter-Specific Quantitative PCR Assays. Syst. Appl. Microbiol. 2013, 36, 235–243. [Google Scholar] [CrossRef]
- Isidro, J.; Ferreira, S.; Pinto, M.; Domingues, F.; Oleastro, M.; Gomes, J.P.; Borges, V. Virulence and antibiotic resistance plasticity of Arcobacter butzleri: Insights on the genomic diversity of an emerging human pathogen. Infect. Genet. Evol. 2020, 80, 104213. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Piro, K.M.; Xu, W.; Hansen, S.; Lewis, K.; Brennan, R.G. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science 2009, 323, 396–401. [Google Scholar] [CrossRef]
- Szczepanowski, R.; Linke, B.; Krahn, I.; Gartemann, K.H.; Gutzkow, T.; Eichler, W.; Puhler, A.; Schulter, A. Detection of 140 clinically relevant antibiotic- resistance genes in the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to selected antibiotics. Microbiology 2009, 155, 2306–2323. [Google Scholar] [CrossRef]
- Tennstedt, T.; Szczepanowski, R.; Krahn, I.; Pühler, A.; Schlüter, A. Sequence of the 68,869 bp IncP-1alpha plasmid pTB11 from a wastewater treatment plant reveals a highly conserved backbone, a Tn402-like integron and other transposable elements. Plasmid 2005, 53, 218–238. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, S.Y.; Son, J.S.; Han, J.E.; Jun, J.W.; Shin, S.P.; Choresca, C., Jr.; Choi, Y.J.; Park, Y.H.; Park, S.C. Molecular characterization of tetracycline- and quinolone-resistant Aeromonas salmonicida isolated in Korea. J. Vet. Sci. 2011, 12, 41–48. [Google Scholar] [CrossRef]
- Han, J.E.; Kim, J.H.; Choresca, C.H., Jr.; Shin, S.P.; Jun, J.W.; Chai, J.Y.; Park, S.C. First description of ColE-type plasmid in Aeromonas spp. carrying quinolone resistance (qnrS2) gene. Lett. Appl. Microbiol. 2012, 55, 290–294. [Google Scholar] [CrossRef]
- Schmidt, A.S.; Bruun, M.S.; Dalsgaard, I.; Larsen, J.L. Incidence, distribution, and spread of tetracycline resistance determinants and integron-associated antibiotic resistance genes among motile aeromonads from a fish farming environment. Appl. Environ. Microbiol. 2001, 67, 5675–5682. [Google Scholar] [CrossRef]
- Figueira, V.; Vaz-Moreira, I.; Silva, M.; Manaia, C.M. Diversity and antibiotic resistance of Aeromonas spp. in drinking and waste water treatment plants. Water Res. 2011, 45, 5599–5611. [Google Scholar] [CrossRef]
- Nwaiwu, O.; Aduba, C.C. An in silico analysis of acquired antimicrobial resistance genes in Aeromonas plasmids. AIMS Microbiol. 2020, 6, 75–91. [Google Scholar]
- Igbinosa, I.H.; Okoh, A.I. Antibiotic susceptibility profile of Aeromonas species isolated from wastewater treatment plant. Sci. World J. 2012, 764563. [Google Scholar]
- Pellegrini, C.; Celenza, G.; Segatore, B.; Bellio, P.; Setacci, D.; Amicosante, G.; Perilli, M. Occurrence of class 1 and 2 integrons in resistant Enterobacteriaceae collected from a urban wastewater treatment plant: First report from central Italy. Microb. Drug. Resist. 2010, 17, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ndi, O.L.; Barton, M.D. Incidence of class 1 integron and other antibiotic resistance determinants in Aeromonas spp. from rainbow trout farms in Australia. J. Fish Dis. 2011, 34, 589–599. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, D.; Cunningham, M.; Ji, B.; Fekete, F.A.; Parry, E.M.; Clark, S.E.; Zalinger, Z.B.; Gilg, I.C.; Danner, G.R.; Johnson, K.A.; et al. Transferable, multiple antibiotic and mercury resistance in Atlantic Canadian isolates of Aeromonas salmonicida subsp. salmonicida is associated with carriage of an IncA/C plasmid similar to the Salmonella enterica plasmid pSN254. J. Antimicrob. Chemother. 2008, 61, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Henriques, I.S.; Fonseca, F.; Alves, A.; Saavedra, M.J.; Correia, A. Occurrence and diversity of integrons and β-lactamase genes among ampicillin-resistant isolates from estuarine waters. Res. Microbiol. 2006, 157, 938–947. [Google Scholar] [CrossRef]
- Gordon, L.; Cloeckaert, A.; Doublet, B.; Schwarz, S.; Bouju-Albert, A.; Ganière, J.P.; Le Bris, H.; Le Flèche-Matéos, A.; Giraud, E. Complete sequence of the floR-carrying multiresistance plasmid pAB5S9 from freshwater Aeromonas bestiarum. J. Antimicrob. Chemother. 2008, 62, 65–71. [Google Scholar] [CrossRef]
- EFSA. Panel on Biological Hazards (BIOHAZ); scientific opinion on the public health risks of bacterial strains producing extendedspectrum β-lactamases and/or AmpC β-lactamases in food and foodproducing animals. EFSA J. 2011, 9, 2322–2417. [Google Scholar] [CrossRef]
- Shaheen, B.W.; Nayak, R.; Boothe, D.M. Emergence of a New Delhi metallo-β-lactamase (NDM-1)-encoding gene in clinical Escherichia coli isolates recovered from companion animals in the United States. Antimicrob. Agents Chemother. 2013, 57, 2902–2903. [Google Scholar] [CrossRef]
- Yang, R.S.; Feng, Y.; Lv, X.Y.; Duan, J.H.; Chen, J.; Fang, L.X.; Xia, J.; Liao, X.P.; Sun, J.; Liu, Y.H. Emergence of NDM-5- and MCR-1-producing Escherichia coli clones ST648 and ST156 from a single Muscovy duck (Cairina moschata). Antimicrob. Agents Chemother. 2016, 60, 6899–6902. [Google Scholar] [CrossRef]
- Fischer, J.; San José, M.; Roschanski, N.; Schmoger, S.; Baumann, B.; Irrgang, A.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Spread and persistence of VIM-1 carbapenemase-producing Enterobacteriaceae in three German swine farms in 2011 and 2012. Vet. Microbiol. 2017, 200, 118–123. [Google Scholar] [CrossRef]
- Al Bayssari, C.; Olaitan, A.O.; Dabboussi, F.; Hamze, M.; Rolain, J.M. Emergence of OXA-48-producing Escherichia coli clone ST38 in fowl. Antimicrob. Agents Chemother. 2015, 59, 745–746. [Google Scholar] [CrossRef]
- Pulss, S.; Semmler, T.; Prenger-Berninghoff, E.; Bauerfeind, R.; Ewers, C. First report of an Escherichia coli strain from swine carrying an OXA-181 carbapenemase and the colistin resistance determinant MCR-1. Int. J. Antimicrob. Agents 2017, 50, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A.; Muggeo, A.; El Garch, F.; Moyaert, H.; de Champs, C.; Guillard, T. Characterization of quinolone resistance mechanisms in Enterobacteriaceae isolated from companion animals in Europe (ComPath II study). Vet. Microbiol. 2018, 216, 159–167. [Google Scholar] [CrossRef]
- González-Zorn, B.; Teshager, T.; Casas, M.; Porrero, M.C.; Moreno, M.A.; Courvalin, P.; Domínguez, L. armA and aminoglycoside resistance in Escherichia coli. Emerg. Infect. Dis. 2005, 11, 954–956. [Google Scholar] [CrossRef]
- Silver, L.L. Fosfomycin: Mechanism and resistance. Cold Spring Harb. Perspect. Med. 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Huang, X.; Deng, Y.; He, L.; Yang, T.; Zeng, Z.; Chen, Z.; Liu, J.H. Dissemination of the fosfomycin resistance gene fosA3 with CTXM β-lactamase genes and rmtB carried on IncFII plasmids among Escherichia coli isolates from pets in China. Antimicrob. Agents Chemother. 2012, 56, 2135–2138. [Google Scholar] [CrossRef]
- Shin, S.W.; Shin, M.K.; Jung, M.; Belaynehe, K.M.; Yoo, H.S. Prevalence of antimicrobial resistance and transfer of tetracycline resistance genes in Escherichia coli isolates from beef cattle. Appl. Environ. Microbiol. 2015, 81, 5560–5566. [Google Scholar] [CrossRef]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef]
- Recchia, G.D.; Hall, R.M. Gene cassettes: A new class of mobile element. Microbiology 1995, 141, 3015–3027. [Google Scholar] [CrossRef]
- Pattishall, K.H.; Acar, J.; Burchall, J.J.; Goldstein, F.W.; Harvey, R.J. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J. Biol. Chem. 1977, 252, 2319–2323. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.L.; Kirchner, M.; Guerra, B.; Granier, S.A.; Lucarelli, C.; Porrero, M.C.; Jakubczak, A.; Threlfall, E.J.; Mevius, D.J. Multiresistant Salmonella enterica serovar 4,[5],12:i:- in Europe: A new pandemic strain? Eurosurveillance 2010, 15, 19580. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Jahn, S.; Schroeter, A.; Malorny, B.; Helmuth, R.; Guerra, B. Extended-spectrum β-lactamases in German isolates belonging to the emerging monophasic Salmonella enterica subsp. Enterica serovar Typhimurium 4,[5],12:i:- European clone. J. Antimicrob. Chemoth. 2012, 67, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Frana, T.S.; Carlson, S.A.; Griffith, R.W. Relative distribution and conservation of genes encoding aminoglycoside-modifying enzymes in Salmonella enterica serotype typhimurium phage type DT104. Appl. Environ. Microbiol. 2001, 67, 445–448. [Google Scholar] [CrossRef]
- Lucarelli, C.; Dionisi, A.M.; Torpdahl, M.; Villa, L.; Graziani, C.; Hopkins, K.; Threlfall, J.; Caprioli, A.; Luzzi, I. Evidence for a second genomic island conferring multidrug resistance in a clonal group of strains of Salmonella enterica serovar Typhimurium and its monophasic variant circulating in Italy, Denmark, and the United Kingdom. J. Clin. Microbiol. 2010, 48, 2103–2109. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Finley, R.; Allen, V.; Ang, L.; Bekal, S.; El Bailey, S.; Haldane, D.; Hoang, L.; Horsman, G.; Louie, M.; et al. Emergence of multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- involving human cases in Canada: Results from the Canadian Integrated Program on Antimicrobial Resistance Surveillance (CIPARS), 2003–2010. J. Antimicrob. Chemother. 2013, 68, 1982–1986. [Google Scholar] [CrossRef]
- He, J.J.; Sun, F.; Sun, D.W.; Wang, Z.Y.; Jin, S.S.; Pan, Z.M.; Xu, Z.Z.; Chen, X.; Jiao, X.A. Multidrug resistance and prevalence of quinolone resistance genes of Salmonella enterica serotypes 4,[5],12:i:- in China. Int. J. Food Microbiol. 2020, 330, 108692. [Google Scholar] [CrossRef]
- Carroll, L.M.; Zurfluh, K.; Jang, H.; Gopinath, G.; Nuesch-Inderbinen, M.; Poirel, L.; Nordmann, P.; Stephan, R.; Guldimann, C. First report of an mcr-1 harboring Salmonella enterica subsp. Enterica serotype 4,5,12:i:- strain isolated from blood of a patient in Switzerland. Int. J. Antimicrob. Agents 2018, 52, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Portes, A.B.; Rodrigues, G.; Leitão, M.P.; Ferrari, R.; Junior, C.A.C.; Panzenhagen, P. Global distribution of plasmid-mediated colistin resistance mcr gene in Salmonella: A systematic review. J. Appl. Microbiol. 2022, 132, 872–889. [Google Scholar] [CrossRef]
- Faja, O.M.; Sharad, A.A.; Yoanis, K.M.; Alwan, M.G.; Mohammed, B.J.; Ahmad, A. Isolation, detection of virulence genes, antibiotic resistance genes, plasmid profile, and molecular typing among Vibrio parahaemolyticus isolated in Malaysian seawater from recreational beaches and fish. Vet. World 2019, 12, 1140–1149. [Google Scholar] [CrossRef]
- Diep, T.T.; Nguyen, N.T.; Nguyen, T.N.; An, H.K.; Nguyen, T.Q.; Nguyen, V.H.; Nguyen, T.V.; Nguyen, T.N.; Izumiya, H.; Ohnishi, M.; et al. Isolation of New Delhi metallo-β-lactamase 1-producing Vibrio cholerae non-O1, non-O139 strain carrying ctxA, st and hly genes in southern Vietnam. Microbiol. Immunol. 2015, 59, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Shivakumaraswamy, S.K.; Deekshit, V.K.; Vittal, R.; Akhila, D.S.; Mundanda, D.M.; Mohan Raj, J.R.; Chakraborty, A.; Karunasagar, I. Phenotypic & genotypic study of antimicrobial profile of bacteria isolates from environmental samples. Indian J. Med. Res. 2019, 149, 232–239. [Google Scholar] [PubMed]
- Ng, L.K.; Martin, I.; Alfa, M.; Mulvey, M. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell. Probes. 2001, 15, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, Z.; Chan, E.W.; Dong, N.; Xia, X.; Chen, S. Molecular Characterization of qnrVC Genes and Their Novel Alleles in Vibrio spp. Isolated from Food Products in China. Antimicrob. Agents Chemother. 2018, 62, e00529-18. [Google Scholar] [CrossRef]
- Baron, S.; Lesne, J.; Jouy, E.; Larvor, E.; Kempf, I.; Boncy, J.; Rebaudet, S.; Piarroux, R. Antimicrobial Susceptibility of Autochthonous Aquatic Vibrio cholerae in Haiti. Front. Microbiol. 2016, 7, 1671. [Google Scholar] [CrossRef]
- Osode, A.N.; Okoh, A.I. Impact of discharged wastewater final effluent on the physicochemical qualities of a receiving watershed in a suburban community of the Eastern Cape Province. Clean-Soil Air Water. 2009, 37, 938–944. [Google Scholar] [CrossRef]
- Igwaran, A.; Okoh, A.I. Occurrence, Virulence and Antimicrobial Resistance-Associated Markers in Campylobacter Species Isolated from Retail Fresh Milk and Water Samples in Two District Municipalities in the Eastern Cape Province, South Africa. Antibiotics 2020, 9, 426. [Google Scholar] [CrossRef]
- Liu, B.T.; Song, F.J.; Zou, M.; Hao, Z.H.; Shan, H. Emergence of Colistin Resistance Gene mcr-1 in Cronobacter sakazakii Producing NDM-9 and in Escherichia coli from the Same Animal. Antimicrob. Agents Chemother. 2017, 61, e01444-16. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Feng, Y.; He, D.; Wang, C.; Zong, Z. Potential Mobilization of mcr-10 by an Integrative Mobile Element via Site-Specific Recombination in Cronobacter sakazakii. Antimicrob. Agents Chemother. 2021, 65, e01717-20. [Google Scholar] [CrossRef]
- Parra-Flores, J.; Holý, O.; Riffo, F.; Lepuschitz, S.; Maury-Sintjago, E.; Rodríguez-Fernández, A.; Cruz-Córdova, A.; Xicohtencatl-Cortes, J.; Mancilla-Rojano, J.; Troncoso, M.; et al. Profiling the Virulence and Antibiotic Resistance Genes of Cronobacter sakazakii Strains Isolated From Powdered and Dairy Formulas by Whole-Genome Sequencing. Front. Microbiol. 2021, 12, 694922. [Google Scholar] [CrossRef]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Brück, W.M.; Allahyari, S.; Rossen, J.W.A.; Mahmoudi, R. Antibiotic Resistance and Molecular Characterization of Cronobacter sakazakii Strains Isolated from Powdered Infant Formula Milk. Foods 2022, 11, 1093. [Google Scholar] [CrossRef]
- Falagas, M.; Athanasaki, F.; Voulgaris, G.; Triarides, N.; Vardakas, K. Resistance to fosfomycin: Mechanisms, frequency and clinical consequences. Int. J. Antimicrob. Agents. 2019, 53, 22–28. [Google Scholar] [CrossRef]
- Parra-Flores, J.; Holý, O.; Bustamante, F.; Lepuschitz, S.; Pietzka, A.; Contreras-Fernández, A.; Castillo, C.; Ovalle, C.; Alarcón-Lavín, M.P.; Cruz-Córdova, A.; et al. Virulence and Antibiotic Resistance Genes in Listeria monocytogenes Strains Isolated from Ready-to-Eat Foods in Chile. Front. Microbiol. 2022, 12, 796040. [Google Scholar] [CrossRef]
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Hanes, R.M.; Huang, Z. Investigation of Antimicrobial Resistance Genes in Listeria monocytogenes from 2010 through to 2021. Int. J. Environ. Res. Public Health 2022, 19, 5506. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.K.R.; Edwards, T.A.; O’Neill, A.J. ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio 2016, 7, e01975-15. [Google Scholar] [CrossRef]
- Hua, M.; Huang, W.; Chen, A.; Rehmet, M.; Jin, C.; Huang, Z. Comparison of antimicrobial resistance detected in environmental and clinical isolates from historical data for the US. BioMed Res. Int. 2020, 51, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Bi, C.; Mehmood, K.; Ali, F.; Qin, J.; Han, Z. Molecular characterization of multi-drug-resistant Staphylococcus aureus in mastitis bovine milk from a dairy farm in Anhui, China. Front. Vet. Sci. 2022, 9, 966533. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Vanhoof, R.; Godard, C.; Content, J.; Nyssen, H.J.; Hannecart-Pokorni, E. Detection by polymerase chain reaction of genes encoding aminoglycoside-modifying enzymes in methicillin-resistant Staphylococcus aureus isolates of epidemic phage types. Belgian Study Group of Hospital Infections (GDEPIH/GOSPIZ). J. Med. Microbiol. 1994, 41, 282–290. [Google Scholar] [CrossRef]
- Yadegar, A.; Sattari, M.; Mozafari, N.A.; Goudarzi, G.R. Prevalence of the genes encoding aminoglycoside-modifying enzymes and methicillin resistance among clinical isolates of Staphylococcus aureus in Tehran, Iran. Microb. Drug. Resist. 2009, 15, 109–113. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, T.; Lee, B.B.; Kim, U.S.; Park, S.W.; Shin, J.W.; Oh, M.-D.; Kim, E.-C.; Lee, Y.-S.; Kim, B.-S.; et al. Frequency of resistance to aminoglycoside antibiotics in Staphylococcus aureus isolates from tertiary hospitals. Korean J. Infect. Dis. 2002, 34, 39–46. [Google Scholar]
- Duran, N.; Ozer, B.; Duran, G.G.; Onlen, Y.; Demir, C. Antibiotic resistance genes & susceptibility patterns in staphylococci. Indian. J. Med. Res. 2012, 135, 389–396. [Google Scholar]
- Weisblum, B. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 1995, 39, 577–585. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M. Mechanisms of Resistance to Macrolide Antibiotics among Staphylococcus aureus. Antibiotics 2021, 10, 1406. [Google Scholar] [CrossRef]
- Lienen, T.; Grobbel, M.; Tenhagen, B.A.; Maurischat, S. Plasmid-Coded Linezolid Resistance in Methicillin-Resistant Staphylococcus aureus from Food and Livestock in Germany. Antibiotics 2022, 11, 1802. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.M.; Donlan, R.M.; Shin, D.H.; Jensen, B.; Clark, N.C.; McDougal, L.K.; Zhu, W.; Musser, K.A.; Thompson, J.; Kohlerschmidt, D.; et al. High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob. Agents Chemother. 2007, 51, 231–238. [Google Scholar] [CrossRef]
- Mahey, N.; Tambat, R.; Chandal, N.; Verma, D.K.; Thakur, K.G.; Nandanwar, H. Repurposing Approved Drugs as Fluoroquinolone Potentiators to Overcome Efflux Pump Resistance in Staphylococcus aureus. Microbiol. Spectr. 2021, 9, e0095121. [Google Scholar] [CrossRef] [PubMed]
- Maranan, M.C.; Moreira, B.; Boyle-Vavra, S.; Daum, R.S. Antimicrobial resistance in staphylococci. Epidemiology, molecular mechanisms, and clinical relevance. Infect. Dis. Clin. N. Am. 1997, 11, 813–849. [Google Scholar] [CrossRef] [PubMed]
- Dechêne-Tempier, M.; Marois-Créhan, C.; Libante, V.; Jouy, E.; Leblond-Bourget, N.; Payot, S. Update on the Mechanisms of Antibiotic Resistance and the Mobile Resistome in the Emerging Zoonotic Pathogen Streptococcus suis. Microorganisms 2021, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.; Radhouani, H.; Coelho, C.; Gonçalves, A.; Carvalho, E.; Carvalho, J.A.; Ruiz-Larrea, F.; Torres, C.; Igrejas, G.; Poeta, P. Prevalence and mechanisms of erythromycin resistance in Streptococcus agalactiae from healthy pregnant women. Microb. Drug. Resist. 2009, 15, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, L.; Li, D.; Wang, M.; Du, F.; Gao, Y.; Wu, Z.; Wang, L. Emergence of a vanG-carrying and multidrug resistant ICE in zoonotic pathogen Streptococccus suis. Vet. Microbiol. 2018, 222, 109–113. [Google Scholar] [CrossRef]
- Du, F.; Lv, X.; Duan, D.; Wang, L.; Huang, J. Characterization of a Linezolid- and Vancomycin-Resistant Streptococcus suis Isolate That Harbors optrA and vanG Operons. Front. Microbiol. 2019, 10, 2026. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Song, L.; Liu, Y.; He, T.; Liu, H.; Wu, C.; Schwarz, S.; Shen, J. First Report of the Multiresistance Gene cfr in Streptococcus suis. Antimicrob. Agents Chemother. 2013, 57, 4061–4063. [Google Scholar] [CrossRef]
- Shang, Y.; Li, D.; Hao, W.; Schwarz, S.; Shan, X.; Liu, B.; Zhang, S.-M.; Li, X.-S.; Du, X.-D. A Prophage and Two ICESa2603-Family Integrative and Conjugative Elements (ICEs) Carrying optrA in Streptococcus suis. J. Antimicrob. Chemother. 2019, 74, 2876–2879. [Google Scholar] [CrossRef]
- Imwattana, K.; Knight, D.R.; Kullin, B.; Collins, D.A.; Putsathit, P.; Kiratisin, P.; Riley, T.V. Antimicrobial resistance in Clostridium difcile ribotype 017. Expert. Rev. Anti Infect. Ther. 2020, 8, 17–25. [Google Scholar] [CrossRef]
- Marsh, J.W.; Pacey, M.P.; Ezeonwuka, C.; Ohm, S.L.; Snyder, D.; Cooper, V.S.; Harrison, L.H.; Doi, Y.; Mustapha, M.M. Clostridioides difcile: A potential source of npmA in the clinical environment. J. Antimicrob. Chemother. 2019, 74, 521–525. [Google Scholar] [CrossRef]
- Spigaglia, P.; Mastrantonio, P.; Barbanti, F. Antibiotic Resistances of Clostridium difcile; Springer: Cham, Switzerland, 2018; pp. 137–159. [Google Scholar]
- Hong, S.; Knight, D.R.; Riley, T.V. The impact of antimicrobial resistance on induction, transmission and treatment of Clostridium difcile infection. Microbiology 2019, 40, 77–81. [Google Scholar]
- Mackin, K.E.; Elliott, B.; Kotsanas, D.; Howden, B.P.; Carter, G.P.; Korman, T.M.; Riley, T.V.; Rood, J.I.; Jenkin, G.A.; Lyras, D. Molecular characterization and antimicrobial susceptibilities of Clostridium difcile clinical isolates from Victoria, Australia. Anaerobe 2015, 34, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Jin, D.; Kim, H.B.; Stratton, C.W.; Wu, B.; Tang, Y.W.; Sun, X. Update on antimicrobial resistance in Clostridium diffcile: Resistance mechanisms and antimicrobial susceptibility testing. J. Clin. Microbiol. 2017, 55, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Deshpande, A.; Hevener, K.E.; Endres, B.T.; Garey, K.W.; Palmer, K.L.; Hurdle, J.G. Constitutive expression of the cryptic vanGCd operon promotes vancomycin resistance in Clostridioides difcile clinical isolates. J. Antimicrob. Chemother. 2020, 75, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.M.; Lynch, T.; McCorrister, S.; Kibsey, P.; Miller, M.; Gravel, D.; Westmacott, G.R.; Mulvey, M.R.; the Canadian Nosocomial Infection Surveillance Program (CNISP). Proteomic analysis of a NAP1 Clostridium difficile clinical isolate resistant to metronidazole. PLoS ONE 2014, 9, e82622. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Chong, P.; Zhang, J.; Hizon, R.; Du, T.; Graham, M.R.; Beniac, D.R.; Booth, T.F.; Kibsey, P.; Miller, M.; et al. Characterization of a stable, metronidazole-resistant Clostridium difficile clinical isolate. PLoS ONE 2013, 8, e53757. [Google Scholar] [CrossRef]
- Kuehne, S.A.; Dempster, A.W.; Collery, M.M.; Joshi, N.; Jowett, J.; Kelly, M.L.; Cave, R.; Longshaw, C.M.; Minton, N.P. Characterization of the impact of rpoB mutations on the in vitro and in vivo competitive ftness of Clostridium difcile and susceptibility to fdaxomicin. J. Antimicrob. Chemother. 2018, 73, 973–980. [Google Scholar] [CrossRef]
- Schwanbeck, J.; Riedel, T.; Laukien, F.; Schober, I.; Oehmig, I.; Zimmermann, O.; Overmann, J.; Groß, U.; Zautner, A.E.; Bohne, W. Characterization of a clinical Clostridioides difcile isolate with markedly reduced fdaxomicin susceptibility and a V1143D mutation in rpoB. J. Antimicrob. Chemother. 2019, 74, 6–10. [Google Scholar] [CrossRef]
- Baines, S.D.; Wilcox, M.H. Antimicrobial resistance and reduced susceptibility in Clostridium difcile: Potential consequences for induction, treatment, and recurrence of C. difcile infection. Antibiotics 2015, 4, 267–298. [Google Scholar] [CrossRef]
- Spigaglia, P.; Barbanti, F.; Mastrantonio, P.; on behalf of the European Study Group on Clostridium difficile (ESGCD); Ackermann, G.; Balmelli, C.; Barbut, F.; Bouza, E.; Brazier, J.; Delmée, M.; et al. Multidrug resistance in European Clostridium difcile clinical isolates. J. Antimicrob. Chemother. 2011, 66, 2227–2234. [Google Scholar] [CrossRef]
- Carlson, T.J.; Endres, B.T.; Bassères, E.; Gonzales-Luna, A.J.; Garey, K.W. Ridinilazole for the treatment of Clostridioides diffcile infection. Expert Opin. Investig. Drugs 2019, 28, 303–310. [Google Scholar] [CrossRef]
- De Palma, G.Z.; Mendiondo, N.; Wonaga, A.; Viola, L.; Ibarra, D.; Campitelli, E.; Salim, N.; Corti, R.; Goldman, C.; Catalano, M. Occurrence of mutations in the antimicrobial target genes related to levofloxacin, clarithromycin, and amoxicillin resistance in Helicobacter pylori isolates from Buenos Aires City. Microb. Drug Res. 2017, 23, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.J.; Sheikh, A.F.; Goodarzi, H.; Yadyad, M.J.; Seyedian, S.S.; Aslani, S.; Assarzadegan, M.A. Genetic basis for metronidazole and clarithromycin resistance in Helicobacter pylori strains isolated from patients with gastroduodenal disorders. Infect. Drug Res. 2019, 12, 535–543. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Cook, N. Foodborne Pathogens. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 83–86. [Google Scholar]
- Liu, D. Aeromonas. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 1099–1110. [Google Scholar]
- Tekedar, H.C.; Arick, M.A.; Hsu, C.-Y.; Thrash, A.; Blom, J.; Lawrence, M.L.; Abdelhamed, H. Identification of Antimicrobial Resistance Determinants in Aeromonas veronii Strain MS-17-88 Recovered From Channel Catfish (Ictalurus punctatus). Front. Cell. Infect. Microbiol. 2020, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- De Silva, L.A.D.S.; Wickramanayake, M.V.K.S.; Heo, G.J. Virulence and antimicrobial resistance potential of Aeromonas spp. associated with shellfish. Lett. Appl. Microbiol. 2021, 73, 176–186. [Google Scholar] [CrossRef]
- Ghenghesh, K.S.; El-Mohammady, H.; Levin, S.Y.; Zorgani, A.; Tawil, K. Antimicrobial resistance profile of Aeromonas species isolated from Libya. Libyan J. Med. 2013, 8, 21320. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.L.; Ko, W.C.; Wu, C.J. Complexity of β-lactamases among clinical Aeromonas isolates and its clinical implications. J. Microbiol. Immunol. Infect. 2012, 45, 398–403. [Google Scholar] [CrossRef]
- Bello-López, J.M.; Cabrero-Martínez, O.A.; Ibáñez-Cervantes, G.; Hernández-Cortez, C.; Pelcastre-Rodríguez, L.I.; Gonzalez-Avila, L.U.; Castro-Escarpulli, G. Horizontal Gene Transfer and Its Association with Antibiotic Resistance in the Genus Aeromonas spp. Microorganisms 2019, 7, 363. [Google Scholar] [CrossRef]
- Enciso-Martínez, Y.; González-Aguilar, G.A.; Martínez-Téllez, M.A.; González-Pérez, C.J.; Valencia-Rivera, D.E.; Barrios-Villa, E.; Ayala-Zavala, J.F. Relevance of tracking the diversity of Escherichia coli pathotypes to reinforce food safety. Int. J. Food Microbiol. 2022, 374, 109736. [Google Scholar] [CrossRef]
- Asadi, Z.; Ghanbarpour, R.; Kalantar-Neyestana, D.; Alizade, H. Determination of extended-spectrum β-lactamase producing and hybrid pathotypes of Escherichia coli isolates from diarrheic samples. Gene Rep. 2022, 27, 101583. [Google Scholar] [CrossRef]
- Lindstedt, B.A.; Finton, M.D.; Porcellato, D.; Brandal, L.T. High frequency of hybrid Escherichia coli strains with combined Intestinal Pathogenic Escherichia coli (IPEC) and Extraintestinal Pathogenic Escherichia coli (ExPEC) virulence factors isolated from human faecal samples. BMC Infect. Dis. 2018, 18, 544. [Google Scholar] [CrossRef] [PubMed]
- Nüesch-Inderbinen, M.; Cernela, N.; Wüthrich, D.; Egli, A.; Stephan, R. Genetic characterization of Shiga toxin producing Escherichia coli belonging to the emerging hybrid pathotype O80:H2 isolated from humans 2010–2017 in Switzerland. Int. J. Med. Microbiol. 2018, 308, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Moreno, E.; Caporal-Hernandez, L.; Mendez-Pfeiffer, P.A.; Enciso-Martinez, Y.; De la Rosa López, R.; Valencia, D.; Arenas-Hernández, M.M.P.; Ballesteros-Monrreal, M.G.; Barrios-Villa, E. Characterization of Diarreaghenic Escherichia coli Strains Isolated from Healthy Donors, including a Triple Hybrid Strain. Antibiotics 2022, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, N.; Berger, M.; Kouzel, I.U.; Mellmann, A.; Berger, P. Comparative virulence characterization of the Shiga toxin phage-cured Escherichia coli O104:H4 and enteroaggregative Escherichia coli. Int. J. Med. Microbiol. 2018, 308, 912–920. [Google Scholar] [CrossRef]
- Alegría, Á.; Arias-Temprano, M.; Fernández-Natal, I.; Rodríguez-Calleja, J.M.; García-López, M.L.; Santos, J.A. Molecular Diversity of ESBL-Producing Escherichia coli from Foods of Animal Origin and Human Patients. Int. J. Environ. Res. Public Health 2020, 17, 1312. [Google Scholar] [CrossRef]
- Dembélé, R.; Konaté, A.; Traoré, O.; Kaboré, W.A.D.; Soulama, I.; Kagambèga, A.; Traoré, A.S.; Guessennd, N.K.; Aidara-Kane, A.; Gassama-Sow, A.; et al. Extended spectrum beta-lactamase and fluoroquinolone resistance genes among Escherichia coli and Salmonella isolates from children with diarrhea, Burkina Faso. BMC Pediatr. 2020, 20, 459. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Yang, G.; Lei, T.; Chen, M.; Ye, Q.; Wang, J.; Gu, Q.; Wei, X.; Zhang, J.; et al. High prevalence of multidrug-resistant Escherichia coli and first detection of IncHI2/IncX4-plasmid carrying mcr-1 E. coli in retail ready-to-eat foods in China. Int. J. Food Microbiol. 2021, 355, 109349. [Google Scholar] [CrossRef]
- Findlay, J.; Perreten, V.; Poirel, L.; Nordmann, P. Molecular analysis of OXA-48-producing Escherichia coli in Switzerland from 2019 to 2020. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1355–1360. [Google Scholar] [CrossRef]
- Irrgang, A.; Hammerl, J.A.; Falgenhauer, L.; Guiral, E.; Schmoger, S.; Imirzalioglu, C.; Fischer, J.; Guerra, B.; Chakraborty, T.; Käsbohrer, A. Diversity of CTX-M-1-producing E. coli from German food samples and genetic diversity of the blaCTX-M-1 region on IncI1 ST3 plasmids. Vet. Microbiol. 2018, 221, 98–104. [Google Scholar] [CrossRef]
- Sivakumar, M.; Abass, G.; Vivekanandhan, R.; Anukampa; Singh, D.K.; Bhilegaonkar, K.; Kumar, S.; Grace, M.R.; Dubal, Z. Extended-spectrum beta-lactamase (ESBL) producing and multidrug-resistant Escherichia coli in street foods: A public health concern. J. Food Sci. Technol. 2021, 58, 1247–1261. [Google Scholar] [CrossRef]
- Canizalez-Roman, A.; Gonzalez-Nuñez, E.; Vidal, J.E.; Flores-Villaseñor, H.; León-Sicairos, N. Prevalence and antibiotic resistance profiles of diarrheagenic Escherichia coli strains isolated from food items in northwestern Mexico. Int. J. Food Microbiol. 2013, 164, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Ardiyati, T.; Rifa’i, M. Widodo Detection of class 1 integron-associated gene cassettes and tetracycline resistance genes in Escherichia coli isolated from ready to eat vegetables. Ann. Med. Surg. 2020, 55, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paredes, D.; de Janon, S.; Villavicencio, F.; Ruales, K.J.; De La Torre, K.; Villacís, J.E.; Wagenaar, J.A.; Matheu, J.; Bravo-Vallejo, C.; Fernandez-Moreira, E.; et al. Broiler farms and carcasses are an important reservoir of multi-drug resistant Escherichia coli in Ecuador. Front. Vet. Sci. 2020, 7, 979. [Google Scholar] [CrossRef]
- Samy, A.A.; Mansour, A.S.; Khalaf, D.D.; Khairy, E.A. Development of multidrug-resistant Escherichia coli in some Egyptian veterinary farms. Vet. World 2022, 15, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Talib, M.A.A.; Radu, S.; Kqueen, C.Y.; Ghazali, F.M. Salmonella: The Critical Enteric Foodborne Pathogen. In Enterobacteria; Bhardwaj, S.B., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Eady, M.; Park, B. The Influence of Environmental Growth Conditions on Salmonella Spectra Obtained from Hyperspectral Microscope Images. Food Anal. Methods 2019, 12, 2638–2646. [Google Scholar] [CrossRef]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef]
- Krzyżewska-Dudek, E.; Kotimaa, J.; Kapczyńska, K.; Rybka, J.; Meri, S. Lipopolysaccharides and outer membrane proteins as main structures involved in complement evasion strategies of non-typhoidal Salmonella strains. Mol. Immunol. 2022, 150, 67–77. [Google Scholar] [CrossRef]
- Fatica, M.K.; Schneider, K.R. Salmonella and produce: Survival in the plant environment and implications in food safety. Virulence 2011, 2, 573–579. [Google Scholar] [CrossRef]
- Marchello, C.S.; Birkhold, M.; Crump, J.A. Vacc-iNTS Consortium Complications and mortality of non-typhoidal Salmonella invasive disease: A global systematic review and meta-analysis. Lancet Infect. Dis. 2022, 22, 692–705. [Google Scholar] [CrossRef]
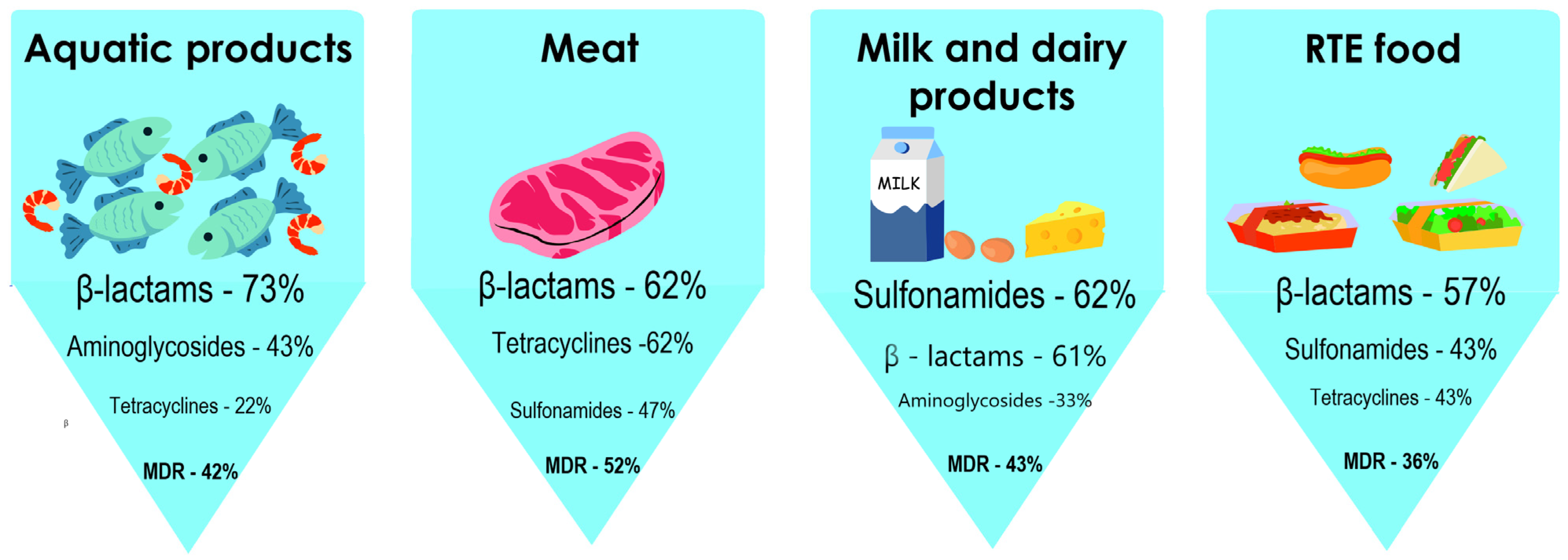
- Ge, H.; Wang, Y.; Zhao, X. Research on the drug resistance mechanism of foodborne pathogens. Microb. Pathog. 2022, 162, 105306. [Google Scholar] [CrossRef]
- Jacob, J.J.; Solaimalai, D.; Rachel, T.; Pragasam, A.K.; Sugumar, S.; Jeslin, P.; Anandan, S.; Veeraraghavan, B. A secular trend in invasive non-typhoidal Salmonella in South India, 2000-2020: Identification challenges and antibiogram. Indian J. Med. Microbiol. 2022, 40, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fang, T.; Zhou, X.; Zhang, D.; Shi, X.; Shi, C. IncHI2 Plasmids Are Predominant in Antibiotic-Resistant Salmonella Isolates. Front. Microbiol. 2016, 7, 1566. [Google Scholar] [CrossRef] [PubMed]
- Nadi, Z.R.; Salehi, T.Z.; Tamai, I.A.; Foroushani, A.R.; Sillanpaa, M.; Dallal, M.M.S. Evaluation of Antibiotic Resistance and Prevalence of Common Salmonella enterica Serovars Isolated from Foodborne Outbreaks. Microchem. J. 2020, 155, 104–660. [Google Scholar] [CrossRef]
- Kariuki, S.; Gordon, M.A.; Feasey, N.; Parry, C.M. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 2015, 33, C21–C29. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, Q.; Alali, W.Q.; Wang, J.; Meng, L.; Xiao, Y.; Yang, H.; Chen, S.; Cui, S.; Yang, B. Characterization of extended-spectrum β-lactamases (ESBLs)-producing Salmonella in retail raw chicken carcasses. Int. J. Food Microbiol. 2017, 248, 72–81. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Xu, X.; Fu, Y.; Xiong, Z.; Zhang, L.; Qu, X.; Zhang, H.; Wei, Y.; Zhan, Z.; et al. High-levels of resistance to quinolone and cephalosporin antibiotics in MDR-ACSSuT Salmonella enterica serovar Enteritidis mainly isolated from patients and foods in Shanghai, China. Int. J. Food Microbiol. 2018, 286, 190–196. [Google Scholar] [CrossRef]
- Yukawa, S.; Tamura, Y.; Tanaka, K.; Uchida, I. Rapid detection of Salmonella enterica serovar Typhimurium DT104 strains by the polymerase chain reaction. Acta Vet. Scand. 2015, 57, 59. [Google Scholar] [CrossRef]
- Mølbak, K.; Baggesen, D.L.; Aarestrup, F.M.; Ebbesen, J.M.; Engberg, J.; Frydendahl, K.; Gerner-Smidt, P.; Petersen, A.M.; Wegener, H.C. An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica serotype typhimurium DT104. N. Engl. J. Med. 1999, 341, 1420–1425. [Google Scholar] [CrossRef]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne Pathogenic Vibrios: Antimicrobial Resistance. Front. Microbiol. 2021, 12, 638331. [Google Scholar] [CrossRef]
- Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity 2022, 14, 97. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Okoh, A.I. Emerging Vibrio species: An unending threat to public health in developing countries. Res. Microbiol. 2008, 159, 495–506. [Google Scholar] [CrossRef]
- Le Roux, F.; Wegner, K.M.; Baker-Austin, C.; Vezzulli, L.; Osorio, C.R.; Amaro, C.; Ritchie, J.M.; Defoirdt, T.; Destoumieux-Garzón, D.; Blokesch, M.; et al. The emergence of Vibrio pathogens in Europe: Ecology, evolution, and pathogenesis. Front. Microbiol. 2015, 6, 830. [Google Scholar] [PubMed]
- Xu, F.; Gonzalez-Escalona, N.; Haendiges, J.; Myers, R.A.; Ferguson, J.; Stiles, T.; Hickey, E.; Moore, M.; Hickey, J.M.; Schillaci, C.; et al. Sequence type 631 Vibrio parahaemolyticus, an emerging foodborne pathogen in North America. J. Clin. Microbiol. 2017, 55, 645–648. [Google Scholar] [CrossRef]
- Elmahdi, S.; Parveen, S.; Ossai, S.; DaSilva, L.V.; Jahncke, M.; Bowers, J.; Jacobs, J. Vibrio parahaemolyticus and Vibrio vulnificus recovered from oysters during an oyster relay study. Appl. Environ. Microbiol. 2018, 84, e01790. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, J.; Li, H.; Tan, S.; Chen, Y.; Yu, H. Prevalence, Antibiotic Susceptibility and Diversity of Vibrio parahaemolyticus Isolates in Seafood from South China. Front. Microbiol. 2018, 8, 2566. [Google Scholar] [CrossRef] [PubMed]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Rukayadi, Y.; Hasan, H.; Thung, T.Y.; Lee, E.; Rollon, W.D.; Hara, H.; Kayali, A.Y.; Nishibuchi, M.; Radu, S. Prevalence and antibiotic resistance patterns of Vibrio parahaemolyticus isolated from different types of seafood in Selangor, Malaysia. Saudi J. Biol. Sci. 2020, 27, 1602–1608. [Google Scholar] [CrossRef]
- Guk, J.H.; Woo, J.H.; Song, H.; Kim, W.H.; Kim, J.; Ryu, S.; Cho, S. Hyper-aerotolerant Campylobacter coli, an emerging foodborne pathogen, shows differential expressions of oxidative stress-related genes. Vet. Microbiol. 2022, 264, 109308. [Google Scholar] [CrossRef]
- Thames, H.T.; Sukumaran, A.T. A Review of Salmonella and Campylobacter in Broiler Meat: Emerging Challenges and Food Safety Measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Kounnoun, A.; Bougtaib, H.; Belmehdi, O.; Senhaji, N.S.; Abrini, J. Prevalence and antimicrobial profiling of Campylobacter spp. isolated from meats, animal, and human feces in Northern of Morocco. Int. J. Food Microbiol. 2021, 349, 109202. [Google Scholar] [CrossRef]
- Allos, B.M. Campylobacter jejuni Infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [PubMed]
- Mota-Gutierrez, J.; Lis, L.; Lasagabaster, A.; Nafarrate, I.; Ferrocino, I.; Cocolin, L.; Rantsiou, K. Campylobacter spp. prevalence and mitigation strategies in the broiler production chain. Food Microbiol. 2022, 104, 103998. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.M.; Balan, K.V.; Hiett, K.L.; Babu, U.S. Current methodologies and future direction of Campylobacter isolation and detection from food matrices, clinical samples, and the agricultural environment. J. Microbiol. Methods 2022, 201, 106562. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Ebrahimi, M.; Luangtongkum, T. The worldwide trend of Campylobacter spp., infection from duck-related isolates and associated phenotypic and genotypic antibiotic resistance, since 1985: Identifying opportunities and challenges for prevention and control. Poult. Sci. 2021, 100, 101213. [Google Scholar] [CrossRef]
- Lazou, T.P.; Serafeim, C.C. Comparison of disk diffusion and broth microdilution methods for antimicrobial susceptibility testing of Campylobacter isolates of meat origin. J. Microbiol. Methods 2023, 204, 106649. [Google Scholar] [CrossRef]
- Paintsil, E.K.; Ofori, L.A.; Adobea, S.; Akenten, C.W.; Phillips, R.O.; Maiga-Ascofare, O.; Lamshöft, M.; May, J.; Obiri Danso, K.; Krumkamp, R.; et al. Prevalence and Antibiotic Resistance in Campylobacter spp. Isolated from Humans and Food-Producing Animals in West Africa: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 140. [Google Scholar] [CrossRef]
- Carvalho, G.G.; Calarga, A.P.; Zorgi, N.E.; Astudillo-Trujillo, C.A.; Gontijo, M.T.P.; Brocchi, M.; Giorgio, S.; Kabuki, D.Y. Virulence and DNA sequence analysis of Cronobacter spp. isolated from infant cereals. Int. J. Food Microbiol. 2022, 376, 109745. [Google Scholar] [CrossRef]
- Gan, X.; Li, M.; Xu, J.; Yan, S.; Wang, W.; Li, F. Emerging of Multidrug-Resistant Cronobacter sakazakii Isolated from Infant Supplementary Food in China. Microbiol. Spectr. 2022, 10, e0119722. [Google Scholar] [CrossRef]
- Quaglia, N.C.; Dambrosio, A. Helicobacter pylori: A foodborne pathogen? World J. Gastroenterol. 2018, 24, 3472–3487. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Q.; Yuan, Y.; Gong, Y. The antibiotic resistance of Helicobacter pylori to five antibiotics and influencing factors in an area of China with a high risk of gastric cancer. BMC Microbiol. 2019, 19, 152. [Google Scholar] [CrossRef]
- Alba, C.; Blanco, A.; Alarcón, T. Antibiotic resistance in Helicobacter pylori. Curr. Opin. Infect. Dis. 2017, 30, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Nestegard, O.; Moayeri, B.; Halvorsen, F.A.; Tønnesen, T.; Sørbye, S.W.; Paulssen, E.; Johnsen, K.M.; Goll, R.; Florholmen, J.R.; Melby, K.K. Helicobacter pylori resistance to antibiotics before and after treatment: Incidence of eradication failure. PLoS ONE 2022, 17, e0265322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, J.; Wang, Z.; Li, H.; Wan, C. Antibiotic Resistance of Helicobacter pylori Strains Isolated From Pediatric Patients in Southwest China. Front. Microbiol. 2021, 11, 621791. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Ye, D.; Hu, C.; Peng, K.; Zhao, H.; Li, H.; Jiang, M. Alarming antibiotics resistance of Helicobacter pylori from children in Southeast China over 6 years. Sci. Rep. 2022, 12, 17754. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Bian, L.; Zhang, Y.; Li, Q.; Xu, Y.; She, Q.; Yan, C.; Lu, G.; Wu, J.; et al. Antibiotic Resistance of Helicobacter pylori and Related Risk Factors in Yangzhou, China: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 816. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Microbe Profile: Listeria monocytogenes: A paradigm among intercellular bacterial pathogens. Microbiology 2019, 165, 719–721. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, L.M. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Wiktorczyk-Kapischke, N.; Skowron, K.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Korkus, J.; Gospodarek-Komkowska, E. Adaptive Response of Listeria monocytogenes to the Stress Factors in the Food Processing Environment. Front. Microbiol. 2021, 12, 710085. [Google Scholar] [CrossRef]
- Nightingale, K.K.; Windham, K.; Wiedmann, M. Evolution and Molecular Phylogeny of Listeria monocytogenes Isolated from Human and Animal Listeriosis Cases and Foods. J. Bacteriol. 2005, 187, 5537–5551. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Sun, T.; Gorris, L.G.M.; Wang, X.; Liu, B.; Dong, Q. The prevalence of Listeria monocytogenes in meat products in China: A systematic literature review and novel meta-analysis approach. Int. J. Food Microbiol. 2020, 312, 108358. [Google Scholar] [CrossRef]
- Skowron, K.; Wiktorczyk, N.; Grudlewska, K.; Wałecka-Zacharska, E.; Paluszak, Z.; Kruszewski, S.; Gospodarek-Komkowska, E. Phenotypic and genotypic evaluation of Listeria monocytogenes strains isolated from fish and fish processing plants. Ann. Microbiol. 2019, 69, 469–482. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Prevent Listeria. Available online: https://www.cdc.gov/listeria/prevention.html (accessed on 13 December 2022).
- European Food Safety Authority (EFSA). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar]
- Smith, A.M.; Tau, N.P.; Smouse, S.L.; Allam, M.; Ismail, A.; Ramalwa, N.R.; Disenyeng, B.; Ngomane, M.; Thomas, J. Outbreak of Listeria monocytogenes in South Africa, 2017-2018: Laboratory activities and experiences associated with whole-genome sequencing analysis of isolates. Foodborne Pathog. Dis. 2019, 16, 524–530. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Listeriosis—Australia. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/09-april-2018-listeriosis-australia-en (accessed on 13 December 2022).
- Centers of Disease Control and Prevention (CDC). Timeline of events: Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado. Available online: https://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/timeline.html (accessed on 13 December 2022).
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Di Ciccio, P.; Meloni, D.; Festino, A.R.; Conter, M.; Zanardi, E.; Ghidini, S.; Vergara, A.; Mazzette, R.; Ianieri, A. Longitudinal study on the sources of Listeria monocytogenes contamination in cold-smoked salmon and its processing environment in Italy. Int. J. Food Microbiol. 2012, 158, 79–84. [Google Scholar] [CrossRef]
- Ramires, T.; Kleinubing, N.R.; Iglesias, M.A.; Vitola, H.R.S.; Núncio, A.S.P.; Kroning, I.S.; Moreira, G.M.S.G.; Fiorentini, M.; da Silva, W.P. Genetic diversity, biofilm and virulence characteristics of Listeria monocytogenes in salmon sushi. Food Res. Int. 2020, 140, 109871. [Google Scholar] [CrossRef]
- Lundén, J.M.; Autio, T.J.; Sjöberg, A.-M.; Korkeala, H.J. Persistent and Nonpersistent Listeria monocytogenes Contamination in Meat and Poultry Processing Plants. J. Food Prot. 2003, 66, 2062–2069. [Google Scholar] [CrossRef]
- Unrath, N.; McCabe, E.; Macori, G.; Fanning, S. Application of Whole Genome Sequencing to Aid in Deciphering the Persistence Potential of Listeria monocytogenes in Food Production Environments. Microorganisms 2021, 9, 1856. [Google Scholar] [CrossRef]
- Magalhães, R.; Ferreira, V.; Brandão, T.; Palencia, R.C.; Almeida, G.; Teixeira, P. Persistent and non-persistent strains of Listeria monocytogenes: A focus on growth kinetics under different temperature, salt, and pH conditions and their sensitivity to sanitizers. Food Microbiol. 2016, 53, 103–108. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 13 December 2022).
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef]
- Maury, M.M.; Tsai, Y.H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Hof, H. An update on the medical management of listeriosis. Expert Opin. Pharmacother 2004, 5, 1727–1735. [Google Scholar]
- Poyart-Salmeron, C.; Carlier, C.; Trieu-Cuot, P.; Courtieu, A.L.; Courvalin, P. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 1990, 335, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Morvan, A.; Moubareck, C.; Leclercq, A.; Hervé-Bazin, M.; Bremont, S.; Lecuit, M.; Courvalin, P.; Le Monnier, A. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob. Agents Chemother. 2010, 54, 2728–2731. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Saei-Dehkordi, S.S.; Mahzounieh, M. Occurrence and antibiotic resistance profiles of Listeria monocytogenes isolated from seafood products and market and processing environments in Iran. Food Control 2013, 34, 630–636. [Google Scholar] [CrossRef]
- Korsak, D.; Borek, A.; Daniluk, S.; Grabowska, A.; Pappelbaum, K. Antimicrobial susceptibilities of Listeria monocytogenes strains isolated from food and food processing environment in Poland. Int. J. Food Microbiol. 2012, 158, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, E.; Ojagh, S.M.; Hosseini, H.; Ghaemi, E.A.; Irajian, G.; Naghizadeh Heidarlo, M. Antimicrobial resistance of Listeria monocytogenes isolated from seafood and humans in Iran. Microb. Pathog. 2016, 100, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yeh, E.; Hall, G.; Cripe, J.; Bhagwat, A.A.; Meng, J. Characterization of Listeria monocytogenes isolated from retail foods. Int. J. Food Microbiol. 2007, 113, 47–53. [Google Scholar] [CrossRef]
- Sakaridis, I.; Soultos, N.; Iossifidou, E.; Papa, A.; Ambrosiadis, I.; Koidis, P. Prevalence and antimicrobial resistance of Listeria monocytogenes isolated in chicken slaughterhouses in northern Greece. J. Food Protect. 2011, 74, 1017–1021. [Google Scholar] [CrossRef]
- Wu, S.; Wu, Q.; Zhang, J.; Chen, M.; Yan, Z.A.; Hu, H. Listeria monocytogenes prevalence and characteristics in retail raw foods in China. PLoS ONE 2015, 10, e0136682. [Google Scholar] [CrossRef]
- Lee, D.Y.; Ha, J.H.; Lee, M.K.; Cho, Y.S. Antimicrobial susceptibility and serotyping of Listeria monocytogenes isolated from ready-to-eat seafood and food processing environments in Korea. Food Sci. Biotechnol. 2017, 26, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. GMR 2003, 2, 63–76. [Google Scholar] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. BioMed Res. Int. 2014, 827965. [Google Scholar] [CrossRef]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Heaton, C.J.; Gerbig, G.R.; Sensius, L.D.; Patel, V.; Smith, T.C. Staphylococcus aureus Epidemiology in Wildlife: A Systematic Review. Antibiotics 2020, 9, 89. [Google Scholar] [CrossRef]
- Benkerroum, N. Staphylococcal enterotoxins and enterotoxin-like toxins with special reference to dairy products: An overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 1943–1970. [Google Scholar] [CrossRef] [PubMed]
- Grispoldi, L.; Karama, M.; Armani, A.; Hadjicharalambous, C.; Cenci-Goga, B.T. Staphylococcus aureus enterotoxin in food of animal origin and staphylococcal food poisoning risk assessment from farm to table. Ital. J. Anim. Sci. 2021, 20, 677–690. [Google Scholar] [CrossRef]
- Sergelidis, D.; Angelidis, A.S. Methicillin-resistant Staphylococcus aureus: A controversial food-borne pathogen. Lett. Appl. Microbiol. 2017, 64, 409–418. [Google Scholar] [CrossRef]
- Wißmann, J.E.; Kirchhoff, L.; Brüggemann, Y.; Todt, D.; Steinmann, J.; Steinmann, E. Persistence of Pathogens on Inanimate Surfaces: A Narrative Review. Microorganisms 2021, 9, 343. [Google Scholar] [CrossRef]
- Ou, Q.; Peng, Y.; Lin, D.; Bai, C.; Zhang, T.; Lin, J.; Ye, X.; Yao, Z. A Meta-Analysis of the Global Prevalence Rates of Staphylococcus aureus and Methicillin-Resistant S. aureus Contamination of Different Raw Meat Products. J. Food Prot. 2017, 80, 763–774. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Zhu, C.; Ma, K.; Fang, M.; Li, B.; Wang, W.; Xue, T. Antibiotics Resistance and Virulence of Staphylococcus aureus Isolates Isolated from Raw Milk from Handmade Dairy Retail Stores in Hefei City, China. Foods 2022, 11, 2185. [Google Scholar] [CrossRef]
- Lv, G.; Jiang, R.; Zhang, H.; Wang, L.; Li, L.; Gao, W.; Zhang, H.; Pei, Y.; Wei, X.; Dong, H.; et al. Molecular Characteristics of Staphylococcus aureus From Food Samples and Food Poisoning Outbreaks in Shijiazhuang, China. Front. Microbiol. 2021, 12, 652276. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.-L.; Mahyudin, N.A.; Amin-Nordin, S.; Radu, S.; Abdul-Mutalib, N.A. Antimicrobial resistance of Staphylococcus aureus among cooked food and food handlers associated with their occupational information in Klang Valley, Malaysia. Food Control 2021, 124, 107872. [Google Scholar] [CrossRef]
- Komodromos, D.; Kotzamanidis, C.; Giantzi, V.; Pappa, S.; Papa, A.; Zdragas, A.; Angelidis, A.; Sergelidis, D. Prevalence, Infectious Characteristics and Genetic Diversity of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus (MRSA) in Two Raw-Meat Processing Establishments in Northern Greece. Pathogens 2022, 11, 1370. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, E.; Porada, K.; Wesołowska, M.; Łęska, B. Occurrence and Characteristics of Staphylococcus aureus Isolated from Dairy Products. Molecules 2022, 27, 4649. [Google Scholar] [CrossRef] [PubMed]
- Mahros, M.A.; Abd-Elghany, S.M.; Sallam, K.I. Multidrug-, methicillin-, and vancomycin-resistant Staphylococcus aureus isolated from ready-to-eat meat sandwiches: An ongoing food and public health concern. Int. J. Food Microbiol. 2021, 346, 109165. [Google Scholar] [CrossRef] [PubMed]
- Saber, T.; Samir, M.; El-Mekkawy, R.M.; Ariny, E.; El-Sayed, S.R.; Enan, G.; Abdelatif, S.H.; Askora, A.; Merwad, A.M.A.; Tartor, Y.H. Methicillin- and Vancomycin-Resistant Staphylococcus aureus From Humans and Ready-To-Eat Meat: Characterization of Antimicrobial Resistance and Biofilm Formation Ability. Front. Microbiol. 2022, 12, 735494. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.H.; Nazir, K.H.M.N.H.; El Zowalaty, M.E.; Kabir, A.; Sarker, S.A.; Siddique, M.P.; Ashour, H.M. Molecular detection of vancomycin and methicillin resistance in Staphylococcus aureus isolated from food processing environments. One Health 2021, 13, 100276. [Google Scholar] [CrossRef]
- Wongnak, P.; Wiratsudakul, A.; Nuanualsuwan, S. A risk assessment of pathogenic Streptococcus suis in pork supply chains and markets in Thailand. Food Control 2020, 118, 107432. [Google Scholar] [CrossRef]
- Kerdsin, A.; Segura, M.; Fittipaldi, N.; Gottschalk, M. Sociocultural Factors Influencing Human Streptococcus suis Disease in Southeast Asia. Foods 2022, 11, 1190. [Google Scholar] [CrossRef]
- Takeuchi, D.; Kerdsin, A.; Akeda, Y.; Chiranairadul, P.; Loetthong, P.; Tanburawong, N.; Areeratana, P.; Puangmali, P.; Khamisara, K.; Pinyo, W.; et al. Impact of a Food Safety Campaign on Streptococcus suis Infection in Humans in Thailand. Am. J. Trop. Med. Hyg. 2017, 96, 1370–1377. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Sroka, J.; Zając, V.; Wasiński, B.; Cisak, E.; Sawczyn, A.; Kloc, A.; Wójcik-Fatla, A. Streptococcus suis: A re-emerging pathogen associated with occupational exposure to pigs or pork products. Part I—Epidemiology. Ann. Agric. Environ. Med. 2017, 24, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; García, C.; Fraile, L.; Tommassen, J.; Arenas, J. How Streptococcus suis escapes antibiotic treatments. Vet. Res. 2022, 53, 91. [Google Scholar] [CrossRef] [PubMed]
- Yongkiettrakul, S.; Maneerat, K.; Arechanajan, B.; Malila, Y.; Srimanote, P.; Gottschalk, M.; Visessanguan, W. Antimicrobial susceptibility of Streptococcus suis isolated from diseased pigs, asymptomatic pigs, and human patients in Thailand. BMC Vet. Res. 2019, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Nedbalcova, K.; Kucharovicova, I.; Zouharova, M.; Matiaskova, K.; Kralova, N.; Brychta, M.; Simek, B.; Pecha, T.; Plodkova, H.; Matiasovic, J. Resistance of Streptococcus suis Isolates from the Czech Republic during 2018–2022. Antibiotics 2022, 11, 1214. [Google Scholar] [CrossRef]
- Dong, C.-L.; Che, R.-X.; Wu, T.; Qu, Q.-W.; Chen, M.; Zheng, S.-D.; Cai, X.-H.; Wang, G.; Li, Y.-H. New Characterization of Multi-Drug Resistance of Streptococcus suis and Biofilm Formation from Swine in Heilongjiang Province of China. Antibiotics 2023, 12, 132. [Google Scholar] [CrossRef]
- Wetzel, D.; McBride, S.M. The Impact of pH on Clostridioides difficile Sporulation and Physiology. Appl. Environ. Microbiol. 2020, 86, e02706-19. [Google Scholar] [CrossRef]
- Candel-Pérez, C.; Ros-Berruezo, G.; Martínez-Graciá, C. A review of Clostridioides [Clostridium] difficile occurrence through the food chain. Food Microbiol. 2019, 77, 118–129. [Google Scholar] [CrossRef]
- Lund, B.M.; Peck, M.W. A possible route for foodborne transmission of Clostridium difficile? Foodborne Pathog. Dis. 2015, 12, 177–182. [Google Scholar] [CrossRef]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Borgmann, S.; Kline, T.R.; LeJeune, J.T. Clostridium difficile in foods and animals: History and measures to reduce exposure. Anim. Health Res. Rev. 2013, 14, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Jafri, F.; Stuhr, D.; Knoll, B.M.; Lim, S.H. A contemporary review of Clostridioides difficile infections in patients with haematologic diseases. J. Intern. Med. 2021, 289, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther. Adv. Infect. Dis. 2016, 3, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Wickramage, I.; Spigaglia, P.; Sun, X. Mechanisms of antibiotic resistance of Clostridioides difficile. J. Antimicrob. Chemother. 2021, 76, 3077–3090. [Google Scholar] [CrossRef]
- Peng, Z.; Addisu, A.; Alrabaa, S.; Sun, X. Antibiotic Resistance and Toxin Production of Clostridium difficile Isolates from the Hospitalized Patients in a Large Hospital in Florida. Front. Microbiol. 2017, 8, 2584. [Google Scholar] [CrossRef]
- Heidari, H.; Sedigh Ebrahim-Saraie, H.; Amanati, A.; Motamedifar, M.; Hadi, N.; Bazargani, A. Toxin profiles and antimicrobial resistance patterns among toxigenic clinical isolates of Clostridioides (Clostridium) difficile. Iran. J. Basic Med. Sci. 2019, 22, 813–819. [Google Scholar]
- Sholeh, M.; Krutova, M.; Forouzesh, M.; Mironov, S.; Sadeghifard, N.; Molaeipour, L.; Maleki, A.; Kouhsari, E. Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 158. [Google Scholar] [CrossRef]

| Causative Agent | Foodborne Outbreaks | Cases of Illness | Hospitalization | Deaths | Food Vehicles Causing Strong-Evidence Outbreaks | |
|---|---|---|---|---|---|---|
| Salmonella spp. | 773 (143) * | 6755 | 1123 | 1 | eggs and egg products (39) **, mixed food (24), bakery products (15), pig meat and products thereof (14), vegetables and juices and other products thereof (11) | |
| Campylobacter spp. | 249 (20) | 1051 | 134 | 6 | broiler meat and products thereof (7), mixed food (5), bovine meat and products thereof (3), other mixed or unspecified poultry meat and products thereof (2) | |
| Escherichia coli | STEC | 31 (5) | 275 | 47 | 0 | bovine meat and products thereof (2), milk (1), vegetables and juices and other products thereof (1), meat and meat products unspecified (1) |
| other than STEC | 27 (4) | 327 | 44 | 0 | vegetables and juices and other products thereof | |
| Listeria monocytogenes | 23 (8) | 104 | 48 | 12 | fish and fish products (4), meat and meat products unspecified (2), other or mixed red meat and products thereof (1), broiler meat and products thereof (1) | |
| Vibrio cholera (non-toxigenic) | 1 (1) | 47 | 1 | 0 | mixed food, crustaceans, shellfish, mollusks and products thereof | |
| Vibrio parahaemolyticus | 3 (1) | 10 | 0 | 0 | ||
| Aeromonas spp. | 1 (1) | 19 | 0 | 0 | mixed foods | |
| Cronobacter sakazakii | 1 (1) | 4 | 4 | 1 | hospital-mixed probiotic formula for infants | |
| Staphylococcus aureus toxins | 61 (20) | 640 | 51 | 0 | mixed foods, other, mixed and/or unspecified poultry or red meat and products thereof, fish and fish products, dairy products, vegetables and juices and other products thereof | |
| Causative Agent | Cases of Illness | Hospitalization | Deaths | Food Vehicles |
|---|---|---|---|---|
| Salmonella Thompson | 115 | 20 | 0 | seafood |
| Salmonella Oranienburg | 1040 | 260 | 0 | whole, fresh onions |
| Salmonella Typhimurium | 31 | 4 | 0 | packaged salad greens |
| Salmonella | 9 | 3 | 0 | frozen cooked shrimp |
| Weltevreden | ||||
| Salmonella | 20 | 5 | 0 | cashew brie |
| Duisburg | ||||
| E. coli O157:H7 | 47 | 19 | 1 | spinach, |
| packaged salad, | ||||
| unknown | ||||
| E. coli O121 | 16 | 7 | 0 | cake mix |
| Listeria monocytogenes | 28 | 26 | 4 | packaged salad |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudlewska-Buda, K.; Bauza-Kaszewska, J.; Wiktorczyk-Kapischke, N.; Budzyńska, A.; Gospodarek-Komkowska, E.; Skowron, K. Antibiotic Resistance in Selected Emerging Bacterial Foodborne Pathogens—An Issue of Concern? Antibiotics 2023, 12, 880. https://doi.org/10.3390/antibiotics12050880
Grudlewska-Buda K, Bauza-Kaszewska J, Wiktorczyk-Kapischke N, Budzyńska A, Gospodarek-Komkowska E, Skowron K. Antibiotic Resistance in Selected Emerging Bacterial Foodborne Pathogens—An Issue of Concern? Antibiotics. 2023; 12(5):880. https://doi.org/10.3390/antibiotics12050880
Chicago/Turabian StyleGrudlewska-Buda, Katarzyna, Justyna Bauza-Kaszewska, Natalia Wiktorczyk-Kapischke, Anna Budzyńska, Eugenia Gospodarek-Komkowska, and Krzysztof Skowron. 2023. "Antibiotic Resistance in Selected Emerging Bacterial Foodborne Pathogens—An Issue of Concern?" Antibiotics 12, no. 5: 880. https://doi.org/10.3390/antibiotics12050880
APA StyleGrudlewska-Buda, K., Bauza-Kaszewska, J., Wiktorczyk-Kapischke, N., Budzyńska, A., Gospodarek-Komkowska, E., & Skowron, K. (2023). Antibiotic Resistance in Selected Emerging Bacterial Foodborne Pathogens—An Issue of Concern? Antibiotics, 12(5), 880. https://doi.org/10.3390/antibiotics12050880






