Compounds That Have an Anti-Biofilm Effect against Common Bacteria at Very Low Concentrations and Their Antibiotic Combination Effect
Abstract
1. Introduction
2. Results
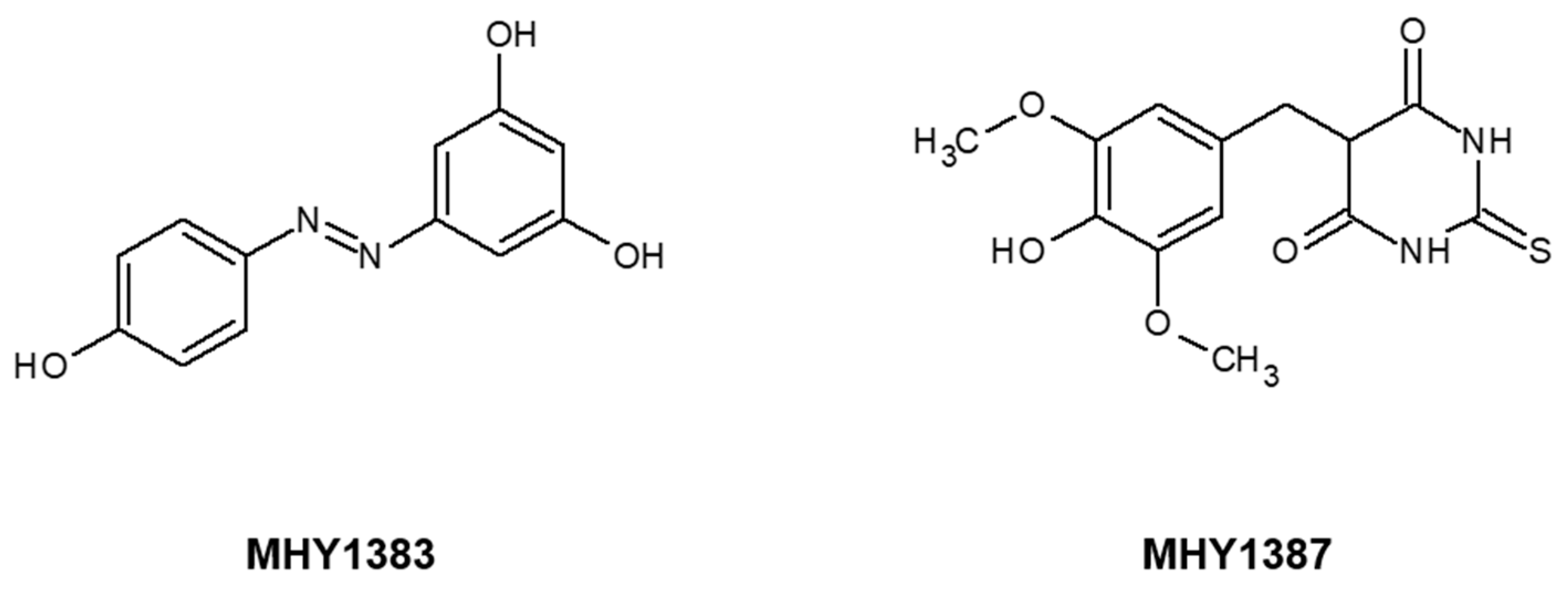
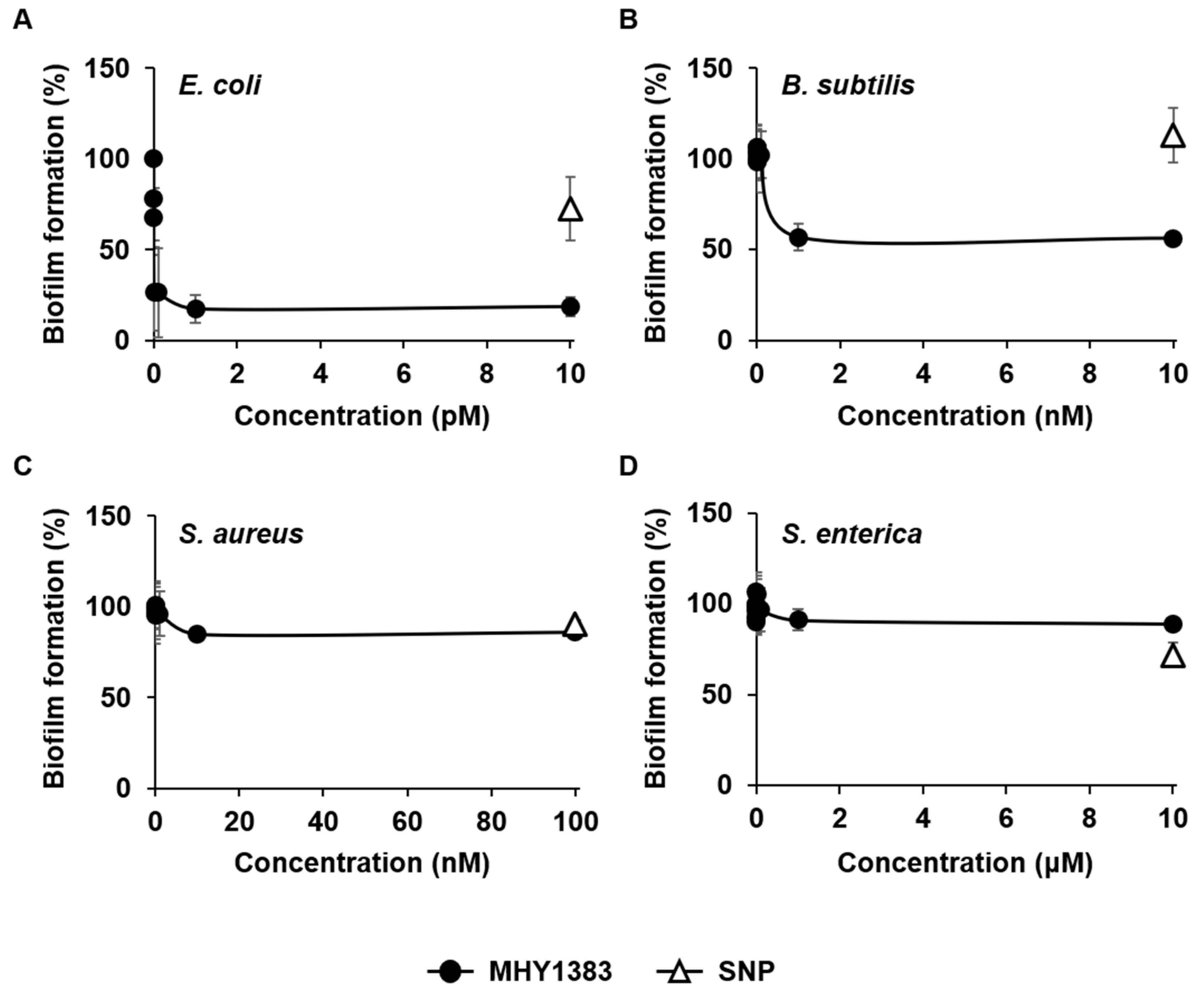
2.1. MHY1383 Had Anti-Biofilm Effects on Common Bacteria at Very Low Concentrations
2.2. MHY1387 Also Effectively Inhibited the Biofilm Formation of a Wide Range of Bacteria at Very Low Concentrations
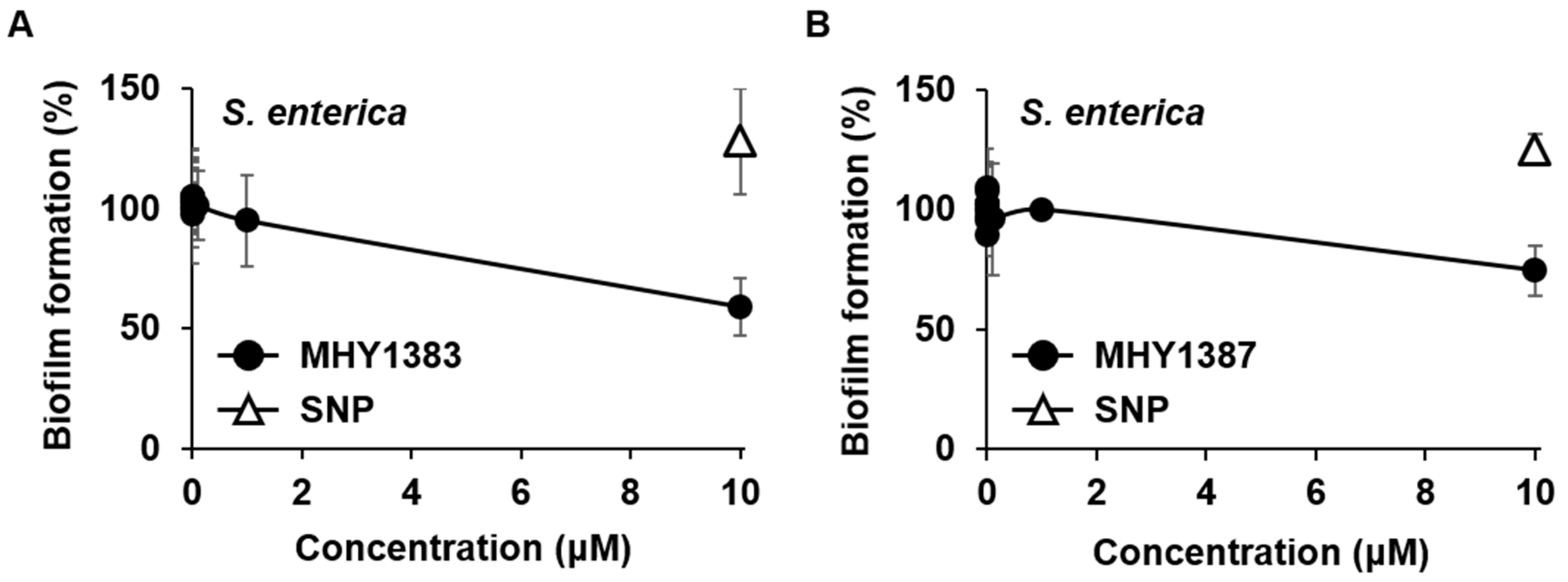
2.3. The Biofilm Inhibitory Effect of MHY1383 and MHY1387 on S. enterica May Vary Depending on the Medium
2.4. MHY1383 and MHY1387 Mostly Little Affect the Minimum Inhibitory Concentration (MIC) of Antibiotics against Bacteria
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Biofilm Assay
4.3. Bacterial Susceptibility to Antibiotics (MIC Measurement)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Lee, J.-H. Antibiofilm agents: A new perspective for antimicrobial strategy. J. Microbiol. 2017, 55, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Kim, S.-K.; Lee, J.-H. Biofilm dispersion in Pseudomonas aeruginosa. J. Microbiol. 2016, 54, 71–85. [Google Scholar] [CrossRef]
- Mishra, M.; Byrd, M.S.; Sergeant, S.; Azad, A.K.; Parsek, M.R.; McPhail, L.; Schlesinger, L.S.; Wozniak, D.J. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell. Microbiol. 2011, 14, 95–106. [Google Scholar] [CrossRef]
- Ghosh, A.; Jayaraman, N.; Chatterji, D. Small-Molecule Inhibition of Bacterial Biofilm. ACS Omega 2020, 5, 3108–3115. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Abdulabbas, H.T.; Marzi, M.; Ghazy, E.; Ekrahi, M.; Pezeshki, B.; Ghasemian, A.; Moawad, A.A. Nickel Nanoparticles: Applications and Antimicrobial Role against Methicillin-Resistant Staphylococcus aureus Infections. Antibiotics 2022, 11, 1208. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Choi, H.; Hong, S.; Moon, H.R.; Lee, J.-H. Antipathogenic Compounds That Are Effective at Very Low Concentrations and Have Both Antibiofilm and Antivirulence Effects against Pseudomonas aeruginosa. Microbiol. Spectr. 2021, 9, e00249-21. [Google Scholar] [CrossRef]
- Song, Y.M.; Ha, Y.M.; Kim, J.-A.; Chung, K.W.; Uehara, Y.; Lee, K.J.; Chun, P.; Byun, Y.; Chung, H.Y.; Moon, H.R. Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorganic Med. Chem. Lett. 2012, 22, 7451–7455. [Google Scholar] [CrossRef]
- Moon, K.M.; Lee, B.; Jeong, J.W.; Kim, D.H.; Park, Y.J.; Kim, H.R.; Park, J.Y.; Kim, M.J.; An, H.J.; Lee, E.K.; et al. Thio-barbiturate-derived compounds are novel antioxidants to prevent LPS-induced inflammation in the liver. Oncotarget 2017, 8, 91662–91673. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Kim, S.-K.; Lee, J.-H. Anti-biofilm effects of anthranilate on a broad range of bacteria. Sci. Rep. 2017, 7, 8604. [Google Scholar] [CrossRef] [PubMed]
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric Oxide Signaling in Pseudomonas aeruginosa Biofilms Mediates Phosphodiesterase Activity, Decreased Cyclic Di-GMP Levels, and Enhanced Dispersal. J. Bacteriol. 2009, 191, 7333–7342. [Google Scholar] [CrossRef]
- Kim, S.-K.; Park, H.-Y.; Lee, J.-H. Anthranilate Deteriorates the Structure of Pseudomonas aeruginosa Biofilms and Antagonizes the Biofilm-Enhancing Indole Effect. Appl. Environ. Microbiol. 2015, 81, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H. Perspectives towards antibiotic resistance: From molecules to population. J. Microbiol. 2019, 57, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Riddle of Biofilm Resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Li, X.-H.; Kim, S.-K.; Lee, J.-H. Anthranilate Acts as a Signal to Modulate Biofilm Formation, Virulence, and Antibiotic Tolerance of Pseudomonas aeruginosa and Surrounding Bacteria. Microbiol. Spectr. 2022, 10, e01463-21. [Google Scholar] [CrossRef]
- Kaplan, J. Biofilm Dispersal: Mechanisms, Clinical Implications, and Potential Therapeutic Uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Rzechowicz, M.; Oh, H.-S.; Saeidi, N.; Hui, L.J.; Winters, H.; Fane, A.G.; Chong, T.H. The efficacy of tannic acid in controlling biofouling by Pseudomonas aeruginosa is dependent on nutrient conditions and bacterial density. Int. Biodeterior. Biodegrad. 2015, 104, 74–82. [Google Scholar] [CrossRef]
- Jardeleza, C.; Foreman, A.; Baker, L.; Paramasivan, S.; Field, J.; Tan, L.W.; Wormald, P.-J. The effects of nitric oxide on Staphylococcus aureus biofilm growth and its implications in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2011, 1, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Regev-Shoshani, G.; Ko, M.; Miller, C.; Av-Gay, Y. Slow Release of Nitric Oxide from Charged Catheters and Its Effect on Biofilm Formation by Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Henares, B.M.; Xu, Y.; Boon, E.M. A Nitric Oxide-Responsive Quorum Sensing Circuit in Vibrio harveyi Regulates Flagella Production and Biofilm Formation. Int. J. Mol. Sci. 2013, 14, 16473–16484. [Google Scholar] [CrossRef] [PubMed]
- Plate, L.; Marletta, M.A. Nitric Oxide Modulates Bacterial Biofilm Formation through a Multicomponent Cyclic-di-GMP Signaling Network. Mol. Cell 2012, 46, 449–460. [Google Scholar] [CrossRef]
- Schreiber, F.; Beutler, M.; Enning, D.; Lamprecht-Grandío, M.; Zafra, O.; González-Pastor, J.E.; De Beer, D. The role of nitric-oxide-synthase-derived nitric oxide in multicellular traits of Bacillus subtilis 3610: Biofilm formation, swarming, and dispersal. BMC Microbiol. 2011, 11, 111. [Google Scholar] [CrossRef]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas aeruginosauses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef]
- Corrigan, R.M.; Abbott, J.C.; Burhenne, H.; Kaever, V.; Gründling, A. c-di-AMP Is a New Second Messenger in Staphylococcus aureus with a Role in Controlling Cell Size and Envelope Stress. PLoS Pathog. 2011, 7, e1002217. [Google Scholar] [CrossRef]
- Holland, L.M.; O’Donnell, S.T.; Ryjenkov, D.A.; Gomelsky, L.; Slater, S.R.; Fey, P.D.; Gomelsky, M.; O’Gara, J.P. A Staphylococcal GGDEF Domain Protein Regulates Biofilm Formation Independently of Cyclic Dimeric GMP. J. Bacteriol. 2008, 190, 5178–5189. [Google Scholar] [CrossRef]
- Nair, D.; Memmi, G.; Hernandez, D.; Bard, J.; Beaume, M.; Gill, S.; Francois, P.; Cheung, A.L. Whole-Genome Sequencing of Staphylococcus aureus Strain RN4220, a Key Laboratory Strain Used in Virulence Research, Identifies Mutations That Affect Not Only Virulence Factors but Also the Fitness of the Strain. J. Bacteriol. 2011, 193, 2332–2335. [Google Scholar] [CrossRef]
- Pearson, J.P.; Pesci, E.; Iglewski, B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997, 179, 5756–5767. [Google Scholar] [CrossRef]
- Perrin, C.; Briandet, R.; Jubelin, G.; Lejeune, P.; Mandrand-Berthelot, M.-A.; Rodrigue, A.; Dorel, C. Nickel Promotes Biofilm Formation by Escherichia coli K-12 Strains That Produce Curli. Appl. Environ. Microbiol. 2009, 75, 1723–1733. [Google Scholar] [CrossRef] [PubMed]


| MIC (μg/mL) | ||||
|---|---|---|---|---|
| Strains | Antibiotics | MIC Alone | MIC in Combination with 100 pM MHY1383 | MIC in Combination with 5 μM MHY1383 |
| P. aeruginosa | Carbenicillin | 13.67 ± 1.53 | 14.67 ± 0.58 | 13.67 ± 1.53 |
| Gentamicin | 2.88 ± 0.48 | 2.63 ± 0.25 | 2.63 ± 0.85 | |
| Tobramycin | 3.25 ± 0.50 | 3.38 ± 0.63 | 3.25 ± 0.50 | |
| Polymyxin B | 3.50 ± 1.00 | 4.13 ± 1.31 | 3.00 ± 0.00 | |
| E. coli | Carbenicillin | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 |
| Gentamicin | 5.00 ± 1.00 | 6.00 ± 2.00 | 4.50 ± 1.32 | |
| Tobramycin | 8.00 ± 0.00 | 8.33 ± 0.58 | 8.00 ± 1.00 | |
| Polymyxin B | 0.83 ± 0.05 | 0.88 ± 0.10 | 0.75 ± 0.10 | |
| B. subtilis | Carbenicillin | 2.25 ± 1.77 | 2.00 ± 1.41 | 1.60 ± 1.27 |
| Gentamicin | 3.83 ± 1.15 | 4.17 ± 1.44 | 4.00 ± 1.32 | |
| Tobramycin | 3.63 ± 1.25 | 3.75 ± 1.19 | 3.75 ± 1.50 | |
| Polymyxin B | 15.00 ± 0.00 | 15.00 ± 0.00 | 18.33 ± 5.77 | |
| S. enterica | Carbenicillin | 4.00 ± 0.00 | 3.50 ± 1.00 | 4.00 ± 0.00 |
| Gentamicin | 9.50 ± 1.00 | 9.50 ± 1.00 | 9.00 ± 1.63 | |
| Tobramycin | 14.75 ± 2.50 | 14.75 ± 2.50 | 13.75 ± 2.06 | |
| Polymyxin B | 3.00 ± 0.00 | 3.00 ± 0.50 | 2.83 ± 0.29 | |
| S. aureus | Carbenicillin | 1.50 ± 0.71 | 1.35 ± 0.92 | 1.00 ± 0.00 |
| Gentamicin | 5.50 ± 0.00 | 5.50 ± 0.00 | 5.50 ± 0.00 | |
| Tobramycin | 11.00 ± 0.00 | 10.67 ± 0.58 | 11.00 ± 0.00 | |
| Polymyxin B | 350.00 ± 0.00 | 400.00 ± 50.00 | 350.00 ± 50.00 | |
| MIC (μg/mL) | ||||
|---|---|---|---|---|
| Strains | Antibiotics | MIC Alone | MIC in Combination with 100 pM MHY1387 | MIC in Combination with 5 μM MHY1387 |
| P. aeruginosa | Carbenicillin | 10.00 ± 0.00 | 12.00 ± 0.00 | 10.00 ± 0.00 |
| Gentamicin | 2.50 ± 0.00 | 2.63 ± 0.25 | 2.13 ± 0.25 | |
| Tobramycin | 3.50 ± 0.00 | 3.13 ± 0.25 | 3.50 ± 0.00 | |
| Polymyxin B | 5.13 ± 0.25 | 5.00 ± 0.00 | 4.13 ± 0.25 | |
| E. coli | Carbenicillin | 6.50 ± 1.00 | 6.50 ± 1.00 | 6.50 ± 1.00 |
| Gentamicin | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | |
| Tobramycin | 10.00 ± 0.00 | 10.25 ± 0.50 | 10.25 ± 0.50 | |
| Polymyxin B | 1.00 ± 0.00 | 1.03 ± 0.05 | 1.03 ± 0.05 | |
| B. subtilis | Carbenicillin | 2.50 ± 1.00 | 1.00 ± 0.00 | 0.70 ± 0.00 |
| Gentamicin | 3.63 ± 0.25 | 3.50 ± 0.00 | 4.00 ± 0.00 | |
| Tobramycin | 4.13 ± 0.25 | 4.00 ± 0.00 | 4.13 ± 0.25 | |
| Polymyxin B | 20.00 ± 0.00 | 20.00 ± 0.00 | 20.00 ± 0.00 | |
| S. enterica | Carbenicillin | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 |
| Gentamicin | 5.25 ± 0.50 | 5.00 ± 0.00 | 5.00 ± 0.00 | |
| Tobramycin | 12.00 ± 0.00 | 9.25 ± 0.50 | 9.25 ± 0.50 | |
| Polymyxin B | 3.63 ± 0.25 | 4.50 ± 0.00 | 2.50 ± 0.00 | |
| S. aureus | Carbenicillin | 0.40 ± 0.00 | 0.48 ± 0.15 | 0.20 ± 0.00 |
| Gentamicin | 4.00 ± 0.00 | 4.63 ± 0.25 | 3.50 ± 0.00 | |
| Tobramycin | 6.00 ± 0.00 | 5.25 ± 0.50 | 5.25 ± 0.50 | |
| Polymyxin B | 212.50 ± 25.00 | 212.50 ± 25.00 | 350.00 ± 0.00 | |
| Name | Genotype | References |
|---|---|---|
| Escherichia coli | ||
| MG1655 | A wild type strain of E. coli K-12 | Lab. collection |
| Bacillus subtilis | ||
| ATCC6051 | A wild type strain of B. subtilis | Lab. collection |
| Salmonella enterica | ||
| SL1344 | A type of S. enterica serovar Typhimurium | Lab. collection |
| Staphylococcus aureus | ||
| RN4220 | A wild type strain of S. aureus | [29] |
| Pseudomonas aeruginosa | ||
| PAO1 | A wild type strain of P. aeruginosa | [30] |
| The Concentration Range for MIC Assay (μg/mL) | ||||
|---|---|---|---|---|
| Strains/Antibiotics | Carbenicillin | Gentamicin | Tobramycin | Polymyxin B |
| P. aeruginosa | 0.2–14 | 0.5–5.5 | 0.5–5.5 | 0.5–5.5 |
| E. coli | 0.5–5.5 | 1–11 | 0.1–1.1 | |
| B. subtilis | 0.5–5.5 | 0.5–5.5 | 5–55 | |
| S. enterica | 1–11 | 1–11 | 0.5–5.5 | |
| S. aureus | 0.5–5.5 | 1–11 | 50–550 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-J.; Li, D.-d.; Lee, J.; Kang, M.K.; Moon, H.R.; Lee, J.-H. Compounds That Have an Anti-Biofilm Effect against Common Bacteria at Very Low Concentrations and Their Antibiotic Combination Effect. Antibiotics 2023, 12, 853. https://doi.org/10.3390/antibiotics12050853
Hwang H-J, Li D-d, Lee J, Kang MK, Moon HR, Lee J-H. Compounds That Have an Anti-Biofilm Effect against Common Bacteria at Very Low Concentrations and Their Antibiotic Combination Effect. Antibiotics. 2023; 12(5):853. https://doi.org/10.3390/antibiotics12050853
Chicago/Turabian StyleHwang, Hyeon-Ji, Dan-dan Li, Jieun Lee, Min Kyung Kang, Hyung Ryong Moon, and Joon-Hee Lee. 2023. "Compounds That Have an Anti-Biofilm Effect against Common Bacteria at Very Low Concentrations and Their Antibiotic Combination Effect" Antibiotics 12, no. 5: 853. https://doi.org/10.3390/antibiotics12050853
APA StyleHwang, H.-J., Li, D.-d., Lee, J., Kang, M. K., Moon, H. R., & Lee, J.-H. (2023). Compounds That Have an Anti-Biofilm Effect against Common Bacteria at Very Low Concentrations and Their Antibiotic Combination Effect. Antibiotics, 12(5), 853. https://doi.org/10.3390/antibiotics12050853







