Synergistic Inhibition of Methicillin-Resistant Staphylococcus aureus (MRSA) by Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils in Association with Oxacillin
Abstract
1. Introduction
2. Results
2.1. Qualitative and Semi-Quantitative Analysis of Essential Oils
2.2. Antibacterial Susceptibility and Fractional Inhibitory (FIC) Index Determination
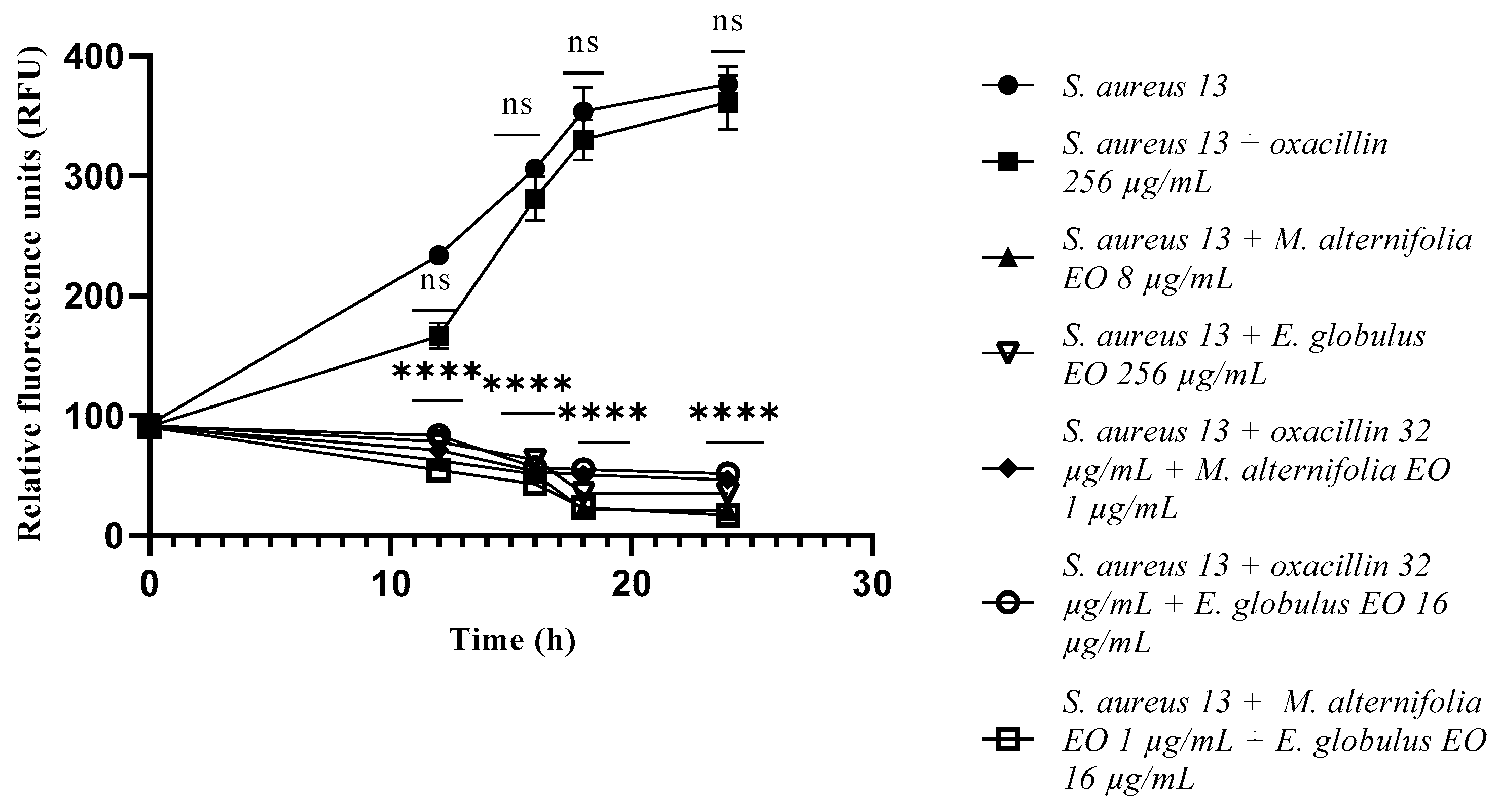
2.3. Growth Kinetics Study via Fluorescence Assay
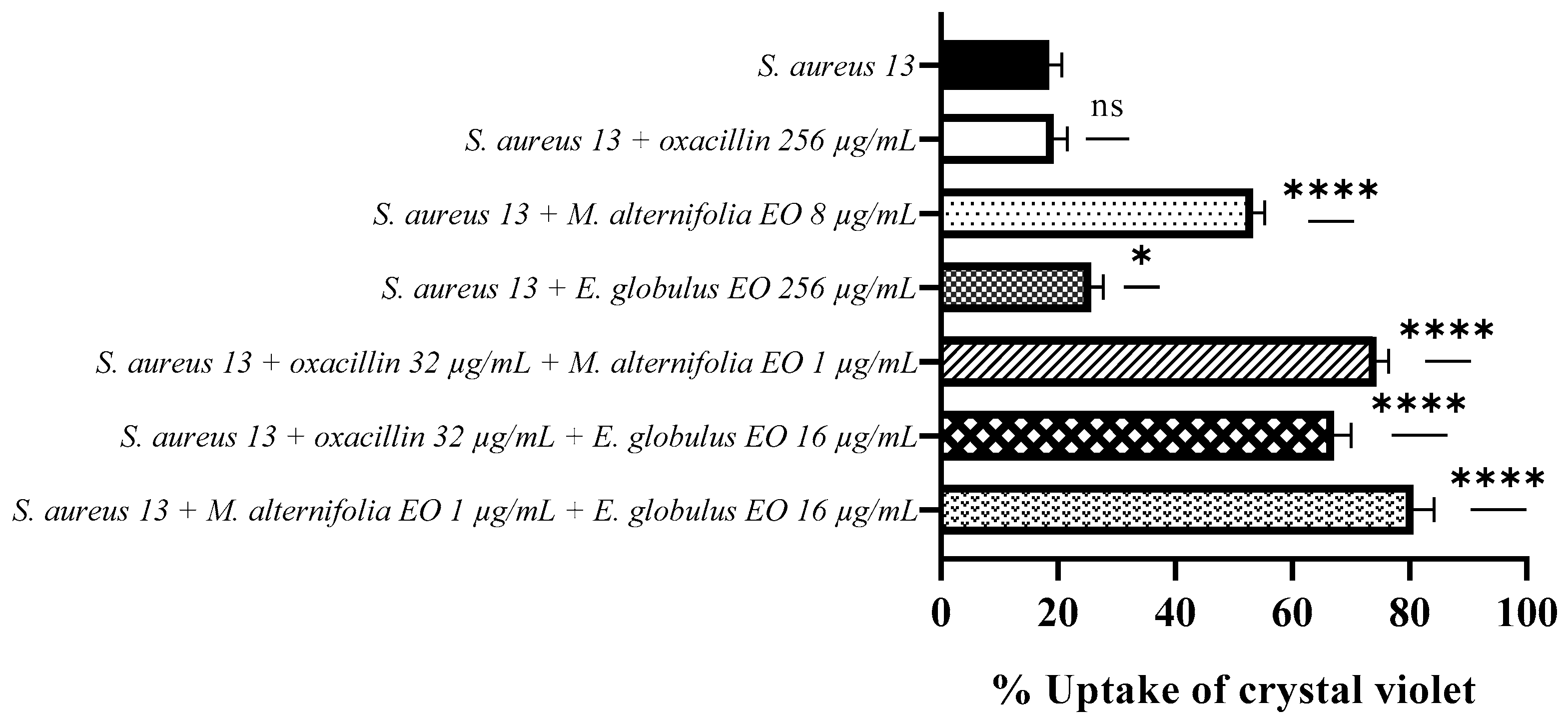
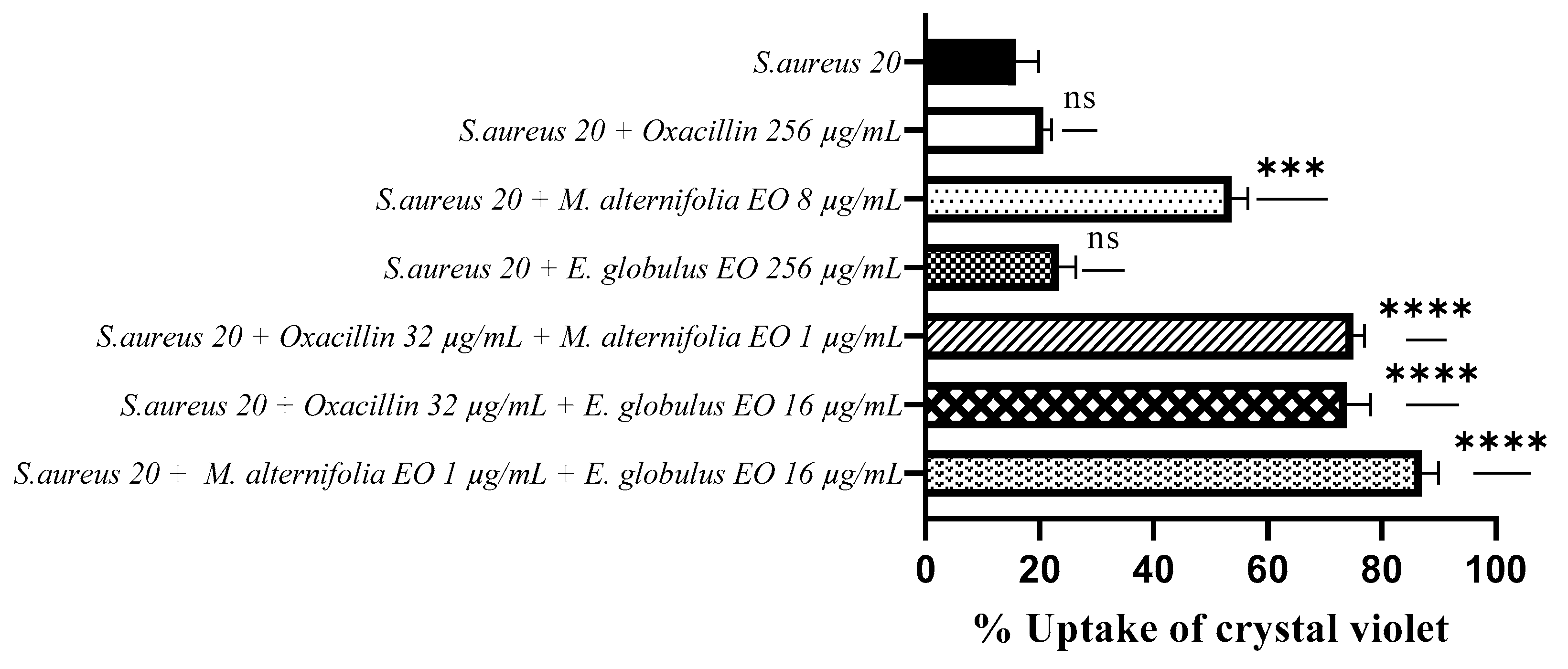
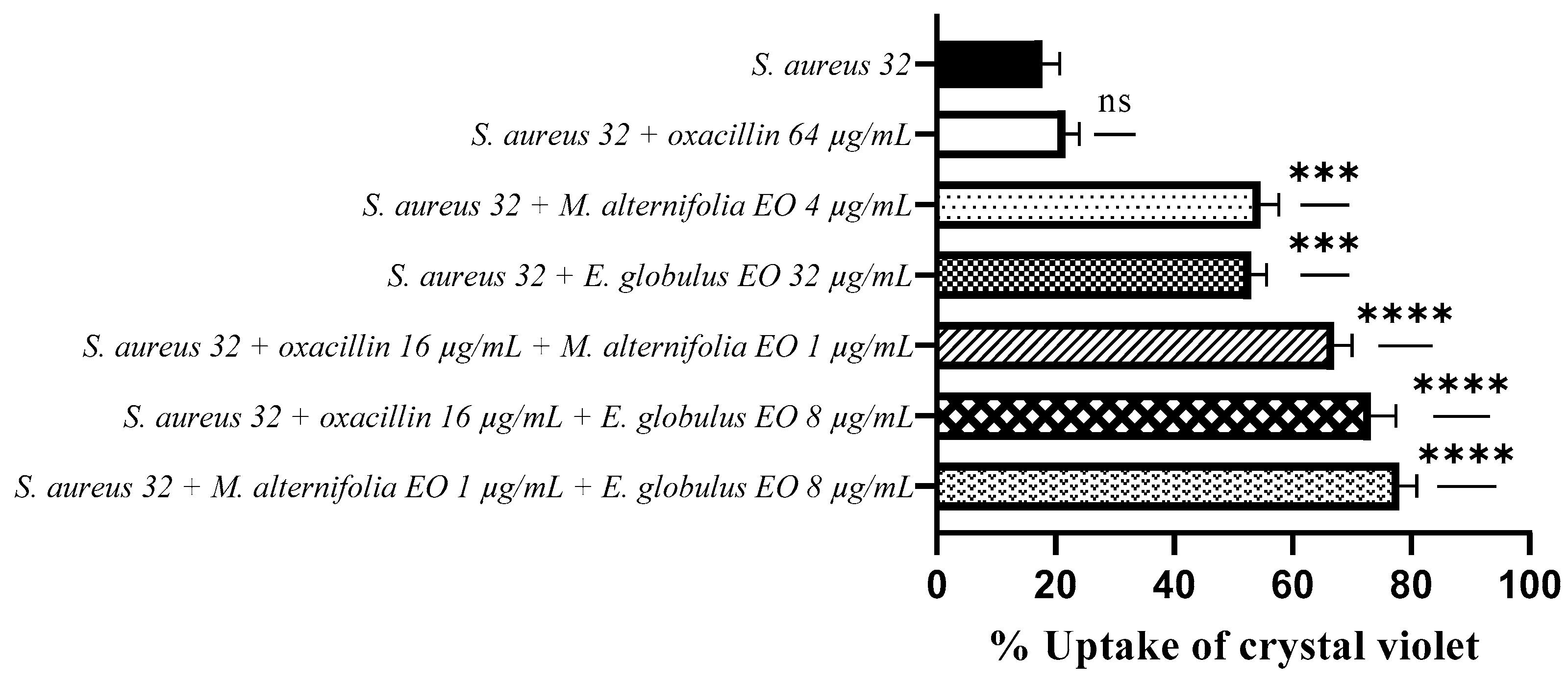
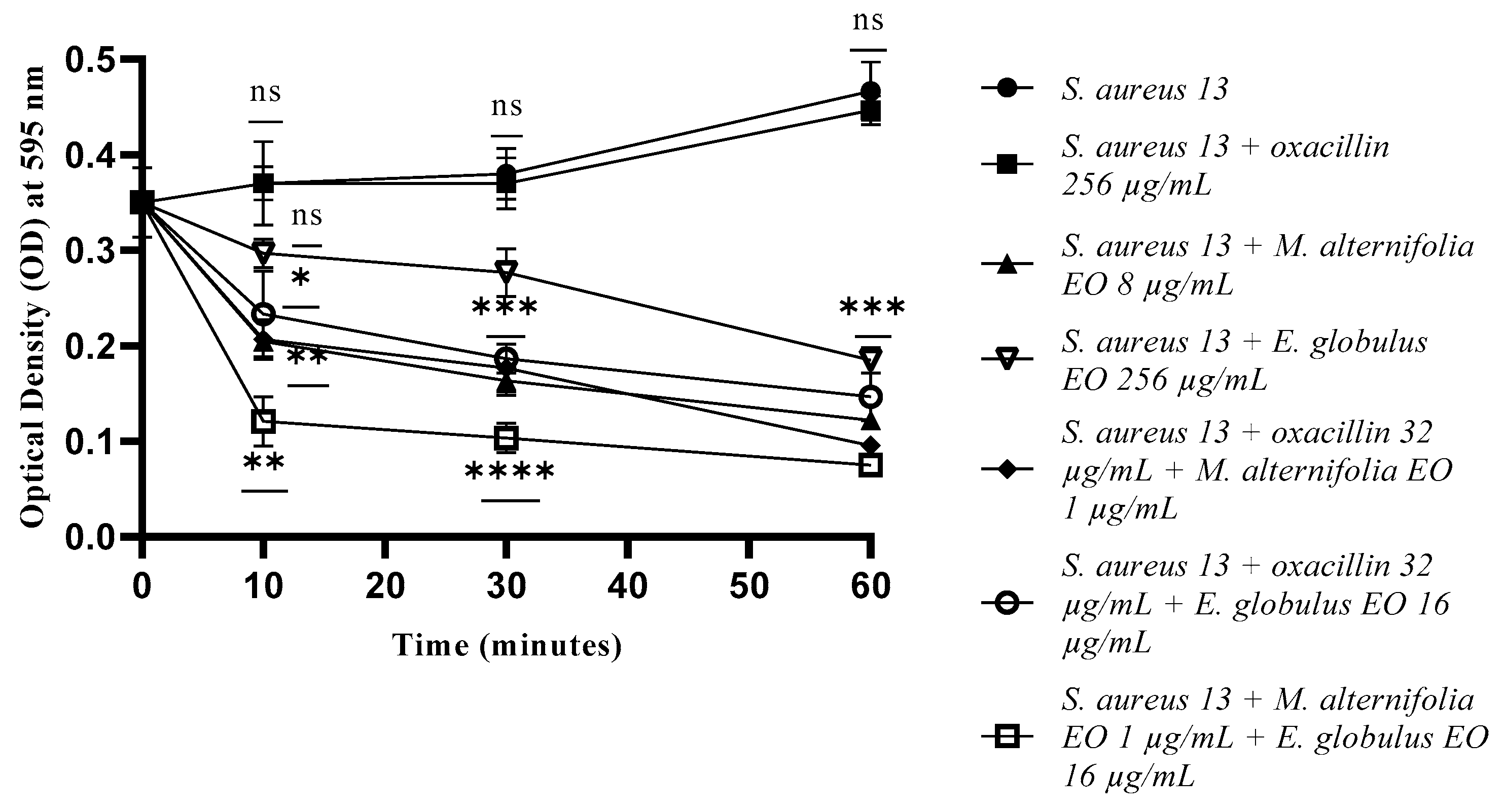
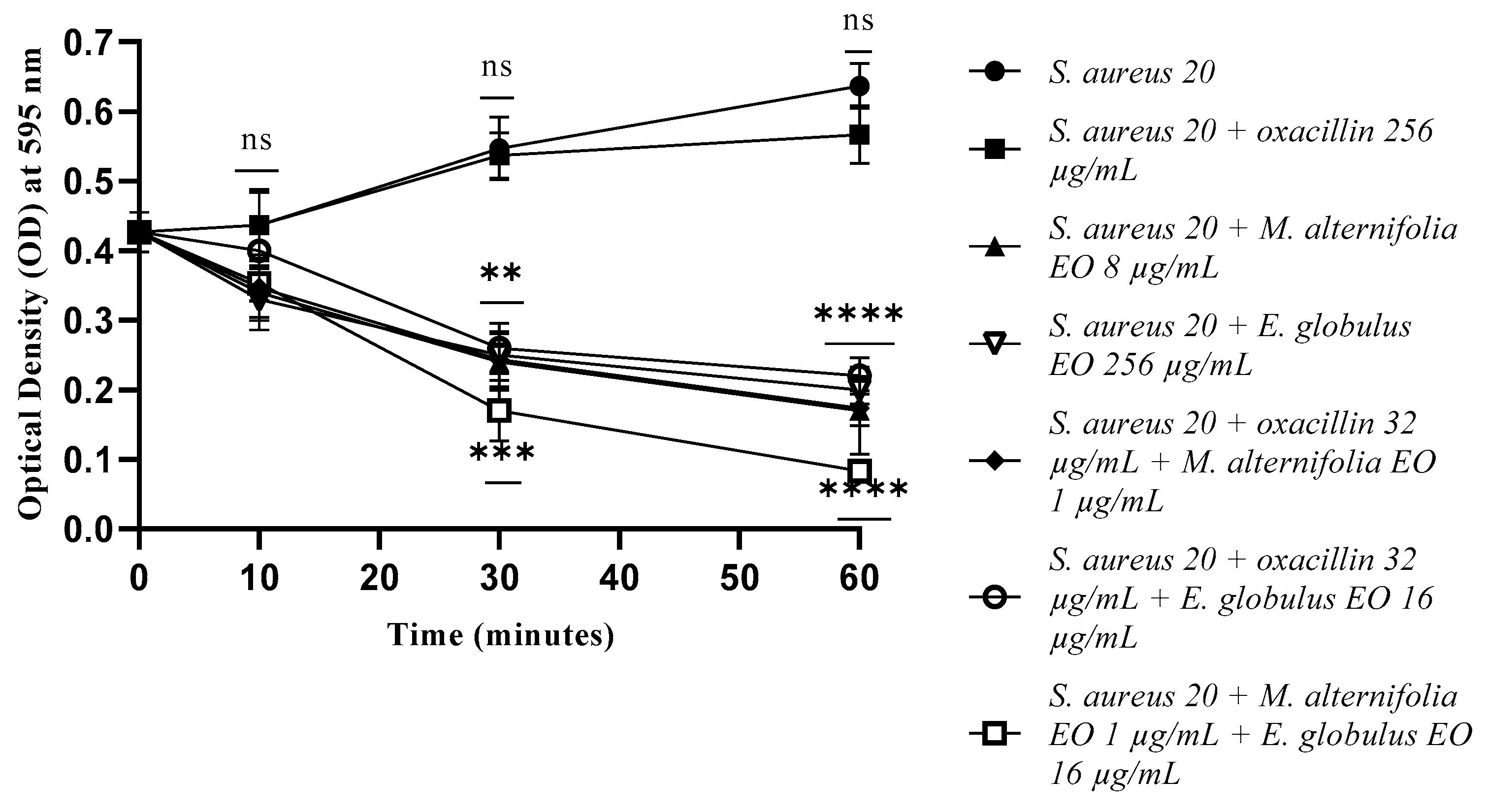
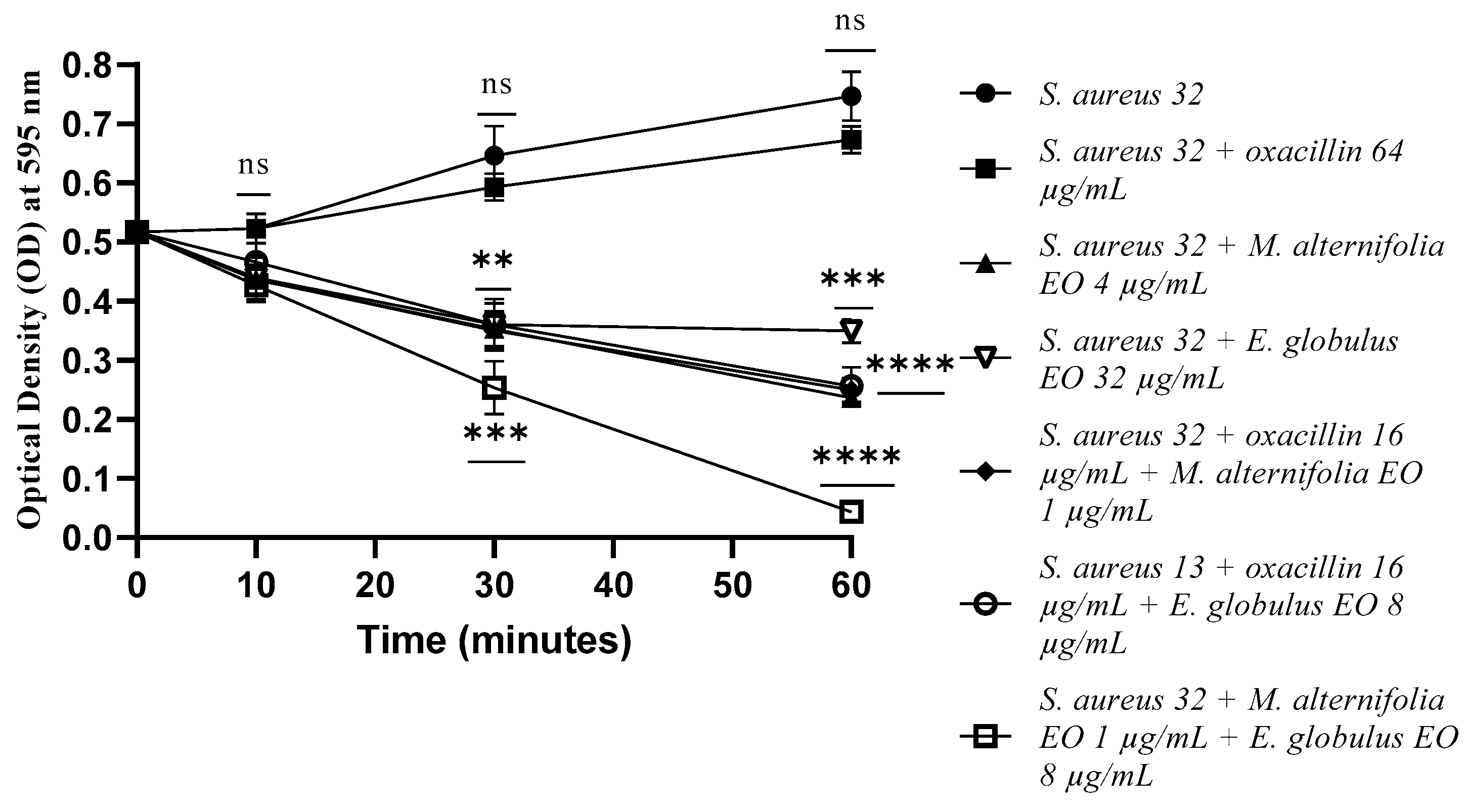
2.4. Determination of Membrane Permeability Alteration via a Cristal Violet Assay
2.5. Membrane Permeability Test via an Addition of SDS 0.1%
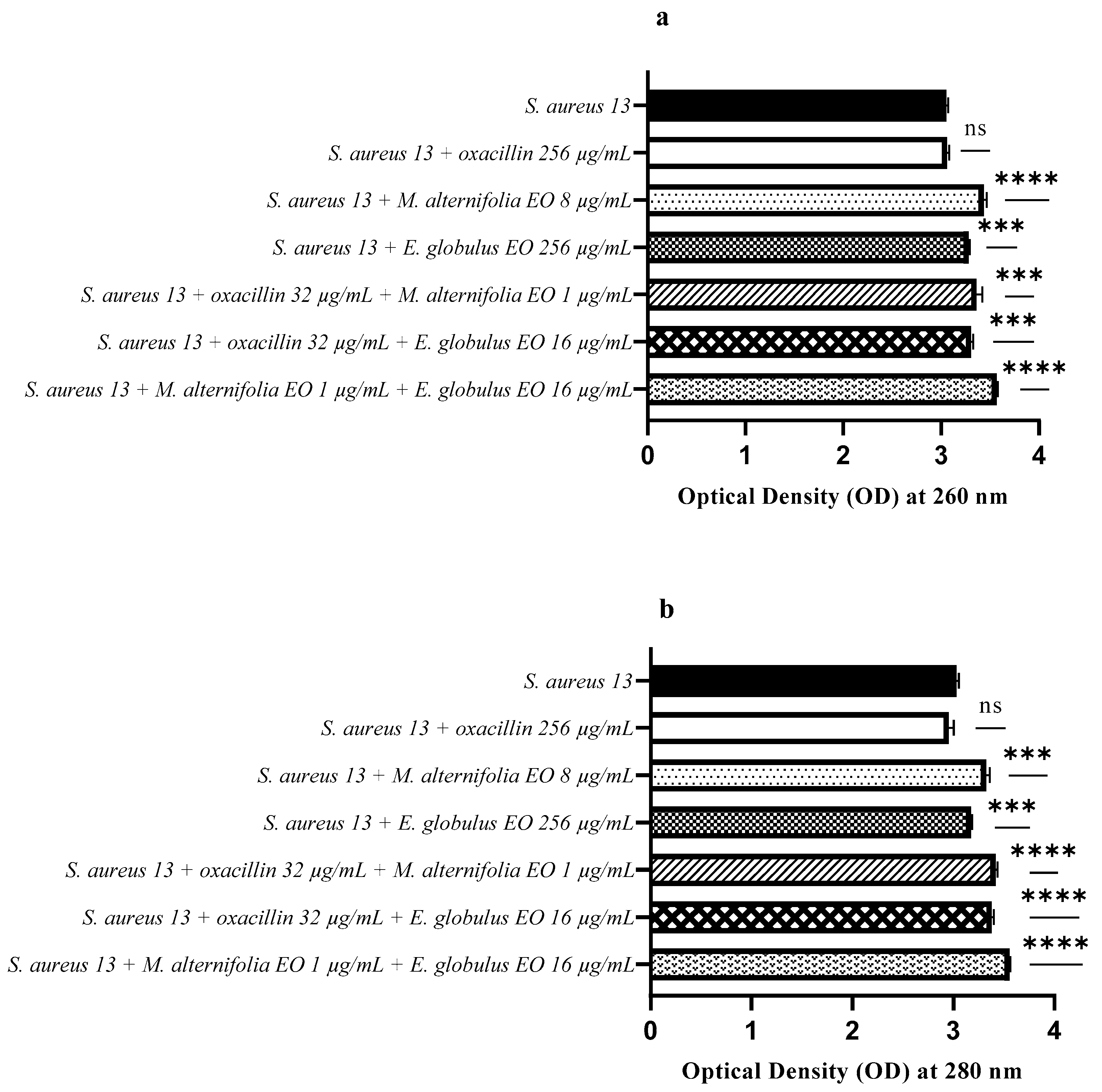
2.6. Release of Nucleic Acids and Proteins
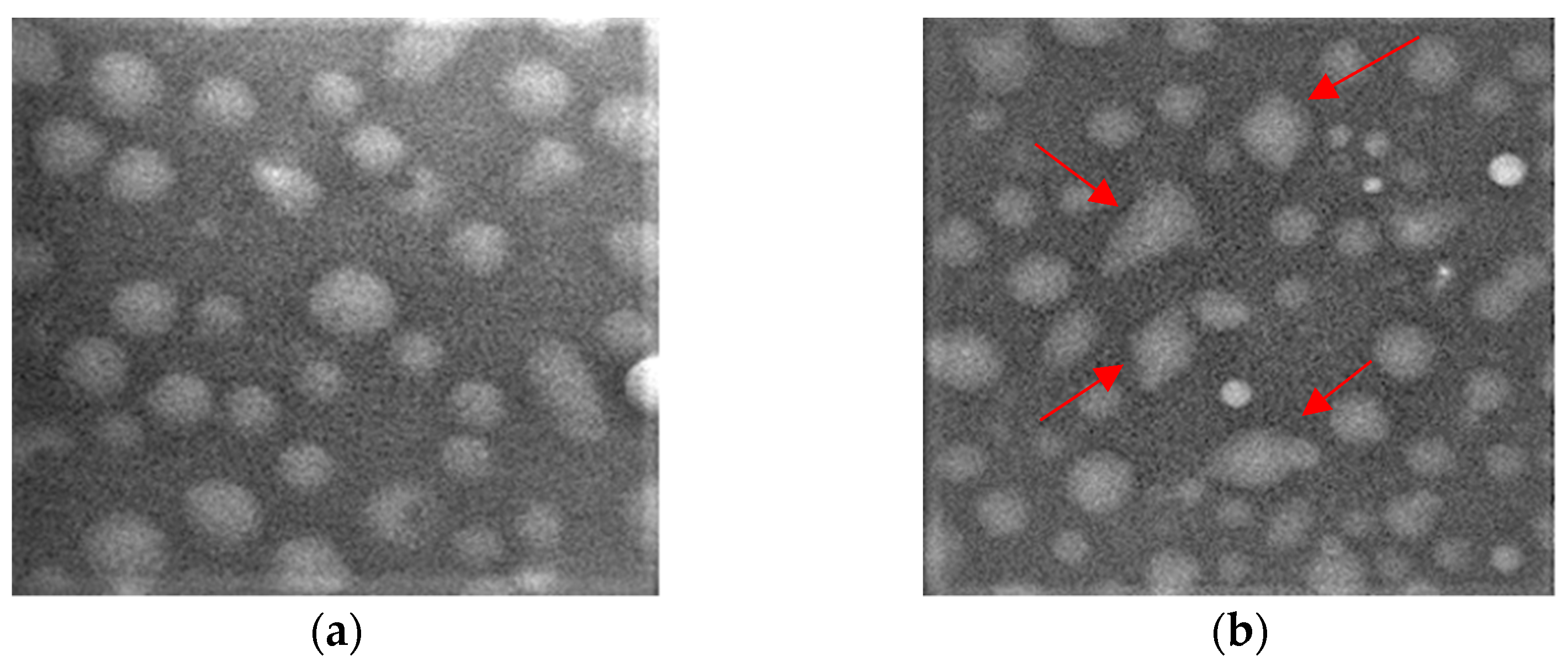
2.7. Scanning Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Microbial Strains and Essential Oils
4.2. Antimicrobial Susceptibility Testing and Detection of Methicillin-Resistant Staphylococcus aureus (MRSA) Genes
4.3. Determination of MIC (Minimum Inhibitory Concentration) and FIC Index (Fractional Inhibitory Concentration)
4.4. Growth Kinetics Study via Fluorescence Assay
4.5. Determination of Membrane Permeability Alteration via a Cristal Violet Assay
4.6. Membrane Permeability Test via an Addition of SDS 0.1%
4.7. Release of Nucleic Acids and Proteins
4.8. Scanning Electron Microscopy
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owen, L.; Laird, K. Synchronous application of antibiotics and essential oils: Dual mechanisms of action as a potential solution to antibiotic resistance. Crit. Rev. Microbiol. 2018, 44, 414–435. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tian, J.; Zhu, J.; Chen, J.; Li, L.; Yang, C.; Chen, J.; Chen, D. Photodynamic and photothermal co-driven CO-enhanced multi-mode synergistic antibacterial nanoplatform to effectively fight against biofilm infections. Chem. Eng. J. 2021, 426, 131919. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, J.; Hu, Y.; Miao, W.; Huang, H. Wrapping collagen-based nanoparticle with macrophage membrane for treating multidrug-resistant bacterial infection. J. Leather Sci. Eng. 2022, 4, 31. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Chen, J.; Shen, T.; Jin, T.; Zeng, B.; Li, L.; Yang, C.; Mu, Z.; Deng, H.; et al. An all-in-one CO gas therapy-based hydrogel dressing with sustained insulin release, anti-oxidative stress, antibacterial, and anti-inflammatory capabilities for infected diabetic wounds. Acta Biomater. 2022, 146, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, L.; Mei, A.; Xu, Y.; Ruan, X.; Wang, W.; Shao, J.; Yang, D.; Dong, X. Recent nanotechnologies to overcome the bacterial biofilm matrix barriers. Small 2023, 19, 2206220. [Google Scholar] [CrossRef]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
- Oliva, A.; Costantini, S.; De Angelis, M.; Garzoli, S.; Božović, M.; Mascellino, M.T.; Vullo, V.; Ragno, R. High potency of Melaleuca alternifolia essential oil against multi-drug resistant Gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Molecules 2018, 23, 2584. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. An overview of the potential therapeutic applications of essential oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine 2009, 16, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Yusoff, K.; Mai, C.W.; Lim, W.M.; Yap, W.S.; Lim, S.H.E.; Lai, K.S. Additivity vs synergism: Investigation of the additive interaction of Cinnamon bark oil and meropenem in combinatory therapy. Molecules 2017, 22, 1733. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Firoozbahr, M.; Kingshott, P.; Palombo, E.A.; Zaferanloo, B. Recent advances in using natural antibacterial additives in bioactive wound dressings. Pharmaceutics 2023, 15, 644. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca genus as antimicrobial agents: From farm to pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef]
- Kwieciński, J.; Eick, S.; Wójcik, K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents 2009, 33, 343–347. [Google Scholar] [CrossRef]
- Brun, P.; Bernabè, G.; Filippini, R.; Piovan, A. In vitro antimicrobial activities of commercially available Tea Tree (Melaleuca alternifolia) essential oils. Curr. Microbiol. 2019, 76, 108–116. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the antimicrobial actions of Tea Tree oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture-in vitro studies on antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential oils: A natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef]
- Falci, S.P.; Teixeira, M.A.; Chagas, P.F.; Martinez, B.B.; Loyola, A.B.; Ferreira, L.M.; Veiga, D.F. Antimicrobial activity of Melaleuca sp. oil against clinical isolates of antibiotics resistant Staphylococcus aureus. Acta Cir. Bras. 2015, 30, 401–406. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. A systematic and comprehensive review on current understanding of the pharmacological actions, molecular mechanisms, and clinical implications of the genus Eucalyptus. Phytomed. Plus 2021, 1, 100089. [Google Scholar] [CrossRef]
- Elangovan, S.; Mudgil, P. Antibacterial properties of Eucalyptus globulus essential oil against MRSA: A systematic review. Antibiotics 2023, 12, 474. [Google Scholar] [CrossRef]
- Denu, R.A.; Patel, D.; Becker, B.J.; Shiffler, T.; Kleinschmidt, P. MRSA septicemia with septic arthritis and prostatic, intraretinal, periapical, and lung abscesses. WMJ 2020, 119, 62–65. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2020; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Bai, Z.; Chen, M.; Lin, Q.; Ye, Y.; Fan, H.; Wen, K.; Zeng, J.; Huang, D.; Mo, W.; Lei, Y.; et al. Identification of methicillin-resistant Staphylococcus aureus from methicillin-sensitive Staphylococcus aureus and molecular characterization in Quanzhou, China. Front. Cell Dev. Biol. 2021, 9, 629681. [Google Scholar] [CrossRef]
- Verma, A.K.; Ahmed, S.F.; Hossain, M.S.; Bhojiya, A.A.; Mathur, A.; Upadhyay, S.K.; Srivastava, A.K.; Vishvakarma, N.K.; Barik, M.; Rahaman, M.M.; et al. Molecular docking and simulation studies of flavonoid compounds against PBP-2a of methicillin-resistant Staphylococcus aureus. J. Biomol. Struct. Dyn. 2022, 40, 10561–10577. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.-H.E.; Chong, C.-M.; Lai, K.-S. Membrane disruption properties of essential oils—A double-edged sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Benvenuti, S.; Truzzi, E.; Messi, P. Effects of Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. essential oils on antibiotic-resistant bacterial biofilms. Molecules 2023, 28, 1671. [Google Scholar] [CrossRef]
- Laxminarayan, R. The overlooked pandemic of antimicrobial resistance. Lancet 2022, 399, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Beck, W.D.; Berger-Bächi, B.; Kayser, F.H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J. Bacteriol. 1986, 165, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Wielders, C.L.; Fluit, A.C.; Brisse, S.; Verhoef, J.; Schmitz, F.J. mecA gene is widely disseminated in Staphylococcus aureus population. J. Clin. Microbiol. 2002, 40, 3970–3975. [Google Scholar] [CrossRef]
- García-Álvarez, L.; Holden, M.T.; Lindsay, H.; Webb, C.R.; Brown, D.F.; Curran, M.D.; Walpole, E.; Brooks, K.; Pickard, D.J.; Teale, C.; et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect. Dis. 2011, 11, 595–603. [Google Scholar] [CrossRef]
- Kinross, P.; Petersen, A.; Skov, R.; Van Hauwermeiren, E.; Pantosti, A.; Laurent, F.; Voss, A.; Kluytmans, J.; Struelens, M.J.; Heuer, O.; et al. Livestock-associated meticillin-resistant Staphylococcus aureus (MRSA) among human MRSA isolates, European Union/European Economic Area countries, 2013. Eurosurveillance 2017, 22, 16-00696. [Google Scholar] [CrossRef]
- Kevorkijan, B.K.; Petrovič, Ž.; Kocuvan, A.; Rupnik, M. MRSA diversity and the emergence of LA-MRSA in a large teaching hospital in Slovenia. Acta Microbiol. Immunol. Hung. 2019, 66, 235–246. [Google Scholar] [CrossRef]
- Chang, S.; Sievert, D.M.; Hageman, J.C.; Boulton, M.L.; Tenover, F.C.; Downes, F.P.; Shah, S.; Rudrik, J.T.; Pupp, G.R.; Brown, W.J.; et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 2003, 348, 1342–1347. [Google Scholar] [CrossRef]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Noble, W.C.; Virani, Z.; Cree, R.G. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol. Lett. 1992, 72, 195–198. [Google Scholar] [CrossRef] [PubMed]
- de Niederhäusern, S.; Bondi, M.; Messi, P.; Iseppi, R.; Sabia, C.; Manicardi, G.; Anacarso, I. Vancomycin-resistance transferability from VanA enterococci to Staphylococcus aureus. Curr. Microbiol. 2011, 62, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Ayari, S.; Shankar, S.; Follett, P.; Hossain, F.; Lacroix, M. Potential synergistic antimicrobial efficiency of binary combinations of essential oils against Bacillus cereus and Paenibacillus amylolyticus-Part A. Microb. Pathog. 2020, 141, 104008. [Google Scholar] [CrossRef] [PubMed]
- Johansen, B.; Duval, R.E.; Sergere, J.C. First evidence of a combination of terpinen-4-ol and α-terpineol as a promising tool against ESKAPE pathogens. Molecules 2022, 27, 7472. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Chanda, S.; Rakholiya, K. Combination therapy: Synergism between natural plant extracts and antibiotics against infectious diseases. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; FORMATEX: Badajoz, Spain, 2011; Volume 1, pp. 520–529. [Google Scholar]
- Rosato, A.; Sblano, S.; Salvagno, L.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. Anti-biofilm inhibitory synergistic effects of combinations of essential oils and antibiotics. Antibiotics 2020, 9, 637. [Google Scholar] [CrossRef]
- Yap, P.S.; Yiap, B.C.; Ping, H.C.; Lim, S.H. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Chapter 6—Efficacy of polymer-based wound dressings in chronic wounds. In Modeling and Control of Drug Delivery Systems; Azar, A.T., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 79–110. [Google Scholar]
- Sroczyk, E.A.; Berniak, K.; Jaszczur, M.; Stachewicz, U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chem. Eng. J. 2022, 429, 132256. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; Volume 39. [Google Scholar]
- Khosravi, A.D.; Jenabi, A.; Montazeri, E.A. Distribution of genes encoding resistance to aminoglycoside modifying enzymes in methicillin-resistant Staphylococcus aureus (MRSA) strains. Kaohsiung J. Med. Sci. 2017, 33, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Marri, L.; Dallai, R.; Marchini, D. The novel antibacterial peptide ceratotoxin A alters permeability of the inner and outer membrane of Escherichia coli K-12. Curr. Microbiol. 1996, 33, 40–43. [Google Scholar] [CrossRef]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef]




| Strains | Oxacillin (µg/mL) | M. alternifolia EO (µg/mL) | E. globulus EO (µg/mL) |
|---|---|---|---|
| S. aureus 13 | 256 | 8 | 256 |
| S. aureus 20 | 256 | 8 | 256 |
| S. aureus 32 | 64 | 4 | 32 |
| Strains | Oxacillin– M. alternifolia EO | Oxacillin– E. globulus EO | M. alternifolia EO– E. globulus EO |
|---|---|---|---|
| S. aureus 13 | 0.25 | 0.19 | 0.19 |
| S. aureus 20 | 0.25 | 0.19 | 0.19 |
| S. aureus 32 | 0.5 | 0.5 | 0.5 |
| Primer | Nucleotide Sequence (5′ to 3′) | Product Size (bp) |
|---|---|---|
| mecA | 5′-CCTAGTAAAGCTCCGGAA-3′ 5′-CTAGTCCATTCGGTCCA-3′ | 314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iseppi, R.; Condò, C.; Messi, P. Synergistic Inhibition of Methicillin-Resistant Staphylococcus aureus (MRSA) by Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils in Association with Oxacillin. Antibiotics 2023, 12, 846. https://doi.org/10.3390/antibiotics12050846
Iseppi R, Condò C, Messi P. Synergistic Inhibition of Methicillin-Resistant Staphylococcus aureus (MRSA) by Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils in Association with Oxacillin. Antibiotics. 2023; 12(5):846. https://doi.org/10.3390/antibiotics12050846
Chicago/Turabian StyleIseppi, Ramona, Carla Condò, and Patrizia Messi. 2023. "Synergistic Inhibition of Methicillin-Resistant Staphylococcus aureus (MRSA) by Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils in Association with Oxacillin" Antibiotics 12, no. 5: 846. https://doi.org/10.3390/antibiotics12050846
APA StyleIseppi, R., Condò, C., & Messi, P. (2023). Synergistic Inhibition of Methicillin-Resistant Staphylococcus aureus (MRSA) by Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils in Association with Oxacillin. Antibiotics, 12(5), 846. https://doi.org/10.3390/antibiotics12050846








