Antibiotic Consumption in a Cohort of Hospitalized Adults with Viral Respiratory Tract Infection
Abstract
1. Introduction
2. Results
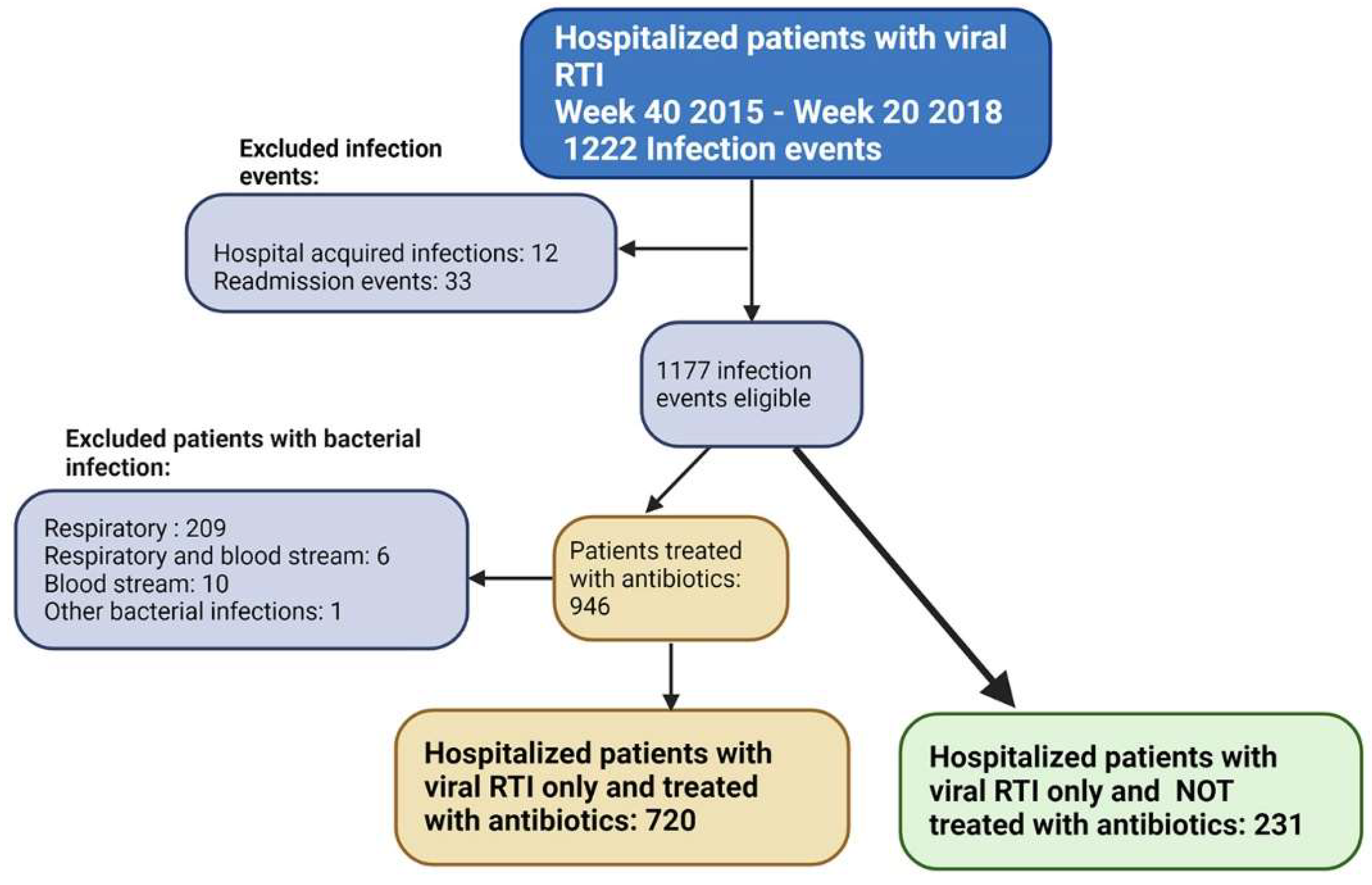
2.1. Infection Events (IE)
2.2. Respiratory Virus Findings
2.3. Antimicrobial Drug Treatment
2.3.1. Antibiotic Treatment during Hospital Stay
2.3.2. Antibiotic Types Prescribed
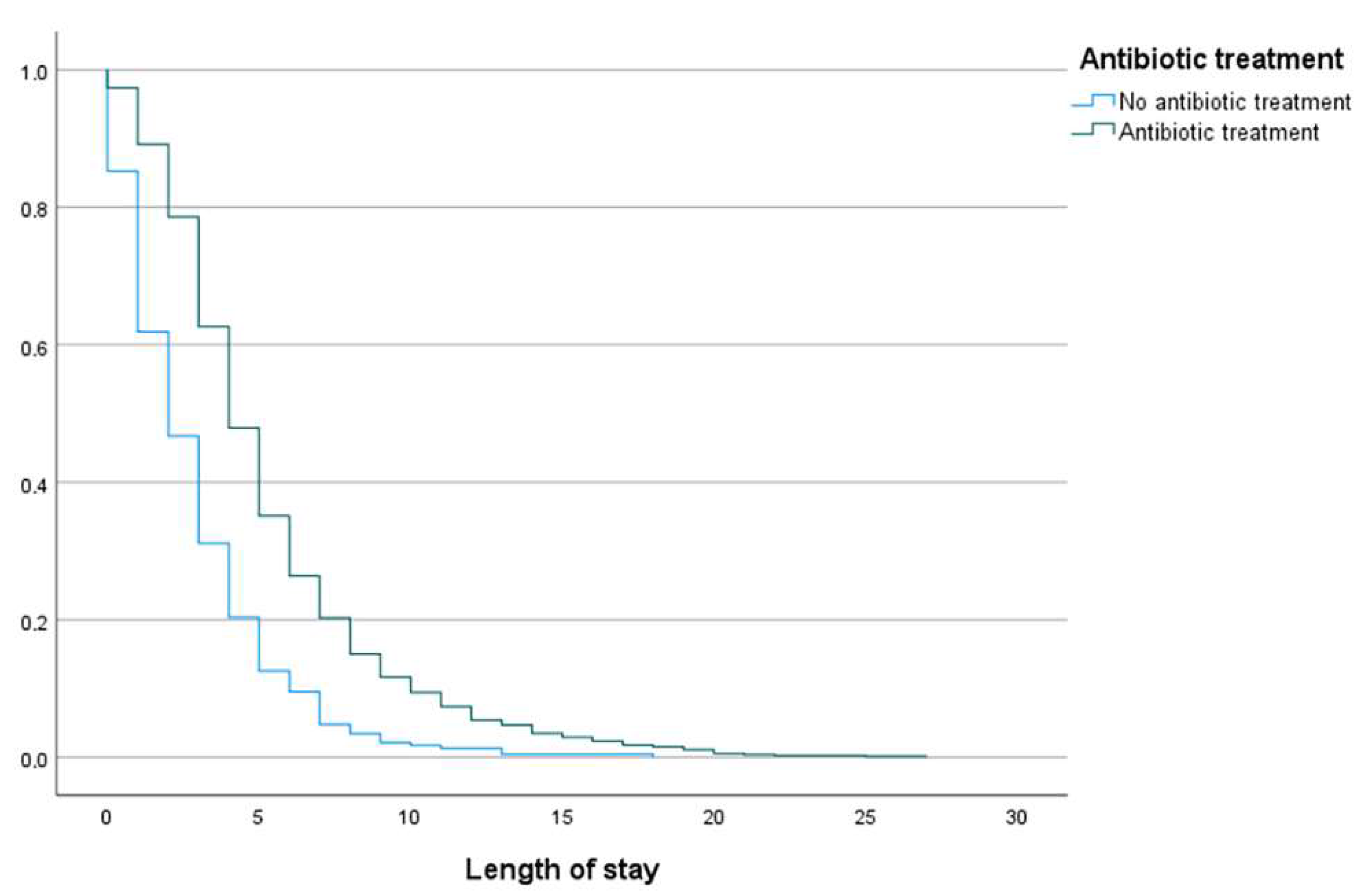
2.3.3. Length and Days of Antibiotic Treatment
2.3.4. Discontinuation of Antibiotic Treatment
2.3.5. Evaluation Score
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Laboratory Findings and Data on Antibiotic Prescription
4.3. Antibiotic Prescription Factors—Evaluation Score
- Chest X-ray/Chest CT compatible with or susceptible of pneumonia (1 point);
- CRP level > 60 mg/L at admission (1 point);
- Oxygen saturation < 90% at admission (1 point);
- Respiratory rate > 20/min at admission (1 point);
- Heart rate > 100 at admission (1 point).
4.4. Ethics Permission
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-resistant Gram-negative bacteria: A systematic review of current epidemiology, prognosis and treatment options. J. Antimicrob. Chemother. 2020, 75, 271–282. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance Geneva. 2014. Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 28 September 2022).
- Jee, Y.; Carlson, J.; Rafai, E.; Musonda, K.; Huong, T.T.G.; Daza, P.; Sattayawuthipong, W.; Yoon, T. Antimicrobial resistance: A threat to global health. Lancet Infect. Dis. 2018, 18, 939–940. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ten Threats to Global Health in 2019. 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 30 August 2022).
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance Genva. 2015. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 6 September 2022).
- Guillemot, D.; Carbon, C.; Balkau, B.; Geslin, P.; Lecoeur, H.; Vauzelle-Kervroëdan, F.; Bouvenot, G.; Eschwége, E. Low dosage and long treatment duration of beta-lactam: Risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 1998, 279, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Viasus, D.; Vecino-Moreno, M.; De La Hoz, J.M.; Carratalà, J. Antibiotic stewardship in community-acquired pneumonia. Expert Rev. Anti-Infect. Ther. 2017, 15, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Alam Tumpa, M.A.; Zehravi, M.; Sarker, M.T.; Yamin, M.; Islam, M.R.; Harun-Or-Rashid, M.; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An Overview of Antimicrobial Stewardship Optimization: The Use of Antibiotics in Humans and Animals to Prevent Resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. ECDC Country Visit to Norway to Discuss Antimicrobial Issues 12–16 March 2018 Stockholm. 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-country-visit-norway.pdf (accessed on 25 September 2022).
- NORM/NORM-VET 2021; Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Norwegian Institute of Public Health Oslo, University Hospital of North Norway: Tromsø, Norway, 2022.
- Walter, J.M.; Wunderink, R.G. Severe Respiratory Viral Infections: New Evidence and Changing Paradigms. Infect. Dis. Clin. N. Am. 2017, 31, 455–474. [Google Scholar] [CrossRef]
- Ruuskanen, O.; Lahti, E.; Jennings, L.C.; Murdoch, D.R. Viral pneumonia. Lancet 2011, 377, 1264–1275. [Google Scholar] [CrossRef]
- Torres, A.; Blasi, F.; Peetermans, W.E.; Viegi, G.; Welte, T. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: A literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1065–1079. [Google Scholar] [CrossRef]
- Lanks, C.W.; Musani, A.I.; Hsia, D.W. Community-acquired Pneumonia and Hospital-acquired Pneumonia. Med. Clin. N. Am. 2019, 103, 487–501. [Google Scholar] [CrossRef]
- Musher, D.M.; Thorner, A.R. Community-acquired pneumonia. N. Engl. J. Med. 2014, 371, 1619–1628. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Chappell, J.D. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- Oslo University Hospital. Methods in Infectious Diseases Oslo. 2023. Available online: https://metodebok.no/index.php?action=topic&item=TRkJXbbw (accessed on 4 April 2023).
- World Health Organization (WHO). WHO Access, Watch, Reserve (AWaRe) Classification of Antibiotics for Evaluation and Monitoring of Use, 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Simonsen, G.S.B.J.; Grave, K.; Hauge, K.; Juvet, L.K.; Lunestad, B.T.; Riisberg, I.; Rørtveit, G.; Urdahl, C.O.A.Å. Antimicrobial Resistance—Knowledge Gaps, Challenges and Relevant Measures. Status 2020 Oslo: The Norwegian Institute of Public Health. 2020. Available online: https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2020/amr-kunnskapshull-rapport.pdf (accessed on 1 September 2022).
- The Norwegian Health Directorate. National Guidelines for Use of Antimicrobial Agents in Hospital Care Oslo. 2020. Available online: https://www.helsedirektoratet.no/retningslinjer/antibiotika-i-sykehus (accessed on 8 September 2022).
- The Centre for Antibiotic Treatment in Primary Care (ASP). Antibiotic Treatment in Primary Care (HDIR)—Pneumonia: The Norwegian Health Directorate. Available online: https://antibiotikaiallmennpraksis.no/index.php?action=topic&item=f6000288c623ddf6aa4c (accessed on 20 September 2022).
- Lim, W.S.; Baudouin, S.V.; George, R.C.; Hill, A.T.; Jamieson, C.; Le Jeune, I.; Macfarlane, J.T.; Read, R.C.; Roberts, H.J.; Levy, M.L.; et al. BTS guidelines for the management of community acquired pneumonia in adults: Update 2009. Thorax 2009, 64 (Suppl. S3), iii1–iii55. [Google Scholar] [CrossRef]
- Lee, K.H.; Gordon, A.; Foxman, B. The role of respiratory viruses in the etiology of bacterial pneumonia: An ecological perspective. Evol. Med. Public Health 2016, 2016, 95–109. [Google Scholar] [CrossRef]
- Bakaletz, L.O. Viral–bacterial co-infections in the respiratory tract. Curr. Opin. Microbiol. 2017, 35, 30–35. [Google Scholar] [CrossRef]
- Falsey, A.R.; Becker, K.L.; Swinburne, A.J.; Nylen, E.S.; Formica, M.A.; Hennessey, P.A.; Criddle, M.M.; Peterson, D.R.; Baran, A.; Walsh, E.E. Bacterial Complications of Respiratory Tract Viral Illness: A Comprehensive Evaluation. J. Infect. Dis. 2013, 208, 432–441. [Google Scholar] [CrossRef]
- Shoar, S.; Musher, D.M. Etiology of community-acquired pneumonia in adults: A systematic review. Pneumonia 2020, 12, 11. [Google Scholar] [CrossRef]
- Holter, J.C.; Müller, F.; Bjørang, O.; Samdal, H.H.; Marthinsen, J.B.; Jenum, P.A.; Ueland, T.; Frøland, S.S.; Aukrust, P.; Husebye, E.; et al. Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: A 3-year prospective study in Norway. BMC Infect. Dis. 2015, 15, 64. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrera, L.; Rao, F.; Senarega, R.; Ferrari-Bravo, M. Chest radiological findings of influenza A H1N1 pneumonia. Rev. Port. Pneumol. 2012, 18, 120–127. [Google Scholar] [CrossRef]
- Shiley, K.T.; Van Deerlin, V.M.; Miller, W.T. Chest CT Features of Community-acquired Respiratory Viral Infections in Adult Inpatients with Lower Respiratory Tract Infections. J. Thorac. Imaging 2010, 25, 68–75. [Google Scholar] [CrossRef]
- Miller, W.T.; Mickus, T.J.; Barbosa, E.; Mullin, C.; Van Deerlin, V.M.; Shiley, K.T. CT of Viral Lower Respiratory Tract Infections in Adults: Comparison among Viral Organisms and between Viral and Bacterial Infections. Am. J. Roentgenol. 2011, 197, 1088–1095. [Google Scholar] [CrossRef]
- van de Maat, J.S.; Garcia Perez, D.; Driessen, G.J.A.; van Wermeskerken, A.M.; Smit, F.J.; Noordzij, J.G.; Tramper-Stranders, G.; Obihara, C.C.; Punt, J.; Moll, H.A.; et al. The influence of chest X-ray results on antibiotic prescription for childhood pneumonia in the emergency department. Eur. J. Pediatr. 2021, 180, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, V.; Neven, A.K.; van den Broek, P.J.; Assendelft, W.J. Diagnostic value of C reactive protein in infections of the lower respiratory tract: Systematic review. BMJ 2005, 331, 26. [Google Scholar] [CrossRef] [PubMed]
- Flanders, S.A.; Stein, J.; Shochat, G.; Sellers, K.; Holland, M.; Maselli, J.; Drew, W.; Reingold, A.L.; Gonzales, R. Performance of a bedside c-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am. J. Med. 2004, 116, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Branche, A.; Neeser, O.; Mueller, B.; Schuetz, P. Procalcitonin to guide antibiotic decision making. Curr. Opin. Infect. Dis. 2019, 32, 130–135. [Google Scholar] [CrossRef]
- Yunus, I.; Fasih, A.; Wang, Y. The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics. PLoS ONE 2018, 13, e0206527. [Google Scholar] [CrossRef]
- Serigstad, S.; Ritz, C.; Faurholt-Jepsen, D.; Markussen, D.; Ebbesen, M.H.; Kommedal, Ø.; Bjørneklett, R.O.; Heggelund, L.; Clark, T.W.; van Werkhoven, C.H.; et al. Impact of rapid molecular testing on diagnosis, treatment and management of community-acquired pneumonia in Norway: A pragmatic randomised controlled trial (CAPNOR). Trials 2022, 23, 622. [Google Scholar] [CrossRef]
- van Brummelen, S.; Tramper-Stranders, G.; Jonkman, K.; de Boer, G.; In ’t Veen, J.; Braunstahl, G.J. Antibiotic Prescriptions in Hospitalized Patients with an Exacerbation COPD and a Proven Influenza or RS Virus Infection. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 1261–1267. [Google Scholar] [CrossRef]
- Shiley, K.T.; Lautenbach, E.; Lee, I. The use of antimicrobial agents after diagnosis of viral respiratory tract infections in hospitalized adults: Antibiotics or anxiolytics? Infect. Control Hosp. Epidemiol. 2010, 31, 1177–1183. [Google Scholar] [CrossRef]
- Cohen, R.; Babushkin, F.; Geller, K.; Finn, T. Characteristics of hospitalized adult patients with laboratory documented Influenza A, B and Respiratory Syncytial Virus—A single center retrospective observational study. PLoS ONE 2019, 14, e0214517. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.; Reyzábal, S.G.; Salillas, J.; Gómez, M.R.L.; Rodríguez-Zurita, M.E.; Torralba, M. Respiratory syncytial virus infection among adults during influenza season: A frequently overlooked diagnosis. J. Med. Virol. 2019, 91, 1679–1683. [Google Scholar] [CrossRef]
- Christensen, I.; Haug, J.B.; Berild, D.; Bjørnholt, J.V.; Skodvin, B.; Jelsness-Jørgensen, L.-P. Factors Affecting Antibiotic Prescription among Hospital Physicians in a Low-Antimicrobial-Resistance Country: A Qualitative Study. Antibiotics 2022, 11, 98. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44 (Suppl. S2), S27–S72. [Google Scholar] [CrossRef]
- Markussen, D.L.; Grewal, H.M.S.; Knoop, S.T.; Serigstad, S.; Kommedal, Ø.; Ebbesen, M.; Ulvestad, E.; Bjørneklett, R.; The CAPNOR Study Group. Comparison of rapid molecular testing methods for detecting respiratory viruses in emergency care: A prospective study. Infect. Dis. 2022, 54, 247–254. [Google Scholar] [CrossRef]
- Debes, S.; Haug, J.B.; de Blasio, B.F.; Jonassen, C.M.; Dudman, S.G. Etiology of viral respiratory tract infections in hospitalized adults, and evidence of the high frequency of prehospitalization antibiotic treatment in Norway. Health Sci. Rep. 2021, 4, e403. [Google Scholar] [CrossRef]
- Debes, S.; Haug, J.B.; de Blasio, B.F.; Lindstrøm, J.C.; Jonassen, C.M.; Dudman, S.G. Clinical Outcome of Viral Respiratory Tract Infections in Hospitalized Adults in Norway: High Degree of Inflammation and Need of Emergency Care for Cases with Respiratory Syncytial Virus. Front. Med. 2022, 9, 866494. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef]
- Nguyen, M.T.N.; Saito, N.; Wagatsuma, Y. The effect of comorbidities for the prognosis of community-acquired pneumonia: An epidemiologic study using a hospital surveillance in Japan. BMC Res. Notes 2019, 12, 817. [Google Scholar] [CrossRef]
- Royal College of Physicians. National Early Warning Score (NEWS): Standardising the Assessment of Acute Illness Severity in the NHS. Report of a Working Party; Royal College of Physicians: London, UK, 2012. [Google Scholar]
- Bauer, T.T.; Ewig, S.; Marre, R.; Suttorp, N.; Welte, T.; the CAPNETZ Study Group. CRB-65 predicts death from community-acquired pneumonia. J. Intern. Med. 2006, 260, 93–101. [Google Scholar] [CrossRef]
| Total N = 951 | Antibiotic Treated n = 720 | Not Antibiotic Treated n = 231 | p-Value * | |
|---|---|---|---|---|
| Female, n (%) Male, n (%) | 506 (53.2) 445 (46.8) | 385 (53.5) 335 (46.5) | 121 (52.4) 110 (47.6) | 0.772 |
| Age, years median (range) | 73 (18–103) | 73 (18–103) | 72 (18–97) | 0.037 |
| Age ≥ 65 years (%) | 662 (69.6) | 518 (71.9) | 144 (62.3) | 0.006 |
| Charlson Score, median (range) | 2 (0–9) | 2 (0–9) | 1 (0–8) | 0.037 |
| Chronic obstructive pulmonary disease, n (%) | 330 (34.7) | 283 (39.3) | 47 (20.3) | <0.001 |
| Immunosuppressed, n (%) | 209 (22) | 169 (23.5) | 40 (17.3) | 0.049 |
| CRB65 category, median (range) Mean (±SD) | 2 (1–3) 1.79 (±0.50) | 2 (1–3) 1.83 (±0.50) | 2 (1–3) 1.67 (±0.49) | <0.001 |
| Patients with temperature ≥ 38 C, n (%) | 497 (52.3) | 408 (56.7) | 89 (38.5) | <0.001 |
| Oxygen saturation at admission, median (range) | 94 (57–100) Nd = 1 | 94 (57–100) | 96 (79–100) Nd = 1 | <0.001 |
| Patients with oxygen saturation < 90%, n (%) | 167 (17.6) Nd = 1 | 149 (20.7) | 18 (7.8) Nd = 1 | <0.001 |
| CRP level at admission, median (range) | 50 (1–589) Nd = 1 | 61.5 (1–589) | 24 (1–307) Nd = 1 | <0.001 |
| Patients with CRP level > 60 mg/L, n (%) | 401 (42.2) Nd = 1 | 362 (50.3) | 39 (16.9) Nd = 1 | <0.001 |
| Patients with WBC level ≥ 11.1 109/L, n (%) | 222 (23.3) Nd = 3 | 199 (27.6) Nd = 1 | 23 (10.0) Nd = 2 | <0.001 |
| NEWS score, median (range) | 4 (0–13) Nd = 90 | 4 (0–13) Nd = 44 | 2 (0–10) Nd = 46 | <0.001 |
| NEWS ≥ 5 points, n (%) | 331 (34.8) Nd = 90 | 301 (41.8) Nd = 44 | 30 (13.0) Nd = 46 | <0.001 |
| Non-invasive ventilation (CPAP, BiPAP), n (%) | 100 (10.5) | 95 (13.2) | 5 (2.2) | <0.001 |
| Mechanical ventilation, n (%) | 5 (0.5) | 5 (0.7) | 0 (0) | 0.344 |
| Admitted to ICU, n (%) | 112 (11.7) | 107 (14.9) | 5 (2.2) | <0.001 |
| Antiviral treatment (oseltamivir), n (%) | 143 (15.0) | 105 (14.6) | 38 (16.5) | 0.490 |
| Median length of stay, days (range) | 4 (0–27) | 4 (0–27) | 2 (0–18) | <0.001 |
| Length of stay ≥ 5 days | 394 (41.4) | 346 (48.1) | 48 (20.8) | <0.001 |
| Death during hospital stay, n (%) | 30 (3.2) | 27 (3.8) | 3 (1.3) | 0.064 |
| All cause mortality 30 days after discharge, n (%) | 23 (2.5) | 17 (2.5) | 6 (2.6) | 0.881 |
| N = 1200 | % | AWaRe * | |
|---|---|---|---|
| J 01C E Beta-lactamase Sensitive Penicillins a | 336 | 46.7 | Access |
| J 01C A Extended spectrum Penicillins b | 308 | 42.8 | Access |
| J01D B First generation Cephalosporins c | 2 | 0.3 | Access |
| J01D C/D Second- and third generation cephalosporins d | 164 | 22.8 | Watch |
| J01G B03 Gentamicin | 135 | 18.8 | Access |
| J01F A Macrolides e | 117 | 16.3 | Watch |
| J01 A A02 Doxycycline | 41 | 5.7 | Access |
| J01C R05 Piperacillin and beta-lactamase inhibitor f | 24 | 3.3 | Watch |
| J01C R02 Amoxicillin and beta-lactamase inhibitor g | 3 | 0.4 | Access |
| J01M A02 Ciprofloxacin | 26 | 3.6 | Watch |
| J01X D Metronidazole | 10 | 1.4 | Access |
| J01F F Clindamycin | 16 | 2.2 | Access |
| J01D H Carbapenems h | 13 | 1.8 | Watch |
| J01X A01 Vancomycin | 4 | 0.6 | Watch |
| J01C F Beta-lactamase Resistant Penicillins i | 1 | 0.1 | Access |
| Total N = 951 | Antibiotic Treated n = 720 | Not Antibiotic Treated n = 231 | p-Value * | |
|---|---|---|---|---|
| Chest X-ray/Chest CT compatible or susceptible of pneumonia, n (%) | 362 (37.0) Nd = 27 | 330 (45.8) Nd = 8 | 32 (13.9) Nd = 19 | <0.001 |
| CRP level > 60 mg/L, n (%) | 401 (42.2) Nd = 1 | 362 (50.3) | 39 (16.9) Nd = 1 | <0.001 |
| Oxygen saturation < 90%, n (%) | 167 (17.6) Nd = 1 | 149 (20.7) | 18 (7.8) Nd = 1 | <0.001 |
| Heart rate > 100/min, n (%) | 295 (31.0) | 242 (33.6) | 53 (22.9) | 0.002 |
| Respiratory rate > 20/min, n (%) | 474 (49.8) | 392 (54.4) | 82 (35.5) | <0.001 |
| Number of evaluation score points | ||||
| 0 points | 148 (15.6) | 67 (9.3) | 81 (35.1) | |
| 1 point | 272 (28.6) | 179 (24.9) | 93 (40.3) | |
| 2 points | 267 (28.1) | 223 (31.0) | 44 (19.0) | |
| 3 points | 178 (18.7) | 169 (23.5) | 9 (3.9) | |
| 4 points | 71 (7.5) | 67 (9.3) | 4 (1.7) | |
| 5 points | 15 (1.6) | 15 (2.1) | 0 (0) | |
| Evaluation score median (range) | 2 (0–5) | 2 (0–5) | 1 (0–4) | <0.001 |
| Evaluation score ≥ 2 points | 531 (55.8) | 474 (65.8) | 57 (24.7) | <0.001 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.012 | 1.002–1.022 | 0.022 |
| Gender female | 0.948 | 0.674–1.332 | 0.757 |
| Chest X-ray/chest CT compatible or susceptible of pneumonia | 3.658 | 2.399–5.580 | <0.001 |
| CRP level > 60 mg/L | 4.686 | 3.118–7.043 | <0.001 |
| Oxygen saturation < 90% | 2.107 | 1.210–3.669 | 0.008 |
| Heart rate > 100/min | 1.537 | 1.031–2.290 | 0.035 |
| Respiratory rate > 20/min | 1.762 | 1.236–2.512 | 0.002 |
| Constant | 0.400 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debes, S.; Haug, J.B.; De Blasio, B.F.; Lindstrøm, J.C.; Jonassen, C.M.; Dudman, S.G. Antibiotic Consumption in a Cohort of Hospitalized Adults with Viral Respiratory Tract Infection. Antibiotics 2023, 12, 788. https://doi.org/10.3390/antibiotics12040788
Debes S, Haug JB, De Blasio BF, Lindstrøm JC, Jonassen CM, Dudman SG. Antibiotic Consumption in a Cohort of Hospitalized Adults with Viral Respiratory Tract Infection. Antibiotics. 2023; 12(4):788. https://doi.org/10.3390/antibiotics12040788
Chicago/Turabian StyleDebes, Sara, Jon Birger Haug, Birgitte Freiesleben De Blasio, Jonas Christoffer Lindstrøm, Christine Monceyron Jonassen, and Susanne Gjeruldsen Dudman. 2023. "Antibiotic Consumption in a Cohort of Hospitalized Adults with Viral Respiratory Tract Infection" Antibiotics 12, no. 4: 788. https://doi.org/10.3390/antibiotics12040788
APA StyleDebes, S., Haug, J. B., De Blasio, B. F., Lindstrøm, J. C., Jonassen, C. M., & Dudman, S. G. (2023). Antibiotic Consumption in a Cohort of Hospitalized Adults with Viral Respiratory Tract Infection. Antibiotics, 12(4), 788. https://doi.org/10.3390/antibiotics12040788









