Abstract
Antimicrobial resistance (AMR) in Citrobacter freundii poses a serious challenge as this species is one of the sources of nosocomial infection and causes diarrheal infections in humans. Ducks could be the potential source of multidrug-resistant (MDR) C. freundii; however, AMR profiles in C. freundii from non-human sources in Bangladesh have remained elusive. This study aimed to detect C. freundii in domestic ducks (Anas platyrhynchos domesticus) in Bangladesh and to determine their phenotypic and genotypic antibiotic susceptibility patterns. A total of 150 cloacal swabs of diseased domestic ducks were screened using culturing, staining, biochemical, polymerase chain reaction (PCR), and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) to detect C. freundii. Phenotypic and genotypic antibiotic susceptibility patterns were done by the disk diffusion method and PCR, respectively. In total, 16.67% (25/150) of the samples were positive for C. freundii. C. freundii isolates showed a range of 20% to 96% resistance to cefotaxime, gentamicin, levofloxacin, ciprofloxacin, cotrimoxazole, tetracycline, ampicillin, and cephalexin. More than 60% of the isolates were phenotypically MDR, and the index of multiple antibiotic resistance ranged from 0.07 to 0.79. Genes encoding resistance to beta-lactams [blaTEM-1-88% (22/25), blaCMY-2-56% (14/25), blaCMY-9-8% (2/25), and blaCTX-M-14-20% (5/25)], sulfonamides [sul1-52% (13/25), sul2-24% (6/25)], tetracyclines [tetA-32% (8/25) and tetB-4% (1/25)], aminoglycosides [aacC4-16% (4/25)], and fluoroquinolones [qnrA-4% (1/25), qnrB-12% (3/25), and qnrS-4% (1/25)] were detected in the isolated C. freundii. To the best of our knowledge, this is the first study in Bangladesh to detect MDR C. freundii with their associated resistance genes from duck samples. We suggest addressing the burden of diseases in ducks and humans and associated AMR issues using the One Health approach.
1. Introduction
Antimicrobial resistance (AMR) is a major global issue that jeopardizes human, animal, and environmental health [1]. If nothing is done to curb AMR by 2050, it is expected to inflict hundreds of millions of fatalities worldwide, enormous financial consequences, and a significant decline in animal production [2,3]. Citrobacter freundii has become more resistant as a direct result of the widespread usage of antibiotics with a broad spectrum of activity [4]. Antimicrobials such as fluoroquinolones, aminoglycosides, nitrofurantoins, carbapenems, and cephalosporins are the typical classes of antibiotics used to treat infections caused by C. freundii [5]. However, the concern is rising because C. freundii has developed resistance to multiple antibiotics. Moreover, it is possible that low-virulent Citrobacter spp., which are able to survive in the host for a long time, could impact the evolution of pathogens by accumulating genes that code for resistance to multiple classes of antimicrobials [6]. The acquisition of resistance genes that confer resistance to multiple antibiotic classes from external sources, such as the environment or other bacteria, can cause multidrug resistance in Citrobacter species [7].
Antimicrobial-resistant bacteria may be present in duck droppings, contaminating the environment [8]. Ducks are possible carriers of important antimicrobial-resistant pathogens that might spread to humans because of their interactions with humans [9]. Humans can acquire C. freundii from ducks through contact with infected eggs, raw or undercooked meat, and duck carcasses at the slaughterhouse [10]. C. freundii infections can be fatal, with death rates ranging from 33 to 48% overall, including 30% mortality in children [11]. The central nervous system of survivor infants may be severely affected, resulting in extreme mental impairment, convulsions, and hemiparesis [12].
The poultry industry in Bangladesh has grown and become a successful agricultural business that makes a significant contribution to the country’s overall gross domestic product and provides a valuable source of protein [13,14]. Duck farming is a profitable livestock sector around the world because of the eggs, meat, and feathers it produces [15]. In Bangladesh, duck farming is important in its rural economy, second only to chicken production [16]. Ducks are typically reared in small-scale farming, either indoors or outdoors, or in an integrated farming system in Bangladesh [17], where they come or stay in close contact with humans. However, the greatest barrier to large-scale duck farming in Bangladesh is infectious disease outbreaks, including duck viral enteritis, duck viral hepatitis, avian influenza, botulism, duck cholera, etc. [18,19].
One of the bacterial pathogens found in duck droppings is Citrobacter spp. However, very little is known about the role of Citrobacter spp. as the source of infections in duck populations. C. freundii is the most prevalent among all Citrobacter species that causes infections in humans and animals [20]. C. freundii was isolated from young ducks having salpingitis [21]. The most common symptoms of C. freundii infection in ducks are discharge from nostrils, leg weakness, whitish diarrhea, recumbency, headshaking, and even sudden death [22].
In Bangladesh, the issue is compounded by the fact that poultry farmers come into direct contact with ducks during the rearing process, particularly when raising domestic ducks (Anas platyrhynchos domesticus) in their own homes. This direct interaction between humans and ducks increases the risk of transmission of C. freundii from domestic ducks to children, creating an even greater cause for concern. Human cases of C. freundii have been recorded in Bangladesh, India, and other Asian countries [23,24,25]; however, C. freundii cases in non-humans are not well described in these regions. In fact, this bacterium has not been well characterized in animals from any South Asian countries. The aim of this study was to detect C. freundii from cloacal swabs of ducks and determine their phenotypic and genotypic antibiotic resistance patterns to elucidate their potential negative impacts on human health.
2. Results
2.1. Occurrence of C. freundii Isolates
In the polymerase chain reaction (PCR) test, 25 of 150 samples were positive for Citrobacter spp. (16.67%, 95% CI: 11.55–23.45%) (Table 1). In MALDI-TOF analysis, all the Citrobacter spp. were detected as C. freundii. The occurrence of C. freundii in cloacal swabs of ducks was higher but not significant in the Kishoreganj district (22%, 95% CI: 12.75–35.24%) compared to that of Mymensingh (18%, 95% CI: 9.77–30.80%) and Netrokona (10%, 95% CI: 4.35–21.36%) districts (Table 1).

Table 1.
Prevalence of C. freundii isolated from cloacal swabs of ducks from different districts of Bangladesh.
2.2. Phenotypic Antibiogram Profiles of Isolated C. freundii
In the antibiotic susceptibility test (AST), C. freundii isolates showed the highest resistance to cephalexin (96%, 95% CI: 80.46–99.80%), followed by ampicillin (76%, 95% CI: 56.57–88.50%), azithromycin (56%, 95% CI: 37.07–73.33%), tetracycline (44%, 95% CI: 26.67–62.93%), cotrimoxazole (40%, 95% CI: 23.40–59.26%), ciprofloxacin and levofloxacin (36%, 95% CI: 20.25–55.48%), gentamicin (24%, 95% CI: 11.50–43.43%), cefotaxime (20%, 95% CI: 8.86–39.13%), ceftriaxone and ceftazidime (12%, 95% CI: 4.17–29.96%), and fosfomycin (4%, 95% CI: 0.21–19.54%) (Figure 1). In addition, 100% of the isolates exhibited sensitivity to nitrofurantoin and chloramphenicol (Figure 1).
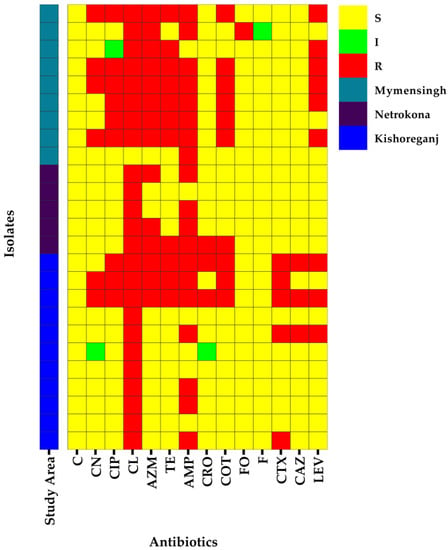
Figure 1.
Heatmap showing the distribution of antibiogram profiles in 25 C. freundii isolates detected from cloacal swabs of ducks, LEV = levofloxacin; CAZ = ceftazidime; CTX = cefotaxime; F = nitrofurantoin; FO = fosfomycin; COT = cotrimoxazole; CRO = ceftriaxone; AMP = ampicillin; TE = tetracycline; AZM = azithromycin; CL = Cephalexin; CIP = ciprofloxacin; CN = gentamycin; C = chloramphenicol, S = Sensitive, I = Intermediate, R = Resistant.
In bivariate analysis, we observed very high positive significant correlations between resistance patterns against cotrimoxazole and tetracycline (p < 0.001); cotrimoxazole and ciprofloxacin (p < 0.001); tetracycline and ciprofloxacin (p < 0.001); tetracycline and azithromycin (p < 0.001); ceftazidime and cefotaxime (p < 0.001); cotrimoxazole and azithromycin (p < 0.001); cotrimoxazole and gentamycin (p < 0.001); levofloxacin and tetracycline (p < 0.001); azithromycin and ciprofloxacin (p < 0.001); levofloxacin and ciprofloxacin (p < 0.001); tetracycline and gentamycin (p < 0.01); ceftazidime and ceftriaxone (p < 0.01); levofloxacin and cotrimoxazole (p < 0.01); and levofloxacin and gentamycin (p < 0.01) (Supplementary Table S1). We also found moderate-to-lower significant positive correlations between resistance patterns of C. freundii isolates against different antibiotics (Supplementary Table S1).
2.3. MDR and MAR Profiles of C. freundii
The majority of the C. freundii isolates (15/25, 60%, 95% CI: 40.74–76.60%) were phenotypically MDR and showed a multiple antibiotic resistance (MAR) index of more than 0.2. Fourteen antibiotic resistance patterns were observed, and eleven of them were MDR. The most common MDR pattern was no. 3 (gentamicin-ciprofloxacin-cephalexin-azithromycin-tetracycline-ampicillin-cotrimoxazole-levofloxacin), which was 26.67% (4/15) of the MDR C. freundii isolates. One isolate was resistant to 11 antibiotics (out of 14 tested antibiotics) from seven different classes (out of ten classes) (Table 2). Moreover, the MAR index of C. freundii isolates varied from 0.07 to 0.79 (Table 2).

Table 2.
Phenotypic multidrug resistance profiles and multiple antibiotic resistance index profiles of C. freundii isolated from cloacal swabs of ducks.
2.4. Genotypic Resistance Profiles of C. freundii Isolates
Upon PCR analysis, out of 25 C. freundii isolates, beta-lactamase genes blaTEM-1, blaCMY-2, blaCMY-9, and blaCTX-M-14 were detected in 88% (95% CI: 70.04–95.83%), 56% (95% CI: 37.07–73.33%), 8% (95% CI: 1.42–24.97%), and 20% (95% CI: 8.86–39.13%) of the isolates, respectively (Figure 2). Genes conferring resistance to sulfonamides [sul1-52% (13/25), 95% CI: 33.49–69.97%; sul2-24% (6/25), 95% CI: 11.49–43.43%], tetracyclines [tetA-32% (8/25), 95% CI: 17.21–51.59%; tetB-4% (1/25), 95% CI: 0.21–19.54%], fluoroquinolones [qnrA-4% (1/25), 95% CI: 0.21–19.54%; qnrB-12% (3/25), 95% CI: 4.17–29.96%; qnrS-4% (1/25), 95% CI: 0.21–19.54%], and aminoglycosides [aacC4-16% (4/25), 95% CI: 6.40–34.65%] were also detected in the isolated C. freundii. No isolates harbored blaSHV-1, blaCTX-M-1, blaCTX-M-2, tetC, and aacC2 genes (Figure 2).
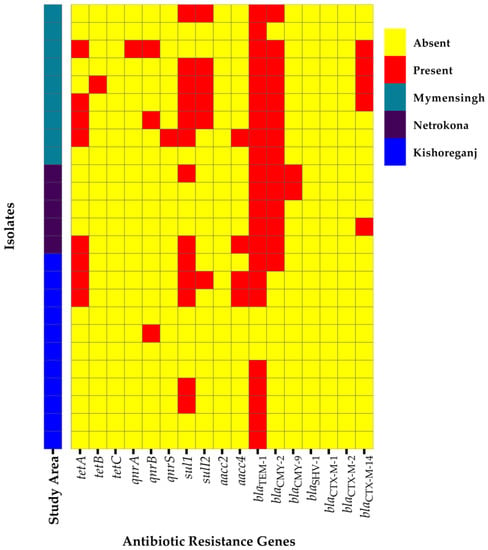
Figure 2.
Heatmap showing the distribution of various antibiotic resistance genes of C. freundii isolated from cloacal swabs of ducks in Bangladesh.
In the bivariate analysis, a high positive significant correlation was observed between the presence of antibiotic resistance genes aac4 and tetA (Pearson correlation coefficient, ρ = 0.636; p = 0.001), qnrA and qnrB (ρ = 0.553; p = 0.004), and sul1 and sul2 (ρ = 0.540; p = 0.005) (Supplementary Table S2). Moreover, moderate-to-low positive significant correlations were also observed between the presence of resistance genes tetA and sul1 (ρ = 0.487; p = 0.013), qnrS and aacC4 (ρ = 0.468; p = 0.018), blaCMY-2 and blaCTX-M-14 (ρ = 0.443; p = 0.026), blaCTX-M-14 and sul2 (ρ = 0.421; p = 0.036), aac4 and sul1 (ρ = 0.419; p = 0.037), blaTEM-1 and blaCMY-2 (ρ = 0.417; p = 0.038), tetB and blaCTX-M-14 (ρ = 0.408; p = 0.043), and qnrA and blaCTX-M-14 (ρ = 0.408; p = 0.043) (Supplementary Table S2).
2.5. Comparison of Phenotypic and Genotypic Resistance Profiles of Isolated C. freundii
In bivariate analysis, a positive significant correlation was observed between phenotypic and genotypic resistance profiles of C. freundii isolates against tetracyclines (ρ = 0.846; p < 0.001), sulfonamides (ρ = 0.784; p < 0.001), aminoglycosides (ρ = 0.521; p = 0.008), and fluoroquinolones (ρ = 0.417; p = 0.038). Given that all the isolates were phenotypically resistant to at least one beta-lactam antibiotic (constant variable), we could not compute the Pearson correlation coefficient to show the correlation between phenotypic and genotypic resistant profiles of C. freundii isolates against beta-lactams. However, 88% similarity was exhibited between beta-lactam antibiotics (n = 25) and beta-lactamase genes (n = 22) based on the phenotypic (disk diffusion) and genotypic (PCR) assays.
3. Discussion
Ducks have the potential to harbor hazardous bacteria that can cause zoonotic diseases in humans, including salmonellosis, E. coli infections, cholera, psittacosis, and others [9]. In our study, about 16.67% of the duck samples harbored C. freundii. The detection of C. freundii in this study suggests that ducks have the potential to transfer this organism to humans, posing an important threat to public health. In Bangladesh, poultry farmers, surrounding people, and the environment are directly exposed to domestic ducks during the process of rearing them. As a result, people in direct contact with ducks, both on farms and in houses, are at an increased risk of being infected with C. freundii from ducks. Importantly, C. freundii isolates have the ability to cause bacteremia in humans, indicating a high risk to human health [4]. A previous study in South Korea reported that C. freundii bacteremia was the major risk factor for a higher mortality rate in hospitalized patients (aged ≥ 15 years) [26]. Duck droppings can contaminate the agricultural environment and can be a source of infections for crop farmers and other people exposed to the contaminated environment [27].
Over time, C. freundii has the tendency to develop resistance to different classes of broad-spectrum antibiotics, and a major emerging issue is the rapid spread of this antibiotic resistance [26]. The result of the antibiotic susceptibility test showed that C. freundii isolates showed resistance to different classes of broad-spectrum antibiotics. For example, resistance was found to multiple classes of antibiotics, including beta-lactams, sulfonamides, fluoroquinolones, tetracyclines, aminoglycosides, macrolides, and glycopeptides, and these antibiotics are used extensively in both human and veterinary medicine. Our study indicates that domestic ducks foraging in different environmental niches could be a potential carrier of antibiotic resistance. Similar to our study, Olaiton et al. [8] also showed that C. freundii isolated from duck droppings showed resistance to tetracyclines, aminoglycosides, beta-lactams, and sulfonamides.
Cephalosporins are still reliable antibiotics for the treatment of C. freundii infections, and the rapid emergence of cephalosporin resistance in C. freundii is considered a global health problem [4]. In this study, C. freundii isolates showed phenotypic resistance to ampicillin, cephalexin, cefotaxime, ceftriaxone, and ceftazidime. In addition to that, several genotypes associated with cephalosporin resistance were found. For example, blaCMY-2 (56%), blaCMY-9 (8%), and blaCTX-M-14 (20%) are considered clinically associated biomarkers [28]. The widespread distribution of beta-lactam genotypes in humans, animals, and the environment indicates an immediate need for improvement in the treatment of infectious diseases [29]. Resistance to beta-lactam antibiotics in an organism may also be developed due to the acquisition of other beta-lactamase genes, e.g., blaTEM and blaCMY, in humans and animals [29,30]. The presence of these clinical biomarkers in C. freundii isolated from ducks is a clear indication of potential widescale spread from human C. freundii isolates or other members of the Enterobacteriaceae family.
Sulfonamides are a significant class of synthetic bacteriostatic antibiotics that are still widely used to treat bacterial infections in veterinary medicine [31]. In our study, 40% of the C. freundii isolates were resistant to the sulfonamide drug called cotrimoxazole. Resistance to sulfonamides in gram-negative bacteria, including C. freundii, typically results from the acquisition of one of two genes, sul1 or sul2, which encode for dihydropteroate synthase forms that are not inhibited by the drug [32]. Sulfonamide resistance genes sul1 and sul2 were detected in 52% and 24% of the C. freundii isolates, respectively. One possible explanation for the increased occurrence of sulfonamide resistance genes among C. freundii isolates collected from the cloaca of ducks is the widespread abuse of this kind of antibiotic in the poultry industry. The presence of sulfonamide-resistant C. freundii and their corresponding genes in ducks should be concerning because these resistant bacteria have the potential to be transferred to humans via direct or indirect contact [33].
Resistance to fluoroquinolone antibiotics is an urgent problem in both human and veterinary medicine worldwide. More than 44% of C. freundii isolates showed phenotypic resistance to fluoroquinolone antibiotics, either to ciprofloxacin or to levofloxacin, which is an important public health concern as these drugs are widely used for humans and large animals. In general, the fluoroquinolone group of antibiotics is a reliable antimicrobial agent for the treatment of Citrobacter infections, especially C. freundii bacteremia [4]. In this study, we detected a higher rate of fluoroquinolone resistance gene qnrB (12%) than that of other qnr (qnrA and qnrS) genes, which is not unusual. The qnrB genes, which encode proteins liable for reduced susceptibility to fluoroquinolones, are by far the most common and diverse subfamily of qnr genes [34]. These antibiotic-resistant bacteria and their resistance genes have the potential to spread to humans via the food chain [35]. Given that these resistant isolates can be passed from ducks to humans, the use of fluoroquinolones in poultry needs to be closely regulated in order to avoid any further resistance to fluoroquinolones.
Tetracyclines are commonly used as one of the first-line antibiotics against a wide variety of non-life-threatening infections, including C. freundii infections [36]. The development of antibiotic resistance in C. freundii limits the treatment options. We report 44% of the C. freundii isolates were phenotypically resistant to tetracycline. We also detected tetracycline resistance genes tetA (8/25) and tetB (1/25) in the C. freundii isolates; however, none for the tetC gene. The detection of tetA and tetB genes in tetracycline-resistant C. freundii isolates demonstrates that the active efflux system was the initial mechanism of tetracycline resistance in ducks [37,38].
Aminoglycosides are among the most effective antibiotics available for treating serious infections [39]. In the present study, 24% of the C. freundii isolates were phenotypically resistant to the aminoglycoside antibiotic named gentamicin. Moreover, we detected the aminoglycoside resistance gene aacC4 in 16% of the C. freundii isolates. The presence of this gene with significance to public health in ducks highlights the necessity of conducting an additional investigation into duck reservoirs for AMR. One of the important causal agents of community-acquired sepsis is C. freundii [40]. Therefore, the presence of aminoglycoside resistance in C. freundii may limit the treatment options for community-acquired sepsis because combining an aminoglycoside with a beta-lactam antibiotic and metronidazole is a common and effective experimental treatment for community-acquired sepsis [41].
This study found that the phenotypic and genotypic resistance profiles of C. freundii isolates against tetracyclines, sulfonamides, aminoglycosides, and fluoroquinolones were significantly correlated (p < 0.05). However, some isolates exhibited phenotypic susceptibility in the presence of resistance genes, and some isolates appeared phenotypic resistant but did not harbor resistant genes. Over time, several random mutations can occur in the gene sequence of an antibiotic resistance gene, rendering it inactive and transforming it into a resistance pseudogene that does not show the expected resistance characteristics [42]. Moreover, observed variations in susceptibility may be explained by heteroresistance processes such as random tandem gene amplification, uncommon mutation, and environmental manipulation of resistant genes [43]. The antibiotic may also act as a modulator, causing antibiotic-resistant genes to express poorly in vitro [44]. Therefore, whole-genome sequencing-based analyses have the potential to provide a precise genotype-to-phenotype resistance link.
Infections developed by MDR bacteria (with a high MAR index) have the potential to have severe repercussions for both human and animal health [45]. Humans and animals alike are at risk due to the spread of MDR Citrobacter spp. [46]. Infections caused by MDR C. freundii have fewer treatment options. In this study, 60% of the isolates were MDR, indicating an alarming issue in ducks and humans. The transfer of resistance genes from one resistant bacterium to another can lead to the development of MDR in bacteria that normally respond to the related classes of antibiotics [7]. Moreover, 60% of the isolates had a MAR index greater than 0.2, suggesting that these bacterial strains originated from a high-risk source of contamination in an area where antibiotics are frequently used [47]. Ducks can spread these pathogens to humans through the food chain or through direct contact, and they can also spread them to the environment through polluted water or feed [48,49].
4. Materials and Methods
4.1. Study Area
From January 2020 to January 2022, the present study was carried out in Mymensingh (24.7539° N, 90.4073° E), Netrokona (24.8103° N, 90.8656° E), and Kishoreganj (24.4260° N, 90.9821° E) districts of Bangladesh (Figure 3). The locations were chosen because of the high density of ducks in these districts, with massive wetland areas.
Figure 3.
Maps showing study areas in Bangladesh with the number of samples collected from each district.
4.2. Sample Collection and Processing
We collected a total of 150 cloacal swabs of diseased domestic ducks (Anas platyrhynchos domesticus) (50 from each district) from different households (10 households from each district, each household reared domestic ducks with a range of 50–100). The diseased ducks had several symptoms, such as whitish or greenish diarrhea, leg weakness, headshaking, and even sudden death. Moreover, the ducks were reared in a scavenging or semi-scavenging system. The cloacal swab was transferred aseptically to a test tube containing Luria–Bertani (LB) broth (HiMedia, Maharashtra, India). The samples were transferred to the lab in a cold chain and enriched the bacteria by incubating the test tube contents of the samples aerobically for 18 to 24 h at 37 °C.
4.3. Isolation and Molecular Detection of Citrobacter spp.
One loopful (1–2 µL) of the overnight growth culture was streaked on a xylose-lysine deoxycholate (XLD) agar (HiMedia, India) plate, and the plate was then incubated aerobically for 24–48 h at 37 °C. For pure colonies, subcultures were done following the same procedures. Colonies showing yellow color with a black center on the XLD agar plate were assumed to be Citrobacter spp. isolates. Finally, we screened the single pure colonies for further confirmation by Gram’s staining technique and corresponding biochemical tests (such as urease, citrate, catalase, motility, H2S test, methyl red, Voges–Proskauer, and sugar fermentation tests) [50].
We performed a PCR test for the final confirmation of isolated Citrobacter spp. targeting a 16S rRNA gene (F: AGAGTTTGATCMTGGCTCAG, R: TACGGYTACCTTGTTACGACTT) as previously mentioned [51]. For PCR, the DNA from the bacterial genome was extracted by following the boiling and freeze–thawing methods described earlier [52,53]. Each extracted DNA was then amplified using a total of 20 µL of the final volume, including 10 µL of the master mix (2×) (Promega, Madison, WI, USA), 1 µL of each primer (forward and reverse) (100 pmol) (Macrogen, Republic of Korea), 2 µL of nuclease-free water, and 6 µL of genomic DNA. Amplified PCR products were examined on a 1.5% agarose gel (Invitrogen, Waltham, MA, USA) using a gel electrophoresis apparatus (Nippon Genetics, Tokyo, Japan). Amplicon products were observed under an ultraviolet trans-illuminator (Biometra, Göttingen, Germany) after being stained with ethidium bromide. The amplicon size was checked using a 1 kb DNA ladder (Promega, Madison, WI, USA).
4.4. Detection of C. freundii by MALDI-TOF Mass Spectrometry
Citrobacter spp. isolates that were positive in PCR assay were then subjected to matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry to detect C. freundii isolates. The MALDI-TOF analysis was done in the QC Laboratory, Dhaka, Bangladesh, and was followed by the procedures previously described by Kolínská et al. [54].
4.5. Antibiotic Susceptibility Test
4.5.1. Phenotypic Analysis
According to the Clinical and Laboratory Standards Institute [55], the AST of isolated C. freundii was done by a Kirby–Bauer disk diffusion test [56]. In this study, 14 antibiotics from ten classes were chosen based on their availability in Bangladesh: fluoroquinolones (ciprofloxacin—5 μg, levofloxacin—5 μg), aminoglycosides (gentamicin—10 μg), tetracyclines (tetracycline—30 μg), macrolides (azithromycin—15 μg), cephalosporins (ceftriaxone—30 μg, cephalexin—30 μg, cefotaxime—30 μg, ceftazidime—30 μg), penicillins (ampicillin—10 μg), glycopeptides (chloramphenicol—30 μg), sulfonamides (cotrimoxazole- 25 μg), phosphonic acids (fosfomycin—200 μg), nitrofurantoin (nitrofurantoin—100 μg) (HiMedia, India). A multidrug-resistant (MDR) isolate was characterized as one that is resistant to three or more antibiotic classes [57]. We enumerated the multiple antibiotic resistance (MAR) index by the following formula [47]: MAR = w/v; here, w = number of antibiotics to which an isolate is resistant, v = total number of antibiotics used in this study.
4.5.2. Genotypic Analysis
Genes conferring resistance to beta-lactams (blaTEM-1, blaCMY-2, blaCMY-9, blaSHV-1, blaCTX-M-1, blaCTX-M-2, and blaCTX-M-14), sulfonamides (sul1 and sul2), tetracyclines (tetA, tetB, and tetC), fluoroquinolones (qnrA, qnrB, and qnrS), and aminoglycosides (aacC2 and aacC4) were tested by a simplex PCR assay (Table 3).

Table 3.
Primers used in the present study for detecting Citrobacter spp. and different antibiotic resistance genes in C. freundii isolates from cloacal swabs of ducks.
4.6. Statistical Analyses
4.6.1. Descriptive Analysis
We used Microsoft Excel 2013 (Los Angeles, CA, USA) for data entry and the Statistical Package for Social Science (SPSS 25, IBM, Chicago, IL, USA) and GraphPad Prism 8.4.2 (GraphPad Software, Inc., Avenida De La Playa La Jolla, CA, USA) for the data analysis. We performed the chi-square test for relatedness (with a Z-test for proportion) to understand the variations in the prevalence of C. freundii among sampling sites. Statistical significance was defined as a p-value of less than 0.05. The binomial 95% confidence interval (CI) was calculated following the Wilson and Brown Hybrid method [60]. Using GraphPad Prism, we created the heatmap to show the distribution of phenotypic and genotypic antibiotic resistance profiles of C. freundii isolates.
4.6.2. Bivariate Analysis
We performed bivariate analysis in SPSS to determine whether resistance patterns in pairs of antibiotics and antibiotic resistance genes from isolated C. freundii were correlated. Moreover, the correlation between phenotypic and genotypic resistance profiles of C. freundii isolates against different classes of antibiotics was determined using bivariate analysis. A statistically significant p-value was less than 0.05 (p < 0.05).
5. Conclusions
Citrobacter spp. was found to be present in more than 16% of the samples collected from ducks in Bangladesh. We reported a range of 20% to 96% resistance in C. freundii isolates to different important antibiotics. The detection of genes encoding resistance to various classes of antibiotics in C. freundii isolated from ducks indicates a significant risk to human health due to the widespread presence of antibiotic resistance and their associated resistance genes in C. freundii. Given the close relationship between ducks, water, and other environmental components, there is a concern about the spreading of antibiotic-resistant C. freundii to humans. This may pose a potential human health risk. C. freundii should be characterized more elaborately using whole genome sequencing. However, further studies using the One Health approach and developed tools and high technologies are helpful in understanding the potential impact of MDR C. freundii in humans and animals and to minimize the risk of the emergence of MDR C. freundii in both animals and humans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12040769/s1, Table S1: Pearson correlation coefficients assessing correlation between pairs of antibiotics to which C. freundii isolates showed resistance; Table S2: Pearson correlation coefficients assessing correlation between pairs of antibiotic resistance genes in C. freundii isolates from cloacal swabs of ducks.
Author Contributions
T.A., M.S.R.K. and M.N. contributed to the study conception and design. T.A. investigated the study. T.A. and M.S.I. analyzed the data. T.A. and M.S.I. prepared the first draft manuscript. M.S.I., N.H., L.E., B.H., M.N., M.T.R., S.M.L.K. and M.S.R.K. reviewed the draft manuscripts. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by ‘Bangladesh Agricultural Research Council’ through ‘National Agricultural Technology Program Phase II’ project for in Country Ph.D. scholarship (P149553).
Institutional Review Board Statement
The institutional ethics committee (Animal Welfare and Experimentation Ethical Committee) at Bangladesh Agricultural University in Mymensingh gave its approval to the procedures and related protocols in this research (AWEEC/BAU/2020[10]).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
Acknowledgments
We acknowledge the Department of Livestock Services of the Ministry of Fisheries and Livestock of Bangladesh for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Islam, M.; Nayeem, M.; Hasan, M.; Sobur, M.; Ievy, S.; Rahman, S.; Kafi, M.; Ashour, H.M.; Rahman, M. Virulence determinants and multidrug resistance of Escherichia coli isolated from migratory birds. Antibiotics 2021, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Tawyabur, M.; Islam, M.; Sobur, M.; Hossain, M.; Mahmud, M.; Paul, S.; Hossain, M.T.; Ashour, H.M.; Rahman, M. Isolation and characterization of multidrug-resistant Escherichia coli and Salmonella spp. from healthy and diseased turkeys. Antibiotics 2020, 9, 770. [Google Scholar] [CrossRef]
- Urmi, M.R.; Ansari, W.K.; Islam, M.S.; Sobur, M.A.; Rahman, M.; Rahman, M.T. Antibiotic resistance patterns of Staphylococcus spp. isolated from fast foods sold in different restaurants of Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 2021, 8, 274–281. [Google Scholar] [CrossRef]
- Liu, L.H.; Wang, N.Y.; Wu, A.Y.J.; Lin, C.C.; Lee, C.M.; Liu, C.P. Citrobacter freundii bacteremia: Risk factors of mortality and prevalence of resistance genes. J. Microbiol. Immunol. Infect. 2018, 51, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Chang, S.C. Citrobacter Species. 2016. Available online: http://www.antimicrobe.org/b93.asp (accessed on 25 February 2023).
- Pepperell, C.; Kus, J.V.; Gardam, M.A.; Humar, A.; Burrows, L.L. Low-virulence Citrobacter species encode resistance to multiple antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3555–3560. [Google Scholar] [CrossRef]
- Sommer, M.O.; Dantas, G. Antibiotics and the resistant microbiome. Curr. Opin. Microbiol. 2011, 14, 556–563. [Google Scholar] [CrossRef]
- Olaitan, J.O.; Shittu, O.B.; Akinliba, A.A. Antibiotic resistance of enteric bacteria isolated from duck droppings. J. Appl. Biosci. 2011, 45, 3008–3018. [Google Scholar]
- Elmberg, J.; Berg, C.; Lerner, H.; Waldenström, J.; Hessel, R. Potential disease transmission from wild geese and swans to livestock, poultry and humans: A review of the scientific literature from a One Health perspective. Infect. Ecol. Epidemiol. 2017, 7, 1300450. [Google Scholar] [CrossRef]
- Lessenger, J.E. Diseases from Animals, Poultry, and Fish, in Agricultural Medicine; Springer: New York, NY, USA, 2006; pp. 367–382. [Google Scholar]
- Owoseni, M.; Okoh, A. Assessment of chlorine tolerance profile of Citrobacter species recovered from wastewater treatment plants in Eastern Cape, South Africa. Environ. Monit. Assess. 2017, 189, 1–12. [Google Scholar] [CrossRef]
- Doran, T.I. The role of Citrobacter in clinical disease of children. Clin. Infect. Dis. 1999, 28, 384–394. [Google Scholar] [CrossRef]
- Islam, M.S.; Sabuj, A.A.M.; Haque, Z.F.; Pondit, A.; Hossain, M.G.; Saha, S. Seroprevalence and risk factors of avian reovirus in backyard chickens in different areas of Mymensingh district in Bangladesh. J. Adv. Vet. Anim. Res. 2020, 7, 546. [Google Scholar] [CrossRef]
- Mili, S.A.; Islam, S.; Al Momen Sabuj, A.; Haque, Z.F.; Pondit, A.; Hossain, G.; Hassan, J.; Saha, S. A Cross-Sectional Seroepidemiological Study on Infectious Bursal Disease in Backyard Chickens in the Mymensingh District of Bangladesh. Vet. Med. Int. 2022, 2022, 9076755. [Google Scholar] [CrossRef]
- Rajput, D.S.; Singh, S.P.; Sudipta, G.; Nema, R.P. Duck farming, fascinating option in India. J. Vet. Sci. Technol. 2014, 5, 181. [Google Scholar]
- Dey, R.K.; Khan, M.S.R.; Nazir, K.H.M.N.H.; Islam, M.A.; Belal, S.M.S.H. Epidemiological investigation on Duck Salmonellosis in some selected areas of Bangladesh. Bangladesh J. Vet. Med. 2016, 14, 149–160. [Google Scholar] [CrossRef]
- Islam, M.A.; Howlider, M.A.R.; Alam, M.A.; Heyamet, M.A.; Debnath, M. Present status, problem and prospect of duck farming in rural areas of Mymensingh district, Bangladesh. Asian J. Med. Biol. Res. 2016, 2, 202–212. [Google Scholar] [CrossRef]
- Hoque, M.A.; Skerratt, L.F.; Rahman, M.A.; Rabiul Alam Beg, A.B.M.; Debnath, N.C. Factors limiting traditional household duck production in Bangladesh. Trop. Anim. Health Prod. 2010, 42, 1579–1587. [Google Scholar] [CrossRef]
- Khatun, A.; Giasuddin, M.; Islam, K.M.; Khanom, S.; Samad, M.A.; Islam, M.R.; Noor, M.; Bhuiyan, J.U.; Kim, W.I.; Eo, S.K.; et al. Surveillance of avian influenza virus type A in semi-scavenging ducks in Bangladesh. BMC Vet. Res. 2013, 9, 196. [Google Scholar] [CrossRef]
- Bai, L.; Xia, S.; Lan, R.; Liu, L.; Ye, C.; Wang, Y.; Jin, D.; Cui, Z.; Jing, H.; Xiong, Y.; et al. Isolation and characterization of cytotoxic, aggregative Citrobacter freundii. PLoS ONE 2012, 7, e33054. [Google Scholar] [CrossRef]
- Bisgaard, M. Salpingitis in web-footed birds: Prevalence, aetiology and significance. Avian Pathol. 1995, 24, 443–452. [Google Scholar] [CrossRef]
- Haruna, E.S.; Usman, M.; Ahmed, S.; Shaibu, J.S.; Makinde, A.A.; Lombin, L.H.; Henton, M.M. Isolation of Citrobacter murliniae from clinically ill and dead quail, ducks and chickens. Vet. Rec. 2004, 154, 119. [Google Scholar] [CrossRef]
- Rahman, A.; Shamsuzzaman, S.M.; Dola, N.Z. Antimicrobial Susceptibility Pattern and Virulence Genes Detection in Citrobacter freundii Isolated from Patients of a Tertiary Care Hospital, Bangladesh: Antimicrobial susceptibility pattern and virulence genes detection in Citrobacter freundii. Int. Arab. J. Antimicrob. Agents 2022, 12, 1. [Google Scholar] [CrossRef]
- Mohanty, S.; Singhal, R.; Sood, S.; Dhawan, B.; Kapil, A.; Das, B.K. Citrobacter infections in a tertiary care hospital in Northern India. J. Infect. 2007, 54, 58–64. [Google Scholar] [CrossRef]
- Farjana, N.E.; Islam, M.A.; Zerin, T.; Begum, M.A. Bacterial association in urinary tract infection and their drug resistance among patients in Rajshahi, Bangladesh. Int. J. Community Med. Public Health 2021, 8, 2144. [Google Scholar] [CrossRef]
- Kim, B.N.; Woo, J.H.; Ryu, J.; Kim, Y.S. Resistance to extended-spectrum cephalosporins and mortality in patients with Citrobacter freundii bacteremia. Infection 2003, 31, 202–207. [Google Scholar] [CrossRef]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef]
- Sidjabat, H.E.; Paterson, D.L.; Qureshi, Z.A.; Adams-Haduch, J.M.; O’Keefe, A.; Pascual, A.; Rodrçguez-Bano, J.; Doi, Y. Clinical features and molecular epidemiology of CMY-type β-lactamase–producing Escherichia coli. Clin. Infect. Dis. 2009, 48, 739–744. [Google Scholar] [CrossRef]
- Islam, M.; Sobur, M.; Rahman, S.; Ballah, F.M.; Ievy, S.; Siddique, M.P.; Rahman, M.; Kafi, M.; Rahman, M. Detection of blaTEM, blaCTX-M, blaCMY, and blaSHV Genes Among Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Isolated from Migratory Birds Travelling to Bangladesh. Microb. Ecol. 2022, 83, 942–950. [Google Scholar] [CrossRef]
- Ngaiganam, E.P.; Pagnier, I.; Chaalal, W.; Leangapichart, T.; Chabou, S.; Rolain, J.M.; Diene, S.M. Investigation of urban birds as source of β-lactamase-producing Gram-negative bacteria in Marseille city, France. Acta Vet. Scand. 2019, 61, 1–7. [Google Scholar] [CrossRef]
- Daeseleire, E.; Van Pamel, E.; Van Poucke, C.; Croubels, S. Veterinary Drug Residues in Foods. In Chemical Contaminants and Residues in Food, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 117–153. [Google Scholar]
- Botts, R.T.; Apffel, B.A.; Walters, C.J.; Davidson, K.E.; Echols, R.S.; Geiger, M.R.; Guzman, V.L.; Haase, V.S.; Montana, M.A.; La Chat, C.A.; et al. Characterization of four multidrug resistance plasmids captured from the sediments of an urban coastal wetland. Front. Microbiol. 2017, 8, 1922. [Google Scholar] [CrossRef]
- Hasan, M.S.; Sultana, M.; Hossain, M.A. Complete genome arrangement revealed the emergence of a poultry origin superbug Citrobacter portucalensis strain NR-12. J. Glob. Antimicrob. Resist. 2019, 18, 126–129. [Google Scholar] [CrossRef]
- Ribeiro, T.G.; Novais, Â.; Branquinho, R.; Machado, E.; Peixe, L. Phylogeny and comparative genomics unveil independent diversification trajectories of qnrB and genetic platforms within particular Citrobacter species. Antimicrob. Agents Chemother. 2015, 59, 5951–5958. [Google Scholar] [CrossRef]
- Samtiya, M.; Matthews, K.R.; Dhewa, T.; Puniya, A.K. Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods 2022, 11, 2966. [Google Scholar] [CrossRef]
- Truong, R.; Tang, V.; Grennan, T.; Tan, D.H.S. A systematic review of the impacts of oral tetracycline class antibiotics on antimicrobial resistance in normal human flora. JAC Antimicrob. Resist. 2022, 4, dlac009. [Google Scholar] [CrossRef]
- Roberts, M.C. Tetracycline resistance determinants: Mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 1996, 19, 1–24. [Google Scholar] [CrossRef]
- Roy, K.; Islam, M.S.; Paul, A.; Ievy, S.; Talukder, M.; Sobur, M.A.; Ballah, F.M.; Khan, M.S.R.; Rahman, M.T. Molecular detection and antibiotyping of multi-drug resistant Enterococcus faecium from healthy broiler chickens in Bangladesh. Vet. Med. Sci. 2022, 8, 200–210. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Ferranti, M.; Cicogna, G.T.; Sattin, A.; Alaibac, M. Citrobacter freundii sepsis in an immunosuppressed patient with pemphigus vulgaris. BMJ Case Rep. CP 2018, 11, e227091. [Google Scholar] [CrossRef]
- Norskov-Lauritsen, N.; Sandvang, D.; Hedegaard, J.; Fussing, V.; Mortensen, K.K.; Sperling-Petersen, H.U.; Schonheyder, H.C. Clonal origin of aminoglycoside-resistant Citrobacter freundii isolates in a Danish county. J. Med. Microbiol. 2001, 50, 636–641. [Google Scholar] [CrossRef]
- Davis, M.A.; Besser, T.E.; Orfe, L.H.; Baker, K.N.; Lanier, A.S.; Broschat, S.L.; New, D.; Call, D.R. Genotypic-phenotypic discrepancies between antibiotic resistance characteristics of Escherichia coli isolates from calves in management settings with high and low antibiotic use. Appl. Environ. Microbiol. 2011, 77, 3293–3299. [Google Scholar] [CrossRef]
- Urmi, U.L.; Nahar, S.; Rana, M.; Sultana, F.; Jahan, N.; Hossain, B.; Alam, M.S.; Mosaddek, A.S.; McKimm, J.; Rahman, N.A.; et al. Genotypic to Phenotypic Resistance Discrepancies Identified Involving β-Lactamase Genes, blaKPC, blaIMP, blaNDM-1, and blaVIM in Uropathogenic Klebsiella pneumoniae. Infect. Drug Resist. 2020, 13, 2863–2875. [Google Scholar] [CrossRef]
- Depardieu, F.; Podglajen, I.; Leclercq, R.; Collatz, E.; Courvalin, P. Modes and modulations of antibiotic resistance gene expression. Clin. Microbiol. Rev. 2007, 20, 79–114. [Google Scholar] [CrossRef]
- Talukder, M.; Islam, M.S.; Ievy, S.; Sobur, M.A.; Ballah, F.M.; Najibullah, M.; Rahman, M.B.; Rahman, M.T.; Khan, M.F.R. Detection of multidrug-resistant Salmonella spp. from healthy and diseased broilers having potential public health significance. J. Adv. Biotechnol. Exp. 2021, 4, 248–255. [Google Scholar] [CrossRef]
- Liu, L.; Qin, L.; Hao, S.; Lan, R.; Xu, B.; Guo, Y.; Jiang, R.; Sun, H.; Chen, X.; LV, X.; et al. Lineage, Antimicrobial Resistance and Virulence of Citrobacter spp. Pathogens 2020, 9, 195. [Google Scholar] [CrossRef]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- Kang, X.; Wang, M.; Meng, C.; Li, A.; Jiao, X.; Pan, Z. Prevalence and whole–genome sequencing analysis of Salmonella reveal its spread along the duck production chain. Poult. Sci. 2022, 101, 101993. [Google Scholar] [CrossRef]
- Fong, I.W. Animals and Mechanisms of Disease Transmission. In Emerging Zoonoses: A Worldwide Perspective; Fong, I.W., Ed.; Springer: Toronto, ON, Canada, 2017; pp. 15–38. [Google Scholar]
- Brenner, D.J.; O’Hara, C.M.; Grimont, P.A.; Janda, J.M.; Falsen, E.; Aldova, E.; Ageron, E.; Schindler, J.; Abbott, S.L.; Steigerwalt, A.G. Biochemical identification of Citrobacter species defined by DNA hybridization and description of Citrobacter gillenii sp. nov.(formerly Citrobacter genomospecies 10) and Citrobacter murliniae sp. nov.(formerly Citrobacter genomospecies 11). J. Clin. Microbiol. 1999, 37, 2619–2624. [Google Scholar] [CrossRef]
- Hashim, M.H.; AlKhafaji, M.H. Isolation and identification of Citrobacter freundii from chicken meat samples using cultural and molecular techniques. Iraqi J. Sci. 2018, 59, 1216–1224. [Google Scholar]
- Ievy, S.; Islam, M.; Sobur, M.; Talukder, M.; Rahman, M.; Khan, M.F.R.; Rahman, M. Molecular detection of avian pathogenic Escherichia coli (APEC) for the first time in layer farms in Bangladesh and their antibiotic resistance patterns. Microorganisms 2020, 8, 1021. [Google Scholar] [CrossRef]
- Islam, M.S.; Paul, A.; Talukder, M.; Roy, K.; Sobur, M.A.; Ievy, S.; Nayeem, M.M.H.; Rahman, S.; Nazir, K.N.H.; Hossain, M.T.; et al. Migratory birds travelling to Bangladesh are potential carriers of multi-drug resistant Enterococcus spp., Salmonella spp., and Vibrio spp. Saudi J. Biol. Sci. 2021, 28, 5963–5970. [Google Scholar] [CrossRef]
- Kolínská, R.; Španělová, P.; Dřevínek, M.; Hrabák, J.; Žemličková, H. Species identification of strains belonging to genus Citrobacter using the biochemical method and MALDI-TOF mass spectrometry. Folia Microbiol. 2015, 60, 53–59. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, M100-S30; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Bauer, A.T.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, S.; White, D.G.; Schroeder, C.M.; Lu, R.; Yang, H.; McDermott, P.F.; Ayers, S.; Meng, J. Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Appl. Environ. Microbiol. 2004, 70, 1–7. [Google Scholar] [CrossRef]
- Azeez, D.A.; Findik, D.; Hatice, T.Ü.R.K.; Arslan, U. Plasmid-mediated fluoroquinolone resistance in clinical isolates of Escherichia coli in Konya, Turkey. Cukurova Med. J. 2018, 43, 295–300. [Google Scholar] [CrossRef]
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval estimation for a binomial proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).