Phentolamine Significantly Enhances Macrolide Antibiotic Antibacterial Activity against MDR Gram-Negative Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Antimicrobial Agents
2.3. MIC Determinations and Fractional Inhibitory Concentration (FIC) Index Assay
2.4. In Vitro Time–Kill Curves
2.5. Galleria mellonella Infection Model
2.6. SEM Analysis
2.7. Outer Membrane Permeability
2.8. Intracellular pH Assay
2.9. Intracellular ATP
2.10. EtBr Accumulation Assay
2.11. Statistical Analysis
3. Results
3.1. In Vitro Interaction Assessments and Time–Kill Curves
3.2. Efficacy of Phentolamine/Erythromycin Combinations for G. mellonella
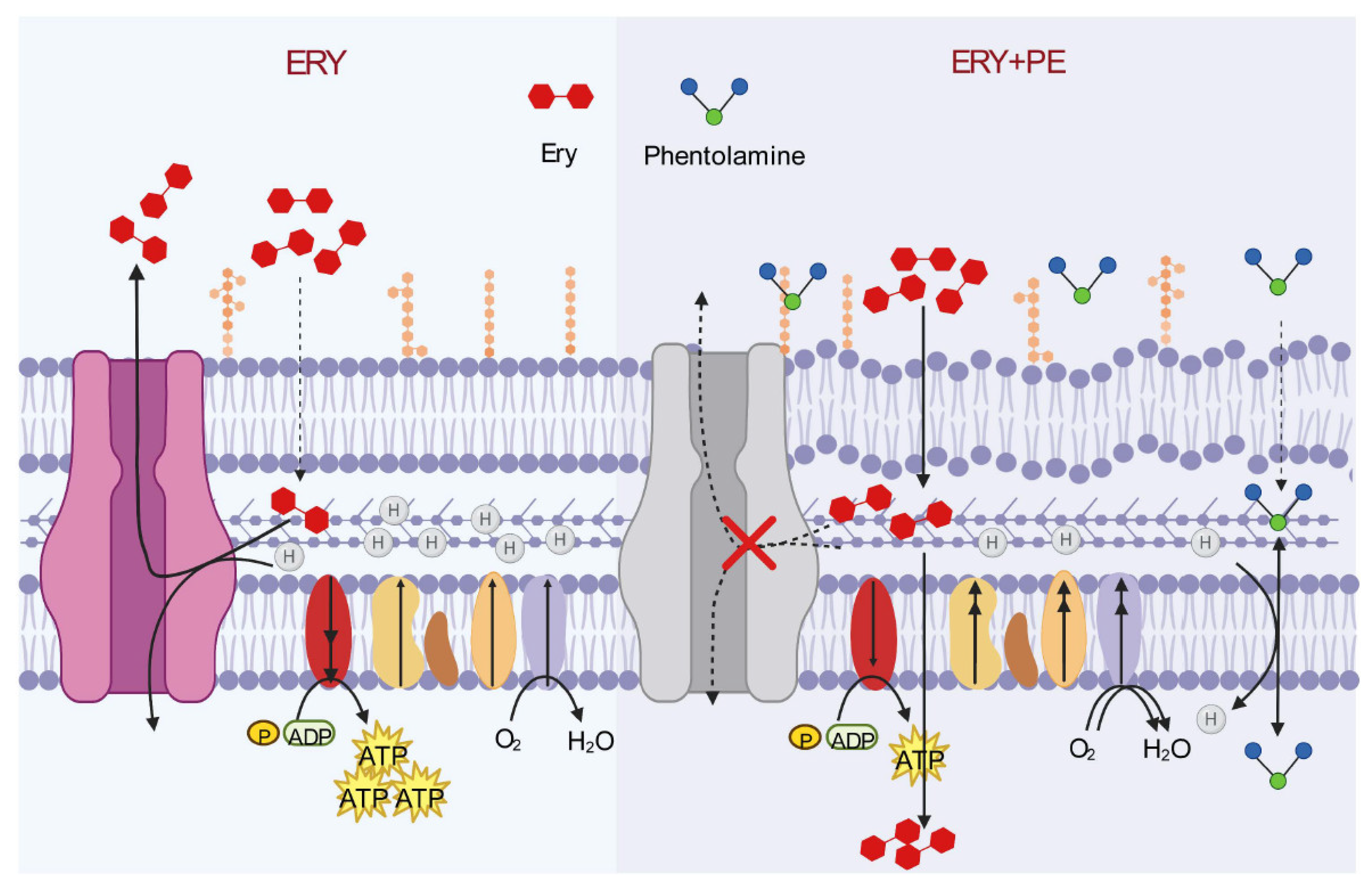
3.3. Mechanism of Synergistic Activity of Phentolamine Combination with Macrolide Antibiotics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bassetti, M.; Garau, J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76, iv23–iv37. [Google Scholar] [CrossRef] [PubMed]
- Scannell, J.W.; Bosley, J.; Hickman, J.A.; Dawson, G.R.; Truebel, H.; Ferreira, G.S.; Richards, D.; Treherne, J.M. Predictive validity in drug discovery: What it is, why it matters and how to improve it. Nat. Rev. Drug Discov. 2022, 21, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.N.; Drown, B.S.; Geddes, E.J.; Lee, H.Y.; Ismail, N.; Lau, G.W.; Hergenrother, P.J. Implementation of permeation rules leads to a FabI inhibitor with activity against Gram-negative pathogens. Nat. Microbiol. 2019, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Novelli, A.; Del Giacomo, P.; Rossolini, G.M.; Tumbarello, M. Meropenem/vaborbactam: A next generation beta-lactam beta-lactamase inhibitor combination. Expert. Rev. Anti. Infect. Ther. 2020, 18, 643–655. [Google Scholar] [CrossRef]
- Seiple, I.B.; Zhang, Z.; Jakubec, P.; Langlois-Mercier, A.; Wright, P.M.; Hog, D.T.; Yabu, K.; Allu, S.R.; Fukuzaki, T.; Carlsen, P.N.; et al. A platform for the discovery of new macrolide antibiotics. Nature 2016, 533, 338–345. [Google Scholar] [CrossRef]
- Parra-Millán, R.; Vila-Farrés, X.; Ayerbe-Algaba, R.; Varese, M.; Sánchez-Encinales, V.; Bayó, N.; Pachón-Ibáñez, M.E.; Teixidó, M.; Vila, J.; Pachón, J.; et al. Synergistic activity of an OmpA inhibitor and colistin against colistin-resistant Acinetobacter baumannii: Mechanistic analysis and in vivo efficacy. J. Antimicrob. Chemother. 2018, 73, 3405–3412. [Google Scholar] [CrossRef]
- Brochado, A.R.; Telzerow, A.; Bobonis, J.; Banzhaf, M.; Mateus, A.; Selkrig, J.; Huth, E.; Bassler, S.O.; Beas, J.Z.; Zietek, M.; et al. Species-specific activity of antibacterial drug combinations. Nature 2018, 559, 259–263. [Google Scholar] [CrossRef]
- Yan, Z.; Shang, Y.; Li, F.; Xie, F.; Qian, H.; Zhang, Y.; Yue, B. Therapeutic efficacy of phentolamine in the management of severe hand, foot and mouth disease combined with pulmonary edema. Exp. Ther. Med. 2017, 13, 1403–1407. [Google Scholar] [CrossRef]
- Lucyk, S.N. Acute Cardiovascular Toxicity of Cocaine. Can. J. Cardiol. 2022, 38, 1384–1394. [Google Scholar] [CrossRef]
- Singh, V.; Cohen, S.P. Prolonging Sympathetic Blockade for Complex Regional Pain Syndrome: Is Botulinum Toxin the Answer? Anesthesiology 2022, 136, 261–264. [Google Scholar] [CrossRef]
- Shrestha, N.; Acharya, U.; Shrestha, P.S.; Acharya, S.P.; Karki, B.; Dhakal, S.S. Topical nitroglycerin for management of peripheral extravasation of vasopressors: A case report. Oxf. Med. Case Rep. 2020, 2020, omaa066. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Liu, P.; Zhang, C.J.; Liao, X.P.; Sun, J.; Liu, Y.H. Colistin Combined with Tigecycline: A Promising Alternative Strategy to Combat Escherichia coli Harboring blaNDM–5 and mcr-1. Front. Microbiol. 2020, 10, 2957. [Google Scholar] [CrossRef]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Zhong, Z.-X.; Cui, Z.-H.; Li, X.-J.; Tang, T.; Zheng, Z.-J.; Ni, W.-N.; Fang, L.-X.; Zhou, Y.-F.; Yu, Y.; Liu, Y.-H.; et al. Nitrofurantoin Combined with Amikacin: A Promising Alternative Strategy for Combating MDR Uropathogenic Escherichia coli. Front. Cell Infect. Microbiol. 2020, 10, 608547. [Google Scholar] [CrossRef]
- Gomara, M.; Ramon-Garcia, S. The FICI paradigm: Correcting flaws in antimicrobial in vitro synergy screens at their inception. Biochem. Pharmacol. 2019, 163, 299–307. [Google Scholar] [CrossRef]
- She, P.; Liu, Y.; Xu, L.; Li, Y.; Li, Z.; Liu, S.; Hussain, Z.; Wu, Y. SPR741, Double- or Triple-Combined with Erythromycin and Clarithromycin, Combats Drug-Resistant Klebsiella pneumoniae, Its Biofilms, and Persister Cells. Front. Cell Infect. Microbiol. 2022, 12, 858606. [Google Scholar] [CrossRef]
- Zhou, N.; Cheng, Z.; Zhang, X.; Lv, C.; Guo, C.; Liu, H.; Dong, H.; Zhang, Y.; Liu, C.; Chang, Y.-F.; et al. Global antimicrobial resistance: A system-wide comprehensive investigation using the Global One Health Index. Infect. Dis. Poverty 2022, 11, 92. [Google Scholar] [CrossRef]
- Yahav, D.; Tau, N.; Shepshelovich, D. Assessment of Data Supporting the Efficacy of New Antibiotics for Treating Infections Caused by Multidrug-resistant Bacteria. Clin. Infect. Dis. 2021, 72, 1968–1974. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Lewis, K. Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 1992, 89, 8938–8942. [Google Scholar] [CrossRef]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Wistrand-Yuen, P.; Nielsen, E.I.; Friberg, L.; Sandegren, L.; Lagerbäck, P.; Tängdén, T. Efficacy of Antibiotic Combinations against Multidrug-Resistant Pseudomonas aeruginosa in Automated Time-Lapse Microscopy and Static Time-Kill Experiments. Antimicrob. Agents Chemother. 2020, 64, e02111-19. [Google Scholar] [CrossRef] [PubMed]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Jelic, D.; Antolovic, R. From Erythromycin to Azithromycin and New Potential Ribosome-Binding Antimicrobials. Antibiotics 2016, 5, 29. [Google Scholar] [CrossRef]
- Novis, I.; Le, E.; Novis, H.; Sorour, K. The Injection of Intraarterial Vasodilator for the Treatment of Vasopressor Extravasation. Am. J. Case Rep. 2022, 10, 197–198. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, H.; Ren, H.; Song, W.; Yang, Z. Comprehensive Perspectives for Erectile Dysfunction Pharmacotherapy: From Mechanism to Application. Curr. Med. Chem. 2022, 29, 6276–6287. [Google Scholar] [CrossRef]
- Alenazy, R. Drug Efflux Pump Inhibitors: A Promising Approach to Counter Multidrug Resistance in Gram-Negative Pathogens by Targeting AcrB Protein from AcrAB-TolC Multidrug Efflux Pump from Escherichia coli. Biology 2022, 11, 1328. [Google Scholar] [CrossRef]
- Nikaido, H.; Pages, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef]
- Egan, A.J.F. Bacterial outer membrane constriction. Mol Microbiol 2018, 107, 676–687. [Google Scholar] [CrossRef]
- Petrosillo, N.; Ioannidou, E.; Falagas, M.E. Colistin monotherapy vs. combination therapy: Evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 2008, 14, 816–827. [Google Scholar] [CrossRef]
- Falagas, M.E.; Rafailidis, P.I.; Kasiakou, S.K.; Hatzopoulou, P.; Michalopoulos, A. Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin-meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin Microbiol Infect 2006, 12, 1227–1230. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Zhang, J. Efficacy of phentolamine combined with ambroxol aerosol inhalation in the treatment of pediatric severe pneumonia and its effect on serum IL-10 and CRP levels. Transl. Pediatr. 2022, 11, 33–40. [Google Scholar] [CrossRef]


| ERY (mg/L) | CLA (mg/L) | AZM (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test Strains | Species | PE (mM) | ERY alone | 1/4MIC PE | 1/2MIC PE | CLA alone | 1/4MIC PE | 1/2MIC PE | AZM alone | 1/4MIC PE | 1/2MIC PE |
| BW25113 | E. coli | 1 | 32 | 8 | 2` | 32 | 8 | 2 | 1 | 0.25 | 0.0625 |
| ATCC 14028 | S. typhimurium | 2 | 32 | 8 | 2` | 32 | 8 | 2 | 1 | 0.25 | 0.0625 |
| ATCC 19606 | A. baumannii | 2 | 8 | 1 | 0.25 | 8 | 1 | 0.125 | 8 | 1 | 0.25 |
| ATCC 27853 | P. aeruginosa | 4 | 128 | 64 | 64 | 128 | 128 | 64 | 32 | 32 | 16 |
| ATCC 700603 | K. Pneumoniae | 2 | 128 | 32 | 16 | 128 | 32 | 16 | 16 | 4 | 1 |
| BW 25113 ΔTolC | E. coli | 1 | 1 | 0.5 | 0.125 | 2 | 1 | 0.0625 | 0.5 | 0.125 | 0.03125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.-H.; He, H.-L.; Zheng, Z.-J.; Yuan, Z.-Q.; Chen, Y.; Huang, X.-Y.; Ren, H.; Zhou, Y.-F.; Zhao, D.-H.; Fang, L.-X.; et al. Phentolamine Significantly Enhances Macrolide Antibiotic Antibacterial Activity against MDR Gram-Negative Bacteria. Antibiotics 2023, 12, 760. https://doi.org/10.3390/antibiotics12040760
Cui Z-H, He H-L, Zheng Z-J, Yuan Z-Q, Chen Y, Huang X-Y, Ren H, Zhou Y-F, Zhao D-H, Fang L-X, et al. Phentolamine Significantly Enhances Macrolide Antibiotic Antibacterial Activity against MDR Gram-Negative Bacteria. Antibiotics. 2023; 12(4):760. https://doi.org/10.3390/antibiotics12040760
Chicago/Turabian StyleCui, Ze-Hua, Hui-Ling He, Zi-Jian Zheng, Zhao-Qi Yuan, Ying Chen, Xin-Yi Huang, Hao Ren, Yu-Feng Zhou, Dong-Hao Zhao, Liang-Xing Fang, and et al. 2023. "Phentolamine Significantly Enhances Macrolide Antibiotic Antibacterial Activity against MDR Gram-Negative Bacteria" Antibiotics 12, no. 4: 760. https://doi.org/10.3390/antibiotics12040760
APA StyleCui, Z.-H., He, H.-L., Zheng, Z.-J., Yuan, Z.-Q., Chen, Y., Huang, X.-Y., Ren, H., Zhou, Y.-F., Zhao, D.-H., Fang, L.-X., Yu, Y., Liu, Y.-H., Liao, X.-P., & Sun, J. (2023). Phentolamine Significantly Enhances Macrolide Antibiotic Antibacterial Activity against MDR Gram-Negative Bacteria. Antibiotics, 12(4), 760. https://doi.org/10.3390/antibiotics12040760






