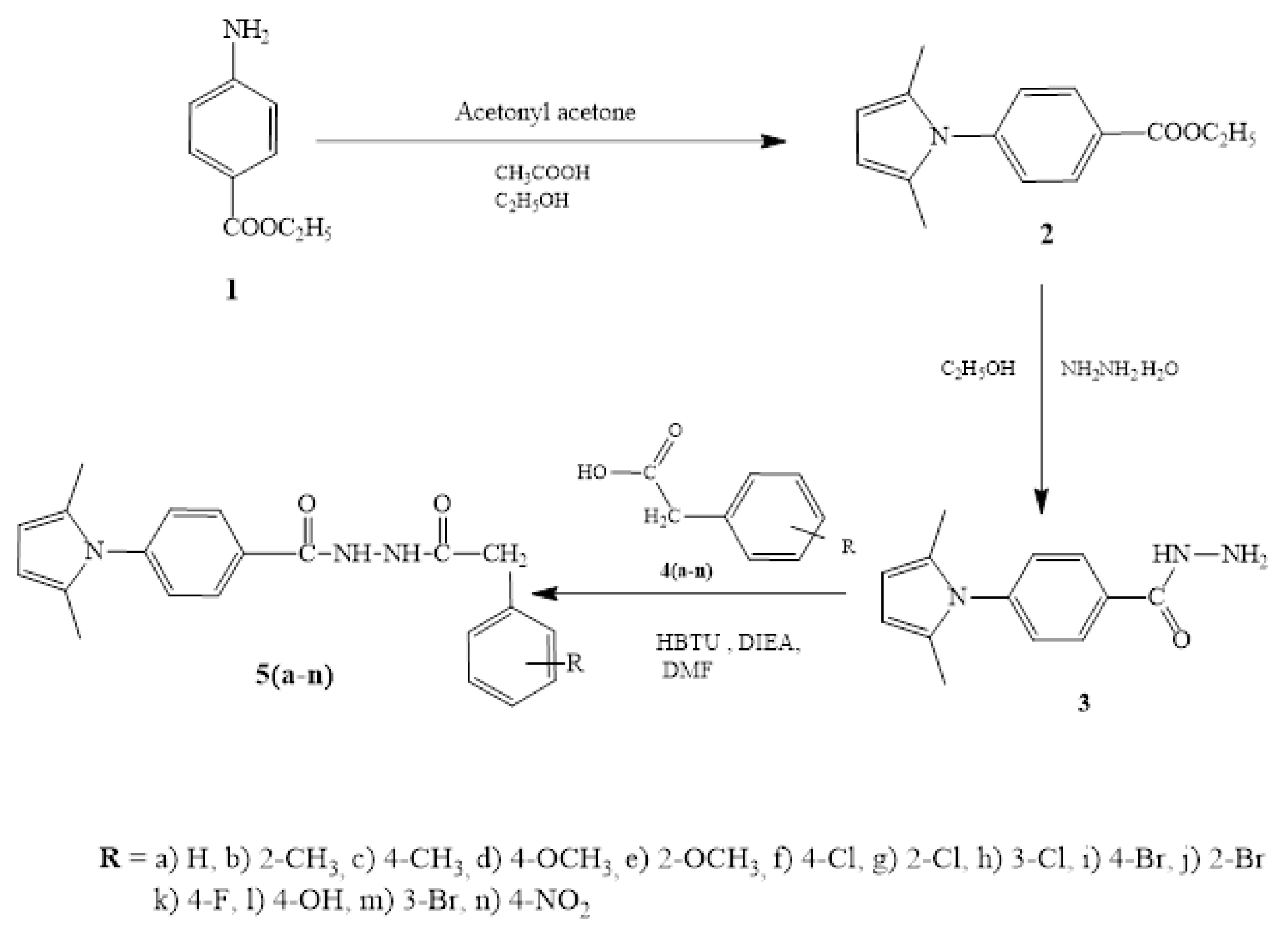
Synthesis, Molecular Docking Study, and Biological Evaluation of New 4-(2,5-Dimethyl-1H-pyrrol-1-yl)-N’-(2-(substituted)acetyl)benzohydrazides as Dual Enoyl ACP Reductase and DHFR Enzyme Inhibitors
Abstract
1. Introduction
2. Results and Discussion
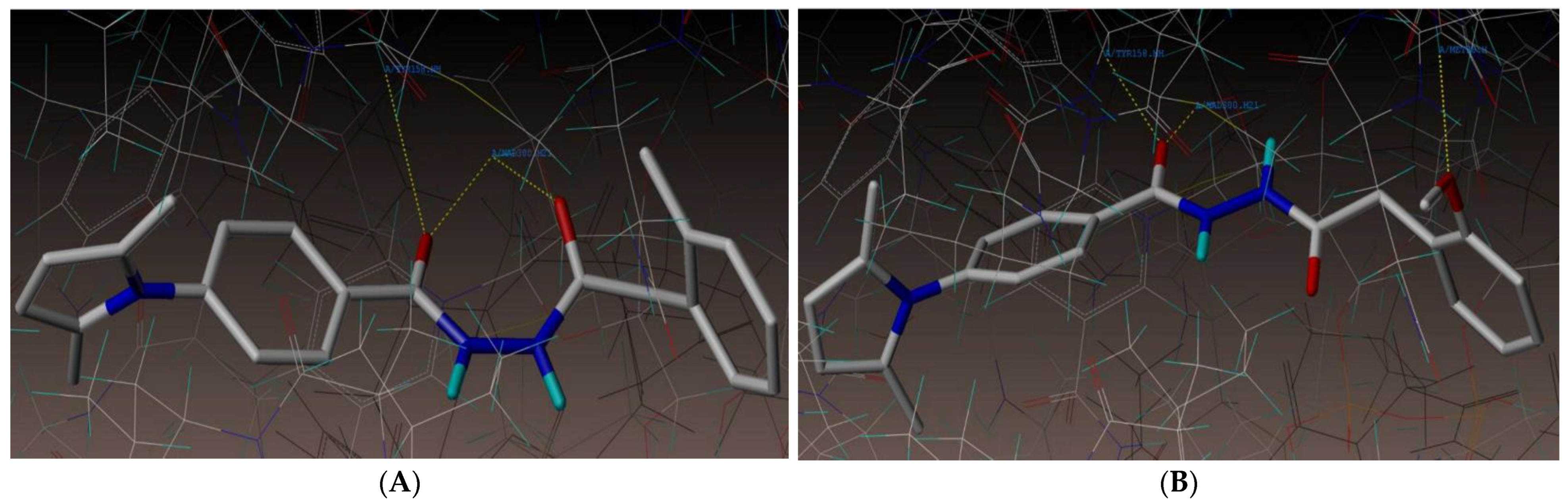
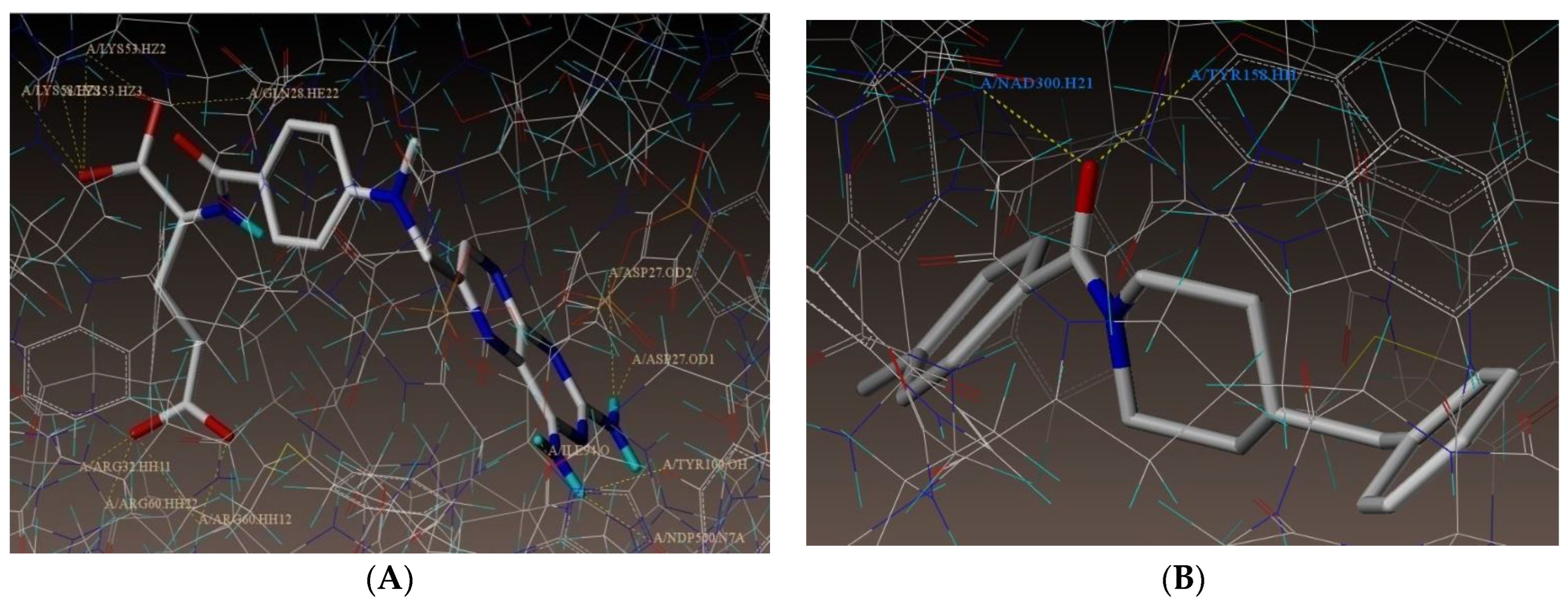
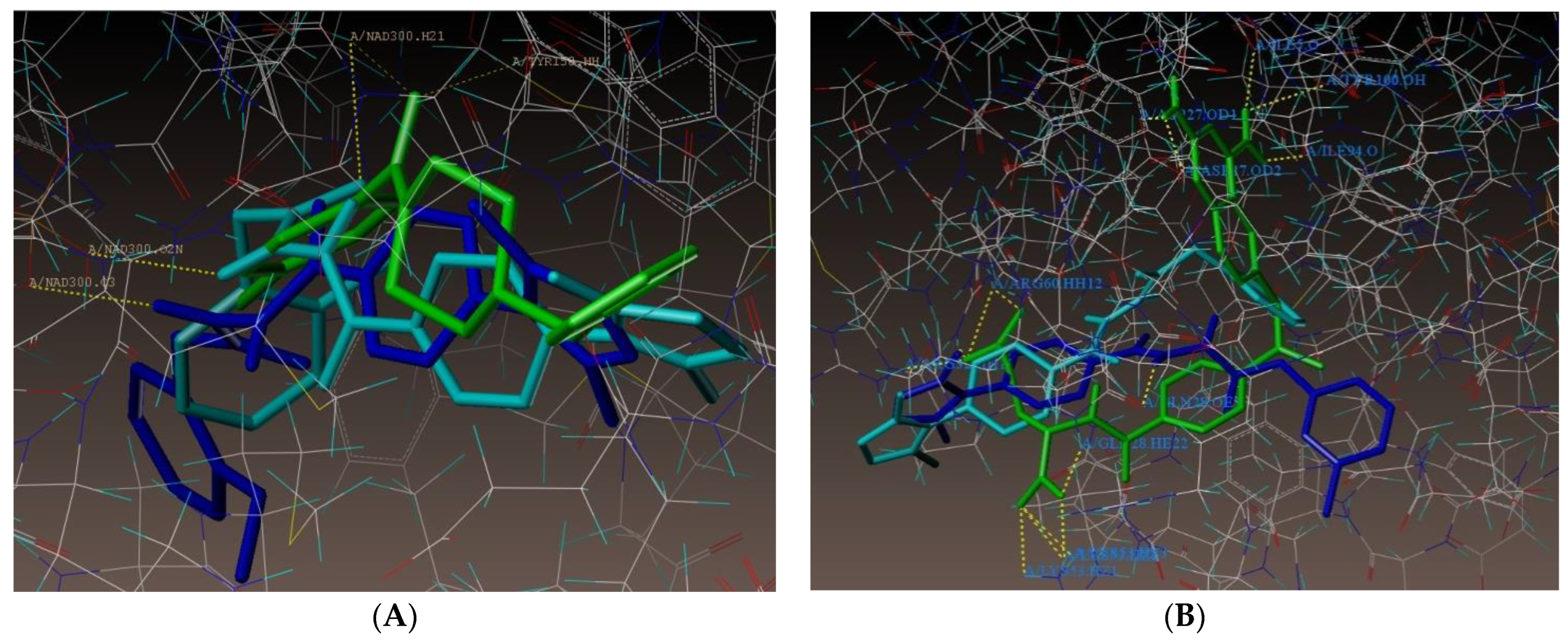
2.1. Molecular Docking
2.2. Antitubercular and Antibacterial Activities
2.3. MtDHFR Inhibitory Activity
2.4. ADME Studies
3. Experimental Section
3.1. Chemicals
3.2. Instruments
3.3. General Procedure for the Synthesis of Dimethylpyrrolylbenzohydrazide Derivatives 5(a-n)
3.3.1. Synthesis of (5a): 4-(2,5-Dimethyl-1H-pyrrol-1-yl)-N′-(2-phenylacetyl)benzohydrazide
3.3.2. Synthesis of (5b): 4-(2, 5-Dimethyl-1H-pyrrol-1-yl)-N′-(2-(o-tolyl)acetyl)benzohydrazide
3.3.3. Synthesis of (5c): 4-(2,5-Dimethyl -1H-pyrrol-1-yl)-N′-(2-(p-tolyl)acetyl)benzohydrazide
3.3.4. Synthesis of (5d): 4-(2,5-Dimethyl-1H-pyrrol-1-yl)-N′-(2-(4-methoxyphenyl)acetyl)benzohydrazide
3.3.5. Synthesis of (5e): 4-(2,5-Dimethyl-1H-pyrrol-1-yl)-N′-(2-(2-methoxyphenyl)acetyl)benzohydrazide
3.3.6. Synthesis of (5f): N′-(2-(4-Chlorophenyl)acetyl)-4-(2,5-dimethyl-1H-pyrrol-1-yl)benzohydrazide
3.3.7. Synthesis of (5g): N′-(2-(2-Chlorophenyl)acetyl)-4-(2,5-dimethyl-1H-pyrrol-1-yl)benzohydrazide
3.3.8. Synthesis of (5h): N′-(2-(3-Chlorophenyl)acetyl)-4-(2,5-dimethyl-1H-pyrrol-1-yl)benzohydrazide
3.3.9. Synthesis of (5i): N′-(2-(4-Bromophenyl)acetyl)-4-(2,5-dimethyl-1H-pyrrol-1-yl)benzohydrazide
3.3.10. Synthesis of (5j): N′-(2-(2-Bromophenyl)acetyl)-4-(2,5-dimethyl-1H-pyrrol-1-yl)benzohydrazide
3.3.11. Synthesis of (5k): 4-(2, 5-Dimethyl-1H-pyrrol-1-yl)-N′-(2-(4-flurophenyl)acetyl)benzohydrazide
3.3.12. Synthesis of (5l): 4-(2, 5-Dimethyl-1H-pyrrol-1-yl)-N′-(2-(4-hydroxyphenyl)acetyl)benzohydrazide
3.3.13. Synthesis of (5m): 4-(2, 5-Dimethyl-1H-pyrrol-1-yl)-N′-(2-(3-bromophenyl)acetyl)benzohydrazide
3.3.14. Synthesis of (5n): 4-(2, 5-Dimethyl-1H-pyrrol-1-yl)-N′-(2-(4-nitrophenyl)acetyl)benzohydrazide
3.4. Molecular Docking Using Surflex-Dock
3.5. ADMET Studies
3.6. MTT-Based Cytotoxicity Activity
3.7. Antitubercular Activity
3.8. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Tuberculosis Report, World Health Organization. 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 25 January 2023).
- Tomioka, H. Current Status and Perspective on Drug Targets in Tubercle Bacilli and Drug Design of Antituberculous Agents Based on Structure-Activity Relationship. Curr. Pharm. Des. 2014, 20, 4305–4306. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.R.; Carmosin, R.J.; Pitis, P.M.; Vaught, J.L.; Almond, H.R.; Stables, J.P.; Wolf, H.H.; Swinyard, E.A.; White, H.S. Aroyl(aminoacyl)pyrroles, a New Class of Anticonvulsant Agents. J. Med. Chem. 1997, 40, 1578–1584. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Ali, S.A.; Abdelaziz, D.H.A.; Fathallah, S.S. Synthesis and Evaluation of Novel Pyrroles and Pyrrolopyrimidines as Anti-Hyperglycemic Agents. BioMed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- El-Hameed, R.H.A.; Sayed, A.I.; Ali, S.M.; Mosa, M.A.; Khoder, Z.M.; Fatahala, S.S. Synthesis of novel pyrroles and fused pyrroles as antifungal and antibacterial agents. J. Enzym. Inhib. Med. Chem. 2021, 36, 2183–2198. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.D.; Vinayak, S.; Prem Kumar, S.R.; Dixit, S.R. Synthesis and antitubercular activity of some N′-substituted ben-zoyl-4-(2,5- dimethyl-1H-pyrrolyl)benzohydrazide derivatives. Indian J. Hetero. Chem. 2018, 28, 195–200. [Google Scholar]
- Venugopala, K.N.; Uppar, V.; Chandrashekharappa, S.; Abdallah, H.H.; Pillay, M.; Deb, P.K.; Morsy, M.A.; Aldhubiab, B.E.; Attimarad, M.; Nair, A.B.; et al. Cytotoxicity and Antimycobacterial Properties of Pyrrolo [1,2-a]quinoline Derivatives: Molecular Target Identification and Molecular Docking Studies. Antibiotics 2020, 9, 233. [Google Scholar] [CrossRef]
- Joshi, S.D.; Devendra, K.; Prem Kumar, S.R. Synthesis and antitubercular activity of some novel N′-substituted benzene sulphonamide derivatives. Indian J. Hetero. Chem. 2018, 28, 441–446. [Google Scholar]
- Global Alliance for TB Drug Development (TB Alliance). Handbook of Anti-Tuberculosis Agents. Tuberculosis. 2008, 88, 1–169. [Google Scholar]
- Emmerich, C.H.; Gamboa, L.M.; Hofmann, M.C.J.; Bonin-Andresen, M.; Arbach, O.; Schendel, P.; Gerlach, B.; Hempel, K.; Bespalov, A.; Dirnagl, U.; et al. Improving target assessment in biomedical research: The GOT-IT recommendations. Nat. Rev. Drug Discov. 2020, 20, 64–81. [Google Scholar] [CrossRef]
- Lage, O.M.; Ramos, M.C.; Calisto, R.; Almeida, E.; Vasconcelos, V.; Vicente, F. Current Screening Methodologies in Drug Discovery for Selected Human Diseases. Mar. Drugs 2018, 16, 279. [Google Scholar] [CrossRef]
- Chakravarti, B.; Mallik, B.; Chakravarti, D.N. Proteomics and Systems Biology: Application in Drug Discovery and Development. Syst. Biol. Drug Discov. Dev. Methods Protoc. 2010, 662, 3–28. [Google Scholar] [CrossRef]
- More, U.A.; Joshi, S.D.; Aminabhavi, T.M.; Gadad, A.K.; Nadagouda, M.N.; Kulkarni, V.H. Design, synthesis, molecular docking and 3D-QSAR studies of potent inhibitors of enoyl-acyl carrier protein reductase as potential antimycobacterial agents. Eur. J. Med. Chem. 2013, 71, 199–218. [Google Scholar] [CrossRef]
- Kumar, S.P.; Shaikh, I.A.; Mahnashi, M.H.; Alshahrani, M.A.; Dixit, S.R.; Kulkarni, V.H.; Lherbet, C.; Gadad, A.K.; Aminabhavi, T.M.; Joshi, S.D. Design, synthesis and computational approach to study novel pyrrole scaffolds as active inhibitors of enoyl ACP reductase (InhA) and Mycobacterium tuberculosis antagonists. J. Indian Chem. Soc. 2022, 99. [Google Scholar] [CrossRef]
- Biava, M.; Porretta, G.C.; Poce, G.; Battilocchio, C.; Alfonso, S.; de Logu, A.; Manetti, F.; Botta, M. Developing Pyr-role-Derived Antimycobacterial Agents: A Rational Lead Optimization Approach. Chem. Med. Chem. 2011, 6, 593–599. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Kittur, B.; Prem Kumar, S.R.; Alqahtani, A.S.; Al Ali, A.; Alshahrani, M.A.; Shaikh, I.A.; Joshi, S.D. In Silico and in Vitro Evaluation of a New N′-(2-Arylacetyl)-4-(1H-Pyrrol-1-Yl) Benzohydrazides as Potential Antimicrobial Agents. Indian J. Hetero. Chem. 2022, 32, 523–530. [Google Scholar]
- Valencia, J.; Rubio, V.; Puerto, G.; Vasquez, L.; Bernal, A.; Mora, J.R.; Cuesta, S.A.; Paz, J.L.; Insuasty, B.; Abonia, R.; et al. QSAR Studies, Molecular Docking, Molecular Dynamics, Synthesis, and Biological Evaluation of Novel Quinolinone-Based Thiosemicarbazones against Mycobacterium tuberculosis. Antibiotics 2022, 12, 61. [Google Scholar] [CrossRef]
- Adeniji, S.E.; Uba, S.; Uzairu, A. Theoretical modeling for predicting the activities of some active compounds as potent inhibitors against Mycobacterium tuberculosis using GFA-MLR approach. J. King Saud Univ. Sci. 2018, 32, 575–586. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Guidetti, B.; Chamayou, A.; André-Barrès, C.; Madacki, J.; Korduláková, J.; Mori, G.; Orena, B.S.; Chiarelli, L.R.; Pasca, M.R.; et al. Mechanochemical Synthesis and Biological Evaluation of Novel Isoniazid Derivatives with Potent Antitubercular Activity. Molecules 2017, 22, 1457. [Google Scholar] [CrossRef]
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-Based Virtual Screening: Advances and Applications in Drug Discovery. Front. Pharmacol. 2018, 9, 1275. [Google Scholar] [CrossRef]
- Caputo, A.; Sartini, S.; Levati, E.; Minato, I.; Elisi, G.M.; Di Stasi, A.; Guillou, C.; Goekjian, P.G.; Garcia, P.; Gueyrard, D.; et al. An Optimized Workflow for the Discovery of New Antimicrobial Compounds Targeting Bacterial RNA Polymerase Complex Formation. Antibiotics 2022, 11, 1449. [Google Scholar] [CrossRef]
- Coates, A.R.; Halls, G.; Hu, Y. Novel Classes of Antibiotics or More of the Same? Br. J. Pharm. 2011, 163, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Salo-Ahen, O.M.H.; Alanko, I.; Bhadane, R.; Bonvin, A.M.J.J.; Honorato, R.V.; Hossain, S.; Juffer, A.H.; Kabedev, A.; Lahtela-Kakkonen, M.; Larsen, A.S.; et al. Molecular Dynamics Simulations in Drug Discovery and Pharmaceutical Development. Processes 2020, 9, 71. [Google Scholar] [CrossRef]
- Maiolini, M.; Gause, S.; Taylor, J.; Steakin, T.; Shipp, G.; Lamichhane, P.; Deshmukh, B.; Shinde, V.; Bishayee, A.; Deshmukh, R.R. The War against Tuberculosis: A Review of Natural Compounds and Their Derivatives. Molecules 2020, 25, 3011. [Google Scholar] [CrossRef] [PubMed]
- Allué-Guardia, A.; García, J.I.; Torrelles, J.B. Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment. Front. Microbiol. 2021, 12, 612675. [Google Scholar] [CrossRef]
- Mase, S.R.; Chorba, T. Treatment of Drug-Resistant Tuberculosis. Clin. Chest Med. 2019, 40, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Maqbul, M.S.; Alshabi, A.M.; Khan, A.A.; Iqubal, S.S.; Mohammed, T.; Shaikh, I.A.; Dawoud, A.; Muddapur, U.M.; Hussain, M.S.; Singh, S. Comparison of e-test Values for Standard Antibiotics and Conventional Antimicrobial Assay Values for Ethanoic Acids against Nosocomial Multidrug-resistant Pseudomonas aeruginosa. J. Pure Appl. Microbiol. 2020, 14, 255–260. [Google Scholar] [CrossRef]
- Joshi, S.D.; More, Y.; Vagdevi, H.M.; Vaidya, V.P.; Gadaginamath, G.S.; Kulkarni, V.H. Synthesis of new 4-(2,5dimethylpyrrol-1-yl)/4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial, antifungal and antitubercular agents. Med. Chem. Res. 2013, 22, 1073–1089. [Google Scholar] [CrossRef]
- Jain, A.N. Surflex: Fully Automatic Flexible Molecular Docking Using a Molecular Similarity-Based Search Engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef]
- TRIPOS Associates, Inc. Sybyl-X Molecular Modeling Software Packages, Version 2.0; TRIPOS Associates, Inc.: St. Louis, MO, USA, 2012.
- Jain, A.N. Scoring noncovalent protein-ligand interactions: A continuous differentiable function tuned to compute binding affinities. J. Comput. Mol. Des. 1996, 10, 427–440. [Google Scholar] [CrossRef]
- Pratama, M.R.F.; Poerwono, H.; Siswodiharjo, S. ADMET properties of novel 5-O-benzoylpinostrobin derivatives. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 1–13. [Google Scholar] [CrossRef]
- Lourenco, M.C.S.; de Souza, M.V.N.; Pinheiro, A.C.; Ferreira, D.M.; Goncalves, B.R.; Nogneira, T.C.M. Evaluation of anti-tubercular activity of nicotinic and isoniazid analogues. Arkivoc 2007, 15, 181–191. [Google Scholar] [CrossRef]
- Goto, S.K.; Jo, K.T.; Kawakita, T.S.; Mitsuhashi, S.T.; Nishino, T.N.; Ohsawa, N.; Tanami, H. Method of minimum in-hibitory concentration (MIC) determination. Chemotherapy 1981, 29, 76–79. [Google Scholar]
- Zawadzka, K.; Felczak, A.; Głowacka, I.E.; Piotrowska, D.G.; Lisowska, K. Evaluation of the Antimicrobial Potential and Toxicity of a Newly Synthesised 4-(4-(Benzylamino)butoxy)-9H-carbazole. Int. J. Mol. Sci. 2021, 22, 12796. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Total Score a | Crash Score b | Polar Score c | D Score d | PMF Score e | G Score f | Chem Score g |
|---|---|---|---|---|---|---|---|
| 2NSD_ligand | 9.25 | −0.93 | 1.54 | −150.083 | −63.091 | −250.959 | −46.922 |
| 5m | 6.73 | −1.45 | 0.00 | −154.008 | −47.31 | −254.69 | −35.77 |
| 5l | 6.66 | −1.40 | 0.00 | −150.09 | −49.357 | −243.120 | −33.421 |
| 5b | 6.64 | −1.40 | 0.00 | −150.813 | −67.397 | −275.03 | −38.55 |
| 5h | 6.60 | −1.32 | 0.02 | −152.344 | −47.406 | −251.930 | −35.59 |
| 5d | 6.58 | −1.43 | 0.00 | −152.248 | −151.700 | −253.195 | −35.191 |
| 5a | 5.77 | −1.94 | 0.00 | −147.496 | −48.955 | −259.388 | −34.870 |
| 5e | 5.57 | −2.26 | 2.20 | −152.202 | −81.681 | −249.402 | −43.879 |
| 5k | 5.51 | −350 | 1.09 | −121.605 | −70.322 | −254.814 | −32.838 |
| 5i | 5.33 | −202 | 0.40 | −148.863 | −52.545 | −248.550 | −36.981 |
| 5n | 5.23 | −161 | 0.78 | −109.058 | −49.383 | −220.034 | −30.758 |
| 5c | 5.09 | −1.90 | 0.67 | −115.009 | −60.352 | −228.411 | −29.464 |
| 5g | 4.64 | −1.53 | 0.00 | −158.311 | −42.321 | −254.298 | −35.355 |
| 5j | 4.52 | −3.58 | 1.75 | −137.888 | −69.052 | −268.596 | −40.945 |
| 5f | 4.44 | −3.21 | 1.79 | −160.561 | −68.462 | −260.375 | −41.004 |
| Compounds | C Score a | Crash Score b | Polar Score c | D Score d | PMF Score e | G Score f | Chem Score g |
|---|---|---|---|---|---|---|---|
| Ligand | 13.76 | −1.32 | 8.92 | −229.875 | −138.104 | −353.514 | −38.494 |
| 5i | 6.09 | −0.80 | 1.24 | −74.154 | −46.492 | −203.793 | −23.832 |
| 5j | 6.07 | −1.60 | 1.12 | −140.353 | −40.058 | −212.293 | −30.808 |
| 5n | 5.87 | −1.06 | 5.71 | −65.226 | −50.507 | −138.374 | −31.331 |
| 5e | 5.76 | −1.66 | 1.01 | −108.460 | −32.896 | −206.830 | −29.186 |
| 5b | 5.72 | −1.92 | 1.13 | −135.734 | −48.681 | −242.893 | −31.229 |
| 5a | 5.68 | −1.82 | 1.78 | −76.626 | −51.678 | −205.484 | −26.926 |
| 5f | 5.52 | −1.05 | 0.34 | −79.996 | −40.690 | −202.029 | −25.188 |
| 5c | 5.50 | −2.09 | 1.09 | −117.301 | −42.304 | −215.813 | −29.720 |
| 5k | 5.40 | −2.17 | 1.08 | −116.983 | −44.793 | −218.472 | −29.380 |
| 5m | 4.86 | −1.06 | 0.00 | −85.927 | −64.808 | −211.509 | −26.216 |
| 5g | 4.84 | −1.73 | 0.00 | −92.589 | −53.332 | −243.476 | −28.948 |
| 5h | 4.68 | −1.01 | 2.52 | −59.255 | −62.042 | −146.737 | −24.631 |
| 5d | 4.55 | −2.34 | 0.66 | −117.427 | −47.229 | −225.918 | −27.805 |
| 5i | 4.29 | −1.31 | 2.78 | −62.632 | −41.743 | −139.089 | −23.044 |
| Compound | M. tuberculosis H37Rv MIC Values in µg mL−1 (µmol mL−1) | S. aureus (Gram +ve) | E. coli (Gram –ve) | IC50 (µM) MtDHFR | % Inhibition of InhA at 50 µM |
|---|---|---|---|---|---|
| MIC in µg mL−1 (µmol mL−1) | MIC in µg mL−1 (µmol mL−1) | ||||
| 5a | 25 (71.95) | 50 (143.91) | 50 (143.91) | 146 | - |
| 5b | 25 (69.16) | 0.8 (2.21) | 50 (138.33) | 125 | - |
| 5c | 25 (69.16) | 3.12(8.63) | 50 (138.33) | 118 | 20 |
| 5d | 3.25 (8.61) | 3.12 (8.2) | 100 (264.94) | 85 | 32 |
| 5e | 6.25 (16.55) | 6.25 (16.55) | 100 (264.94) | 78 | - |
| 5f | 1.6 (4.19) | 1.6 (4.19) | 50 (130.93) | 25 | 76 |
| 5g | 3.25 (8.51) | 3.12 (8.17) | 50 (130.93) | 26 | - |
| 5h | 3.25 (8.51) | 6.25 (16.36) | 50 (130.93) | 30 | - |
| 5i | 1.6 (3.75) | 6.25 (14.66) | 50 (117.28) | 18 | 72 |
| 5j | 1.6 (3.75) | 6.25 (14.66) | 50 (117.28) | 15 | - |
| 5k | 0.8 (2.18) | 1.6 (4.37) | 50 (136.41) | 10 | 88 |
| 5l | 3.25 (8.94) | 6.25 (17.19) | 50 (137.58) | 30 | - |
| 5m | 3.25 (7.64) | 12.5 (29.40) | 50 (117.62) | 18 | - |
| 5n | 1.6 (4.07) | 12.5 (31.85) | 50 (127.41) | 15 | - |
| Pyrazinamide | 3.125 | - | - | - | |
| Streptomycin | 6.25 | - | - | - | |
| Ciprofloxacin | - | 2 | 2 | - | |
| TMP | - | - | - | 92 | - |
| TCL | - | - | - | - | >99 |
| Compound | Log P | Molar Refractivity | TPSA | HBA | HBD | RB | GI Absorption | BBB Permeant | Log Kp cm/s | Solubility | CYP Inhibitor | Lipinski Violation | Synthetic Accessibility | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A2 | 2C19 | 2C9 | 2D6 | 3A4 | |||||||||||||
| 5a | 2.62 | 100.99 | 63.13 | 2 | 2 | 7 | High | Yes | −5.93 | Moderately soluble | No | Yes | Yes | Yes | Yes | 0 | 2.65 |
| 5b | 2.80 | 105.96 | 63.13 | 2 | 2 | 7 | High | Yes | −5.76 | Moderately soluble | No | Yes | Yes | Yes | Yes | 0 | 2.81 |
| 5c | 3.09 | 105.96 | 63.13 | 2 | 2 | 7 | High | Yes | −5.76 | Moderately soluble | No | No | Yes | Yes | Yes | 0 | 2.76 |
| 5d | 3.03 | 107.48 | 72.36 | 3 | 2 | 8 | High | Yes | −6.14 | Moderately soluble | No | Yes | Yes | Yes | Yes | 0 | 2.74 |
| 5e | 3.02 | 107.48 | 72.36 | 3 | 2 | 8 | High | Yes | −6.14 | Moderately soluble | No | Yes | Yes | Yes | Yes | 0 | 2.82 |
| 5f | 2.96 | 106.00 | 63.13 | 2 | 2 | 7 | High | Yes | −5.70 | Moderately soluble | Yes | Yes | Yes | Yes | Yes | 0 | 2.70 |
| 5g | 3.35 | 106.00 | 63.13 | 2 | 2 | 7 | High | Yes | −5.70 | Moderately soluble | Yes | Yes | Yes | Yes | Yes | 0 | 2.77 |
| 5h | 3.16 | 106.00 | 63.13 | 2 | 2 | 7 | High | Yes | −5.70 | Moderately soluble | Yes | Yes | Yes | Yes | Yes | 0 | 2.70 |
| 5i | 3.16 | 108.69 | 63.13 | 2 | 2 | 7 | High | Yes | −5.93 | Moderately soluble | Yes | Yes | Yes | Yes | Yes | 0 | 2.75 |
| 5j | 3.40 | 108.69 | 63.13 | 2 | 2 | 7 | High | Yes | −5.93 | Moderately soluble | Yes | Yes | Yes | Yes | Yes | 0 | 2.83 |
| 5k | 2.71 | 100.95 | 63.13 | 3 | 2 | 7 | High | Yes | −5.97 | Moderately soluble | No | No | Yes | Yes | Yes | 0 | 2.69 |
| 5l | 2.63 | 103.02 | 83.36 | 3 | 3 | 7 | High | No | −6.28 | Moderately soluble | Yes | No | Yes | Yes | No | 0 | 2.64 |
| 5m | 3.11 | 108.69 | 63.13 | 2 | 2 | 7 | High | Yes | −5.93 | Moderately soluble | Yes | Yes | Yes | Yes | Yes | 0 | 2.83 |
| 5n | 2.48 | 109.81 | 108.95 | 4 | 2 | 8 | High | No | −6.33 | Moderately soluble | No | Yes | Yes | No | Yes | 0 | 2.90 |
| Compound Code | LD50 mg/kg | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity | Aryl Hydrocarbon Receptor | Androgen Receptor (AR) | Androgen Receptor Ligand Binding Domain | Aromatase | Estrogen Receptor Ligand Binding Domain | Peroxisome Proliferator Activated Receptor Gamma | Nuclear Factor | Heat Shock Factor Response Element | Mitochondrial Membrane Potential | Phosphoprotein | ATPase Family AAA Domain Containing Protein 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 150 | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5b | 150 | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5c | 150 | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5d | 150 | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5e | 700 | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5f | 700 | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5g | 150 | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5h | 150 | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5i | 150 | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5j | 150 | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5k | 150 | Active | Active | Inactive | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5l | 150 | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5m | 150 | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 5n | 150 | Active | Active | Inactive | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Compound | IC50 (µM) a | |
|---|---|---|
| MV Cell Lines b | A549 c | |
| 5a | - | - |
| 5b | - | |
| 5c | 221 ± 0.8 | 219 ± 0.7 |
| 5d | 214 ± 0.6 | 214 ± 0.2 |
| 5e | - | - |
| 5f | 215 ± 0.2 | 216 ± 0.5 |
| 5g | - | - |
| 5h | - | - |
| 5i | 218 ± 0.3 | 214 ± 0.4 |
| 5j | ||
| 5k | 221 ± 0.3 | 219 ± 0.4 |
| 5l | - | - |
| 5m | - | - |
| 5n | - | - |
| INH | >450 | >450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahnashi, M.H.; Koganole, P.; S. R., P.K.; Ashgar, S.S.; Shaikh, I.A.; Joshi, S.D.; Alqahtani, A.S. Synthesis, Molecular Docking Study, and Biological Evaluation of New 4-(2,5-Dimethyl-1H-pyrrol-1-yl)-N’-(2-(substituted)acetyl)benzohydrazides as Dual Enoyl ACP Reductase and DHFR Enzyme Inhibitors. Antibiotics 2023, 12, 763. https://doi.org/10.3390/antibiotics12040763
Mahnashi MH, Koganole P, S. R. PK, Ashgar SS, Shaikh IA, Joshi SD, Alqahtani AS. Synthesis, Molecular Docking Study, and Biological Evaluation of New 4-(2,5-Dimethyl-1H-pyrrol-1-yl)-N’-(2-(substituted)acetyl)benzohydrazides as Dual Enoyl ACP Reductase and DHFR Enzyme Inhibitors. Antibiotics. 2023; 12(4):763. https://doi.org/10.3390/antibiotics12040763
Chicago/Turabian StyleMahnashi, Mater H., Pooja Koganole, Prem Kumar S. R., Sami S. Ashgar, Ibrahim Ahmed Shaikh, Shrinivas D. Joshi, and Ali S. Alqahtani. 2023. "Synthesis, Molecular Docking Study, and Biological Evaluation of New 4-(2,5-Dimethyl-1H-pyrrol-1-yl)-N’-(2-(substituted)acetyl)benzohydrazides as Dual Enoyl ACP Reductase and DHFR Enzyme Inhibitors" Antibiotics 12, no. 4: 763. https://doi.org/10.3390/antibiotics12040763
APA StyleMahnashi, M. H., Koganole, P., S. R., P. K., Ashgar, S. S., Shaikh, I. A., Joshi, S. D., & Alqahtani, A. S. (2023). Synthesis, Molecular Docking Study, and Biological Evaluation of New 4-(2,5-Dimethyl-1H-pyrrol-1-yl)-N’-(2-(substituted)acetyl)benzohydrazides as Dual Enoyl ACP Reductase and DHFR Enzyme Inhibitors. Antibiotics, 12(4), 763. https://doi.org/10.3390/antibiotics12040763








