Cefiderocol Efficacy in a Real-Life Setting: Single-Centre Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Infections Profiles
2.2. Microbiology
2.3. Therapy Variables
2.4. Outcomes
3. Results
3.1. Study Population
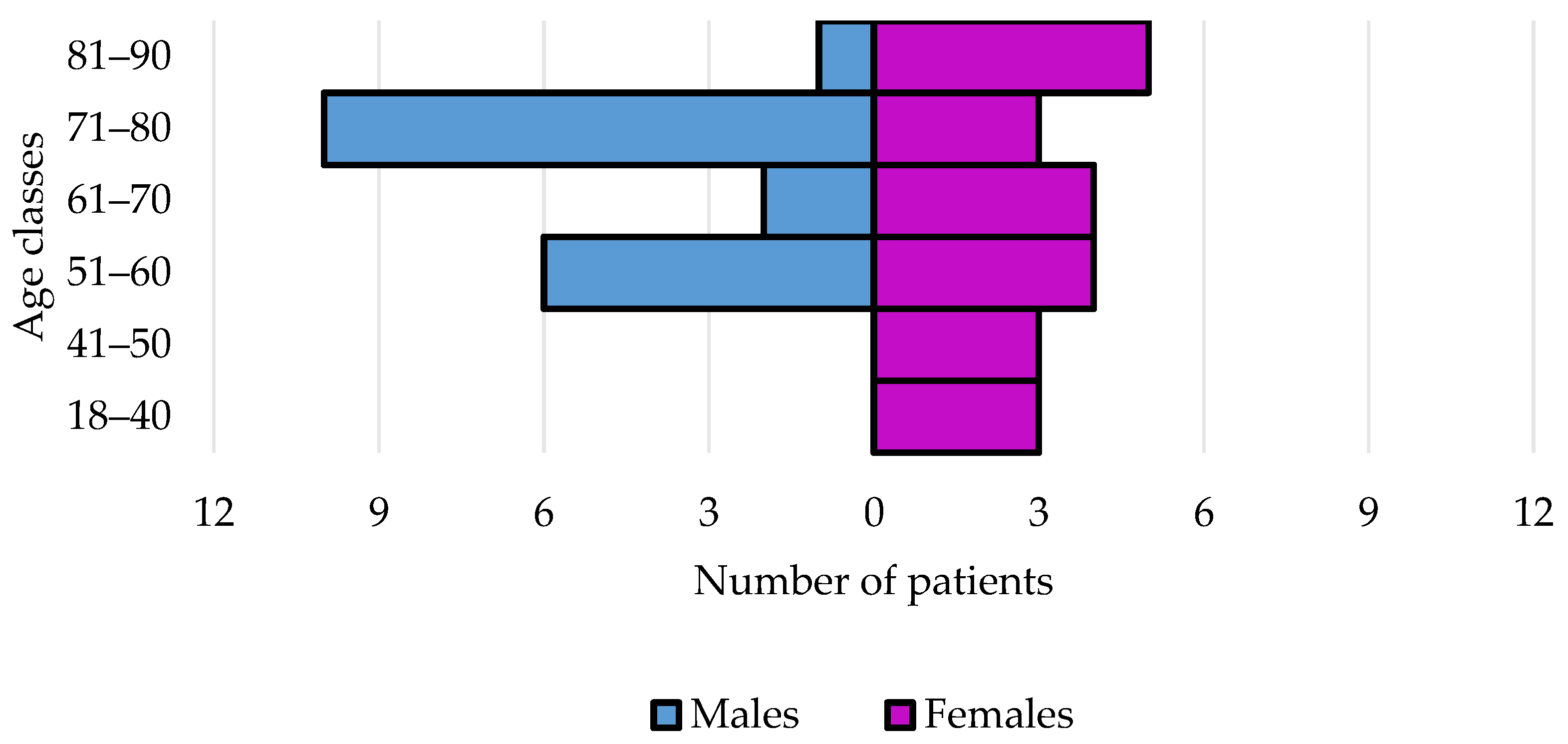
3.1.1. Demographic and Anamnestic Features
3.1.2. Infection Features
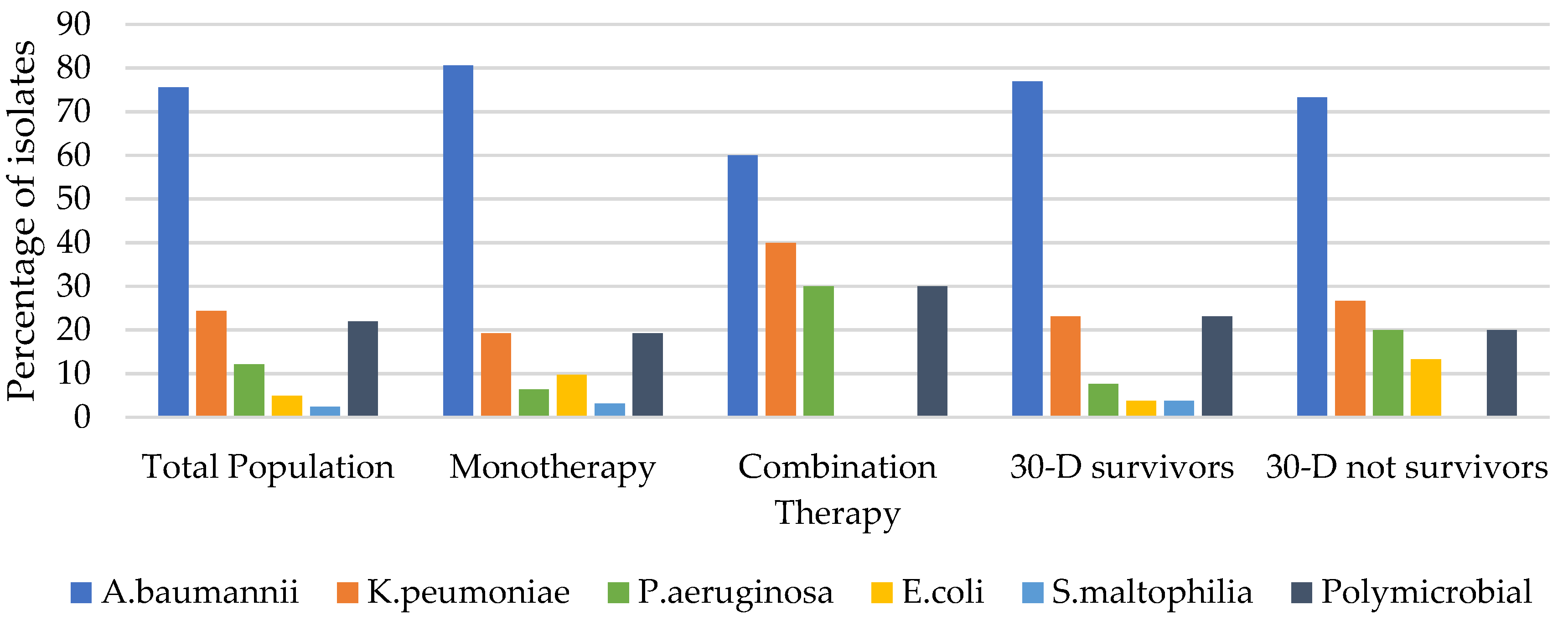
3.1.3. Microbiological Features
3.1.4. Treatment Features
3.1.5. Outcomes
3.2. Subgroup Analyses
3.2.1. Monotherapy vs. Combination Therapy
3.2.2. CRAB Infections
3.2.3. 30-D Survivors vs. Non-Survivors
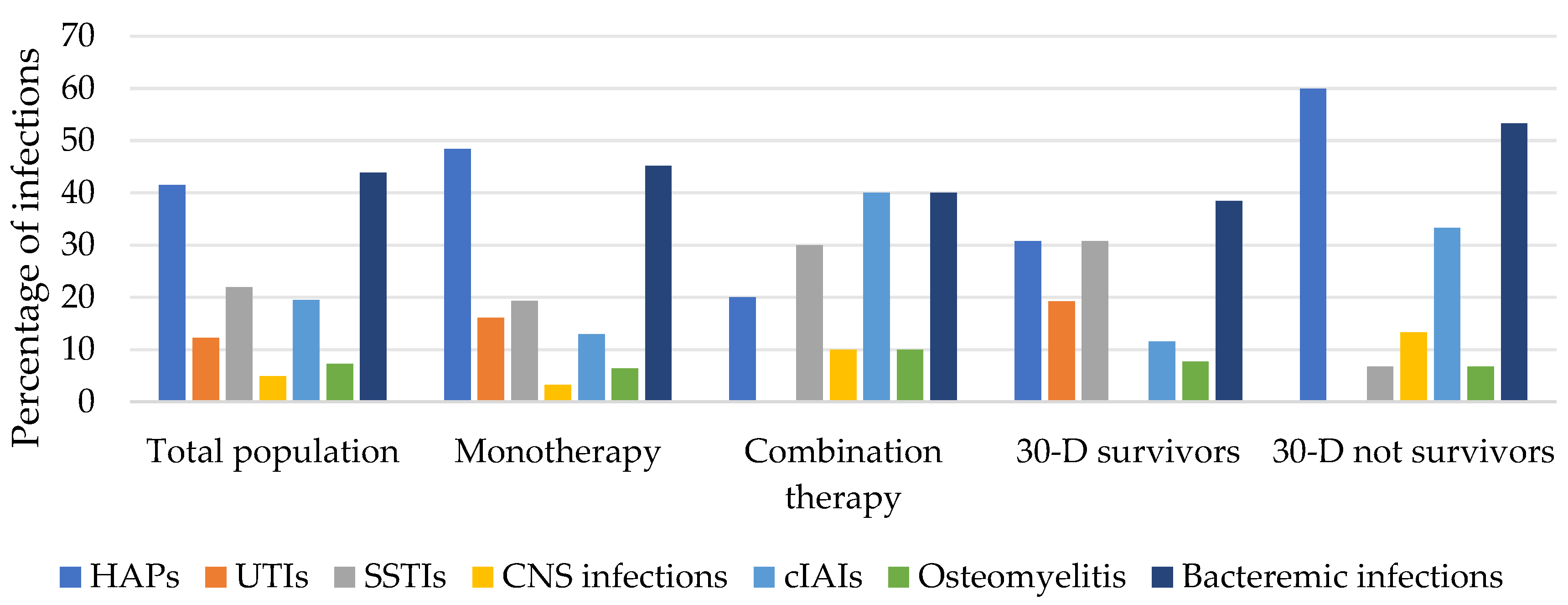
3.2.4. Types of Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrosillo, N.; Giannella, M.; Lewis, R.; Viale, P. Treatment of carbapenem-resistant Klebsiella pneumoniae: The state of the art. Expert Rev. Anti-Infect. Ther. 2013, 11, 159–177. [Google Scholar] [CrossRef]
- EMA Raccomanda L’approvazione di Zavicefta per i Pazienti con Limitate Opzioni di Trattamento. Available online: https://www.aifa.gov.it/-/ema-raccomanda-l-approvazione-di-zavicefta-per-i-pazienti-con-limitate-opzioni-di-trattamento (accessed on 8 September 2022).
- Comitato per i Medicinali per Uso Umano (CHMP) dell’EMA: Highlights del Meeting di Febbraio. Available online: https://www.aifa.gov.it/-/comitato-per-i-medicinali-per-uso-umano-chmp-dell-ema-highlights-del-meeting-di-febbra-4 (accessed on 8 September 2022).
- EMA Raccomanda un Nuovo Medicinale per una Serie di Infezioni. Available online: https://www.aifa.gov.it/-/ema-raccomanda-un-nuovo-medicinale-per-una-serie-di-infezioni (accessed on 8 September 2022).
- El-Lababidi, R.M.; Rizk, J.G. Cefiderocol: A Siderophore Cephalosporin. Ann. Pharmacother. 2020, 54, 1215–1231. [Google Scholar] [CrossRef]
- Volpicelli, L.; Venditti, M.; Ceccarelli, G.; Oliva, A. Place in Therapy of the Newly Available Armamentarium for Multi-Drug-Resistant Gram-Negative Pathogens: Proposal of a Prescription Algorithm. Antibiotics 2021, 10, 1475. [Google Scholar] [CrossRef]
- Overview of Carbapenemase-Producing Gram-Negative Bacilli—UpToDate. Available online: https://www.uptodate.com/contents/overview-of-carbapenemase-producing-gram-negative-bacilli (accessed on 8 September 2022).
- Tiseo, G.; Brigante, G.; Giacobbe, D.R.; Maraolo, A.E.; Gona, F.; Falcone, M.; Giannella, M.; Grossi, P.; Pea, F.; Rossolini, G.M.; et al. Diagnosis and management of infections caused by multidrug-resistant bacteria: Guideline endorsed by the Italian Society of Infection and Tropical Diseases (SIMIT), the Italian Society of Anti-Infective Therapy (SITA), the Italian Group for Antimicrobial Stewardship (GISA), the Italian Association of Clinical Microbiologists (AMCLI) and the Italian Society of Microbiology (SIM). Int. J. Antimicrob. Agents 2022, 60, 106611. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase–Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Leonildi, A.; Della Sala, L.; Vecchione, A.; Barnini, S.; Farcomeni, A.; Menichetti, F. Cefiderocol-Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, e0214221. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.; Pasquini, Z.; Bartoletti, M.; Caiazzo, L.; Fornaro, G.; Bussini, L.; Volpato, F.; Marchionni, E.; Rinaldi, M.; Trapani, F.; et al. Cefiderocol treatment for carbapenem-resistant Acinetobacter baumannii infection in the ICU during the COVID-19 pandemic: A multicentre cohort study. JAC-Antimicrob. Resist. 2021, 3, dlab174. [Google Scholar] [CrossRef] [PubMed]
- Hoellinger, B.; Simand, C.; Jeannot, K.; Garijo, C.; Cristinar, M.; Reisz, F.; Danion, F.; Ursenbach, A.; Lefebvre, N.; Boyer, P.; et al. Real world clinical outcome of cefiderocol for treatment of multidrug resistant non-fermenting Gram-negative bacilli infections: A case series. Clin. Microbiol. Infect. 2023, 29, 393–395. [Google Scholar] [CrossRef]
- Weber, C.; Schultze, T.; Göttig, S.; Kessel, J.; Schröder, A.; Tietgen, M.; Besier, S.; Burbach, T.; Häussler, S.; Wichelhaus, T.A.; et al. Antimicrobial Activity of Ceftolozane-Tazobactam, Ceftazidime-Avibactam, and Cefiderocol against Multidrug-Resistant Pseudomonas aeruginosa Recovered at a German University Hospital. Microbiol. Spectr. 2022, 10, e01697-22. [Google Scholar] [CrossRef]
- Corcione, S.; De Benedetto, I.; Pinna, S.M.; Vita, D.; Lupia, T.; Montrucchio, G.; Brazzi, L.; De Rosa, F.G. Cefiderocol use in Gram negative infections with limited therapeutic options: Is combination therapy the key? J. Infect. Public Health 2022, 15, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Meschiari, M.; Volpi, S.; Faltoni, M.; Dolci, G.; Orlando, G.; Franceschini, E.; Menozzi, M.; Sarti, M.; Del Fabro, G.; Fumarola, B.; et al. Real-life experience with compassionate use of cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa (DTR-P) infections. JAC-Antimicrob. Resist. 2021, 3, dlab188. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Belati, A.; Diella, L.; Stufano, M.; Romanelli, F.; Scalone, L.; Stolfa, S.; Ronga, L.; Maurmo, L.; Dell’Aera, M.; et al. Cefiderocol-Based Combination Therapy for “Difficult-to-Treat” Gram-Negative Severe Infections: Real-Life Case Series and Future Perspectives. Antibiotics 2021, 10, 652. [Google Scholar] [CrossRef]
- König, C.; Both, A.; Rohde, H.; Kluge, S.; Frey, O.; Röhr, A.; Wichmann, D. Cefiderocol in Critically Ill Patients with Multi-Drug Resistant Pathogens: Real-Life Data on Pharmacokinetics and Microbiological Surveillance. Antibiotics 2021, 10, 649. [Google Scholar] [CrossRef]
- Gavaghan, V.; Miller, J.L.; Dela-Pena, J. Case series of cefiderocol for salvage therapy in carbapenem-resistant Gram-negative infections. Infection 2022, 51, 475–482. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 1 March 2023).
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar]
- Bassetti, M.; Mularoni, A.; Giacobbe, D.R.; Castaldo, N.; Vena, A. New Antibiotics for Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Semin. Respir. Crit. Care Med. 2022, 43, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Nicolau, D.P.; Rodvold, K.A.; Wunderink, R.G.; Echols, R.; Matsunaga, Y.; Menon, A.; Portsmouth, S.; Wajima, T. Intrapulmonary pharmacokinetic profile of cefiderocol in mechanically ventilated patients with pneumonia. J. Antimicrob. Chemother. 2021, 76, 2902–2905. [Google Scholar] [CrossRef] [PubMed]
- Kufel, W.D.; Abouelhassan, Y.; Steele, J.M.; Gutierrez, R.L.; Perwez, T.; Bourdages, G.; Nicolau, D.P. Plasma and cerebrospinal fluid concentrations of cefiderocol during successful treatment of carbapenem-resistant Acinetobacter baumannii meningitis. J. Antimicrob. Chemother. 2022, 77, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67–75.e1. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wang, Y.; Ma, X.; He, Y.; Zhao, J.; Guan, J.; Li, Y.; Gao, Z. In vitro and in vivo efficacy of cefiderocol plus tigecycline, colistin, or meropenem against carbapenem-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Mutakabbir, J.C.; Nguyen, L.; Maassen, P.T.; Stamper, K.C.; Kebriaei, R.; Kaye, K.S.; Castanheira, M.; Rybak, M.J. In Vitro Antibacterial Activity of Cefiderocol against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, AAC0264620. [Google Scholar] [CrossRef]
- Biagi, M.; Vialichka, A.; Jurkovic, M.; Wu, T.; Shajee, A.; Lee, M.; Patel, S.; Mendes, R.E.; Wenzler, E. Activity of Cefiderocol Alone and in Combination with Levofloxacin, Minocycline, Polymyxin B, or Trimethoprim-Sulfamethoxazole against Multidrug-Resistant Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2020, 64, e00559-20. [Google Scholar] [CrossRef]
- Eucast: Cefiderocol Susceptibility Testing. Available online: https://www.eucast.org/eucast_news/news_singleview/?tx_ttnews%5Btt_news%5D=493&cHash=22779384b74c8cf2c55aa3f7fd69d173 (accessed on 1 March 2023).
- Hobson, C.A.; Cointe, A.; Jacquier, H.; Choudhury, A.; Magnan, M.; Courroux, C.; Tenaillon, O.; Bonacorsi, S.; Birgy, A. Cross-resistance to cefiderocol and ceftazidime–avibactam in KPC β-lactamase mutants and the inoculum effect. Clin. Microbiol. Infect. 2021, 27, 1172.e7–1172.e10. [Google Scholar] [CrossRef]
- Stracquadanio, S.; Bonomo, C.; Marino, A.; Bongiorno, D.; Privitera, G.F.; Bivona, D.A.; Mirabile, A.; Bonacci, P.G.; Stefani, S. Acinetobacter baumannii and Cefiderocol, between Cidality and Adaptability. Microbiol. Spectr. 2022, 10, e02347-22. [Google Scholar] [CrossRef]
- Marino, A.; Stracquadanio, S.; Campanella, E.; Munafò, A.; Gussio, M.; Ceccarelli, M.; Bernardini, R.; Nunnari, G.; Cacopardo, B. Intravenous Fosfomycin: A Potential Good Partner for Cefiderocol. Clinical Experience and Considerations. Antibiotics 2022, 12, 49. [Google Scholar] [CrossRef]
- Mabayoje, D.A.; NicFhogartaigh, C.; Cherian, B.P.; Tan, M.G.M.; Wareham, D.W. Compassionate use of cefiderocol for carbapenem-resistant Acinetobacter baumannii prosthetic joint infection. JAC-Antimicrob. Resist. 2021, 3 (Suppl. 1), i21–i24. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Carbonara, S.; Marino, A.; Di Caprio, G.; Carretta, A.; Mularoni, A.; Mariani, M.F.; Maraolo, A.E.; Scotto, R.; et al. Mortality attributable to bloodstream infections caused by different carbapenem-resistant Gram negative bacilli: Results from a nationwide study in Italy (ALARICO Network). Clin. Infect. Dis. 2023, ciad100. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef] [PubMed]
| Variables | Study Population | Monotherapy | Combination | p Value | 30-D Survivors | 30-D Non-Survivors | p Value | CRAB |
|---|---|---|---|---|---|---|---|---|
| Patients | n = 41 | n = 31 | n = 10 | n = 26 | n = 15 | n = 31 | ||
| Males | 20 (48.8) | 15 (48.4) | 5 (50.0) | 0.93 | 11 (42.3) | 9 (60.0) | 0.27 | 16 (51.6) |
| Females | 21 (51.2) | 16 (51.6) | 5 (50.0) | 0.92 | 15 (57.7) | 6 (40.0) | 0.27 | 15 (48.4) |
| M:F ratio | 0.9:1 | 0.9:1 | 1:1 | 0.7:1 | 1.5:1 | 1.1:1 | ||
| Age, median years (IQR) | 70 (54–75) | 61 (52–76) | 72 (64–74) | 0.23 | 60 (51–75) | 73 (70–78) | 0.02 | 61 (52–73) |
| Age, years > 70 | 19 (46.3) | 12 (38.7) | 7 (70.0) | 0.08 | 8 (30.8) | 11 (73.3) | 0.008 | 11 (35.5) |
| CCI, median (IQR) | 5 (3–6) | 5 (2–6) | 4 (3–5) | 0.92 | 4 (2–6) | 5 (5–6) | 0.07 | 4 (12.9) |
| CCI ≥ 4 | 29 (70.7) | 22 (71.0) | 7 (70.0) | 0.95 | 15 (57.7) | 14 (93.3) | 0.01 | 20 (64.5) |
| Previous in hospital admission * | 20 (48.8) | 14 (45.2) | 6 (60.0) | 0.41 | 11 (42.3) | 9 (60.0) | 0.27 | 13 (41.9) |
| Previous bacterial infections * | 17 (41.5) | 11 (35.5) | 6 (60.0) | 0.17 | 8 (30.8) | 9 (60.0) | 0.60 | 12 (38.7) |
| Previous antibiotic therapy * | 34 (82.9) | 25 (80.6) | 9 (90.0) | 0.9 | 21 (80.7) | 12 (80.0) | 0.70 | 25 (80.6) |
| Unit at infection onset | ||||||||
| Medical ward | 20 (48.8) | 16 (51.6) | 4 (40.0) | 0.52 | 13 (50.0) | 7 (46.7) | 0.83 | 14 (45.2) |
| Surgical ward | 8 (19.5) | 4 (12.9) | 4 (40.0) | 0.06 | 6 (23.1) | 2 (13.3) | 0.44 | 6 (19.3) |
| Intensive care unit | 13 (31.7) | 11 (35.5) | 2 (20.0) | 0.36 | 7 (26.9) | 6 (40.0) | 0.39 | 11 (35.5) |
| Types of infections | ||||||||
| HAPs | 17 (41.5) | 15 (48.4) | 2 (20.0) | 0.11 | 8 (30.8) | 9 (60.0) | 0.05 | 13 (41.9) |
| cUTIs | 5 (12.2) | 5 (16.1) | 0 (0) | 0.17 | 5 (19.2) | 0 (0) | 0.06 | 3 (9.7) |
| cIAIs | 8 (19.5) | 4 (12.9) | 4 (40.0) | 0.06 | 3 (11.5) | 5 (33.3) | 0.08 | 6 (19.3) |
| SSTIs | 9 (21.9) | 6 (19.3) | 3 (30.0) | 0.47 | 8 (30.8) | 1 (6.7) | 0.07 | 8 (25.8) |
| Osteomyelitis | 3 (7.3) | 2 (6.4) | 1 (10.0) | 0.71 | 2 (7.7) | 1 (6.7) | 0.90 | 2 (6.4) |
| CNS infections | 2 (4.9) | 1 (3.2) | 1 (10.0) | 0.39 | 0 (0) | 2 (13.3) | 0.05 | 2 (6.4) |
| Bloodstream infections | 18 (43.9) | 14 (45.2) | 4 (40.0) | 0.77 | 10 (38.5) | 8 (53.3) | 0.35 | 15 (48.4) |
| Septic shock | 14 (34.2) | 11 (35.5) | 3 (30.0) | 0.75 | 4 (15.4) | 10 (66.7) | 0.0008 | 10 (32.2) |
| Bacterial isolates | ||||||||
| Acinetobacter baumannii | 31 (75.6) | 25 (80.6) | 6 (60.0) | 0.18 | 20 (76.9) | 11 (73.3) | 0.79 | |
| Klebsiella pneumoniae | 10 (24.4) | 6 (19.3) | 4 (40.0) | 0.18 | 6 (23.1) | 4 (26.7) | 0.79 | |
| Pseudomonas aeruginosa | 5 (12.2) | 2 (6.4) | 3 (30.0) | 0.047 | 2 (7.7) | 3 (20.0) | 0.41 | |
| Escherichia coli | 3 (7.3) | 3 (9.7) | 0 (0) | 0.31 | 1 (3.8) | 2 (13.3) | 0.26 | |
| Stenotrophomonas maltophilia | 1 (2.4) | 1 (3.2) | 0 (0) | 0.56 | 1 (3.8) | 0 (0) | 0.44 | |
| Polymicrobial isolates | 9 (22.0) | 6 (19.3) | 3 (30.0) | 0.47 | 6(23.1) | 3 (20.0) | 0.47 | |
| No isolates | 1 (2.4) | 1 (3.2) | 0 (0) | 0.31 | 1 (3.8) | 0 (0) | 0.44 |
| Variables | Study Population | Monotherapy | Combination | p Value | 30-D Survivors | 30-D Non-Survivors | p Value | CRAB |
|---|---|---|---|---|---|---|---|---|
| Therapy | n = 41 | n = 31 | n = 10 | n = 26 | n = 15 | n = 31 | ||
| Days before therapy, median (IQR) * | 21 (14–32) | 19 (13–31) | 22 (15–33) | 0.53 | 23 (12–32) | 17 (14–26) | 0.31 | 21 (14–34) |
| Days of therapy, median (IQR) | 9 (6–19) | 9 (5–16) | 13 (6–21) | 0.36 | 10 (7–22) | 7 (3–14) | 0.16 | 9 (7–21) |
| Duration of therapy, >9 days | 19 (46.3) | 13 (41.9) | 6 (60.0) | 0.31 | 14 (53.8) | 5 (33.3) | 0.20 | 15 (48.4) |
| Monotherapy regimen | 31 (75.6) | 20 (76.9) | 11 (73.3) | 0.79 | 25 (80.6) | |||
| Combination therapy regimen | 10 (24.4) | 6 (23.1) | 4 (26.7) | 0.79 | 6 (19.3) | |||
| Outcomes | ||||||||
| Microbiological eradication at EOT | 33 (80.5) | 27 (87.1) | 6 (60.0) | 0.06 | 22 (84.6) | 11 (73.3) | 0.43 | 25 (80.6) |
| Clinical cure at EOT | 23 (56.1) | 18 (58.1) | 5 (50.0) | 0.65 | 20 (64.5) | |||
| Clinical response in first 72 h * | 33 (80.5) | 24 (77.4) | 9 (90.0) | 0.38 | 22 (84.6) | 11 (73.3) | 0.43 | 28 (90.3) |
| Need to switch * | 3 (7.3) | 2 (6.4) | 1 (10.0) | 0.71 | 2 (7.7) | 1 (6.7) | 0.90 | 0 (0) |
| All-causes hospital mortality | 19 (46.3) | 14 (45.2) | 5 (50.0) | 0.78 | 15 (48.4) | |||
| 30-D all-causes mortality | 15 (36.6) | 11 (35.5) | 4 (40.0) | 0.79 | 11 (35.5) |
| Variables | HAPs | cUTIs | cIAIs | SSTIs | Osteomyelitis | CNS Infections | BSIs |
|---|---|---|---|---|---|---|---|
| Patients | n = 17 | n = 5 | n = 8 | n = 9 | n = 3 | n = 2 | n = 18 |
| Bacterial isolates | |||||||
| Acinetobacter baumannii | 13 (76.5) | 3 (60.0) | 6 (75.0) | 8 (88.9) | 2 (66.7) | 2 (100) | 15 (83.3) |
| Klebsiella pneumoniae | 2 (11.8) | 2 (40.0) | 5 (62.5) | 3 (33.3) | 0 (0) | 0 (0) | 5 (27.8) |
| Pseudomonas aeruginosa | 2 (11.8) | 0 (0) | 0 (0) | 2 (22.2) | 1 (33.3) | 0 (0) | 2 (11.1) |
| Escherichia coli | 2 (11.8) | 1 (20.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) |
| Stenotrophomonas maltophilia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) |
| Polymicrobial isolates | 3 (17.6) | 1 (20.0) | 3 (37.5) | 3 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| No isolates | 1 (5.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Therapy | |||||||
| Days of therapy, median (IQR) | 8 (2–14) | 10 (7–10) | 10 (4–24) | 11 (8–22) | 32 (24–37) | 12 (8–16) | 9 (7–18) |
| Monotherapy regimen | 15 (88.2) | 5 (100) | 4 (50.0) | 6 (66.7) | 2 (66.7) | 1 (50.0) | 14 (77.8) |
| Combination therapy regimen | 2 (11.8) | 0 (0) | 4 (50.0) | 3 (33.3) | 1 (33.3) | 1 (50.0) | 4 (22.2) |
| Outcomes | |||||||
| Microbiological eradication at EOT | 15 (88.2) | 5 (100) | 5 (62.5) | 8 (88.9) | 2 (66.7) | 1 (50.0) | 14 (77.8) |
| Clinical cure at EOT | 5 (29.4) | 4 (80.0) | 3 (37.5) | 8 (88.9) | 2 (66.7) | 1 (50.0) | 9 (50.0) |
| Clinical response in first 72 h * | 11 (64.7) | 4 (80.0) | 6 (75.0) | 8 (88.9) | 3 (100) | 2 (100) | 16 (88.9) |
| 30-D all-causes mortality | 9 (52.9) | 0 (0) | 5 (62.5) | 1 (11.1) | 1 (33.3) | 2 (100) | 8 (44.4) |
| Variables | Our Study | Falcone et al. [12] | Pascale et al. [13] | Hoellinger et al. [14] | Weber et al. [15] | Corcione et al. [16] | Meschiari et al. [17] | Bavaro et al. [18] | König et al. [19] | Gavaghan et al. [20] |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 41 | 47 | 42 | 10 | 8 | 18 | 17 | 13 | 5 | 24 |
| Median age (IQR) | 70 (54–75) | 63 (53–75) | 64 (55–73) | 66 (56–71.5) | 64 (39–80) | 54.5 (35–65) | 64 (58–73) | 63 (53–69) | 55 (41–76) | 66.5 (60–74) |
| HAPs/VAPs | 9/17 (52.9) | 7/12 (58.3) | NA/14 | 4/5 (80) | 0/2 (0) | 4/13 (30.8) | 3/8 (37.5) | 1/3 (33.3) | 1/4 (25) | 9/19 (47.4) |
| CRAB | 8/13 (61.5) | 7/12 (58.3) | NA/14 | 1/1 (100) | 0 | 4/13 (30.8) | 1/1 (100) | 1/2 (50) | 1/2 (50) | 5/12 (41.7) |
| K. pneumoniae | 1/2 (50) | NA | 0 | 0 | 0 | 0 | 1/1 (100) | 0 | 0 | 2/3 (66.7) |
| P. aeruginosa | 0/2 (0) | NA | 0 | 1/2 (50) | 0/2 (0) | 0 | 3/8 (37.5) | 0/1 (0) | 0/2 (0) | 3/7 (42.8) |
| Polymicrobial | 2/3 (66.7) | NA | NA | 0 | 0 | 0 | 2/3 (66.7) | 0 | 0 | 2/6 (33.3) |
| cIAIs | 5/8 (62.5) | NA/1 | NA | 0/1 (0) | 1/1 (100) | 0 | 2/4 (50) | 0/2 (0) | 0 | 0 |
| cUTIs | 0/5 (0) | 0 | NA | 2/2 (100) | 0/4 (0) | 0 | 0 | 0 | 0 | 0/1 (0) |
| SSTIs | 1/9 (11.1) | NA/5 | NA | 0 | 0 | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0 | 2/4 (50) |
| Osteomyelitis | 1/3 (33.3) | NA/0 | NA | 0 | 0/1 (0) | 0 | 1/1 (100) | 0 | 0 | 0 |
| CNS infections | 2/2 (100) | NA/1 | NA | 1/1 (100) | 0 | 0 | 0/1 (0) | 0 | 0 | 0 |
| BSIs | 8/18 (44.4) | 7/27 (25.9) | NA/27 | 5/6 (83.3) | 1/1 (100) | 5/15 (33.3) | 1/4 (25) | 3/9 (33.3) | 1/2 (50) | 3/5 (60) |
| CRAB | 7/15 (46.7) | 7/27 (25.9) | NA/27 | 1/1 (100) | 0 | 5/13 (38.5) | 0 | 3/8 (37.5) | 0/1 (0) | 3/3 (100) |
| Septic shock | 10/14 (71.4) | NA/30 | NA/18 | 5/6 (83.3) | 1/1(100) | NA | 3/5 (60) | 1/4 (25) | 2/5 (40) | NA |
| 30-D Mortality | 15/41 (36.6) | 16/47 (34) | 23/42 (55) | 6/10 (60) | 1/8 (12.5) | 5/18 (27.8) | 4/17 (23.5) | 3/13 (23.1) | 2/5 (40) | 10/24 (42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palermo, G.; Medaglia, A.A.; Pipitò, L.; Rubino, R.; Costantini, M.; Accomando, S.; Giammanco, G.M.; Cascio, A. Cefiderocol Efficacy in a Real-Life Setting: Single-Centre Retrospective Study. Antibiotics 2023, 12, 746. https://doi.org/10.3390/antibiotics12040746
Palermo G, Medaglia AA, Pipitò L, Rubino R, Costantini M, Accomando S, Giammanco GM, Cascio A. Cefiderocol Efficacy in a Real-Life Setting: Single-Centre Retrospective Study. Antibiotics. 2023; 12(4):746. https://doi.org/10.3390/antibiotics12040746
Chicago/Turabian StylePalermo, Gabriele, Alice Annalisa Medaglia, Luca Pipitò, Raffaella Rubino, Manuela Costantini, Salvatore Accomando, Giovanni Maurizio Giammanco, and Antonio Cascio. 2023. "Cefiderocol Efficacy in a Real-Life Setting: Single-Centre Retrospective Study" Antibiotics 12, no. 4: 746. https://doi.org/10.3390/antibiotics12040746
APA StylePalermo, G., Medaglia, A. A., Pipitò, L., Rubino, R., Costantini, M., Accomando, S., Giammanco, G. M., & Cascio, A. (2023). Cefiderocol Efficacy in a Real-Life Setting: Single-Centre Retrospective Study. Antibiotics, 12(4), 746. https://doi.org/10.3390/antibiotics12040746







