Abstract
Beta-lactams (BL) are the first line agents for the antibiotic management of critically ill patients with sepsis or septic shock. BL are hydrophilic antibiotics particularly subject to unpredictable concentrations in the context of critical illness because of pharmacokinetic (PK) and pharmacodynamics (PD) alterations. Thus, during the last decade, the literature focusing on the interest of BL therapeutic drug monitoring (TDM) in the intensive care unit (ICU) setting has been exponential. Moreover, recent guidelines strongly encourage to optimize BL therapy using a PK/PD approach with TDM. Unfortunately, several barriers exist regarding TDM access and interpretation. Consequently, adherence to routine TDM in ICU remains quite low. Lastly, recent clinical studies failed to demonstrate any improvement in mortality with the use of TDM in ICU patients. This review will first aim at explaining the value and complexity of the TDM process when translating it to critically ill patient bedside management, interpretating the results of clinical studies and discussion of the points which need to be addressed before conducting further TDM studies on clinical outcomes. In a second time, this review will focus on the future aspects of TDM integrating toxicodynamics, model informed precision dosing (MIPD) and “at risk” ICU populations that deserve further investigations to demonstrate positive clinical outcomes.
1. Interest of Beta-Lactam Therapeutic Drug Monitoring in the Critically Ill Patient
Sepsis and septic shock are one of the most common causes of intensive care unit (ICU) admission and are associated with increased mortality [1]. The scarcity of new antibiotics that arrives in the pipeline to counter the growing concern of antimicrobial resistance led the medical community to better use the current antibiotics available [2].
Beta-lactams (BL) are the most prescribed antibiotic in critically ill patients. BL are hydrophilic antibiotics particularly subjects to unpredictable concentrations in the context of critical illness due to pharmacokinetic (PK) and pharmacodynamic (PD) alterations [3]. Basically, the two main pathophysiological changes are an increased volume of distribution and modified renal and/or hepatic clearance [4]. In addition to dynamic changes in physiological function, ICU therapies such as massive vascular filling and supportive extracorporeal therapies, such as renal replacement therapy and extracorporeal membrane oxygenation, also impact antibiotic concentrations [5]. The highest risk at the early stage of sepsis remains BL underexposure [6]. It has been demonstrated that ICU patients with BL underexposure have a 1.5-fold higher risk of clinical failure and need antibiotic escalation and death [7]. Regarding the therapeutic target, ICU patients frequently faced high-resistance bacteria with higher MICs than in non-ICU patients [8]. Because of the high risk of underdosage and the highest required PD target, optimization of the BL dose in critically ill patients appears essential. Moreover, pathophysiological alterations are a dynamic process in ICU patients and could change every day. From a PK point of view, at the onset of the septic shock, there is high risk of BL underdosing. From a PD point of view, during this early phase, infections are frequently undocumented or documented but without any minimal inhibitory concentration (MIC) determination. Consequently, empirical PK/PD target are mostly based on a “worst-case scenario” and are generally high. Subsequently, PK alterations could be variable, depending on the severity of the septic shock, from renal insufficiency requiring renal replacement therapy to normal renal function for instance. At this stage, infection is generally documented, and MIC could be available. Thus, PK/PD target should be reevaluated. The Figure 1 depicts one example of timeline regarding both PK and PD alterations during the antibiotic course of critically ill patient.
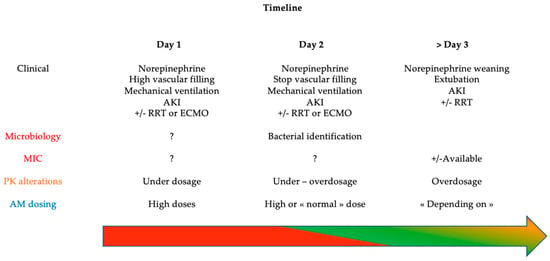
Figure 1.
Timeline of PK/PD alterations in critically ill patients with septic shock. AKI: acute kidney injury, RRT: renal replacement therapy, ECMO: extracorporeal membrane oxygenation, VF: vascular filling, MIC: minimal inhibitory concentration, PK: pharmacokinetics, AM: antimicrobial; Arrow with color: level of inadequate BL concentration risk: red = very high, green = low, orange = variable.
Lastly, recent guidelines strongly encourage optimizing antibiotic prescription based on their PK/PD properties and using therapeutic drug monitoring (TDM) to ensure adequate dose and concentrations [9,10].
2. Barriers to Overcome to Increase BL Therapeutic Drug Monitoring Adherence
According to Watson et al., “Therapeutic Drug Monitoring (TDM) is a measurement made in the laboratory of a parameter which, with appropriate interpretation, will directly influence prescribing procedures” [11]. This definition implies a multidisciplinary approach from the antibiotic concentration measurement (laboratory level) to its interpretation in the clinical context (clinician level). It also supposed a direct link between drug concentration and therapeutic effect (efficacy) and/or toxic effect (toxicity). One last basic principle for TDM concerns the site of measure, mostly the plasma where concentrations are generally higher than in the tissue of interest [12].
The two antibiotic classes that better illustrate TDM principles are aminoglycosides and vancomycin [13]. Indeed, these two antibiotic classes have a narrow therapeutic index and a high risk of toxicity. However, in both cases, toxic thresholds to avoid toxicity and adequate concentrations to ensure efficacy are well known in the literature [14,15,16]. Moreover, concerning TDM access, both classes are usually analyzed using immunoassays that are relatively cheap, not time consuming, not requiring well-trained personal and with good reproducibility [16]. This allows the results of measurement to be rapidly available for clinicians. Lastly, TDM of these two antibiotics are available worldwide and 24/7. Recent surveys showed that TDM for these two classes of antibiotic is well implemented [17,18].
Contrary to aminoglycosides and vancomycin, BL TDM is more challenging for several reasons [19]. The first reason is related to the physicochemical properties of the drug itself. BL are hydrophilic drugs with low to moderate protein binding (except for ceftriaxone and flucloxacillin which exhibit high protein binding) [20]. The most binding protein is albumin. This is important to emphasize for BL TDM interpretation, because only the free fraction of the drug is active. In the critically ill patient, hypoalbuminemia frequently occurs [21]. Unfortunately, most of centers where TDM is available measure the total concentration of BL [22]. In this case, to appropriately interpret the results of BL concentrations, clinicians should correct these concentrations by the free fraction based on the percentage of protein binding published in the literature [23]. Secondly, the optimal PK/PD target remains debated. In critically ill patients, 100% of the time where the free concentration is above the MIC (100% fT > MIC) is often suggested as a therapeutic target [7,10]. Some authors also proposed, in addition to the time spend above the MIC, to add a multiple of the MIC (i.e., 2–5 × MIC) [24,25,26]. The rationale to increase the ratio of MIC target is based on tissue diffusion, technical issues about the uncertainty of MIC and BL measures and resistance suppression [23,27]. However, these propositions come mostly from observational studies showing a better microbiological cure and clinical cure without any impact on the mortality. More research is warranted to determine whether the target should be increased from 100% fT > MIC to 100% fT > k × MIC (where k is a multiple factor strictly above 1). Finally, most of the literature about BL PK/PD target concerns ICU patients infected with bacteria expressing high MICs or target those based on a “worst-case” scenario to justify high doses. No study focused on dose and PK/PD target for the treatment of low MIC bacteria, which account for most patients, including ICU patients [28]. In documented infections, the greatest issue regarding MIC remains the turnaround time. Indeed, 24 h minimum are required to obtain a positive culture, and, depending on the MIC determination method, an additional 24 h may be necessary [29]. This timeline cannot be easily reduced, and an alternative must be taken, such as epidemiological cutoff (ECOFF) based on local ecology.
Secondly, unlike assays for aminoglycosides and vancomycin, no commercially available assay for BL routine monitoring has been implemented [30]. Most centers use in-house methods for BL quantification that utilize chromatographic separation coupled to ultraviolet (UV) or mass spectrometric (MS) detection. Having in-house high-performance liquid chromatography (HPLC) or MS requires expensive equipment and staff expertise that limit its deployment. Moreover, BL are instable at room temperature, and samples should be maintained on ice or frozen until processed to limit degradation. Recent surveys have highlighted that most centers have to export their analyses leading to delays of results and risk of sample degradation [22]. These pre-analytical issues added to the questions on the optimal PK/PD target for BL make BL a class apart.
Table 1 summarizes points to optimize before TDM implementation.

Table 1.
Barriers to counter for a better TDM adherence.
3. Clinical Evidence Supporting BL TDM
During the year 2022, four systematic reviews and meta-analysis have been published about the interest of BL TDM in critically ill patients [19,28,31,32]. Two were focused on the effect of BL TDM on clinical outcome such as mortality and emergence of antimicrobial resistance [31,32]. These two reviews have analyzed 39 studies knowing that some studies were included in both reviews. None of these two reviews have showed an association between BL TDM use and mortality or emergence of antimicrobial resistance. Moreover, according to the authors, there was a high risk of bias (level critical—serious) on items such as “deviation from intended intervention” and “confounding”. These two biases could have significantly influenced the interest of the TDM itself (i.e., when not following the TDM indications) and the impact on mortality with the confounding factors.
In both reviews, there were few prospective randomized clinical trials (RCT) [33,34,35]. Three RCTs were present in the two reviews and totalize 111 patients of various origins: septic patients with normal renal function (n = 41 [35]), neutropenic patients (n = 32 [33]) and burns patients (n = 38 [34]). Therefore, they included highly specific sub population of ICU patients which could question the extrapolation of the results in the general ICU population. Patients with renal replacement therapy (RRT) or augmented renal clearance (ARC), two major sources of BL concentration modifications, were not included. In neutropenic patients, general PK alteration frequently occurred, and, most importantly, the absence of neutrophils altered the bacterial clearance [36] and, thus, changed the PK/PD target to attain. Lastly, burns patients constitute a specific population about their own PK alterations and specific infectious risk because of the loss of protective integument [37].
The most recent RCT about BL TDM use and its impact on mortality was the study from Hagel et al. [38] which was included in the review from Mangalore [32]. This RCT has enrolled the largest sample size (n = 249) and was multicenter, and the population was the most representative of the general ICU population (i.e., 74% of patients had septic shock; the median SOFA score at admission was 12.1 ± 2.8). Only the piperacillin (in piperacillin/tazobactam combination) was evaluated, and the PK/PD target was based on the MIC of Pseudomonas aeruginosa for empirical therapy. The trial was negative on its primary outcome (difference in mean SOFA score with TDM vs. no TDM, p = 0.39). A 4.2% lower mortality and a higher rate of microbiological and clinical cure were observed, but without reaching statistical significance. The rate of target attainment was better with the use of TDM with less underdosing occurrence. Nevertheless, when focusing on target attainment, less than 50% of patients reached the optimal target (without any overdosing) within the five first days with a nadir during the first day. According to the authors, the negative results on mortality could be explained by the high PK/PD target. Indeed, on day one, the PK/PD target was based on MIC from Pseudomonas aeruginosa namely 16 mg/L for piperacillin leading to 19% (control group) and 33.9% (TDM group) attainment. However, as most of the identified bacteria displayed a lowest MIC, this non-target attainment could not result in poor outcomes. They are few studies comparing an a priori PK/PD target attainment (based on worst-case scenario with high MIC mostly for empirical therapy) and a posteriori MIC, after bacteria identification. One study from Leon et al., showed in critically ill patients with intra-abdominal infections requiring surgery an increase in PK/PD target attainment before (33%) and after documentation (71%) [39]. This point highlights the crucial role of the MIC used for the choice of the PK/PD target.
The two other reviews depicted the challenge of clinical studies about BL PK/PD target and the role of TDM [19,28]. They both highlighted two main challenges:
- (i)
- The choice of the optimal PK/PD target is unknown and depends on the administration method of the considered BL.
- (ii)
- The choice of the MIC for the target (“true MIC” or ECOFF-based) and its determination (risk of interstrain and interlaboratory differences) [40].
The recently published study from Magreault and colleagues summarized the points to consider for MIC interpretation. They reviewed MIC microbiological indications and integrated the MIC in a microbiological, pharmacological and clinical situation approach [41]. One aspect which differs in clinical studies from daily routine practice is the delay for TDM results. Whatever the study, TDM results were available a few hours after the samples. One recent survey about routine practice of TDM highlighted the delay response time as a major barrier for TDM implementation [22]. Moreover, interpretation of TDM results was well known by the prescribers because of the available algorithm to adjust the dose, and the included centers were experts in BL TDM. The same survey about routine practice of TDM showed the difficulty regarding TDM results interpretation as the second major barrier, especially in a non-expert center. All in all, reduction in the delay for TDM results and help for interpretation are the two main objectives to translate results from clinical study into the daily routine practice. Figure 1 depicts all the challenges faced by clinical trials aiming at evaluating the interest of TDM on PK/PD target attainment and its impact on clinical outcomes.
Table 2 proposed a new critical review of the four most recent RCTs on BL TDM use and its impact on mortality.

Table 2.
Characteristics of the four most recent PK/PD target randomized control studies.
A focus on PK/PD target according to the choice of the MIC and TDM management was highlighted. Several concerns could be addressed:
- (i)
- The heterogeneity of included population with a lot of confounding factors.
- (ii)
- An imbalance between a priori target (worst-case scenario/MIC of Pseudomonas aeruginosa for empirical treatment) and the actual MIC after bacterial documentation (MIC much lower) without PK/PD target adjustment.
- (iii)
- The variability in algorithm for dose adjustment.
- (iv)
- The approximation of the renal function measurement leading to possible overestimation of the true renal function, which is a confounding factor especially for BL.
- (v)
- The most used concentration of BL was the total fraction, although the PD target was based on the free concentration which needs an estimation.
These concerns should probably partly explain the negative results. Moreover, we must emphasize that, due the interventional design of the study, TDM results were available rapidly, which is not the case for most of centers. Finally, if piperacillin with tazobactam was the most evaluated BL, we need to be cautious about the tazobactam PK/PD changes. According to the review from Monogue [42], there is a lack of data about PK/PD of beta-lactamase inhibitors regarding, among others, the threshold for efficacy for instance. The role of the beta-lactamase inhibitors for the treatment of multidrug-resistant bacteria is crucial and, thus, probably influences the results like the microbiological cure and ultimately, the mortality.
Based on all these challenges, we suggest five key points for designing further studies aiming at assessing the interest of TDM to decrease mortality in ICU patients:
- To evaluate one BL per study: dose and administration method as well as algorithm of dose adjustment must be described.
- To avoid dual therapy to limit confounder: if required, in case of septic shock for instance, the choice of the second antibiotic must be unique with adequate dose and the statistical analysis must consider this variable for the final interpretation.
- To include one origin of infection ideally or infection with or without a source control but not both: indeed, when a source control is possible (i.e., catheter removal, surgical lavage, etc.) the clinical and microbiological cure would depend on the antibiotic adequate concentration and the reduced bacterial load allowed by an external factor. Thus, to include pneumonia and peritonitis for instance could alter comparability of patients.
- To include a homogeneous population of ICU patients to avoid confounding factors: If immunocompromised patients are evaluated, it must be an inclusion criterion. Indeed, as mentioned earlier, the absence of neutrophils in neutropenic patients altered the bacterial clearance that could compromise the microbiological cure [36]. Another frequent confounding factor is renal failure [43] or ARC [44]. These phenomena both frequently occur in ICU patients and should be considered. Nevertheless, it is a pity that almost all PK/PD studies published nowadays used estimation of the renal function with the CKD-EPI or Cockcroft–Gault formula. Indeed, it has been strongly demonstrated that these two formulae are less accurate in critically ill patients compared to the measured renal clearance using urinary collection [45,46].
- To carefully choose the MIC used for PK/PD target: most studies based the PK/PD target attainment on day 1 (empirical phase) on a worst-case scenario considering the MIC of a low-susceptible pathogen, mostly Pseudomonas aeruginosa. Whatever the choice of the MIC for the empirical phase, it must be reconsidered with the documented MIC, a posteriori or based on the local ecology.
Figure 2 lists the point to consider when constructing future studies on PK/PD target attainment (2A, upper panel) and summarizes the five proposed tips (2B, lower panel)
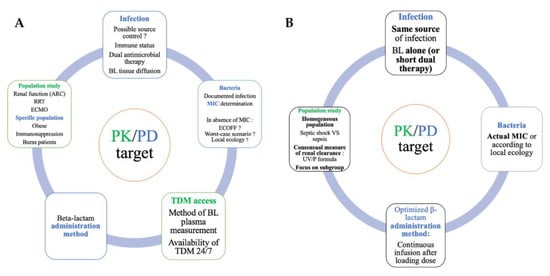
Figure 2.
Keypoints to consider when constructing future studies on PK/PD target attainment ((A), upper panel) and suggested five tips ((B), lower panel). ARC: augmented renal clearance, RRT: renal replacement therapy, ECMO: extracorporeal membrane oxygenation, BL: beta-lactam, MIC: minimal inhibitory concentration, TDM: therapeutic drug monitoring, PK/PD: pharmacokinetics/pharmacodynamics.
4. Beta-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: Time to Consider Toxicity? Role of TDM for Assessing BL Toxicity
A growing body of evidence indicates that BL may cause significant toxicity in specific populations, such as the ICU population [47]. Therefore, TDM is also essential in preventing unnecessary excessively high BL exposure that may lead to toxicities. As the threshold BL concentrations for dose-dependent toxicity are generally high, this allows the use of higher initial empirical dosing regimens that can be subsequently refined with TDM. Recently, some studies have demonstrated the role of TDM to minimize non-BL antimicrobial-related toxicity [48]. In a retrospective study including 93 patients, no excessive drug toxicity associated with higher than licensed doses based on TDM was found for either meropenem or piperacillin tazobactam although mean daily doses were more than 40% higher in the high-dose groups [49]. However, the main barrier to TDM-based dosing adjustment to limit toxicity is the lack of well-established thresholds for BL, and efforts to determine toxicodynamics targets are strongly needed. Some studies have focused on the concentration-neurotoxicity relationship of BL in the intensive care setting. Cefepime trough concentrations above 22 mg/L (when administered by intermittent infusions) or concentrations at steady state above 35 mg/L (when administered by continuous infusion) have been associated with neurotoxicity in 50% of patients [50,51]. Comparatively, the same risk has been reported for trough above 64 mg/L for meropenem, 125 mg/L for flucloxacillin and 360 mg/L for piperacillin (used without tazobactam) [52]. In combination with tazobactam, a plasma steady-state concentration of piperacillin above 157 mg/L is predictive of the occurrence of neurological disorders in ICU patients with a specificity of 97% and a sensitivity of 52% [53]. Finally, when the fCmin normalized to the EUCAST clinical breakpoint for Pseudomonas aeruginosa (i.e., fCmin/MIC P. aeruginosa ratio) exceeded 8, a significant deterioration of the neurological status occurred in approximately half of the ICU patients treated with piperacillin/tazobactam and approximately two-thirds of the ICU patients treated with meropenem [54]. It is of note that an ongoing prospective clinical trial, the OPTIMAL TDM Study (NCT03790631), aims to address the toxicity thresholds of cefepime, imipenem, meropenem, piperacillin, flucloxacillin, amoxicillin and ceftazidime. However, the impact of TDM guided dosing adjustment to prevent harmful BL concentrations and to improve clinical outcome has yet to be determined.
5. Beta-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: Time to Integrate Antimicrobial PK/PD Software?
As seen previously, the traditional approaches of TDM have some limitations. TDM relies on manual data entry, plasma-level determination and interpretation by pharmacists or physicians with PK/PD background. Finally, TDM dosing guidance may become available after several doses, while adequate exposure may be needed right from the start of therapy. To overcome the barriers related to antimicrobial TDM-guided dose optimization, innovative approaches using health record data in real time have been proposed (Figure 3).
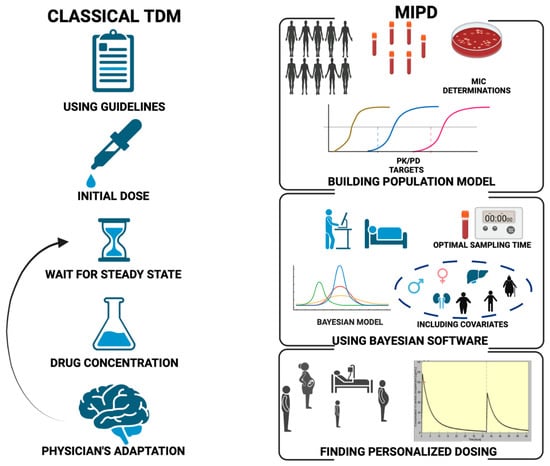
Figure 3.
Comparison of the traditional TDM (left) and MIPD (right) workflows. MIC: minimum inhibitory concentration; MIPD: model-informed precision dosing; PD: pharmacodynamic; PK: pharmacokinetic; TDM: therapeutic drug monitoring.
A computerized approach using dedicated software that can integrate in their algorithm the different variables affecting antimicrobial PK such as weight, renal function or age could allow a more precise dose adaptation [55]. Model informed precision dosing (MIPD) uses a mathematical model to interpret the measured drug concentration. Thus, MIPD is data driven, relying on the individual patient’s current characteristics and clinical data and drug properties. To integrate the necessary data to inform the modelling, interfacing with health record data in real time is crucial. However, MIPD generally requires custom-made software tools as generic modelling software are too complex for clinicians to apply. Until recently, very few studies have focused on individualized and computerized dose adaptation to optimize antimicrobial therapy in ICU patients [56,57,58,59,60,61]. These approaches aim to better understand each patient’s unique pharmacological profile.
5.1. Dosing Software Principles
Recently, complex mathematical modeling and PK models have been embedded into dosing software to assist with drug dosing. According to the model used in the computer program, three categories of dosing software are currently used:
- (a)
- Linear regression model.
The simplest dosing software relies on linear regression model. This method uses a posteriori drug dosing calculations where the patient’s pharmacokinetic parameters are calculated from at least two measured serum concentrations and assume a one compartment model. Based on the PK results, the program will determine the most appropriate dosing regimen for the patient [62]. Several pitfalls of linear regression models have been identified. This approach does not consider a specific PK population and does not predict an initial dose, and each analysis is performed independently without considering any change in the patient’s characteristics over the course of time.
- (b)
- Population PK-based dosing software.
This approach can be compared to an improved nomogram. Unlike the linear regression model, a single measurement is sufficient to generate dose predictions. This method does not consider any change in patient parameters and does not adapt to the previous determinations. The dose recommendation is only based on population PK parameters without using the patient individual PK results. This approach is considered as an a priori dosing method. This limited view results in a likely loss of reliability in patients whose parameters may change significantly during treatment [63,64].
- (c)
- Bayesian forecasting software.
Dosing software implementing Bayesian method combines a given population PK model with the data from an individual patient to determine optimal dose adjustment. Population PK data are used to a priori determine the recommended dose likely to achieve a predefined PK/PD target. When measured drug concentrations become available, these data together with existing population PK data can be used to derive the individual PK parameters using Bayesian estimation and predict individualized dosing regimen. The individual PK parameter estimate is referred to as the maximum a posteriori (MAP) Bayesian estimate. These parameters are derived from the characteristics of the patient for whom the optimal dose is required and will therefore have an important influence on the final simulation. Thus, Bayesian forecasting presents the advantage of using TDM data that strengthen the accuracy of dosing recommendations and consider inter-individual variability. The Bayesian approach offers the advantage that it makes optimal use of all information contained in the population model (a priori) combined with the most current pharmacokinetic information from the patient (a posteriori) to develop the patient’s most precise regimen [65]. Each new serum level collected helps improve the modeling of a patient’s unique PK parameters, further improving dosing recommendations and predictions. This approach can be applied under complicated dosing regimens and non–steady-state conditions and using single concentration measurements and samples taken at flexible times.
Despite these advantages, the implementation of Bayesian methods into healthcare settings has been limited, even though some national guidelines recommend this approach mainly for antimicrobial drugs with easily available TDM, i.e., non-BL antibiotics [66]. Several potential barriers to the widespread implementation of Bayesian methods in clinical practice have been identified. The lack of PK expertise and easy-to-use dosing tools to support clinicians in better tailoring antimicrobial dosing are likely to explain the poor implementation of Bayesian methods. On the other end, the need to integrate individual conditions to increase dosing precision has grown. To maximize the success of these efforts and to further facilitate their implementation into clinical practice, there is a clear need for using friendly software tools.
5.2. Clinical Data Supporting Antimicrobial Dosing Software Use
Available clinical trials supporting BL dosing software tools are summarized in Table 3.

Table 3.
Characteristics of the four clinical trials reporting BL dosing software clinical performance in critically ill patients.
A growing body of evidence indicates, especially in critically ill patients, that beta-lactam TDM-guided dosing and MIPD may maximize efficacy. Felton et al. analyzed the fitness of BestDoseTM software to estimate each individual’s pharmacokinetics by comparison of the observed-versus-predicted piperacillin concentration after 24 h of therapy and its ability to accurately predict piperacillin dosing from the observed piperacillin concentrations [57]. They found that the dose optimization software predicted a mean piperacillin dosage of 4.02 g in the eight patients administered piperacillin doses of 4.00 g when at least two observed piperacillin concentrations were available. Moreover, linear regression of the observed-versus-predicted piperacillin concentrations for the eight individuals including in the study demonstrated an r2 of >0.89. Heil et al. demonstrated that the use of the PK/PD-based antibiotic dosing calculator- ID-ODS resulted in the probability of target attainment of 98% in 50 severely ill patients treated with cefepime, meropenem or piperacillin-tazobactam [67]. Similarly, Chiriac et al. have shown that combining the application of dosing software and consecutive TDM increases therapeutic drug exposure of piperacillin in patients with sepsis and septic shock [68]. Additionally, in 12% of patients with excessive and potentially toxic piperacillin concentrations, subsequent TDM-guided dosing adjustments enabled the adequate reduction of piperacillin dosing and exposure [68]. In this subtherapeutic group, higher mortality was observed compared to the therapeutic group for comparable severity scores. Even though no strong conclusion may be drawn based on these clinical outcome findings, these results confirm the need for dose BL dose optimization with two objectives: achieving therapeutic exposure and avoiding potentially harmful BL concentrations.
However, the DOLPHIN trial, a recent multicenter trial comparing BL TDM in 388 adult ICUs using MIPD and pharmacometrics modeling to TDM according to usual care, did not improve outcomes such as length of stay or mortality [69]. Unexpectedly, the rate of target attainment in the MIPD was quite low (ranging from 55.6% to 71.4%) [70]. This study highlights some important considerations while using MIPD dose optimization:
- -
- First, the importance of early, appropriate and adequately dosed empiric antimicrobials as TDM can only be applied after empiric antimicrobial dose selection, and initial dosing may be more predictive of meaningful outcomes than TDM assisted maintenance dosing only.
- -
- Second, the choice of the dosing software and the PK models is a key determinant for antimicrobial MIPD-guided optimization success. The PK/PD dosing software used in the DOLPHIN trial—InsightRx®- although registered as a CE-labeled medical device with published embedded PK models, failed to predict adequate BL and ciprofloxacin doses in ICU patients. As previously reported, the external evaluation of published population PK models may lead to poor predictive performance when applying to a cohort of ICU patients different from the one used to develop the PK models as shown for meropenem [71,72]. Thus, it is necessary to perform a fit-for-purpose evaluation of the models to assess their predictive performance in the MIPD setting they are intended to be used before any implementation.
- -
- Third, only 61% of patients in the MIPD group had a second TDM sampling. As duration of therapy was short (median duration of therapy: 4 days in MIPD group vs. 3.5 in standard dosing group) together with delayed dosing optimization due to informed consent, the benefit of MIPD-guided dosing on clinical outcomes may be limited by a restricted interventional period.
5.3. Barriers to the Widespread Use of Dosing Software That Need to Be Overcome
Before MIPD becomes common clinical reality, several issues must be addressed [73].
- -
- First, in the near future, the regulatory framework for antimicrobial dosing software tools needs to be reinforced. Software developers may be required to register their dosing software with relevant regulatory bodies before health services are able to incorporate the technology.
- -
- Second, the difficulty of MIPD use for untrained healthcare providers questions the integration of specialized pharmacists or pharmacologists into clinical teams. Indeed, to drive adoption of these tools in clinical practice, it is essential to provide proper education of the intended end-user. The lack of dedicated time for practitioners to use these tools on a larger scale together with a lack of knowledge about the reliability of software outputs, as well as understanding of how the software works, have been identified as influencing trust in dose-prediction software [74]. Implementation of MIPD into dosing advisory service deserves consideration to facilitate translation of Bayesian forecasting dosing recommendations into clinical practice as already demonstrated for vancomycin [74].
- -
- Third, the ability to integrate dose-prediction software within existing hospital electronic medication management systems helps minimizing the need for prescribers to input data and is a key aspect considered by prescribers when deciding to accept software. Providing clinicians with quality-assured user-friendly decision support tools available in application form for personal mobile devices, integrated into electronic hospital record (EHR) prescribing software, is of paramount importance for the widespread implementation of MIPD.
- -
- Fourth, cost-effectiveness of using BL dosing software must be demonstrated. Precision dosing may require additional costs initially for analysis of drug concentration or other biomarkers that provide information necessary for optimal dose selection. These analyses, though theoretically cost-effective, may require a learning curve for clinicians before expenditures are reduced in clinical practice. Another cost associated with precision dosing is the integration of drug dosing software into EHRs. Although favorable cost outcomes from using dosing software for non-BL antibiotics have been reported, the DOLPHIN trial found incremental costs of EUR 5312 with an average decrease in 6 months QALY of 0.03 (range −0.5 to 0.5) in MIPD compared to the standard dosing [69,75].
6. Beta-Lactam Therapeutic Drug Monitoring for the Critically Ill Patient: Time to Focus on Rationale of TDM Use in Special ICU Populations?
Clinical awareness that dosing requires fine-tuning to achieve the desired effects has grown in special critically ill populations.
Obese patients are sometimes excluded from the screening phase, even though these are patients for whom the optimization of drug doses is challenging on a daily basis, especially since their prevalence is increasing dramatically [76]. Therapeutic failures in obese patients are common and responsible for longer hospital stays even in less serious patients [77]. PK variability in obese patients is due to alterations in Vd and clearance resulting from increased adipose tissue and lean muscle mass. A study focusing on piperacillin-tazobactam TDM in severely obese ICU patients emphasized the increased risk of underexposure and high interindividual variability in this population [78].
Renal failure leads to a risk of toxicity by overdosing, proving the interest of anticipating increasing serum concentrations [47]. Although there is extremely high variability of BL exposure in patients with renal failure or those undergoing RRT supports TDM and dose individualization, few studies have investigated whether TDM and subsequent dose adjustment could reduce such variability and improve BL exposure [79]. To date, no randomized study assessing TDM interventions in patients undergoing RRT has been conducted, and the optimal dose adjustment approach (empirical dosing, nomograms or dosing software) remains to be determined. MIPD dosing and innovative approaches such as the integrated dialysis pharmacometrics model that incorporates all RRT samples (pre- and post-filter plasma and effluent), RRT types and settings and drug physiochemical properties to provide quantitative insight into drug clearance in patients receiving RRT should be considered in future studies [80].
On the other hand, critically ill patients exhibiting augmented renal clearance defined as CrCl above 130 mL/min/1.73 m2 are more likely to experience BL underexposure potentially leading to clinical failure when BL dose are not adjusted [81]. A single-center prospective study focusing on unbound BL concentrations in ICU patients highlighted that one third of dosing regimens required a dose increase to achieve therapeutic exposure and showed an increased risk of failure in patients with ARC (OR 2.47) [21,82]. In 215 septic patients with ARC (median CrCl = 178 mL/min), Jacobs et al. found that subtherapeutic concentrations of BL varied from 36% of the cohort when considering Enterobacterales breakpoint up to 55% for Pseudomonas aeruginosa. The proportion of inadequate BL plasma concentrations significantly increases (up to 100% for piperacillin) in the upper range of measured CrCl (240–300 mL/min). However, as weak correlations between measured CrCl and BL plasma concentrations and between measured CrCl and BL clearances were observed in this study, dosing adjustments based on measured CrCl only are unlikely to achieve PK/PD targets. Under these circumstances, TDM should be strongly encouraged to guide BL drug dosing as off-label dosing is often necessary to achieve therapeutic concentrations. Interestingly, the package insert of recently released antibiotics integrates ARC dosing recommendations, as seen for cefiderocol. MIPD may also be valuable in this population but has not been investigated yet. Post hoc analyses of the DOLPHIN trial are currently underway in such population and may inform future trials.
Finally, another challenging situation for BL dosing optimization is infection with less susceptible pathogens requiring higher BL exposure to achieve PK/PD targets. In a recent single center retrospective study, meropenem dose optimization was successfully achieved by dosing adjustment based on real-time TDM for infections caused by KPC with a meropenem MIC ≤ 64 mg/L [83]. Besides, as rapid antimicrobial susceptibility testing is currently being developed, TDM-based dosing adjustments considering true and rapidly available MICs may further improve BL therapy at the early phase of infections with difficult-to-treat pathogens.
7. Future Perspectives
Further well-designed prospective clinical trials will be required to determine the benefit of precision dosing and will be crucial to put the effort of TDM into cost effectiveness. In the near future, a multicenter randomized controlled superiority trial aiming to assess the effectiveness and safety of data driven automated antimicrobial dosing advice and a study combining the use of a Bayesian model with rapid molecular diagnostic test in order to offer an optimal personalized care will provide new insights into optimized TDM and MIPD benefit [84,85].
Artificial intelligence (AI) embedded into dosing software is a promising strategy [86]. AI software uses reinforcement learning to provide recommendations on dose adaptation. AI software analyzes large patient databases to determine interventions that may influence patient outcome. AI algorithms and machine learning allow to continuously refine existing models based on the specific population, thus maximizing the probability to achieve PK/PD target.
8. Conclusions
Beta-lactam dosing optimization is a cornerstone of critically ill patient management. Therapeutic drug monitoring of BL antibiotics and the ability to individualize dosing regimens remains a promising approach with great potential to impact selective patient populations at high risk of inadequate BL exposure. For several reasons, current studies evaluating BL dosing optimization fail to demonstrate a decline in mortality. The combination of Beta-lactam dosing optimization with rapid molecular diagnostic testing to offer an optimal personalized care could be an interesting approach when designing further studies. The benefit of MIPD remains to be determined from safety and cost point of views. In the meantime, efforts must be pursued on an available, simple-to-operate and cost-effective TDM program that provides a short turnaround time in combination with reliable and easy-to-interpret results in the critical care setting.
Author Contributions
E.N., H.M. and C.R. reviewed the available literature, draft, review and edit the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest for this work.
References
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Lizza, B.D.; Raush, N.; Micek, S.T. Antibiotic Optimization in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2022, 43, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Lipman, J.; Akova, M.; Bassetti, M.; Dimopoulos, G.; Kaukonen, M.; Koulenti, D.; Martin, C.; Montravers, P.; Rello, J.; et al. Risk factors for target non-attainment during empirical treatment with beta-lactam antibiotics in critically ill patients. Intensive Care Med. 2014, 40, 1340–1351. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Valenza, G.; Seifert, H.; Decker-Burgard, S.; Laeuffer, J.; Morrissey, I.; Mutters, R. Comparative Activity of Carbapenem Testing (COMPACT) study in Germany. Int. J. Antimicrob. Agents 2012, 39, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.-H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Watson, I.; Potter, J.; Yatscoff, R.; Fraser, A.; Himberg, J.J.; Wenk, M. Editorial. Ther. Drug Monit. 1997, 19, 125. [Google Scholar] [CrossRef]
- Jager, N.G.L.; van Hest, R.M.; Lipman, J.; Roberts, J.A.; Cotta, M.O. Antibiotic exposure at the site of infection: Principles and assessment of tissue penetration. Expert Rev. Clin. Pharmacol. 2019, 12, 623–634. [Google Scholar] [CrossRef]
- Reeves, D.; Lovering, A.; Thomson, A. Therapeutic drug monitoring in the past 40 years of the Journal of Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2016, 71, 3330–3332. [Google Scholar] [CrossRef]
- Wenk, M.; Vožeh, S.; Follath, F. Serum Level Monitoring of Antibacterial Drugs. A review. Clin. Pharmacokinet. 1984, 9, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Begg, E.J.; Barclay, M.L.; Kirkpatrick, C.M. The therapeutic monitoring of antimicrobial agents. Br. J. Clin. Pharmacol. 2001, 52 (Suppl. S1), 35S–43S. [Google Scholar]
- Charmillon, A.; Novy, E.; Agrinier, N.; Leone, M.; Kimmoun, A.; Levy, B.; Demoré, B.; Dellamonica, J.; Pulcini, C. The ANTIBIOPERF study: A nationwide cross-sectional survey about practices for beta-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin. Microbiol. Infect. 2016, 22, 625–631. [Google Scholar] [CrossRef]
- Imani, S.; Alffenaar, J.-W.; Cotta, M.O.; Daveson, K.; van Hal, S.; Lau, C.; Marriott, D.; Penm, J.; Roberts, J.A.; Tabah, A.; et al. Therapeutic drug monitoring of commonly used anti-infective agents: A nationwide cross-sectional survey of Australian hospital practices. Int. J. Antimicrob. Agents 2020, 56, 106180. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.G.; Fernandes, J.; Duarte, A.R.; Fernandes, S.M. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics 2022, 11, 1839. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Pea, F.; Lipman, J. The Clinical Relevance of Plasma Protein Binding Changes. Clin. Pharmacokinet. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef]
- Tritscher, P.; Delannoy, M.; Agrinier, N.; Charmillon, A.; Degand, N.; Dellamonica, J.; Roger, C.; Leone, M.; Scala-Bertola, J.; Novy, E. Assessment of current practice for beta-lactam therapeutic drug monitoring in French ICUs in 2021: A nationwide cross-sectional survey. J. Antimicrob. Chemother. 2022, 77, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit Care 2019, 23, 104. [Google Scholar] [PubMed]
- Aitken, S.; Altshuler, J.; Guervil, D.J.; Hirsch, E.B.; Ostrosky-Zeichner, L.L.; Ericsson, C.D.; Tam, V.H. Cefepime free minimum concentration to minimum inhibitory concentration (fCmin/MIC) ratio predicts clinical failure in patients with Gram-negative bacterial pneumonia. Int. J. Antimicrob. Agents 2015, 45, 541–544. [Google Scholar] [CrossRef]
- Abdul–Aziz, M.H.; Brady, K.; Cotta, M.O.; Roberts, J.A. Therapeutic Drug Monitoring of Antibiotics: Defining the Therapeutic Range. Ther. Drug Monit. 2021, 44, 19–31. [Google Scholar] [CrossRef]
- Wong, G.; Taccone, F.; Villois, P.; Scheetz, M.H.; Rhodes, N.J.; Briscoe, S.; McWhinney, B.; Nunez-Nunez, M.; Ungerer, J.; Lipman, J.; et al. β-Lactam pharmacodynamics in Gram-negative bloodstream infections in the critically ill. J. Antimicrob. Chemother. 2020, 75, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Dilworth, T.J.; Schulz, L.T.; Micek, S.T.; Kollef, M.H.; Rose, W.E. beta-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: Weighing the Challenges and Opportunities to Assess Clinical Value. Crit Care Explor. 2022, 4, e0726. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Fratoni, A.J.; Nicolau, D.P.; Kuti, J.L. A guide to therapeutic drug monitoring of beta-lactam antibiotics. Pharmacotherapy 2021, 41, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Luxton, T.; King, N.; Wälti, C.; Jeuken, L.; Sandoe, J. A systematic review of the effect of therapeutic drug monitoring on patient health outcomes during treatment with penicillins. J. Antimicrob. Chemother. 2022, 77, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Pai Mangalore, R.; Ashok, A.; Lee, S.J.; Romero, L.; Peel, T.N.; Udy, A.A.; Peleg, A.Y. Beta-Lactam Antibiotic Therapeutic Drug Monitoring in Critically Ill Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Roberts, M.S.; Tiong, I.S.; Gardner, J.H.; Lehman, S.; Peake, S.L.; Hahn, U.; Warner, M.S.; Roberts, J.A. Can therapeutic drug monitoring optimize exposure to piperacillin in febrile neutropenic patients with haematological malignancies? A randomized controlled trial. J. Antimicrob. Chemother. 2015, 70, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Eggimann, P.; Pantet, O.; Pagani, J.L.; Dupuis-Lozeron, E.; Pannatier, A.; Sadeghipour, F.; Voirol, P.; Que, Y.-A. Impact of Real-Time Therapeutic Drug Monitoring on the Prescription of Antibiotics in Burn Patients Requiring Admission to the Intensive Care Unit. Antimicrob. Agents Chemother. 2018, 62, e01818-17. [Google Scholar] [CrossRef]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef]
- Guo, B.; Abdelraouf, K.; Ledesma, K.R.; Chang, K.-T.; Nikolaou, M.; Tam, V.H. Quantitative Impact of Neutrophils on Bacterial Clearance in a Murine Pneumonia Model. Antimicrob. Agents Chemother. 2011, 55, 4601–4605. [Google Scholar] [CrossRef]
- Ortwine, J.K.; Pogue, J.M.; Faris, J. Pharmacokinetics and pharmacodynamics of antibacterial and antifungal agents in adult patients with thermal injury: A review of current literature. J. Burn Care Res. 2015, 36, e72–e84. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef]
- Leon, L.; Guerci, P.; Pape, E.; Thilly, N.; Luc, A.; Germain, A.; Butin-Druoton, A.-L.; Losser, M.-R.; Birckener, J.; Scala-Bertola, J.; et al. Serum and peritoneal exudate concentrations after high doses of beta-lactams in critically ill patients with severe intra-abdominal infections: An observational prospective study. J. Antimicrob. Chemother. 2020, 75, 156–161. [Google Scholar] [CrossRef]
- Mouton, J.W.; Meletiadis, J.; Voss, A.; Turnidge, J. Variation of MIC measurements: The contribution of strain and laboratory variability to measurement precision. J. Antimicrob. Chemother. 2018, 73, 2374–2379. [Google Scholar] [CrossRef]
- Magreault, S.; Jaureguy, F.; Carbonnelle, E.; Zahar, J.R. When and How to Use MIC in Clinical Practice? Antibiotics 2022, 11, 1748. [Google Scholar] [CrossRef]
- Monogue, M.L.; Nicolau, D.P. Pharmacokinetics-pharmacodynamics of beta-lactamase inhibitors: Are we missing the target? Expert Rev. Anti-Infect. Ther. 2019, 17, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Egea, A.; Dupuis, C.; de Montmollin, E.; Wicky, P.H.; Patrier, J.; Jaquet, P.; Lefèvre, L.; Sinnah, F.; Marzouk, M.; Sonneville, R.; et al. Augmented renal clearance in the ICU: Estimation, incidence, risk factors and consequences-a retrospective observational study. Ann Intensive Care. 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Neves, M.; Rodrigues, L.; Teixeira, L.; Pinho, J.; Pimentel, J. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J. Nephrol. 2014, 27, 403–410. [Google Scholar] [CrossRef]
- Grootaert, V.; Willems, L.; Debaveye, Y.; Meyfroidt, G.; Spriet, I. Augmented Renal Clearance in the Critically Ill: How to Assess Kidney Function. Ann. Pharmacother. 2012, 46, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef]
- Neely, M.N.; Kato, L.; Youn, G.; Kraler, L.; Bayard, D.; van Guilder, M.; Schumitzky, A.; Yamada, W.; Jones, B.; Minejima, E. Prospective Trial on the Use of Trough Concentration versus Area under the Curve to Determine Therapeutic Vancomycin Dosing. Antimicrob. Agents Chemother. 2018, 62, e02042-17. [Google Scholar] [CrossRef]
- McDonald, C.; Cotta, M.O.; Little, P.J.; McWhinney, B.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Is high-dose beta-lactam therapy associated with excessive drug toxicity in critically ill patients? Minerva Anestesiol. 2016, 82, 957–965. [Google Scholar]
- Lamoth, F.; Buclin, T.; Pascual, A.; Vora, S.; Bolay, S.; Decosterd, L.A.; Calandra, T.; Marchetti, O. High Cefepime Plasma Concentrations and Neurological Toxicity in Febrile Neutropenic Patients with Mild Impairment of Renal Function. Antimicrob. Agents Chemother. 2010, 54, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Huwyler, T.; Lenggenhager, L.; Abbas, M.; Lorenzini, K.I.; Hughes, S.; Huttner, B.; Karmime, A.; Uçkay, I.; von Dach, E.; Lescuyer, P.; et al. Cefepime plasma concentrations and clinical toxicity: A retrospective cohort study. Clin. Microbiol. Infect. 2017, 23, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of beta-lactam concentration-toxicity relationships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Quinton, M.-C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.-S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e00654-17. [Google Scholar] [CrossRef]
- Beumier, M.; Casu, G.S.; Hites, M.; Wolff, F.; Cotton, F.; Vincent, J.L.; Jacobs, F.; Taccone, F.S. Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015, 81, 497–506. [Google Scholar]
- Blondiaux, N.; Wallet, F.; Favory, R.; Onimus, T.; Nseir, S.; Courcol, R.J.; Durocher, A.; Roussel-Delvallez, M. Daily serum piperacillin monitoring is advisable in critically ill patients. Int. J. Antimicrob. Agents 2010, 35, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Csajka, C.; Thoma, Y.; Buclin, T.; Widmer, N. Benchmarking Therapeutic Drug Monitoring Software: A Review of Available Computer Tools. Clin. Pharmacokinet. 2012, 52, 9–22. [Google Scholar] [CrossRef]
- Felton, T.W.; Roberts, J.A.; Lodise, T.P.; Van Guilder, M.; Boselli, E.; Neely, M.N.; Hope, W.W. Individualization of Piperacillin Dosing for Critically Ill Patients: Dosing Software to Optimize Antimicrobial Therapy. Antimicrob. Agents Chemother. 2014, 58, 4094–4102. [Google Scholar] [CrossRef]
- Neely, M.; Margol, A.; Fu, X.; van Guilder, M.; Bayard, D.; Schumitzky, A.; Orbach, R.; Liu, S.; Louie, S.; Hope, W. Achieving Target Voriconazole Concentrations More Accurately in Children and Adolescents. Antimicrob. Agents Chemother. 2015, 59, 3090–3097. [Google Scholar] [CrossRef]
- Hope, W.W.; VanGuilder, M.; Donnelly, J.P.; Blijlevens, N.M.A.; Brüggemann, R.J.M.; Jelliffe, R.W.; Neely, M.N. Software for Dosage Individualization of Voriconazole for Immunocompromised Patients. Antimicrob. Agents Chemother. 2013, 57, 1888–1894. [Google Scholar] [CrossRef]
- Bel Kamel, A.; Bourguignon, L.; Marcos, M.; Ducher, M.; Goutelle, S. Is Trough Concentration of Vancomycin Predictive of the Area Under the Curve? A Clinical Study in Elderly Patients. Ther. Drug Monit. 2017, 39, 83–87. [Google Scholar] [CrossRef]
- Mathew, S.K.; Mathew, B.S.; Neely, M.N.; Naik, G.S.; Prabha, R.; Jacob, G.G.; K, S.; Fleming, D.H. A Nonparametric Pharmacokinetic Approach to Determine the Optimal Dosing Regimen for 30-Minute and 3-Hour Meropenem Infusions in Critically Ill Patients. Ther. Drug Monit. 2016, 38, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Avent, M.L.; Rogers, B.A.; Cheng, A.C.; Paterson, D.L. Current use of aminoglycosides: Indications, pharmacokinetics and monitoring for toxicity. Intern. Med. J. 2011, 41, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Barras, M.A.; Serisier, D.; Hennig, S.; Jess, K.; Norris, R.L.G. Bayesian Estimation of Tobramycin Exposure in Patients with Cystic Fibrosis. Antimicrob. Agents Chemother. 2016, 60, 6698–6702. [Google Scholar] [CrossRef]
- Donagher, J.; Martin, J.H.; Barras, M.A. Individualised medicine: Why we need Bayesian dosing. Intern. Med. J. 2017, 47, 593–600. [Google Scholar] [CrossRef]
- Duffull, S.; Kirkpatrick, C.; Begg, E.J. Comparison of two Bayesian approaches to dose-individualization for once-daily aminoglycoside regimens. Br. J. Clin. Pharmacol. 1997, 43, 125–135. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oda, K.; Shoji, K.; Hanai, Y.; Takahashi, Y.; Fujii, S.; Hamada, Y.; Kimura, T.; Mayumi, T.; Ueda, T.; et al. Clinical Practice Guidelines for Therapeutic Drug Monitoring of Vancomycin in the Framework of Model-Informed Precision Dosing: A Consensus Review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Pharmaceutics 2022, 14, 489. [Google Scholar] [CrossRef]
- Heil, E.L.; Nicolau, D.P.; Farkas, A.; Roberts, J.A.; Thom, K.A. Pharmacodynamic Target Attainment for Cefepime, Meropenem, and Piperacillin-Tazobactam Using a Pharmacokinetic/Pharmacodynamic-Based Dosing Calculator in Critically Ill Patients. Antimicrob. Agents Chemother. 2018, 62, e01008-18. [Google Scholar] [CrossRef]
- Chiriac, U.; Richter, D.; Frey, O.; Röhr, A.; Helbig, S.; Preisenberger, J.; Hagel, S.; Roberts, J.; Weigand, M.; Brinkmann, A. Personalized Piperacillin Dosing for the Critically Ill: A Retrospective Analysis of Clinical Experience with Dosing Software and Therapeutic Drug Monitoring to Optimize Antimicrobial Dosing. Antibiotics 2021, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Muller, A.E.; de Winter, B.C.M.; Hunfeld, N.G.M.; Purmer, I.M.; van Vliet, P.; Wils, E.-J.; Haringman, J.; et al. Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: A multicentre randomised clinical trial. Intensive Care Med. 2022, 48, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.O.; Lipman, J.; De Waele, J. Advancing precision-based antimicrobial dosing in critically ill patients. Intensive Care Med. 2023, 49, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guilhaumou, R.; Blin, O.; Velly, L.; Marsot, A. External evaluation of population pharmacokinetic models for continuous administration of meropenem in critically ill adult patients. Eur. J. Clin. Pharmacol. 2020, 76, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Dhaese, S.A.M.; Farkas, A.; Colin, P.; Lipman, J.; Stove, V.; Verstraete, A.G.; Roberts, J.A.; De Waele, J.J. Population pharmacokinetics and evaluation of the predictive performance of pharmacokinetic models in critically ill patients receiving continuous infusion meropenem: A comparison of eight pharmacokinetic models. J. Antimicrob. Chemother. 2018, 74, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.S.; von Moltke, L. The Impact of Formulation, Delivery, and Dosing Regimen on the Risk of Drug-Drug Interactions. Clin. Pharmacol. Ther. 2019, 105, 1329–1331. [Google Scholar] [CrossRef] [PubMed]
- Stocker, S.L.; Carland, J.E.; Reuter, S.E.; Stacy, A.E.; Schaffer, A.L.; Stefani, M.; Lau, C.; Kirubakaran, R.; Yang, J.J.; Shen, C.F.; et al. Evaluation of a Pilot Vancomycin Precision Dosing Advisory Service on Target Exposure Attainment Using an Interrupted Time Series Analysis. Clin. Pharmacol. Ther. 2020, 109, 212–221. [Google Scholar] [CrossRef]
- van Lent-Evers, N.A.; Mathot, R.A.; Geus, W.P.; van Hout, B.A.; Vinks, A.A. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: A cost-effectiveness analysis. Ther. Drug Monit. 1999, 21, 63–73. [Google Scholar] [CrossRef]
- Meng, L.; Mui, E.; Holubar, M.K.; Deresinski, S.C. Comprehensive Guidance for Antibiotic Dosing in Obese Adults. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 1415–1431. [Google Scholar] [CrossRef]
- Pinner, N.A.; Tapley, N.G.; Barber, K.E.; Stover, K.R.; Wagner, J.L. Effect of Obesity on Clinical Failure of Patients Treated With beta-Lactams. Open Forum Infect. Dis. 2021, 8, ofab212. [Google Scholar] [CrossRef]
- Jung, B.; Mahul, M.; Breilh, D.; Legeron, R.; Signe, J.; Jean-Pierre, H.; Uhlemann, A.-C.; Molinari, N.; Jaber, S. Repeated Piperacillin-Tazobactam Plasma Concentration Measurements in Severely Obese Versus Nonobese Critically Ill Septic Patients and the Risk of Under– and Overdosing*. Crit. Care Med. 2017, 45, e470–e478. [Google Scholar] [CrossRef]
- Matusik, E.; Boidin, C.P.; Friggeri, A.; Richard, J.-C.; Bitker, L.; Roberts, J.A.; Goutelle, S.P. Therapeutic Drug Monitoring of Antibiotic Drugs in Patients Receiving Continuous Renal Replacement Therapy or Intermittent Hemodialysis: A Critical Review. Ther. Drug Monit. 2021, 44, 86–102. [Google Scholar] [CrossRef]
- Broeker, A.; Vossen, M.G.; Thalhammer, F.; Wallis, S.C.; Lipman, J.; Roberts, J.A.; Wicha, S.G. An Integrated Dialysis Pharmacometric (IDP) Model to Evaluate the Pharmacokinetics in Patients Undergoing Renal Replacement Therapy. Pharm. Res. 2020, 37, 96. [Google Scholar] [CrossRef]
- Carrie, C.; Chadefaux, G.; Sauvage, N.; de Courson, H.; Petit, L.; Nouette-Gaulain, K.; Pereira, B.; Biais, M. Increased beta-Lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: A before and after study. Crit Care. 2019, 23, 379. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Taccone, F.S.; Roberts, J.A.; Jacobs, F.; Cotton, F.; Wolff, F.; Creteur, J.; Vincent, J.-L.; Hites, M. β-Lactam Dosage Regimens in Septic Patients with Augmented Renal Clearance. Antimicrob. Agents Chemother. 2018, 62, e02534-17. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Della Siega, P.; Cojutti, P.; Sartor, A.; Crapis, M.; Scarparo, C.; Bassetti, M. Might real-time pharmacokinetic/pharmacodynamic optimisation of high-dose continuous-infusion meropenem improve clinical cure in infections caused by KPC-producing Klebsiella pneumoniae? Int. J. Antimicrob. Agents 2017, 49, 255–258. [Google Scholar] [CrossRef]
- Roggeveen, L.F.; Fleuren, L.M.; Guo, T.; Thoral, P.; De Grooth, H.J.; Swart, E.L.; Klausch, T.L.T.; Van Der Voort, P.H.J.; Girbes, A.R.J.; Bosman, R.J.; et al. Right Dose Right Now: Bedside data-driven personalized antibiotic dosing in severe sepsis and septic shock—Rationale and design of a multicenter randomized controlled superiority trial. Trials 2019, 20, 745. [Google Scholar] [CrossRef] [PubMed]
- Irwin, A.D.; Coin, L.J.M.; Harris, P.N.A.; Cotta, M.O.; Bauer, M.J.; Buckley, C.; Balch, R.; Kruger, P.; Meyer, J.; Shekar, K.; et al. Optimising Treatment Outcomes for Children and Adults Through Rapid Genome Sequencing of Sepsis Pathogens. A Study Protocol for a Prospective, Multi-Centre Trial (DIRECT). Front. Cell. Infect. Microbiol. 2021, 11, 667680. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, M.; Celi, L.A.; Badawi, O.; Gordon, A.C.; Faisal, A.A. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat. Med. 2018, 24, 1716–1720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).