Vancomycin Sequestration in ST Filters: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Stability Studies
2.1.1. In the Sampling Tubes
2.1.2. In the Reservoir (Central Compartment)
2.2. Actual Flow Rate Provided by the Dialyzer
2.3. Elimination from the Central Compartment (CC)
2.3.1. In the Continuous Diafiltration Mode
2.3.2. In the Continuous Dialysis CD Mode
2.4. Summary of Results
3. Discussion
4. Materials and Methods
4.1. Vancomycin Preparation
4.2. Stability Study
4.3. Measurements of Vancomycin Concentrations
4.4. Filter and Dialyzer Device
4.5. Modes of Exposure of the Filter to Vancomycin
4.6. Mode of CRRT
4.7. Samplings
4.8. Assessment of the Routes of Elimination
4.9. Actual Flow Rate Provided by Prismaflex Dialyzer
4.10. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Q.; Gomersall, C.D.; Leung, P.P.; Choi, G.Y.; Joynt, G.M.; Tan, P.E.; Wong, A.S. The adsorption of vancomycin by polyacrylonitrile, polyamide, and polysulfone hemofilters. Artif. Organs 2008, 32, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; Redant, S.; Djimafo, P.; Preseau, T.; Cismas, B.V.; Kaefer, K.; Barreto Gutierrez, L.; Anane, S.; Attou, R.; Gallerani, A.; et al. Membrane adsorption in vancomycin treatment is membrane type dependent in CVVHDF: Dose correction is crucial. Crit. Care 2022, 26, 117. [Google Scholar] [CrossRef]
- Matzke, G.R.; O’Connell, M.B.; Collins, A.J.; Keshaviah, P.R. Disposition of vancomycin during hemofiltration. Clin. Pharmacol. Ther. 1986, 40, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Anandan, J.V.; Touchette, M.A. Vancomycin clearance with high-flux dialysis membranes. Int. J. Artif. Organs. 1998, 21, 131–133. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Xia, Y.; Chu, Y.; Zhong, H.; Li, J.; Liang, P.; Bu, Y.; Zhao, R.; Liao, Y.; et al. Recommendation of Antimicrobial Dosing Optimization During Continuous Renal Replacement Therapy. Front. Pharmacol. 2020, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Schaedeli, F.; Uehlinger, D.E. Urea kinetics and dialysis treatment time predict vancomycin elimination during high-flux hemodialysis. Clin. Pharmacol. Ther. 1998, 63, 26–38. [Google Scholar] [CrossRef]
- Quale, J.M.; O’Halloran, J.J.; DeVincenzo, N.; Barth, R.H. Removal of vancomycin by high-flux hemodialysis membranes. Antimicrob. Agents Chemother. 1992, 36, 1424–1426. [Google Scholar] [CrossRef] [PubMed]
- Baud, F.J.; Houze, P.; Carli, P.; Lamhaut, L. Alteration of the pharmacokinetics of aminoglycosides by adsorption in a filter during continuous renal replacement therapy. An in vitro assessment. Therapie 2020, 76, 415–424. [Google Scholar] [CrossRef]
- Baud, F.J.; Houze, P.; Raphalen, J.H.; Winchenne, A.; Philippe, P.; Carli, P.; Lamhaut, L. Diafiltration flowrate is a determinant of the extent of adsorption of amikacin in renal replacement therapy using the ST150((R))-AN69 filter: An in vitro study. Int. J. Artif. Organs. 2020, 43, 758–766. [Google Scholar] [CrossRef]
- Baud, F.J.; Jullien, V.; Abarou, T.; Pilmis, B.; Raphalen, J.H.; Houze, P.; Carli, P.; Lamhaut, L. Elimination of fluconazole during continuous renal replacement therapy. An in vitro assessment. Int. J. Artif. Organs. 2021, 44, 453–464. [Google Scholar] [CrossRef]
- Pinner, N.A.; Canada, R.B.; Broyles, J.E.; Hudson, J.Q. Evaluation of vancomycin and gentamicin dialysis clearance using in vivo and in vitro systems. Ren. Fail 2012, 34, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.M.; Cardone, K.E.; Nolin, T.D.; Mason, D.L.; Grabe, D.W. Determination of vancomycin and gentamicin clearance in an in vitro, closed loop dialysis system. BMC Nephrol. 2014, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Onichimowski, D.; Ziolkowski, H.; Nosek, K.; Jaroszewski, J.; Rypulak, E.; Czuczwar, M. Comparison of adsorption of selected antibiotics on the filters in continuous renal replacement therapy circuits: In vitro studies. J. Artif. Organs. 2020, 23, 163–170. [Google Scholar] [CrossRef]
- Onichimowski, D.; Nosek, K.; Ziolkowski, H.; Jaroszewski, J.; Pawlos, A.; Czuczwar, M. Adsorption of vancomycin, gentamycin, ciprofloxacin and tygecycline on the filters in continuous renal replacement therapy circuits: In full blood in vitro study. J. Artif. Organs. 2020, 24, 65–73. [Google Scholar] [CrossRef]
- Myers, A.L.; Gaedigk, A.; Dai, H.; James, L.P.; Jones, B.L.; Neville, K.A. Defining risk factors for red man syndrome in children and adults. Pediatr. Infect. Dis. J. 2012, 31, 464–468. [Google Scholar] [CrossRef]
- Kronfol, N.O.; Lau, A.H.; Barakat, M.M. Aminoglycoside binding to polyacrylonitrile hemofilter membranes during continuous hemofiltration. ASAIO Trans. 1987, 33, 300–303. [Google Scholar] [PubMed]
- Purohit, P.J.; Elkomy, M.H.; Frymoyer, A.; Sutherland, S.M.; Drover, D.R.; Hammer, G.B.; Su, F. Antimicrobial Disposition During Pediatric Continuous Renal Replacement Therapy Using an Ex Vivo Model. Crit. Care Med. 2019, 47, e767–e773. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.; Tozer, T.N. Distribution. In Clinical Pharmacokinetics. Concepts and Applications, 3rd ed.; Williams & Wilkins: Baltimore, Maryland, 1995; pp. 137–155. [Google Scholar]
- Baud, F.J.; Houze, P. RE to manuscript ‘In vitro removal of anti-infective agents by a novel cytokine adsorbent system’. Int. J. Artif. Organs. 2019, 42, 528–529. [Google Scholar] [CrossRef]
- Baud, F.J.; Houze, P. Should In Vitro and In Vivo Studies on Antimicrobial Agents during Continuous Renal Replacement Therapy Comply with General Principles of Pharmacokinetics? Antimicrob. Agents. Chemother. 2020, 64, e00388-20. [Google Scholar] [CrossRef]
- Kolbinger, P.; Gruber, M.; Roth, G.; Graf, B.M.; Ittner, K.P. Filter Adsorption of Anidulafungin to a Polysulfone-Based Hemofilter During CVVHD In Vitro. Artif. Organs. 2018, 42, 200–207. [Google Scholar] [CrossRef]
- Derendorf, H.; Schmidt, S. Plasma-to-Blood Concentration ratio. In Rowalnd and Tozer’s Clinical Pharamcokinetics and Pharmacodynamics Concepts and Applications, 5th ed.; Derendorf, H., Schmidt, S., Eds.; Wolters Kluwer: Philadelphia, PN, USA, 2020; pp. 775–776. [Google Scholar]
- Emoto, C.; Johnson, T.N.; McPhail, B.T.; Vinks, A.A.; Fukuda, T. Using a Vancomycin PBPK Model in Special Populations to Elucidate Case-Based Clinical PK Observations. CPT Pharmacomet. Syst. Pharm. 2018, 7, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Hariri, G.; Hodjat Panah, K.; Beneteau-Burnat, B.; Chaquin, M.; Mekinian, A. Ait-Oufella H: Carboxyhemoglobin, a reliable diagnosis biomarker for hemolysis in intensive care unit: A retrospective study. Crit. Care 2021, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, S.; Yang, H.; Lee, F.; Wu, J.T.; Qian, M.G. A novel liquid chromatography/tandem mass spectrometry based depletion method for measuring red blood cell partitioning of pharmaceutical compounds in drug discovery. Rapid Commun. Mass. Spectrom. 2005, 19, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Barg, N.L.; Supena, R.B.; Fekety, R. Persistent staphylococcal bacteremia in an intravenous drug abuser. Antimicrob. Agents. Chemother. 1986, 29, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.J.; Davies, J.G.; Olliff, C.J.; Kingswood, C.; Street, M. Use of in vitro models of haemofiltration and haemodiafiltration to estimate dosage regimens for critically ill patients prescribed cefpirome. J. Clin. Pharm. Ther. 1998, 23, 353–359. [Google Scholar] [CrossRef]
- FDA. Bioanalytical Method Validation Guidance for Industry; Administration, F.D., Ed.; Food & Drug Administration: Baltimore, Maryland, 2018. [Google Scholar]
- Houze, P.; Baud, F.J.; Raphalen, J.H.; Winchenne, A.; Moreira, S.; Gault, P.; Carli, P.; Lamhaut, L. Continuous renal replacement therapy in the treatment of severe hyperkalemia: An in vitro study. Int. J. Artif. Organs. 2020, 43, 87–93. [Google Scholar] [CrossRef]
- Houze, P.; Laforge, M.; Baud, F.J. Lactate blood measurement in acute cyanide poisoning: Effect of preanalytical delay and hydroxocobalamin uses as treatment. Ann. Biol. Clin. 2018, 76, 96–103. [Google Scholar] [CrossRef]
- Baud, F.J.; Seif, V.; Houze, P.; Raphalen, J.H.; Pilmis, B.; Carli, P.; Lamhaut, L. Elimination of three doses of gentamicin over three consecutive days using a polyacrylonitrile-derived filter: An in vitro assessment. Int. J. Artif. Organs. 2021, 44, 641–650. [Google Scholar] [CrossRef]
- Neri, M.; Villa, G.; Garzotto, F.; Bagshaw, S.; Bellomo, R.; Cerda, J.; Ferrari, F.; Guggia, S.; Joannidis, M.; Kellum, J.; et al. Nomenclature for renal replacement therapy in acute kidney injury: Basic principles. Crit. Care 2016, 20, 318. [Google Scholar] [CrossRef]
- Rowland, M.; Tozer, T.N. Intravenous dose. In Clinical Pharmacokinetics Concepts and Applications, 3rd ed.; Rowland, M., Tozer, T.N., Eds.; Williams & Wilkins: Baltimore, Maryland, 1995; pp. 18–33. [Google Scholar]
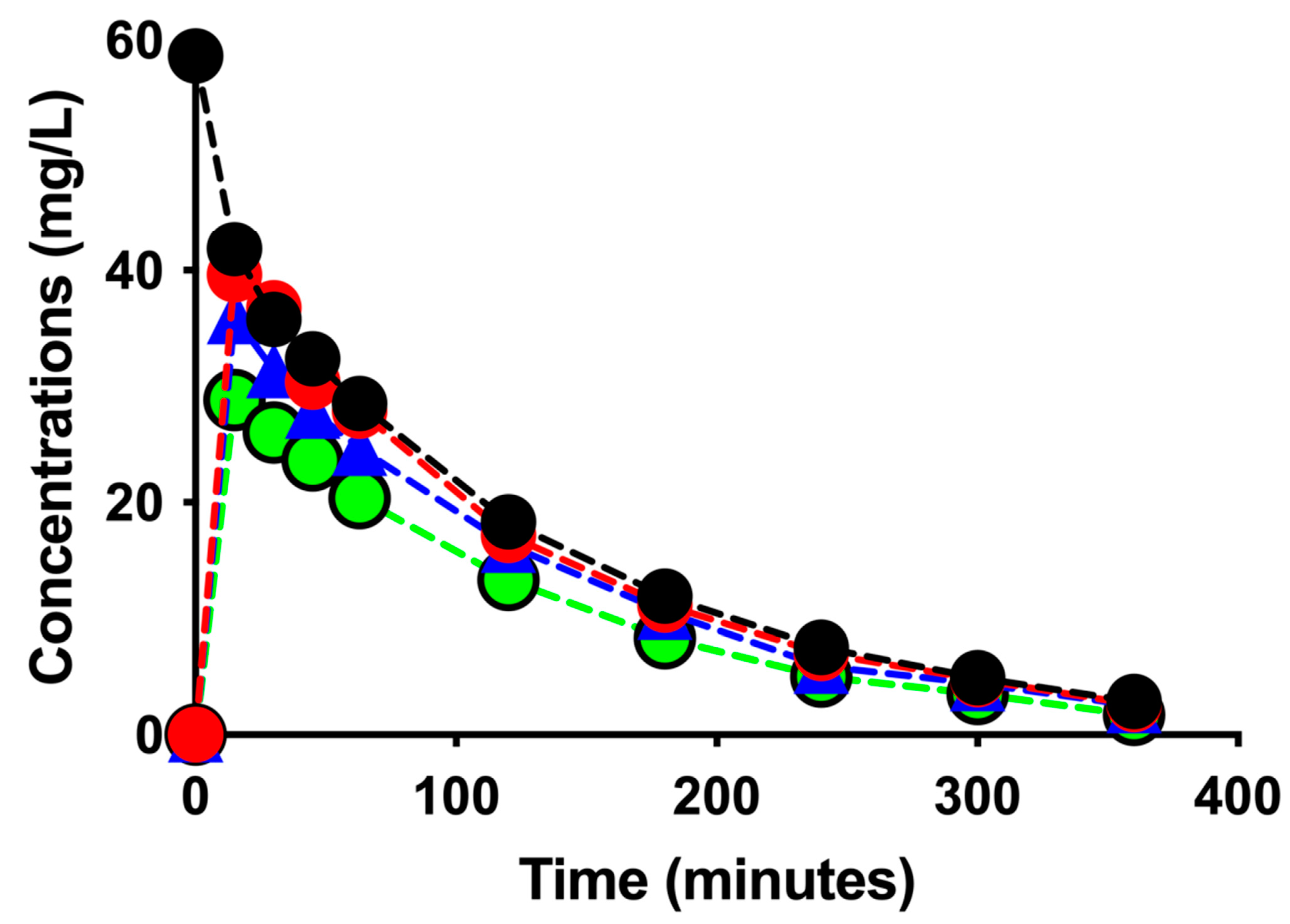
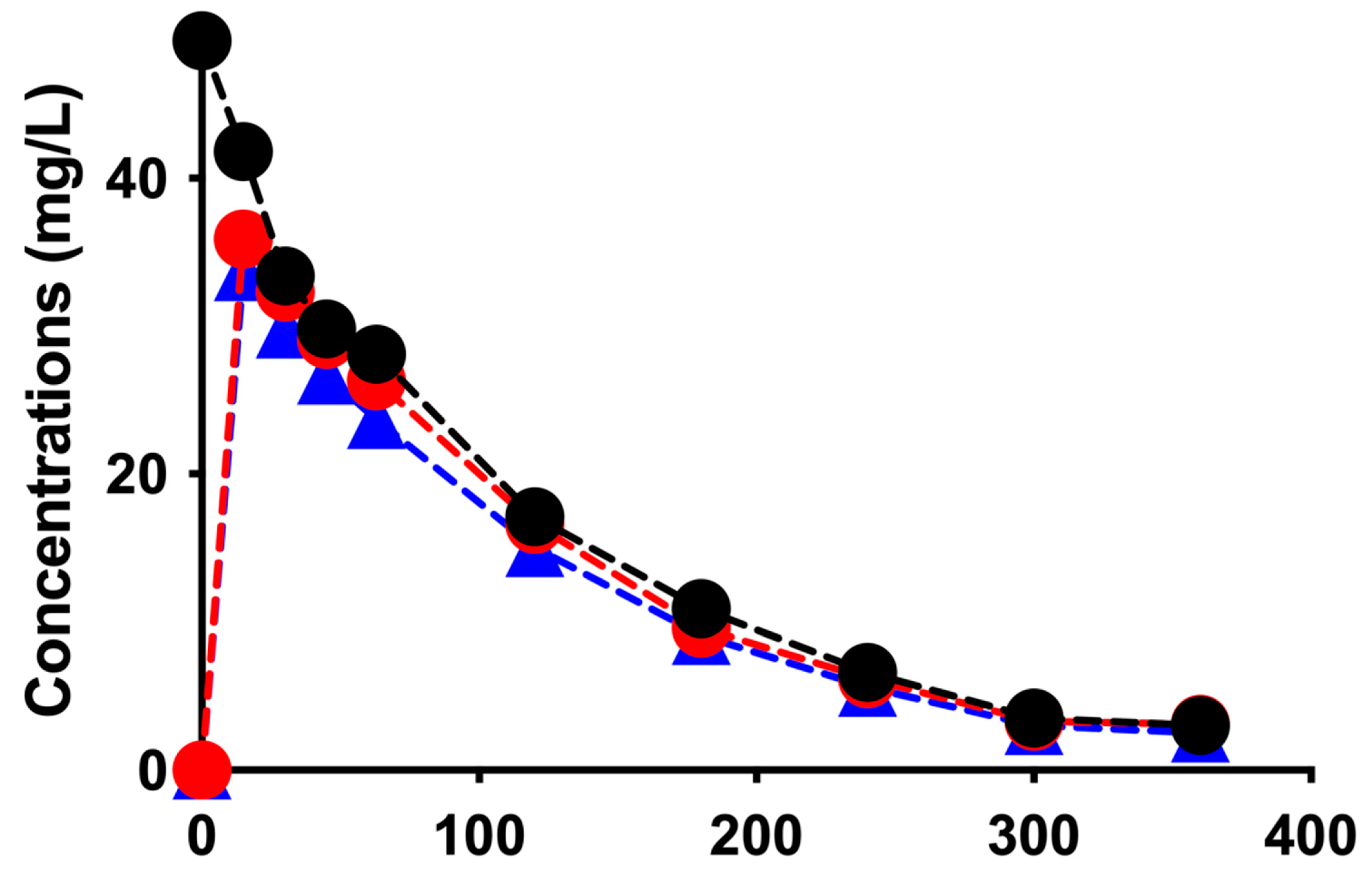
| Sessions/Pharmacokinetic Parameters | CDF * | CD ** | ||
|---|---|---|---|---|
| CDF-1 | CDF-2 | CD-1 | CD-2 | |
| Concentrations in the CC (mg/L): | ||||
| T0 min | 58.6 | 49.3 | 50.7 | 47.0 |
| T360 min | 2.9 | 3.0 | 6.0 | 6.1 |
| AUC0–360 (mg.min/L) | ||||
| CC | 5824 | 5375 | 6968 | 6893 |
| Instantaneous effluents | 3814 | - | 5833 | 5003 |
| Extraction coefficient (%) | 10 +/− 4 | 9 +/− 4 | 12 +/− 3 | 15 +/− 1 |
| % Eliminated from the CC | 95.1 | 93.9 | 86.2 | 87.0 |
| Clearance from CC (L/h) | 2.41 | 2.34 | 2.18 | 2.05 |
| Clearance calculated from AUCefflu instant (L/h) | 4.4 | - | 1.9 | 2.5 |
| % Eliminated by CDF or CD | 114 | 114 | 113 | 102 |
| % Sequestrated | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baud, F.J.; Houzé, P.; Raphalen, J.-H.; Philippe, P.; Lamhaut, L. Vancomycin Sequestration in ST Filters: An In Vitro Study. Antibiotics 2023, 12, 620. https://doi.org/10.3390/antibiotics12030620
Baud FJ, Houzé P, Raphalen J-H, Philippe P, Lamhaut L. Vancomycin Sequestration in ST Filters: An In Vitro Study. Antibiotics. 2023; 12(3):620. https://doi.org/10.3390/antibiotics12030620
Chicago/Turabian StyleBaud, Frédéric J., Pascal Houzé, Jean-Herlé Raphalen, Pascal Philippe, and Lionel Lamhaut. 2023. "Vancomycin Sequestration in ST Filters: An In Vitro Study" Antibiotics 12, no. 3: 620. https://doi.org/10.3390/antibiotics12030620
APA StyleBaud, F. J., Houzé, P., Raphalen, J.-H., Philippe, P., & Lamhaut, L. (2023). Vancomycin Sequestration in ST Filters: An In Vitro Study. Antibiotics, 12(3), 620. https://doi.org/10.3390/antibiotics12030620







