Antibiotic Prophylaxis in One-Stage Revision of Septic Total Knee Arthroplasty: A Scoping Review
Abstract
1. Introduction
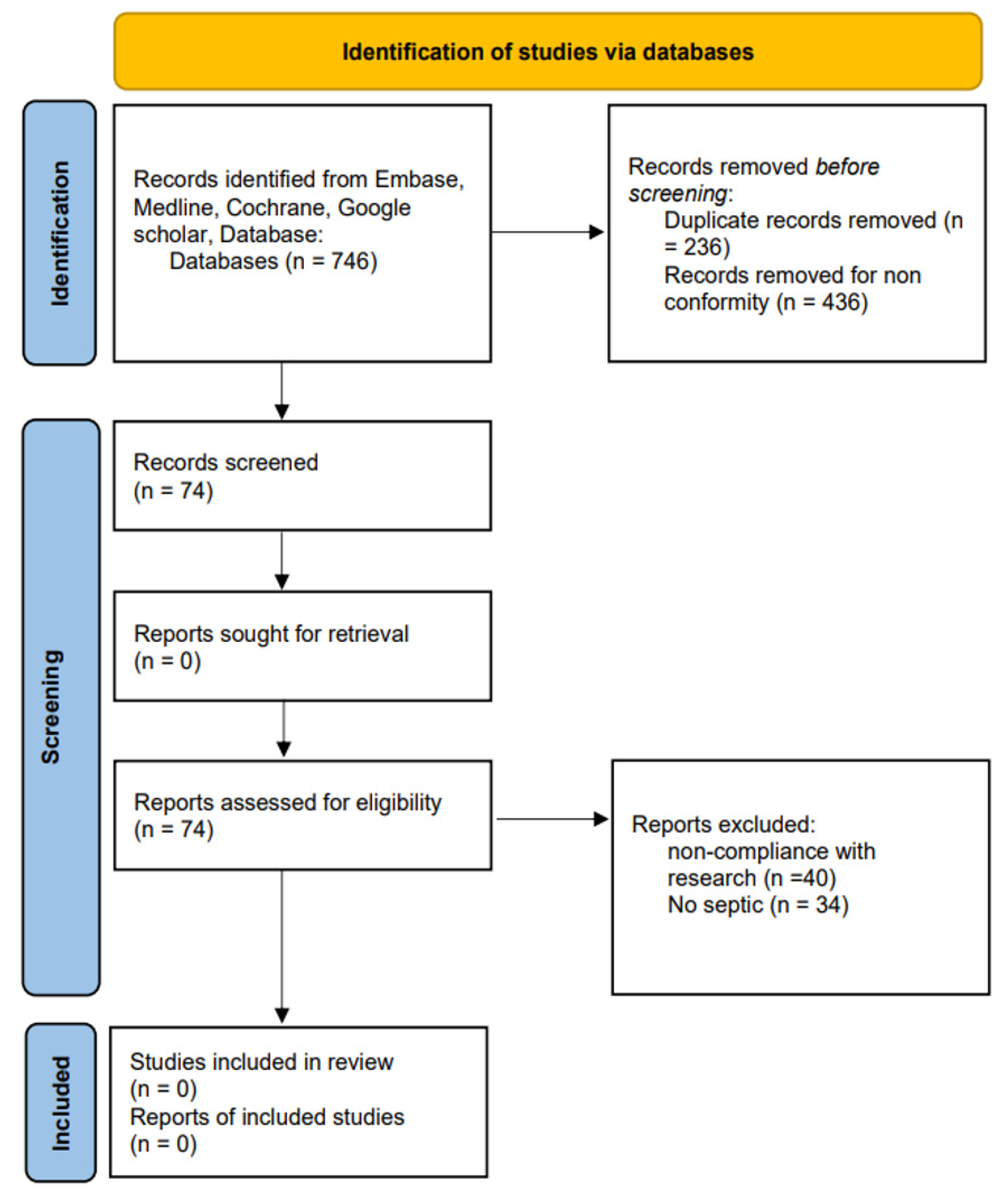
2. Results
3. Discussion
4. Materials and Methods
4.1. Inclusion and Exclusion Criteria
4.2. Literature Research
4.3. Study Selection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ethgen, O.; Bruyere, O.; Richy, F.; D’ardennes, C.; Reginster, J. Health-Related Quality of Life in Total Hip and Total Knee Arthroplasty. J. Bone Jt. Surg. 2004, 86, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.J.; Clement, N.D.; Hamilton, D.; Gaston, P.; Patton, J.T.; Howie, C.R. Predicting the cost-effectiveness of total hip and knee replacement. Bone Jt. J. 2013, 95, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic Burden of Periprosthetic Joint Infection in the United States. J. Arthroplast. 2012, 27, 61–65. [Google Scholar] [CrossRef]
- Wong, E.S. The Price of a Surgical-Site Infection: More Than Just Excess Length of Stay. Infect. Control. Hosp. Epidemiol. 1999, 20, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Salari, P.; Farinelli, L.; D’Anzeo, M.; Onori, N.; Gigante, A. Synovial Biomarkers to Detect Chronic Periprosthetic Joint Infection: A Pilot Study to Compare Calprotectin Rapid Test, Calprotectin ELISA Immunoassay and Leukocyte Esterase Test. J. Arthroplast. 2022, 37, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.K.; Rasouli, M.R.; Parvizi, J. Periprosthetic joint infection: Current concept. Indian J. Orthop. 2013, 47, 10–17. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef]
- Salari, P.; Grassi, M.; Cinti, B.; Onori, N.; Gigante, A. Synovial Fluid Calprotectin for the Preoperative Diagnosis of Chronic Periprosthetic Joint Infection. J. Arthroplast. 2019, 35, 534–537. [Google Scholar] [CrossRef]
- Segawa, H.; Tsukayama, D.T.; Kyle, R.F.; Becker, D.A.; Gustilo, R.B. Infection After Total Knee Arthroplasty. A Retrospective Study of the Treatment of Eighty-One Infections*. J. Bone Jt. Surg. 1999, 81, 1434–1445. [Google Scholar] [CrossRef]
- Kirkland, K.B.; Briggs, J.P.; Trivette, S.L.; Wilkinson, W.E.; Sexton, D.J. The Impact of Surgical-Site Infections in the 1990s: Attributable Mortality, Excess Length of Hospitalization, And Extra Costs. Infect. Control. Hosp. Epidemiol. 1999, 20, 725–730. [Google Scholar] [CrossRef]
- Freeman, M.A.; Sudlow, R.A.; Casewell, M.W.; Radcliff, S.S. The management of infected total knee replacements. J. Bone Jt. Surg. 1985, 67, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Buechel, F.F.; Femino, F.P.; D’Alessio, J. Primary exchange revision arthroplasty for infected total knee replacement: A long-term study. Am. J. Orthop. (Belle Mead N.J.) 2004, 33, 190–198. [Google Scholar]
- Göksan, S.B.; Freeman, M.A. One-stage reimplantation for infected total knee arthroplasty. J. Bone Jt. Surg. 1992, 74, 78–82. [Google Scholar] [CrossRef]
- Rosenberg, A.G. TKA Salvage: The End Game When Reimplantation Won’t Work. Orthopedics 1999, 22, 879–880. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.R.; Insall, J.N.; Windsor, R.E.; Moran, M.C. Survivorship of cemented knee replacements. J. Bone Jt. Surg. 1989, 71, 798–803. [Google Scholar] [CrossRef]
- Razii, N.; Clutton, J.M.; Kakar, R.; Morgan-Jones, R. Single-stage revision for the infected total knee arthroplasty The Cardiff experience. Bone Jt. Open 2021, 2, 305–313. [Google Scholar] [CrossRef]
- Brunt, A.C.C.; Gillespie, M.; Holland, G.; Brenkel, I.; Walmsley, P. Results of ‘two-in-one’ single-stage revision total knee ar-throplasty for infection with associated bone loss PROSPECTIVE FIVE-YEAR FOLLOW UP. Bone Jt. Open 2022, 3, 107–113. [Google Scholar] [CrossRef]
- DeBenedictis, K.J.; Rowan, N.M.; Boyer, B.L. A Double-Blind Study Comparing Cefonicid with Cefazolin as Prophylaxis in Patients Undergoing Total Hip or Knee Replacement. Clin. Infect. Dis. 1984, 6, S901–S904. [Google Scholar] [CrossRef]
- Doyon, F.; Evrard, J.; Mazas, F.; Hill, C. Long-term results of prophylactic cefazolin versus placebo in total hip replacement. Lancet 1987, 329, 860. [Google Scholar] [CrossRef]
- Anderson, D.J.; Podgorny, K.; Berríos-Torres, S.I.; Bratzler, D.W.; Dellinger, E.P.; Greene, L.; Nyquist, A.-C.; Saiman, L.; Yokoe, D.S.; Maragakis, L.L.; et al. Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update. Infect. Control. Hosp. Epidemiol. 2014, 35, 605–627. [Google Scholar] [CrossRef]
- Aboltins, C.A.; Berdal, J.E.; Casas, F.; Corona, P.S.; Cuellar, D.; Ferrari, M.C.; Hendershot, E.; Huang, W.; Kuo, F.-C.; Malkani, A.; et al. Hip and Knee Section, Prevention, Antimicrobials (Systemic): Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2018, 34, S279–S288. [Google Scholar] [CrossRef]
- Kuo, F.-C.; Chang, Y.-H.; Huang, T.-W.; Chen, D.W.-C.; Tan, T.L.; Lee, M.S. Post-operative prophylactic antibiotics in aseptic revision hip and knee arthroplasty: A propensity score matching analysis. Sci. Rep. 2022, 12, 18319. [Google Scholar] [CrossRef]
- Yamada, K.; Matsumoto, K.; Tokimura, F.; Okazaki, H.; Tanaka, S. Are Bone and Serum Cefazolin Concentrations Adequate for Antimicrobial Prophylaxis? Clin. Orthop. Relat. Res. 2011, 469, 3486–3494. [Google Scholar] [CrossRef] [PubMed]
- Stefánsdóttir, A.; Johansson, D.; Knutson, K.; Lidgren, L.; Robertsson, O. Microbiology of the infected knee arthroplasty: Report from the Swedish Knee Arthroplasty Register on 426 surgically revised cases. Scand. J. Infect. Dis. 2009, 41, 831–840. [Google Scholar] [CrossRef]
- Smith, E.B.; Wynne, R.; Joshi, A.; Liu, H.; Good, R.P. Is it time to include vancomycin for routine perioperative antibiotic prophylaxis in total joint arthroplasty patients? J. Arthroplast. 2012, 27, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Magreault, S.; Heym, B.; Salmon, D.; Kitzis, M.-D.; Billaud, E.; Marmor, S.; Jannot, A.-S.; Salomon, L.; Jullien, V. Influence of the clindamycin administration route on the magnitude of clindamycin–rifampicin interaction: A prospective pharmacokinetic study. Clin. Microbiol. Infect. 2021, 27, 1857.e1–1857.e7. [Google Scholar] [CrossRef]
- Hansen, E.; Belden, K.; Silibovsky, R.; Vogt, M.; Arnold, W.V.; Bicanic, G.; Yamada, K. Perioperative antibiotics. J. Arthroplast. 2014, 29 (Suppl. S2), 29–48. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Houck, P.M.; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for sur-gery: Downloaded by: University of Michigan. Copyrighted material. advisory statement from the National Surgical Infection Prevention Project. Am. J. Surg. 2005, 189, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Gehrke, T.; Chen, A.F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt. J. 2013, 95-B, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R.; Hospital Infection Control Practices Advisory Commit-tee. Guideline for prevention of surgical site infection. Bull Am. Coll Surg. 2000, 85, 23–29. [Google Scholar]
- Agodi, A.; Auxilia, F.; Barchitta, M.; Cristina, M.L.; Mura, I.; Nobile, M.; Pasquarella, C. Compliance with guidelines on antibiotic prophylaxis in hip and knee arthroplasty in Italy: Results of the GISIO-ISChIA project. Ann. Ig. 2015, 27, 520–525. [Google Scholar] [PubMed]
- Hanssen, A.D.; Osmon, D.R. The Use of Prophylactic Antimicrobial Agents During and After Hip Arthroplasty. Clin. Orthop. Relat. Res. 1999, 369, 124–138. [Google Scholar] [CrossRef]
- Spangehl, M.J.; Masri, B.A.; O’Connell, J.X.; Duncan, C.P. Prospective analysis of preoperative and intraoperative investiga-tions for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J. Bone Joint. Surg. Am. 1999, 81, 672–683. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999, 27, 97–132; quiz 133–134; discussion 196. [Google Scholar] [CrossRef]
- Tetreault, M.; Wetters, N.G.; Aggarwal, V.; Mont, M.; Parvizi, J.; Della Valle, C.J. The Chitranjan Ranawat Award: Should Prophylactic Antibiotics Be Withheld Before Revision Surgery to Obtain Appropriate Cultures? Clin. Orthop. Relat. Res. 2013, 472, 52–56. [Google Scholar] [CrossRef]
- Burnett, S.R.J.; Aggarwal, A.; Givens, S.A.; McClure, T.J.; Morgan, P.M.; Barrack, R.L. Prophylactic Antibiotics Do Not Affect Cultures in the Treatment of an Infected TKA: A Prospective Trial. Clin. Orthop. Relat. Res. 2010, 468, 127–134. [Google Scholar] [CrossRef]
- Ghanem, E.; Parvizi, J.; Clohisy, J.; Burnett, S.; Sharkey, P.F.; Barrack, R. Perioperative Antibiotics Should Not Be Withheld in Proven Cases of Periprosthetic Infection. Clin. Orthop. Relat. Res. 2007, 461, 44–47. [Google Scholar] [CrossRef]
- Finley, R.; Glass-Kaastra, S.K.; Hutchinson, J.; Patrick, D.M.; Weiss, K.; Conly, J. Declines in Outpatient Antimicrobial Use in Canada (1995–2010). PLoS ONE 2013, 8, e76398. [Google Scholar] [CrossRef]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic Joint Infection: The Incidence, Timing, and Predisposing Factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Rosteius, T.; Jansen, O.; Fehmer, T.; Baecker, H.; Citak, M.; Schildhauer, T.; Geßmann, J. Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. J. Med. Microbiol. 2018, 67, 1608–1613. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Bakhshi, H.; Ecker, N.U.; Parvizi, J.; Gehrke, T.; Kendoff, D. Organism Profile in Periprosthetic Joint Infection: Pathogens Differ at Two Arthroplasty Infection Referral Centers in Europe and in the United States. J. Knee Surg. 2014, 27, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Chang, C.-H.; Lin, Y.-C.; Lee, S.-H.; Hsieh, P.-H.; Chang, Y. Different microbiological profiles between hip and knee prosthetic joint infections. J. Orthop. Surg. 2019, 27, 2309499019847768. [Google Scholar] [CrossRef]
- Roman, M.D.; Bocea, B.-A.; Ion, N.-I.; Vorovenci, A.E.; Dragomirescu, D.; Birlutiu, R.-M.; Birlutiu, V.; Fleaca, S.R. Are There Any Changes in the Causative Microorganisms Isolated in the Last Years from Hip and Knee Periprosthetic Joint Infections? Antimicrobial Susceptibility Test Results Analysis. Microorganisms 2023, 11, 116. [Google Scholar] [CrossRef]
- Nickinson, R.S.J.; Board, T.N.; Gambhir, A.K.; Porter, M.L.; Kay, P.R. The microbiology of the infected knee arthroplasty. Int. Orthop. 2009, 34, 505–510. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Grzega, C.; Sahan, I.; Geipel, U.; Becker, S. Occurrence of Rare Pathogens at the Site of Periprosthetic Hip and Knee Joint Infections: A Retrospective, Single-Center Study. Antibiotics 2021, 10, 882. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Bortolin, M.; Zagra, L.; Romanò, C.L.; Cappelletti, L. Epidemiology and Antibiotic Resistance of Late Prosthetic Knee and Hip Infections. J. Arthroplast. 2017, 32, 2496–2500. [Google Scholar] [CrossRef]
- Page, C.P.; Bohnen, J.M.A.; Fletcher, J.R.; McManus, A.T.; Solomkin, J.S.; Wittmann, D.H. Antimicrobial prophylaxis for sur-gical wounds. Guidelines for clinical care. Arch. Surg. 1993, 128, 79–88. [Google Scholar] [CrossRef]
- Holmes, R.L.; Jorgensen, J.H. Inhibitory Activities of 11 Antimicrobial Agents and Bactericidal Activities of Vancomycin and Daptomycin against Invasive Methicillin-Resistant Staphylococcus aureus Isolates Obtained from 1999 through 2006. Antimicrob. Agents Chemother. 2008, 52, 757–760. [Google Scholar] [CrossRef]
- Wyles, C.C.; Hevesi, M.; Osmon, D.R.; Park, M.A.; Habermann, E.B.; Lewallen, D.G.; Berry, D.J.; Sierra, R.J. 2019 John Charnley Award: Increased risk of prosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin. Bone Jt. J. 2019, 101-B, 9–15. [Google Scholar] [CrossRef]
- Siddiqi, A.; Forte, S.A.; Docter, S.; Bryant, D.; Sheth, N.P.; Chen, A.F. Perioperative Antibiotic Prophylaxis in Total Joint Arthroplasty. J. Bone Jt. Surg. 2019, 101, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Jahoda, D.; Nyc, O.; Pokorný, D.; Landor, I.; Sosna, A. Antibiotic treatment for prevention of infectious complications in joint replacement. Acta Chir. Orthop. Et Traumatol. Cechoslov. 2006, 73, 108–114. [Google Scholar]
- Parsons, T.; French, J.; Oshima, T.; Figueroa, F.; Neri, T.; Klasan, A.; Putnis, S. International Survey of Practice for Prophylactic Systemic Antibiotic Therapy in Hip and Knee Arthroplasty. Antibiotics 2022, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.L.; Shohat, N.; Rondon, A.J.; Foltz, C.; Goswami, K.; Ryan, S.P.; Seyler, T.M.; Parvizi, J. Perioperative Antibiotic Prophylaxis in Total Joint Arthroplasty. J. Bone Jt. Surg. 2019, 101, 429–437. [Google Scholar] [CrossRef]
- Illingworth, K.D.; Mihalko, W.M.; Parvizi, J.; Sculco, T.; McArthur, B.; el Bitar, Y.; Saleh, K.J. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: A multicenter approach: AAOS exhibit selection. J. Bone Joint. Surg. Am. 2013, 95, e50. Available online: https://pubmed.ncbi.nlm.nih.gov/23595076/ (accessed on 25 September 2022). [CrossRef]
- Vårdprogram för Led-och Skelettinfektioner. Sven Infekt (SILF); Sweden. Available online: https://infektion.net/wp-content/uploads/2018/11/2018-vardprogram-led-och-skelettinfektioner-final-2018-11-29.pdf (accessed on 31 August 2022).
- Villa, J.M.; Pannu, T.S.; Riesgo, A.M.; Patel, P.D.; Mont, M.A.; Higuera-Rueda, C.A. Dual Antibiotic Prophylaxis in Total Knee Arthroplasty: Where Do We Stand? J. Knee Surg. 2019, 33, 100–105. [Google Scholar] [CrossRef]
- American Academy of Orthopaedic Surgeons Advisory Statement: Recommendations for the Use of Intravenous Antibiotic Prophylaxis in Primary Total Joint Arthroplasty. Available online: http://www.aaos.org/about/papers/advistmt/1027.asp (accessed on 27 August 2011).
- Sewick, A.; Makani, A.; Wu, C.; O’Donnell, J.; Baldwin, K.D.; Lee, G.C. Does dual antibiotic prophylaxis better prevent sur-gical site infections in total joint arthroplasty? Clin. Orthop. Relat. Res. 2012, 470, 2702–2707. [Google Scholar] [CrossRef]
- Fehring, T.K.; Odum, S.; Calton, T.F.; Mason, J.B. Articulating Versus Static Spacers in Revision Total Knee Arthroplasty for Sepsis. Clin. Orthop. Relat. Res. 2000, 380, 9–16. [Google Scholar] [CrossRef]
- Meek, R.M.D.; Masri, B.A.; Dunlop, D.; Garbuz, D.S.; Greidanus, N.V.; Mcgraw, R.; Duncan, C.P. Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of the prostalac articulating spacer. J. Bone Jt. Surg. 2003, 85, 1888–1892. [Google Scholar] [CrossRef]
- Hofmann, A.A.; Kane, K.R.; Tkach, T.K.; Plaster, R.L.; Camargo, M.P. Treatment of infected total knee arthroplasty using an articu-lating spacer. Clin. Orthop. Relat. Res. 1995, 321, 45–54. [Google Scholar] [PubMed]
- Haddad, F.S.; Masri, B.; Campbell, D.; McGraw, R.W.; Beauchamp, C.P.; Duncan, C.P. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. J. Bone Jt. Surg. 2000, 82, 807–812. [Google Scholar] [CrossRef]
- Goldstein, W.M.; Kopplin, M.; Wall, R.; Berland, K. Temporary articulating methylmethacrylate antibiotic spacer (TAMMAS). A new method of intraoperative manufacturing of a custom articulating spacer. J. Bone Jt. Surg. 2001, 83, S92–S97. [Google Scholar] [CrossRef]
- Freeman, M.G.; Fehring, T.K.; Odum, S.M.; Fehring, K.; Griffin, W.L.; Mason, J.B. Functional Advantage of Articulating Versus Static Spacers in 2-Stage Revision for Total Knee Arthroplasty Infection. J. Arthroplast. 2007, 22, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Insall, J.N.; Thompson, F.M.; Brause, B.D. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J. Bone Joint Surg. Am. 1983, 65, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Piovan, G.; Farinelli, L.; Screpis, D.; Marocco, S.; Motta, L.; Palazzolo, G.; Natali, S.; Zorzi, C. The role of antibiotic calcium sulfate beads in acute periprosthetic knee infection: A retrospective cohort study. Arthroplasty 2022, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, P.R.; Dhotar, H.S.; Sternheim, A.; Gross, A.E.; Safir, O.; Backstein, D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: Tech-niques, controversies, and outcomes. J. Am. Acad. Orthop. Surg. 2014, 22, 153–164. [Google Scholar] [CrossRef]
- von Foerster, G.; Klüber, D.; Käbler, U. Mid- to long-term results after treatment of 118 cases of periprosthetic infections after knee joint replacement using one-stage exchange surgery. Orthopade. 1991, 20, 244–252. (In German) [Google Scholar] [PubMed]
- Romanò, C.L.; Gala, L.; Logoluso, N.; Romanò, D.; Drago, L. Two-stage revision of septic knee prosthesis with articulating knee spacers yields better infection eradication rate than one-stage or two-stage revision with static spacers. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2445–2453. [Google Scholar] [CrossRef]
- Klouche, S.; Leonard, P.; Zeller, V.; Lhotellier, L.; Graff, W.; Leclerc, P.; Sariali, E. Infected total hip arthroplasty revision: One- or two-stage procedure? Orthop. Traumatol. Surg. Res. 2012, 98, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, R.W.; Kay, P.R.; Rawal, A. A case for one-stage revision in infected total knee arthroplasty? Knee 2011, 18, 1–4. [Google Scholar] [CrossRef]
- Zahar, A.; Kendoff, D.O.; Klatte, T.O.; Gehrke, T.A. Can Good Infection Control Be Obtained in One-stage Exchange of the Infected TKA to a Rotating Hinge Design? 10-year Results. Clin. Orthop. Relat. Res. 2016, 474, 81–87. [Google Scholar] [CrossRef]
- Nagra, N.S.; Hamilton, T.W.; Ganatra, S.; Murray, D.W.; Pandit, H. One-stage versus two-stage exchange arthroplasty for infected total knee arthroplasty: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2015, 24, 3106–3114. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Lenguerrand, E.; Blom, A.W.; Beswick, A.D.; Inform Team. Re-Infection Outcomes Following One- And Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151537. [Google Scholar] [CrossRef]
- Pangaud, C.; Ollivier, M.; Argenson, J.-N. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. Efort Open Rev. 2019, 4, 495–502. [Google Scholar] [CrossRef]
- Wroblewski, B.M. One-stage revision of infected cemented total hip arthroplasty. Clin. Orthop. Relat. Res. 1986, 211, 103–107. [Google Scholar] [CrossRef]
- Young, S.W.; Zhang, M.; Moore, G.A.; Pitto, R.P.; Clarke, H.D.; Spangehl, M.J. The John, N. Insall Award: Higher Tissue Concentrations of Vancomycin Achieved With Intraosseous Re-gional Prophylaxis in Revision TKA: A Randomized Controlled Trial. Clin. Orthop. Relat. Res. 2018, 476, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kakis, A.; Nichols, A.; Ries, M.D.; Vail, T.P.; Bozic, K.J. Targeted Use of Vancomycin as Perioperative Prophylaxis Reduces Periprosthetic Joint Infection in Revision TKA. Clin. Orthop. Relat. Res. 2014, 472, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.-C.; Lin, P.-C.; Bell, K.L.; Ko, J.-Y.; Wang, C.-J.; Wang, J.-W. Extended Postoperative Prophylactic Antibiotics with First-Generation Cephalosporin Do Not Reduce the Risk of Periprosthetic Joint Infection following Aseptic Revision Total Knee Arthroplasty. J. Knee Surg. 2019, 33, 597–602. [Google Scholar] [CrossRef]
- Villa, J.M.; Pannu, T.S.; Braaksma, W.; Higuera, C.A.; Riesgo, A.M. Extended Oral Antibiotic Prophylaxis After Aseptic Total Hip or Knee Arthroplasty Revisions: A Preliminary Report. J. Arthroplast. 2022, 38, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Zingg, M.; Kheir, M.M.; Ziemba-Davis, M.; Meneghini, R.M. Reduced Infection Rate After Aseptic Revision Total Knee Arthroplasty With Extended Oral Antibiotic Protocol. J. Arthroplast. 2022, 37, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Renz, N.; Müller, M.; Perka, C.; Trampuz, A. Implant-associated infections—Diagnostics. Chirurg 2016, 87, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Dlaska, C.E.; Perka, C.; Trampuz, A.; Renz, N. Preoperative synovial fluid culture poorly predicts the pathogen causing periprosthetic joint infection. Infection 2020, 49, 427–436. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Ochsner, P.E.; Borens, O.; Bodler, P.-M. Schweizerische Gesellschaft fur Orthopadie und Traumatologie. In Infections of the Musculo-skeletal System: Basic Principles Prevention Diagnosis and Treatment, 1st ed.; Swiss Orthopaedics In-House Publisher: Grandvaux, Switzerland, 2014. [Google Scholar]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Imagama, T.; Nakashima, D.; Seki, K.; Seki, T.; Matsuki, Y.; Yamazaki, K.; Sakai, T. Comparison of bacterial culture results of preoperative synovial fluid and intraoperative specimens in patients with joint infection. J. Infect. Chemother. 2020, 27, 562–567. [Google Scholar] [CrossRef]
- Boyle, K.K.; Kapadia, M.; Chiu, Y.-F.; Khilnani, T.; Miller, A.O.; Henry, M.W.; Lyman, S.; Carli, A.V. The James, A. Rand Young Investigator’s Award: Are Intraoperative Cultures Necessary If the Aspiration Culture Is Positive? A Concordance Study in Periprosthetic Joint Infection. J. Arthroplast. 2021, 36, S4–S10. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Hao, L.; Chai, W.; Jun, F.; Chen, J. The concordance between preoperative aspiration and intraoperative synovial fluid culture results: Intraoperative synovial fluid re-cultures are necessary whether the preoperative aspiration culture is positive or not. Bmc Infect. Dis. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Mont, M.A.; Waldman, B.J.; Hungerford, D.S. Evaluation of Preoperative Cultures Before Second-Stage Reimplantation of a Total Knee Prosthesis Complicated by Infection. J. Bone Jt. Surg. 2000, 82, 1552–1557. [Google Scholar] [CrossRef]
- Burnett, R.S.J.; Kelly, M.A.; Hanssen, A.D.; Barrack, R.L. Technique and Timing of Two-stage Exchange for Infection in TKA. Clin. Orthop. Relat. Res. 2007, 464, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Wilkinson, J.M.; Cooper, J.R.; Kerry, R.M.; Hamer, A.J.; Norman, P.; Stockley, I. Accuracy of Joint Aspiration for the Preoperative Diagnosis of Infection in Total Hip Arthroplasty. J. Arthroplast. 2006, 21, 221–226. [Google Scholar] [CrossRef]
- Meermans, G.; Haddad, F.S. Is There a Role for Tissue Biopsy in the Diagnosis of Periprosthetic Infection? Clin. Orthop. Relat. Res. 2010, 468, 1410. [Google Scholar] [CrossRef] [PubMed]
- Matter-Parrat, V.; Ronde-Oustau, C.; Boéri, C.; Gaudias, J.; Jenny, J.-Y. Agreement between pre-operative and intra-operative bacteriological samples in 85 chronic peri-prosthetic infections. Orthop. Traumatol. Surg. Res. 2017, 103, 301–305. [Google Scholar] [CrossRef]
- Holleyman, R.J.; Deehan, D.; Charlett, A.; Gould, K.; Baker, P.N. Does pre-operative sampling predict intra-operative cultures and antibiotic sensitivities in knee replacements revised for infection?: A study using the NJR dataset. Knee Surg. Sports Traumatol. Arthrosc. 2015, 24, 3056–3063. [Google Scholar] [CrossRef] [PubMed]
- Declercq, P.; Neyt, J.; Depypere, M.; Goris, S.; Van Wijngaerden, E.; Verhaegen, J.; Wauters, J.; Spriet, I. Preoperative joint aspiration culture results and causative pathogens in total hip and knee prosthesis infections: Mind the gap. Acta Clin. Belg. 2019, 75, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Morawietz, L.; Hasart, O.; Strube, P.; Perka, C.; Tohtz, S. Diagnosis of periprosthetic infection following total hip arthroplasty—evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J. Orthop. Surg. Res. 2008, 3, 31. [Google Scholar] [CrossRef]
- Somme, D.; Ziza, J.-M.; Desplaces, N.; Chicheportiche, V.; Chazerain, P.; Leonard, P.; Lhotellier, L.; Jacquenod, P.; Mamoudy, P. Contribution of routine joint aspiration to the diagnosis of infection before hip revision surgery. Jt. Bone Spine 2003, 70, 489–495. [Google Scholar] [CrossRef]
- Buchholz, H.W.; Elson, R.A.; Engelbrecht, E.; Lodenkämper, H.; Röttger, J.; Siegel, A. Management of deep infection of total hip replacement. J. Bone Jt. Surg. 1981, 63, 342–353. [Google Scholar] [CrossRef]
- Le Vavasseur, B.; Zeller, V. Antibiotic Therapy for Prosthetic Joint Infections: An Overview. Antibiotics 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Straus, S.E. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccullo, C.; Neri, T.; Farinelli, L.; Gigante, A.; Philippot, R.; Farizon, F.; Boyer, B. Antibiotic Prophylaxis in One-Stage Revision of Septic Total Knee Arthroplasty: A Scoping Review. Antibiotics 2023, 12, 606. https://doi.org/10.3390/antibiotics12030606
Ciccullo C, Neri T, Farinelli L, Gigante A, Philippot R, Farizon F, Boyer B. Antibiotic Prophylaxis in One-Stage Revision of Septic Total Knee Arthroplasty: A Scoping Review. Antibiotics. 2023; 12(3):606. https://doi.org/10.3390/antibiotics12030606
Chicago/Turabian StyleCiccullo, Carlo, Thomas Neri, Luca Farinelli, Antonio Gigante, Rémi Philippot, Frederic Farizon, and Bertrand Boyer. 2023. "Antibiotic Prophylaxis in One-Stage Revision of Septic Total Knee Arthroplasty: A Scoping Review" Antibiotics 12, no. 3: 606. https://doi.org/10.3390/antibiotics12030606
APA StyleCiccullo, C., Neri, T., Farinelli, L., Gigante, A., Philippot, R., Farizon, F., & Boyer, B. (2023). Antibiotic Prophylaxis in One-Stage Revision of Septic Total Knee Arthroplasty: A Scoping Review. Antibiotics, 12(3), 606. https://doi.org/10.3390/antibiotics12030606






