Abstract
Antimicrobial resistance (AMR) has become a global public health concern in recent decades. Although several investigations evaluated AMR in commensal and pathogenic bacteria from different foods of animal origin in Australia, there is a lack of studies that compared AMR in commensal E. coli isolated from retail table eggs obtained from different laying hen housing systems. This study aimed to determine AMR and differences in AMR patterns among E. coli isolates recovered from retail table eggs sourced from caged and non-caged housing systems in Western Australia. Commensal E. coli isolates were tested for susceptibility to 14 antimicrobials using a broth microdilution method. Clustering analyses and logistic regression models were applied to identify patterns and differences in AMR. Overall, there were moderate to high frequencies of resistance to the antimicrobials of lower importance used in Australian human medicine (tetracycline, ampicillin, trimethoprim, and sulfamethoxazole) in the isolates sourced from the eggs of two production systems. All E. coli isolates were susceptible to all critically important antimicrobials except the very low level of resistance to ciprofloxacin. E. coli isolates from eggs of non-caged systems had higher odds of resistance to tetracycline (OR = 5.76, p < 0.001) and ampicillin (OR = 3.42, p ≤ 0.01) compared to the isolates from eggs of caged systems. Moreover, the number of antimicrobials to which an E. coli isolate was resistant was significantly higher in table eggs from non-caged systems than isolates from caged systems’ eggs. Considering the conservative approach in using antimicrobials in the Australian layer flocks, our findings highlight the potential role of the environment or human-related factors in the dissemination and emergence of AMR in commensal E. coli, particularly in retail table eggs of non-cage system origin. Further comprehensive epidemiological studies are required to better understand the role of different egg production systems in the emergence and dissemination of AMR in commensal E. coli.
1. Introduction
Commercial layer farms play an essential role in providing protein sources by supplying eggs [1]. In Australia, the demand for the production and consumption of eggs is increasing. In the financial year 2021–2022, 6.6 billion eggs were produced by the Australian egg industry, and per capita egg consumption grew to 262 eggs, which was higher than in previous years [2].
Enteric bacteria can contaminate eggs from the colonized gut, the feces of layer hens during or after oviposition, or the infected reproductive organs with probable penetration into the eggshell [3,4]. Escherichia coli (E. coli) is a common commensal organism in humans and warm-blooded animals, such as birds and other livestock, with some opportunistic pathogen strains, which are responsible for a wide range of infections [5]. This organism is regarded as the optimal antimicrobial resistance (AMR) indicator in foods of animal origin [6] due to its ability to carry mobile genetic elements and other antibiotic resistance determinants that might transfer to other strains that reside in the host [7].
The widespread use of antimicrobials in food animal production, including poultry production, raises a significant global public health concern that is often associated with the emergence of resistance against antimicrobials that are commonly used in those animals [8,9,10]. Humans are exposed to resistant microorganisms through the consumption of raw or inadequately cooked eggs added to some desserts and sauces [11] or from cross-contamination during meal preparation [12], which promotes the risk of therapeutic failures if humans are infected with multidrug-resistant bacteria [13,14].
Australia practices a cautious regulatory approach concerning the use of critically important antimicrobials in food-producing animals. For instance, most critical antimicrobials including fluoroquinolones and gentamicin are restricted to being used in food animals. Moreover, the mass administration of third-generation cephalosporins and ceftiofur is limited in livestock and is also not approved for use in the poultry industry [15,16,17]. There is also minimal use of antimicrobials in the Australian commercial layer industry due to the risk of antimicrobial residues in eggs [15]. Only a few therapeutic antibiotics are permitted to be used in layer flocks, with a zero-withdrawal time for eggs, including chlortetracycline, a lincomycin-spectinomycin combination, as well as a brand of amoxicillin [18]. However, despite this admirable stewardship of antimicrobial use, there are still reports of AMR among commensal E. coli isolates in the egg production industry in Australia [19,20].
Different egg production systems, including cage, barn, and free-range, are utilized in commercial layer farms in Australia. Although each of these production systems has both advantages and disadvantages, the development of non-cage production systems (particularly free-range) in commercial layer farms has been driven by Australian consumer demand for reasons related to the birds’ welfare and the quality of eggs [21,22]. Free-range egg production has grown significantly over the last 15 years, and in 2020–2021, it comprised 52% of all retail grocery sales, which is higher than the cage system (36%) [2].
According to previous studies, the free-range egg production system appears to face more challenges related to biosecurity implementation over the control of environmental stressors and vectors [23,24]. The elevated environmental contact of free-range layer birds compared to caged ones may increase the incidence of infectious poultry diseases requiring treatment with antimicrobials [18], which might increase the emergence of resistant commensal and pathogenic bacteria. Therefore, we hypothesize that there might be differences in AMR patterns among commensal E. coli isolated from retail table eggs obtained from the cage and non-cage housing systems.
Differences in AMR determinants in E. coli isolates were previously investigated in different bird species and housing systems in Canada [25] as well as in laying hens and eggs in conventional and organic keeping systems in Germany [26]. Although limited studies have investigated AMR in commensal E. coli from table eggs [19] and layer farm environments in Australia [20], to the best of our knowledge, no published literature has compared the AMR patterns of E. coli isolates sourced from retail table eggs of the cage and non-cage housing systems. Therefore, to address this knowledge gap, this study has aimed to determine the prevalence of AMR and multidrug resistance (MDR) in E. coli isolated from retail table eggs of caged and non-caged origin and assess the association between resistance carriage in E. coli with layer housing systems in Western Australia.
2. Results
2.1. Description of Submissions
A total of 100 E. coli isolates recovered from retail table egg samples of the cage and non-cage origin were selected for antimicrobial susceptibility testing. We combined the isolates recovered from table eggs produced by the barn and free-range housing systems as “non-cage” (n = 68) to enable better statistical power when comparing with the isolates obtained from table eggs sourced from the cage housing system (n = 32).
2.2. Descriptive Analyses
Analysis indicated that 57 (57%) of the 100 E. coli isolates were resistant to at least one antimicrobial agent. The highest resistances observed were to tetracycline (n = 49, 49%), followed by ampicillin (n = 36, 36%), trimethoprim (n = 20, 20%), sulfamethoxazole (n = 18, 18%), ciprofloxacin (n = 2, 2%), and tigecycline (n = 1, 1%). Data indicated that all the isolates were susceptible to azithromycin, meropenem, cefotaxime, ceftazidime, chloramphenicol, nalidixic acid, colistin, and gentamicin (Table 1).

Table 1.
Number and percentage of commensal E. coli isolates (n = 100) from Western Australian retail table eggs of caged and non-caged origin that were resistant to 14 selected antimicrobials.
In the E. coli isolates obtained from the eggs of the cage system, there was a moderate prevalence of resistance (15–39%) to tetracycline and ampicillin, a low frequency of resistance (5–14%) to trimethoprim and sulfamethoxazole, and a very low prevalence of resistance (<5%) to ciprofloxacin (Table 1).
In the non-cage table egg origin E. coli isolates, there was a high prevalence of resistance (≥40%) to tetracycline and ampicillin, a moderate prevalence of resistance (15–39%) to trimethoprim and sulfamethoxazole, and a very low prevalence of resistance (<5%) to ciprofloxacin and tigecycline (Table 1).
Multidrug resistance was detected in 9.4% (95% CI = 1.9–25%) of the E. coli isolates from the eggs of cage housing systems and 26.5% (95% CI = 16.5–38.6%) of the isolates from the eggs of non-cage system. The AMR and MDR patterns of E. coli isolates are shown in Table 2. Two and four MDR profiles (microbiologically resistant to three or more classes of antimicrobials) were identified in the E. coli isolates recovered from retail table eggs of the cage and non-cage housing systems, respectively. Our findings indicated that the most common MDR pattern in the isolates of both caged (2 isolates) and non-caged (13 isolates) source retail table eggs was the concurrent resistance to sulfamethoxazole-trimethoprim-ampicillin-tetracycline (Table 2).

Table 2.
Antimicrobial resistance patterns of commensal E. coli (n = 100) isolated from retail table eggs of the cage and non-cage housing systems in Western Australia.
It is worth mentioning that no resistance to the 14 antimicrobials tested was identified in 43% of the E. coli isolates. Isolates sourced from retail table eggs of the cage systems showed a higher rate of susceptibility (65.6%) compared to the isolates recovered from table eggs of the non-cage housing systems (32.3%) (Figure 1).
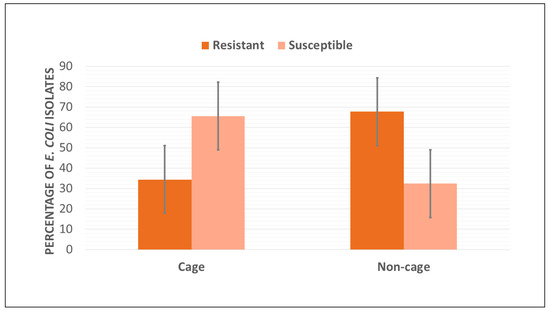
Figure 1.
Percentage of resistant and susceptible E. coli isolates (n = 100) recovered from retail table eggs sourced from cage and non-cage housing systems in Western Australia.
2.3. Cluster Analyses
Single-linkage clustering dendrograms with Jaccard distances for E. coli resistance are represented in Figure 2. In the dendrogram showing the E. coli isolates sourced from the cage system, three main resistance clustering patterns were identified, the patterns of resistance to sulfamethoxazole and tetracycline, trimethoprim and ampicillin, and a cluster of isolates that were susceptible to azithromycin, meropenem, cefotaxime, ceftazidime, chloramphenicol, nalidixic acid, colistin, tigecycline, and gentamicin (Figure 2a). The dendrogram of the E. coli isolates from retail table eggs of non-cage systems also included three main resistance clustering patterns. One cluster showed resistance to sulfamethoxazole and trimethoprim, whereas the other showed resistance to ampicillin and tetracycline. A relatively high proportion (i.e., a cluster) of the isolates were also susceptible to azithromycin, meropenem, cefotaxime, ceftazidime, chloramphenicol, nalidixic acid, colistin, and gentamicin (Figure 2b).
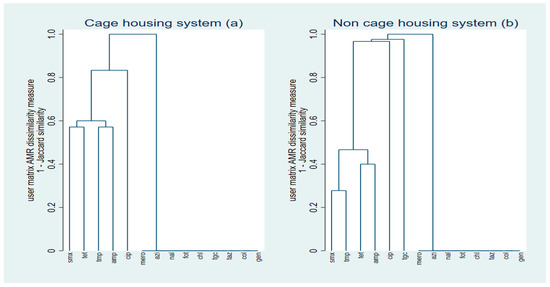
Figure 2.
Single-linkage clustering dendrograms of resistance of egg-sourced E. coli (n = 100) isolates to antimicrobials by production system ((a) cage and (b) non-cage). SMX: sulfamethoxazole; TMP: trimethoprim ampicillin; CIP: ciprofloxacin; NAL: nalidixic acid; TET: tetracycline; MERO: meropenem; AZI: azithromycin; FOT: cefotaxime; TAZ: ceftazidime; CHL: chloramphenicol; TGC: tigecycline; COL: colistin; AMP: ampicillin; GEN: gentamicin.
Multiple correspondence analysis coordinate plots for the first two dimensions of resistance in E. coli isolates from retail egg samples of the cage and non-cage systems are shown in Figure 3. Eight antimicrobials (azithromycin, cefotaxime, ceftazidime, chloramphenicol, meropenem, nalidixic acid, colistin, and gentamicin) were removed from the analysis because not enough variation was identified. For E. coli isolates from eggs of the caged housing system, the first two dimensions explained 79.1% of the variation in antimicrobial resistance (Figure 3a). A high relatedness of resistance to ampicillin and tetracycline (co-resistance cluster) was observed, and a lower degree of relatedness between resistance to trimethoprim and sulfamethoxazole was also noted.
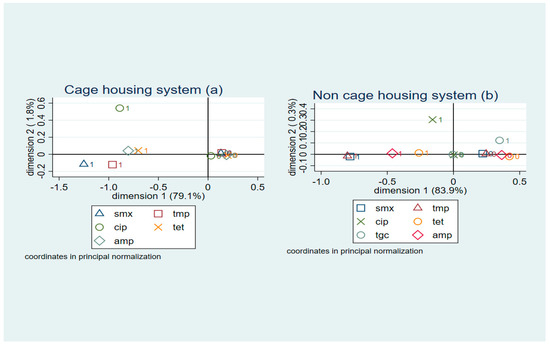
Figure 3.
Multiple correspondence analysis coordinate plot displaying the presence (1) and absence (0) of resistance to six antimicrobials in E. coli isolates (n = 100) from (a) cage and (b) non-cage retail table eggs for the first two dimensions. SMX: sulfamethoxazole; TMP: trimethoprim; CIP: ciprofloxacin; TET: tetracycline; TGC: tigecycline; AMP: ampicillin.
For E. coli isolates obtained from eggs of non-caged systems, the first two dimensions explained 83.9% of the variation in AMR (Figure 3b), and a cluster of co-resistance to trimethoprim and sulfamethoxazole was detected (when observation scores were plotted along dimensions 1 and 2). A lower degree of relatedness between resistance to ampicillin and tetracycline was also identified.
2.4. Logistic Regression
The odds of resistance to tetracycline (Odds Ratio = 5.76, 95% CI = 2.18–15.22, p < 0.001) were almost 6 times higher, and the odds of resistance to ampicillin were 3.4 times higher (OR = 3.42, 95% CI = 1.24–9.37, p = 0.017) in E. coli isolates sourced from retail table eggs of non-caged housing systems compared to the isolates sourced from the egg samples of the caged system.
2.5. Poisson Regression
The number of antimicrobials to which an E. coli isolate was resistant was significantly higher (incidence rate ratio = 2.27, 95% CI = 1.39–3.73, p = 0.001) in the isolates from eggs originating from non-caged housing systems when compared to the isolates from table egg samples of caged system source.
3. Discussion
The present study evaluated AMR in commensal E. coli isolates originating from caged and non-caged retail table eggs in Western Australia, representing a relatively moderate to high frequency of resistance to antimicrobials commonly used to treat bacterial infections in poultry and other livestock. Resistance to the critically important antimicrobials in human medicine was rare among E. coli isolated from retail table eggs in Western Australia. This study identified a higher resistance carriage to some of the antimicrobials in E. coli isolated from table eggs from the non-cage system when compared to the cage system. Our findings have provided helpful baseline data that will promote our understanding of AMR in commensal E. coli originating from retail table eggs sourced from caged and non-caged systems in Western Australia. These findings might aid in achieving “objective three” of Australia’s first national antimicrobial resistance strategy to enhance the nationally coordinated One Health surveillance of antimicrobial resistance and antimicrobial usage.
Given the differences in sampling settings and protocols and the lab and analytical methods employed, direct comparisons between the present study and similar previous investigations should be treated with caution. Our findings indicated high to moderate resistance among E. coli isolates to historically used antimicrobials that are still used in animal production [27], including tetracycline (49%), ampicillin (36%), trimethoprim (20%), and sulfisoxazole (18%). In Australia, E. coli isolates from the commercial egg industry have been identified as being highly resistant to the same antimicrobials [20]. Although low levels of resistance to aminoglycoside (1%) and phenicol (2.4%) were reported in the study mentioned above in Australia, no E. coli isolates were resistant to the same classes of antimicrobials in the present investigation. Despite different sampling settings and frameworks, farm-related factors such as antimicrobial use and health management may play an important role in the variations in the AMR prevalence of commensal E. coli isolates in different studies.
According to our results, the resistance of E. coli isolates recovered from non-caged origin retail table eggs to historically used antimicrobials in poultry production, including tetracycline, ampicillin, trimethoprim, and sulfisoxazole, was considerably higher than the prevalence of resistance to the same antimicrobials in E. coli isolates sourced from egg samples from the caged system.
Previous investigations have demonstrated that when comparing different housing systems, it is evident that freshly laid eggs in the cage housing system have a lower bacterial load than eggs from non-cage housing systems [28,29,30,31]. This could be due to the higher contact of eggs in the non-cage system with a contaminated environment or perhaps due to the higher shedding of bacteria in non-caged layer birds compared to caged birds because of the higher prevalence of environmental stressors [23]. It could be hypothesized that the higher level of bacterial contamination of eggs from the non-caged system might be associated with a higher level of resistant bacteria on the eggs. Further studies at the farm and retail levels are required to confirm or disprove this hypothesis.
On the other hand, our findings might reinforce the previous hypothesis mentioned, in the commercial egg production industry, shifting towards more extensive production systems (i.e., free-range) has promoted the incidence of many diseases, resulting in a return to the use of medications [18]. This might increase the emergence of resistant bacteria in such a housing system, which warrants further comprehensive research. Nonetheless, different levels of biosecurity measures at farms and human-related factors at the retail level also cannot be ignored in relation to the emergence and dissemination of resistant bacteria to humans through the consumption of eggs or egg products. Since Australian egg layers generally discourage the prophylactic use of antimicrobials, more strict levels of biosecurity might be required in the future due to the increasing number of higher-welfare management systems, including free-range and barn systems.
Among the critically important antimicrobials tested in the present study, only a very low level of resistance to ciprofloxacin was identified in E. coli isolates from table eggs sourced from both cage (3.1%) and non-cage (1.5%) systems. However, without complete plasmid DNA sequencing, it cannot be fully concluded that these few isolates were resistant to fluoroquinolone [19]. These results concur with the previous studies, which reported rare resistance to critically important antimicrobials in E. coli isolated from Australian livestock, including from commercial egg layer farms [20], meat chickens [32], pigs [33], and cattle [34]. Our findings, although promising, further highlight the potential role of environmental or human-related factors in detecting non-wild type ciprofloxacin-resistant E. coli in the absence of local antimicrobial selection pressure at layer farms in Australia [19]. Future investigations might be necessary to prove this hypothesis.
The absence of resistance to nearly all of the critically important antimicrobials in our investigation could be due to the presence of a conservative approach in the general use of these antimicrobials in food-producing animals [35], particularly in the commercial egg layer industry [15] in Australia. Based on these promising findings, it is hypothesized that commensal E. coli isolates originating from table eggs sourced from both cage and non-cage systems in Western Australia will continue to be susceptible to critical antimicrobials in human medicine in the future. However, continuous AMR surveillance of table eggs and environmental samples from Western Australia’s egg industry will shed further light on this hypothesis.
Four MDR profiles were identified in the present study, and the most common MDR pattern in E. coli isolates was co-resistance to three classes of antimicrobials, including beta-lactams, folate pathway inhibitors, and tetracycline. Concurrent resistance to the same antimicrobial classes was also recently reported for the MDR E. coli isolates from the Australian commercial egg industry [20]. Our findings are encouraging when compared to the previous study [20], wherein the presence of a few E. coli isolates resistant to four and five antimicrobial classes was also reported, which was not identified in the present study. In our investigation, the higher frequency of MDR in E. coli isolated from table eggs of non-caged systems (26.5%) compared to the E. coli isolated from table eggs of caged systems (9.4%) might highlight the critical role of environmental vectors and stressors in the non-caged egg production system. However, more comprehensive and comparative studies might be needed to confirm our findings and to investigate other effective factors related to the role of housing systems in the development of MDR in commensal E. coli in Western Australia’s commercial egg industry.
Our single-linkage clustering analysis of the E. coli isolates sourced from non-caged retail eggs demonstrated concurrent resistance to sulfamethoxazole and trimethoprim as well as tetracycline and ampicillin. Antimicrobial resistance clusters of E. coli isolates sourced from non-caged retail eggs also consisted of the same antimicrobials, including a cluster of co-resistance to sulfamethoxazole and tetracycline and another cluster of concurrent resistance to trimethoprim and ampicillin, which are widely used antibiotics in human and veterinary medicine. The results also indicated that almost all of the E. coli isolates recovered from the eggs produced by both housing systems were pan-susceptible to the critically important antimicrobials in human medicine, which is an encouraging and expected finding because of the minimal use of these antibiotics in the Australian commercial layers [18]. Our MCA analysis for the E. coli isolates originating from table eggs samples from cage and non-cage systems indicated a high degree of relatedness (e.g., co-resistance) between resistance to folate pathway inhibitors antimicrobials, including sulfamethoxazole and trimethoprim. Similar resistance mechanisms between these two members of the folate pathway inhibitors antimicrobial class [36] might be the reason for this high relatedness. A lower degree of relatedness between resistance to ampicillin and tetracycline was also identified. Our MCA findings for the E. coli isolates sourced from table eggs of the caged housing system were slightly different from our single-linkage clustering, possibly due to the differences between these two clustering methods.
The concurrent resistance of the retail egg E. coli isolates to the commonly used antimicrobials in food animal production highlights the necessity of the judicious use of these antimicrobials in food-producing animals to reduce the development and dissemination of AMR and MDR bacteria at the farm level [37,38]. It is important to highlight that there might be a higher probability of intestinal colonization with resistant bacterial strains than with susceptible strains; however, the underlying reasons for this phenomenon must be investigated by further research. It is also worth mentioning that the absence of relatedness between resistance to quinolones and the other antimicrobial classes is an encouraging finding of the present study. Nonetheless, continuous monitoring through an effective ongoing AMR surveillance program at farms and the retail level is required to maintain the minimum level of quinolone-resistant E. coli in Western Australia’s egg industry.
Our regression models indicated a higher probability of resistance to tetracycline and ampicillin among the E. coli isolates from non-caged produced retail eggs compared to the isolates recovered from eggs of caged systems. The results also indicated that the number of antimicrobials to which an E. coli isolates was resistant was significantly higher in E.coli isolates from the eggs of non-caged systems when compared to the isolates from eggs of the caged system. Differences in AMR between the two main egg production systems (cage and non-cage) might be partly explained by the variations in antimicrobial use and husbandry practices at the cage and non-cage layer farms. Moreover, the role of environmental and human-related factors from farms to the retail stage cannot be ignored. Both hypotheses underscore the need for further research to verify our findings and investigate other related factors in the development of AMR and MDR in commensal E. coli in foods of animal origins.
The limitations of this study include sampling bias, as free-range eggs were purposively oversampled because of their growing demand in Australia; therefore, the higher frequency of E. coli isolates recovered from non-caged (68%) compared to caged eggs (32%), as well as the low overall number of tested E. coli isolates (n = 100) in this study, might have influenced the AMR prevalence of commensal E. coli in both cage and non-cage retail table egg samples.
4. Materials and Methods
4.1. Study Design and Lab Methods
The study design and laboratory methods were described in detail in our previously published study [19]. Briefly, a total of 181 retail egg samples (each containing one dozen eggs) collected from different supermarkets across Perth (Western Australia) were tested for the isolation of commensal E. coli using the ISO 16649-1:2018 standard [39]. The selected colonies were then confirmed to species level using MALDI-TOF (matrix-assisted laser desorption ionization time-of-flight) with research-use-only (RUO) library databases, version claim 4 (microflex instrument, Bruker Diagnostics, Germany).
Antimicrobial susceptibility of confirmed E. coli isolates (n =100) to 14 antimicrobials was conducted in a micro-broth dilution using commercially prepared panels (Sensititre EUVSEC, TREK Diagnostic Systems, Thermo Fisher Scientific) according to the manufacturer’s guidelines, and quality control strains E. coli ATCC25922 were used throughout the testing. Minimum inhibitory concentrations (MICs) were interpreted using microbiological cut-off values (also referred to as ‘Epidemiological Cut-off Values’ or ECOFFs). It is worth mentioning that ECOFFs are not a predictor of clinical success but rather measures of an antimicrobial drug MIC distribution that separate bacterial populations into microbiologically susceptible (wild type) and microbiologically resistant (non-wild type) [20]. In the present investigation, we used ECOFFs values represented by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [40]. Isolates that are microbiologically resistant to three or more antimicrobials classes based on ECOFFs are classified as MDR phenotype [20]. To simplify the explanation of individual and multidrug resistance patterns, we used the words “susceptible” and “resistant” for microbiologically susceptible (wild type) and microbiologically resistant (non-wild type) isolates, respectively.
4.2. Data Analysis
Antimicrobial susceptibility data were transferred into a spreadsheet in Microsoft Excel (Microsoft Office 2016), checked for missing values, and subsequently imported into a statistical software program (STATA Intercooled software Version 17, Stata Corporation, College Station, TX, USA) for analysis.
4.2.1. Descriptive Analysis
For egg samples from caged and non-caged housing systems, estimates of the proportion of E. coli isolates resistant to each tested antimicrobial were determined by dividing the number of isolates resistant to an antimicrobial by the total number of isolates tested for the antimicrobial. Added to that, estimates of the proportion of isolates that demonstrated MDR were calculated by dividing the number of MDR isolates by the total number of tested isolates. It should be noted that a sample was considered to be resistant to an antimicrobial if at least one isolate from that sample was resistant to that antimicrobial. Confidence intervals (CIs) were computed using exact binomial CIs using the Clopper–Pearson method for all proportions.
4.2.2. Cluster Analysis
Cluster analysis, using the Jaccard binary similarity coefficient, was conducted for each variable (eggs from cage and non-cage systems) to compare individual antimicrobials concerning their similarity in the resistance status of E. coli. Dendrograms were constructed using the single-linkage clustering method with the Jaccard distance. The dissimilarity between antimicrobials was measured by Jaccard distance by subtracting the Jaccard binary similarity coefficient from one [41]. Therefore, a high dissimilarity measure shows that relatively few isolates were resistant to both antimicrobials. In contrast, a low dissimilarity measure indicates a relatively high proportion of isolates resistant to both antimicrobials. All isolates were considered susceptible to both antimicrobials when the dissimilarity measure was zero.
Multiple correspondence analysis, using the Burt method with principal normalization [42,43], was constructed for E. coli isolates recovered from table egg samples produced by the cage and non-cage housing systems to identify relationships within the set of six selected antimicrobials in terms of their similarity in E. coli resistance. We included dimensions that explained at least two-thirds of the variation in the data for further analysis. Observation scores were computed and plotted to visualize the distribution of AMR patterns along the first two dimensions.
4.2.3. Logistic Regression
To identify differences in E. coli resistance between housing systems (cage and non-cage), logistic regression was applied; only antimicrobials for which ≥5% of the isolates were resistant were evaluated. Therefore, 4 of 14 antimicrobials were analyzed, including sulfamethoxazole, trimethoprim, ampicillin, and tetracycline. One logistic regression model was made for each antimicrobial. In these univariable models, the dependent variable indicated the prevalence of resistance to the antimicrobial, whereas the independent variable was the production systems (non-cage compared to cage). A p-value ≤ 0.05 on the Wald χ2 test demonstrated a statistically significant association.
Added to that, Poisson regression models were built to identify differences in E. coli MDR between the egg samples produced by two production systems (cage and non-cage). In this model, the independent variable was the production system (cage and non-cage), and the dependent variable was the number of antimicrobials to which an isolate was resistant.
5. Conclusions
Our findings demonstrated moderate to high levels of resistance to antimicrobials of lower importance in human medicine in the E. coli isolates from eggs produced by both housing systems. An exceptionally low level of resistance to critically important antimicrobials (ciprofloxacin) in human medicine was also detected in a few E. coli isolates from the eggs produced by both cage and non-cage systems. In this study, the higher prevalence of AMR and MDR in E. coli isolates recovered from eggs sourced from the non-caged housing systems compared to the isolates recovered from eggs of the caged system highlights the necessity of further research regarding the early detection of AMR in commensal and pathogenic bacteria, specifically in the non-caged egg production system, which is the popular system viewed by the public due to its higher welfare status of laying hens. Further comprehensive epidemiological studies are required to better understand the role of different egg production systems in the emergence and dissemination of AMR in commensal E. coli to meet expectations regarding the safety of food products and humans in Australia’s future.
Author Contributions
Conceptualization, H.R.S. and I.H.; methodology, H.R.S., S.S. and I.H.; investigation, H.R.S.; data analysis, H.R.S. and C.V.; visualization, H.R.S. and C.V.; writing—original draft preparation, H.R.S.; writing—review and editing, H.R.S., C.V., S.S. and I.H.; project administration, I.H.; funding acquisition, I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Ph.D. Scholarship from Murdoch University and a postdoctoral research associate fund from the Department of Pathobiology, College of Veterinary Medicine, University of Illinois Urbana-Champaign given to Hamid Reza Sodagari.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the staff of the Antimicrobial Resistance and Infectious Disease (AMRID) Laboratory at Murdoch University for their assistance in antimicrobial susceptibility testing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jabir, F.; Hague, M.T. Study on production performance of ISA Brown strain at Krishibid Firm Ltd. Trishal, Mymensingh. Bangladesh Res. Publ. J. 2010, 3, 1039–1044. [Google Scholar]
- Australian Eggs. Australian Egg Industry Overview. Available online: https://www.australianeggs.org.au/egg-industry (accessed on 23 February 2023).
- Keller, L.H.; Benson, C.E.; Krotec, K.; Eckroade, R.J. Salmonella enteritidis colonization of the reproductive tract and forming and freshly laid eggs of chickens. Infect. Immun. 1995, 63, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Agabou, A.; Lezzar, N.; Ouchenane, Z.; Khemissi, S.; Satta, D.; Sotto, A.; Lavigne, J.P.; Pantel, A. Clonal relationship between human and avian ciprofloxacin-resistant Escherichia coli isolates in North-Eastern Algeria. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 227–234. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority Panel on Biological Hazards (BIOHAZ); Europena Medicines Agency Committee for Medicinal Products for Veterinary Use (CVMP). ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption in humans and food-producing animals. EFSA J. 2017, 15, e05017. [Google Scholar] [CrossRef]
- Vangchhia, B.; Blyton, M.D.; Collignon, P.; Kennedy, K.; Gordon, D.M. Factors affecting the presence, genetic diversity and antimicrobial sensitivity of Escherichia coli in poultry meat samples collected from Canberra, Australia. Environ. Microbiol. 2018, 20, 1350–1361. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Wegener, H.C.; Collignon, P. Resistance in bacteria of the food chain: Epidemiology and control strategies. Expert Rev. Anti Infect. Ther. 2008, 6, 733–750. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Davis, M.; Leibler, J.H.; Peterson, A.E. One reservoir: Redefining the community origins of antimicrobial resistant infections. Med. Clin. N. Am. 2008, 92, 391–1407. [Google Scholar] [CrossRef]
- Shrestha, R.D.; Agunos, A.; Gow, S.P.; Deckert, A.E.; Varga, C. Associations between antimicrobial resistance in fecal Escherichia coli isolates and antimicrobial use in Canadian turkey flocks. Front. Microbiol. 2022, 13, 954123. [Google Scholar] [CrossRef]
- Adesiyun, A.; Offiah, N.; Seepersadsingh, N.; Rodrigo, S.; Lashley, V.; Musai, L. Frequency and antimicrobial resistance of enteric bacteria with spoilage potential isolated from table eggs. Food Res. Int. 2006, 39, 212–219. [Google Scholar] [CrossRef]
- Adeboye, O.A.; Kwofie, M.K.; Bukari, N. Campylobacter, Salmonella and Escherichia coli Food Contamination Risk in Free-Range Poultry Production System. Adv. Appl. Microbiol. 2020, 10, 525–542. [Google Scholar] [CrossRef]
- Snyder, H.L.; Niebuhr, S.E.; Dickson, J.S. Transfer of methicillinresistant Staphylococcus aureus from retail pork products onto food contact surfaces and the potential summer exposure. J. Food Prot. 2013, 76, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; McEntire, J.C.; Zhang, L.; Li, X.; Doyle, M. The transfer of antibiotic resistance from food to humans: Facts, implications and future directions. Rev. Sci. Tech. 2012, 31, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Australian Veterinary Association (AVA). Veterinary Use of Antibiotics Critical to Human Health. Australian Veterinary Association. 2015. Available online: https://www.ava.com.au/siteassets/resources/fighting-antimicrobial-resistance/veterinary-use-of-antibiotics-critical-to-human-health.pdf (accessed on 23 February 2023).
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460. [Google Scholar] [CrossRef]
- Commonwealth of Australia. Importance Ratings and Summary of Antibacterial Uses in Human and Animal Health in Australia; Australian Strategic and Technical Advisory Group on Antimicrobial Resistance (ASTAG); Department of Agriculture, Water Resources, and the Environment: Canberra, Australia, 2018. Available online: https://www.amr.gov.au/sites/default/files/2022-10/importance-ratings-and-summary-of-antibacterial-uses-in-human-and-animal-health-in-australia.pdf (accessed on 23 February 2023).
- Groves, P.; Underwood, G. Impact of antibiotic use and disease risks on Australian laying hen welfare. Anim. Prod. Sci. 2021, 61, 1037–1041. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Wang, P.; Robertson, I.; Abraham, S.; Sahibzada, S.; Habib, I. Antimicrobial resistance and genomic characterisation of Escherichia coli isolated from caged and non-caged retail table eggs in Western Australia. Int. J. Food Microbiol. 2021, 340, 109054. [Google Scholar] [CrossRef] [PubMed]
- Australian Eggs. Surveillance for Antimicrobial Resistance in Enteric Commensals and Pathogens in the Australian Commercial Egg Industry; Australian Eggs Limited: North Sydney, Australia, 2021; Available online: https://www.australianeggs.org.au/assets/research/documents/Egg-industry-AMR-survey-Final-Report2_May-2021.pdf (accessed on 23 February 2023).
- Abbott, R. Hen housing systems and egg safety. Poult. Int. 2010, 49, 32–33. [Google Scholar]
- Chousalkar, K.; Gast, R.; Martelli, F.; Pande, V. Review of Egg-Related Salmonellosis and Reduction Strategies in the United States, Australia, United Kingdom, and New Zealand. Crit. Rev. Microbiol. 2018, 44, 290–303. [Google Scholar] [CrossRef]
- Gole, V.C.; Woodhouse, R.; Caraguel, C.; Moyle, T.; Rault, J.L.; Sexton, M.; Chousalkar, K. Dynamics of Salmonella shedding and welfare of hens in free-range egg production systems. Appl. Environ. Microbiol. 2017, 83, e03313-16. [Google Scholar] [CrossRef] [PubMed]
- Weeks, C.A.; Lambton, S.L.; Williams, A.G. Implications for welfare, productivity and sustainability of the variation in reported levels of mortality for laying hen flocks kept in different housing systems: A meta-analysis of ten studies. PLoS ONE 2016, 11, e0146394. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Guerin, M.T.; Brash, M.L.; Slavic, D.; Boerlin, P.; Susta, L. Antimicrobial resistance in fecal Escherichia coli and Salmonella enterica isolates: A two-year prospective study of small poultry flocks in Ontario, Canada. BMC Vet. Res. 2019, 15, 464. [Google Scholar] [CrossRef]
- Schwaiger, K.; Schmied, E.M.; Bauer, J. Comparative analysis of antibiotic resistance characteristics of Gram-negative bacteria isolated from laying hens and eggs in conventional and organic keeping systems in Bavaria, germany. Zoonoses Public Health 2008, 55, 331–341. [Google Scholar] [CrossRef]
- WOAH (World Organisation for Animal Health). OIE Annual Report on Antimicrobial Agents Intended for Use in Animal. 2021. Available online: https://www.woah.org/app/uploads/2021/05/a-fifth-annual-report-amr.pdf (accessed on 23 February 2023).
- Vlčková, J.; Tůmová, E.; Ketta, M.; Englmaierová, M.; Chodová, D. Effect of housing system and age of laying hens on eggshell quality, microbial contamination, and penetration of microorganisms into eggs. Czech J. Anim. Sci. 2018, 63, 51–60. [Google Scholar] [CrossRef]
- De Reu, K.; Messens, W.; Heyndrickx, M.; Rodenburg, T.B.; Uyttendaele, M.; Herman, L. Bacterial contamination of table eggs and the influence of housing systems. World’s Poult. Sci. J. 2008, 64, 5–19. [Google Scholar] [CrossRef]
- Mallet, S.; Guesdon, V.; Ahmed, A.M.H.; Nys, Y. Comparison of eggshell hygiene in two housing systems: Standard and furnished cages. Br. Poult. Sci. 2006, 47, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.R.; Chousalkar, K.K. Effect of production system and flock age on egg quality and total bacterial load in commercial laying hens. J. Appl. Poult. Res. 2014, 23, 59–70. [Google Scholar] [CrossRef]
- Abraham, S.; O’Dea, M.; Sahibzada, S.; Hewson, K.; Pavic, A.; Veltman, T.; Abraham, R.; Harris, T.; Trott, D.J.; Jordan, D. Escherichia coli and Salmonella spp. isolated from Australian meat chickens remain susceptible to critically important antimicrobial agents. PLoS ONE 2019, 14, e0224281. [Google Scholar] [CrossRef]
- Kidsley, A.K.; Abraham, S.; Bell, J.M.; O’Dea, M.; Laird, T.J.; Jordan, D.; Mitchell, P.; McDevitt, C.A.; Trott, D.J. Antimicrobial Susceptibility of Escherichia coli and Salmonella spp. Isolates From Healthy Pigs in Australia: Results of a Pilot National Survey. Front. Microbiol. 2018, 9, 1207. [Google Scholar] [CrossRef]
- Barlow, R.S.; McMillan, K.E.; Duffy, L.L.; Fegan, N.; Jordan, D.; Mellor, G.E. Prevalence and Antimicrobial Resistance of Salmonella and Escherichia coli from Australian Cattle Populations at Slaughter. J. Food Prot. 2015, 78, 912–920. [Google Scholar] [CrossRef]
- Veltman, T.; Jordan, D.; McDevitt, C.A.; Bell, J.; Howden, B.P.; Valcanis, M.; O’Dea, M.; Abraham, S.; Scott, P.; Kovac, J.H.; et al. Absence of high priority critically important antimicrobial resistance in Salmonella sp. isolated from Australian commercial egg layer environments. Int. J. Food Microbiol. 2021, 340, 109042. [Google Scholar] [CrossRef]
- Podnecky, N.L.; Rhodes, K.A.; Mima, T.; Drew, H.R.; Chirakul, S.; Wuthiekanun, V.; Schupp, J.M.; Sarovich, D.S.; Currie, B.J.; Keim, P.; et al. Mechanisms of Resistance to Folate Pathway Inhibitors in Burkholderia pseudomallei: Deviation from the Norm. mBio 2017, 8, e01357-17. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- ISO 16649-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 1: Colony-Count Technique at 44 Degrees C Using Membranes and 5-Bromo-4-chloro-3-indolyl Beta-Dglucuronide. 2018. Available online: https://www.iso.org/standard/64951.html (accessed on 12 November 2020).
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 12 November 2020).
- Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data: An Introduction to Cluster Analysis; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Greenacre, M.J. Multiple and Joint Correspondence Analysis. In Correspondence Analysis in the Social Sciences; Greenacre, M.J., Blasius, J., Eds.; Academic Press: London, UK, 1994. [Google Scholar]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 1999. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).