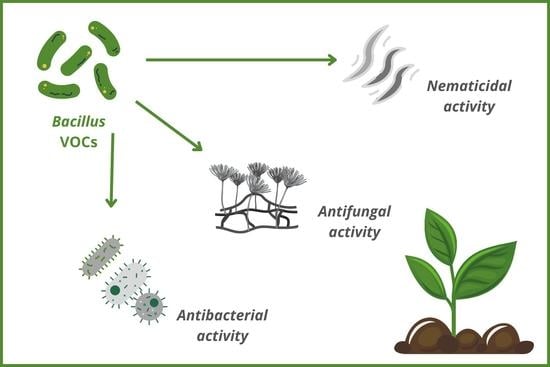
Bacillus VOCs in the Context of Biological Control
Abstract
1. Introduction
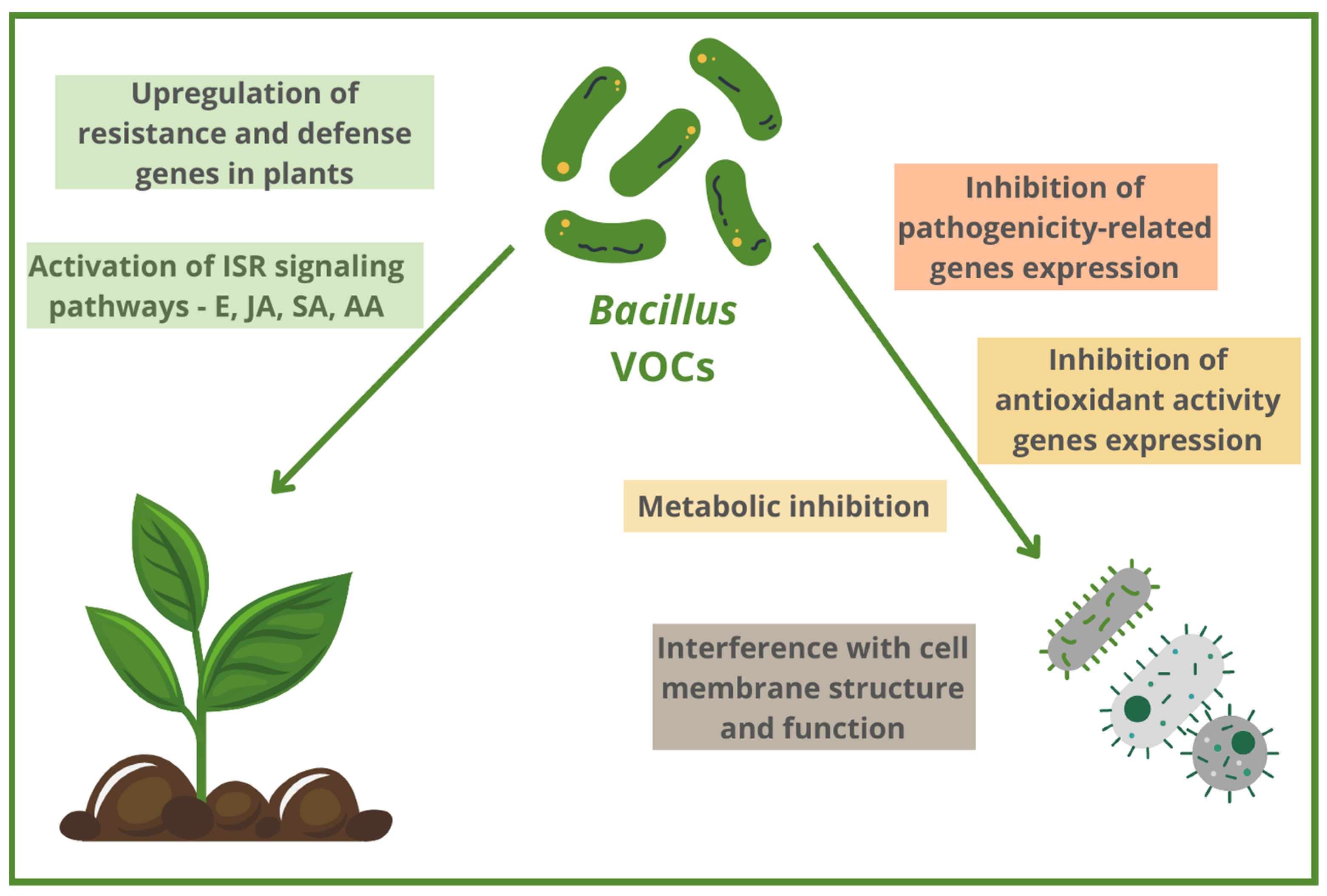
2. Antibacterial Bacillus-Based VOCs and Their Mechanisms of Action
2.1. ISR (Induced Systemic Resistance) Induction via Different Signaling Pathways as the Mechanism of Antibacterial Activity of Bacillus VOCs
2.2. Modulation of Pathogens’ Gene Expression by Antibacterial Bacillus-Based VOCs
2.3. Structural and Functional Changes at Cell Level Caused by Antibacterial Bacillus VOCs
2.4. Effects of Antibacterial Bacillus VOCs Concentration and Treatment Timing on Biocontrol Efficiency
| Bacillus Strain | Plant Pathogen (Disease) | Antibacterial VOCs | Reference |
|---|---|---|---|
| B. subtilis FB17 | Pseudomonas syringae pv. tomato | acetoin | Rudrappa et al. [16] |
| B. subtilis FA26 | Clavibacter michiganensis ssp. sepedonicus (potato bacterial ring rot) | benzaldehyde nonanal benzothiazole acetophenone | Rajer et al. [5] |
| B.s subtilis SYST2 | Ralstonia solanacearum (tobacco bacterial wilt) | albuterol 1,3-propanediol | Tahir et al. [12] |
| B. subtilis GB03 | Pseudomonas syringae pv. lachrymans | 2,3-butanediol | Song et al. [18] |
| B. amyloliquefaciens IN937a | Xanthomonas axonopodis pv. vesicatoria (pepper bacterial spot) | 3-pentanol | Choi et al. [20] |
| B. amyloliquefaciens T-5 | Ralstonia solanacearum (tomato bacterial wilt) | mixture of VOCs | Raza et al. [22] |
| B. amyloliquefaciens SQR-9 | Ralstonia solanacearum (tomato bacterial wilt) | mixture of VOCs | Raza et al. [21] |
| 2-nonanone 2-undecanone nonanal xylene benzothiazole butylated hydroxy toluene | Raza et al. [23] | ||
| B. megaterium BmBP17 | Ralstonia solanacearum | pyrazine, 2-ethyl-3-methyl pyrazine, 2-ethyl- pyrazine, 2, 5-dimethyl pyrazine, 2-methyl | Munjal et al. [27] |
| B. cereus D13 | Xanthomonas oryzae pv. oryzae (rice bacterial leaf blight) Xanthomonas oryzae pv. oryzicola Pseudomonas syringae pv. tomato Ralstonia solanacearum | decyl alcohol 3,5,5-trimethylhexanol | Xie et al. [25] Xie et al. [4] |
| B. atrophaeus JZB120050 | Ralstonia solanacearum Pseudomonas tolaasii Pseudomonas syringae pv. lachrymans | mixture of VOCs | Ni et al. [28] |
| B. subtilis GB03 B. amyloliquefaciens IN937a | Erwinia carotovora subsp. carotovora | 2,3-butanediol acetoin | Ryu et al. [15] |
| B. amyloliquefaciens FZB42 B. atrophaeus LSSC22 | Ralstonia solanacearum (tobacco bacterial wilt) | benzaldehyde 1,2-benzisothiazol-3(2H)-one 1,3-butadiene | Tahir et al. [24] |
| B. velezensis X5-2 B. megaterium X6-3 Pseudomonas orientalis X2-1P | Xanthomonas campestris pv. campestris (black rot of winter oilseed rape) | mixture of VOCs | Jelušić et al. [26] |
| B. velezensis JCK-1618 B. velezensis JCK-1696 | Xanthomonas arboricola pv. pruni | mixture of VOCs | Han et al. [25] |
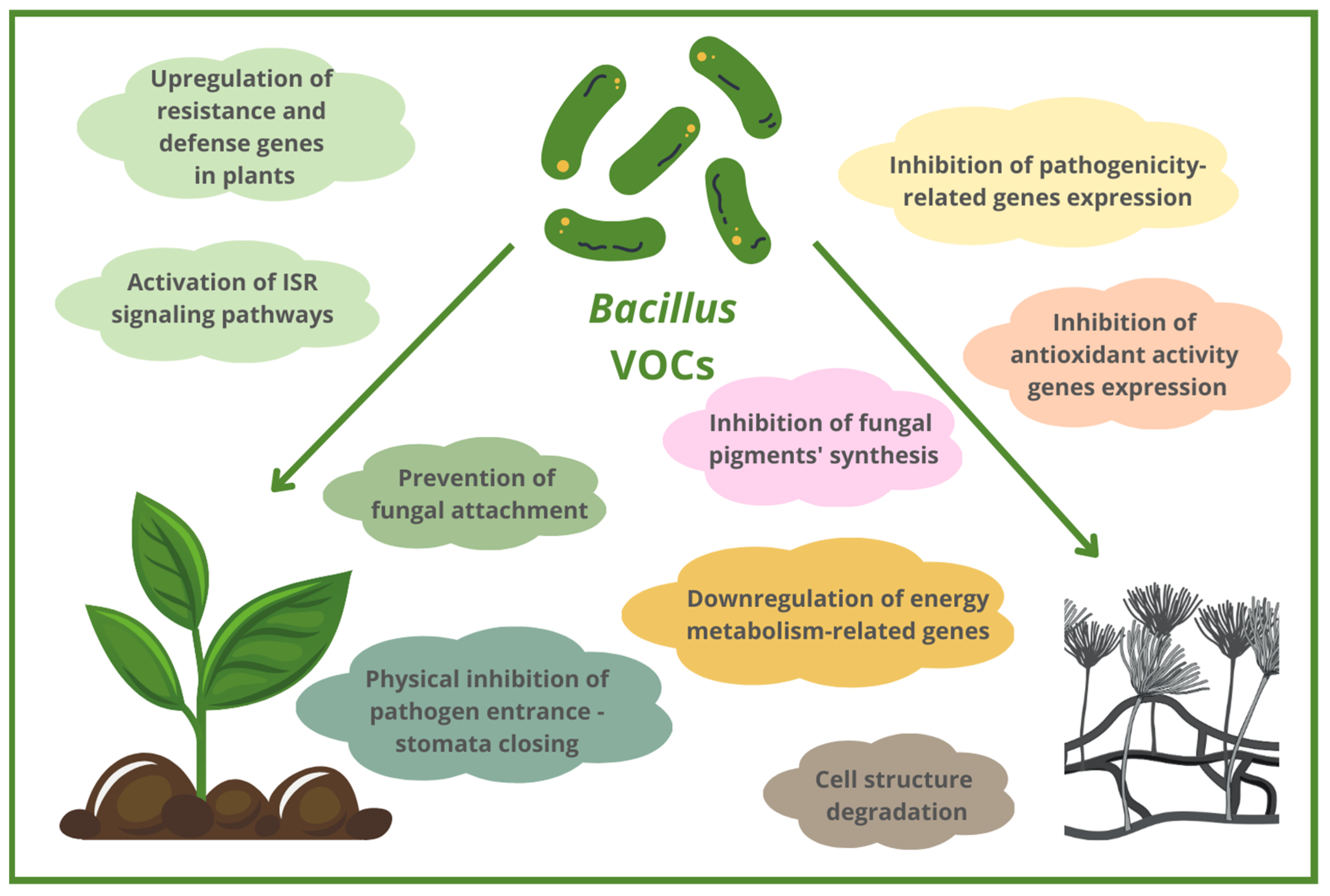
3. Mechanisms of Action of Antifungal Bacillus-Based VOCs
3.1. Cultivation and Treatment Variables Affecting Antifungal Efficiency of Bacillus VOCs
3.2. Wide Spectrum of Bacillus VOCs Antifungal Activity
3.3. Morphological and Ultrastructural Abnormalities in Fungal Cells Caused by Bacillus VOCs
3.4. Inhibition of Different Fungal Growth Stages by Bacillus VOCs
3.5. Prevention of Fungal Plant Attachment and Colonization by Bacillus VOCs
3.6. Altering the Expression of Genes Related to Pathogenicity, Metabolism and Antioxidant Activity of Fungal Pathogens
3.7. ISR Induced by Bacillus VOCs as Antifungal Mechanism of Action
3.8. Inhibition of Fungal Pigments Production by Bacillus VOCs
3.9. In Vivo Application of Bacillus VOCs in Biocontrol of Fungal Diseases
3.9.1. Fungal Diseases Caused by Colletotrichum gloeosporioides
3.9.2. Fungal Diseases Caused by Fusarium spp.
3.9.3. Fungal Diseases Caused by Sclerotinia spp.
3.9.4. Fungal Diseases Caused by Monilinia spp.
3.9.5. Fungal Diseases Caused by Alternaria spp.
3.9.6. Fungal Diseases Caused by Botrytis cinerea
3.9.7. Suppression of Mycotoxigenic Fungi by Bacillus-Based VOCs
3.9.8. Suppression of Other Fungal Diseases by Bacillus-Based VOCs
| Bacillus Strain | Plant Pathogen (Disease) | Antifungal VOCs | Reference |
|---|---|---|---|
| B. subtilis 155 | Penicillium digitatum Sacc. (green mold of citrus fruit) | mixture of VOCs | Leelasuphakul et al. [43] |
| B. subtilis G8 | Sclerotinia sclerotiorum Botrytis cinerea Alternaria brassicae Alternaria solani Alternaria citrulina Fusarium oxysporum Cercospora kikuchii Chupp Rhizoctonia solani | mixture of VOCs | Liu et al. [47] |
| B. subtilis | Sclerotinia sclerotiorum (white mold of Lactuca sativa) | mixture of VOCs | Monteiro et al. [58] |
| B. subtilis GB03 | Botrytis cinerea | mixture of VOCs | Sharifi and Ryu [84] |
| B. subtilis ACB-AP3 B. subtilis ACB-83 | Phyllosticta citricarpa (orange black spot) | mixture of VOCs | Kupper et al. [107] |
| B. subtilis IBFCBF-4 | Fusarium oxysporum f. sp. niveum (watermellon Fusarium wilt) | mixture of VOCs | Zhu et al. [59] |
| B. subtilis JY-7-2L | Sclerotium rolfsii (southern blight of Aconitum carmichaelii Debx.) | mixture of VOCs | Zou et al. [100] |
| B. subtilis C9 | Rhizoctonia solani | acetylbutanediol | Islam et al. [48] |
| B. subtilis G-1 | Sclerotium rolfsii (stem rot or white mould of groundnut) | tridecane | Shifa et al. [98] |
| B. subtilis M29 | Botrytis cinerea | 1-butanol acetic acid butyl ester 1-heptylene-4-alcohol 3-methyl-3-hexanol furan-tetrahydro-2,5-dimethyl 2,6-diisocyanato-1-methyl-benzene 1-propoxy-2-propanol benzophenone | Mu et al. [61] |
| B. subtilis CF-3 | Monilinia fructicola Colletotrichum gloeosporioides | 1-octanol 2,4-di-tert-butylthiophenol | Gao et al., 2017 [37] |
| Botrytis cinerea (strawberry gray mold) Colletotrichum gloeosporioides (litchi antrachnose) Penicillium expansum (blue mold of apple) Monilinia fructicola (peach brown rot) Alternaria alternata (Alternaria rot and black spot of jujube) | 2,4-di-tert-butylthiophenol benzothiazole 1-octanol benzoic acid benzaldehyde 3-methylbutanal | Gao et al., 2018 [36] | |
| Monilinia fructicola Colletotrichum gloeosporioides | mixture of VOCs | Wu et al. [102] | |
| Monilinia fructicola | benzothiazole | Zhou et al. [77] | |
| Colletotrichum gloeosporioides | 2,4-di-tert-butylphenol | Zhao et al. [89] Wang et al. [88] | |
| B. subtilis CL2 | Mucor circinelloides Fusarium arcuatisporum Alternaria iridiaustralis Colletotrichum fioriniae | 2,3-butanedione 3-methylbutyric acid | Ling et al. [72] |
| B. subtilis BTK1 | Sarocladium oryzae (rice sheath rot) | 3-heptanone, 5-ethyl-4-methyl- butanoic acid, 2-methyl 1-propanol 2,2-dimethyl acetate | Surya et al. [108] |
| B. subtilis DZSY21 | Curvularia lunata (maize leaf spot) | 2-methylbutyric acid 2-heptanone isopentyl acetate | Xie et al. [83] |
| B. subtilis ZD01 | Alternaria solani (potato early blight) | acetophenone 2-nonanone m-tolunitrile 2-ethylhexanol 2-heptanone benzylacetone 6-methyl-2-heptanone benzothiazole 5-methyl-2-hexanone | Zhang et al. [71] |
| 6-methyl-2-heptanone | Zhang et al., [68] | ||
| B. subtilis BS-01 | Alternaria solani (tomato Alternaria blight) | triphenylphosphine oxide pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(phenylmethyl) n-hexadecanoic acid n-tridecan-1-ol octadecane octadecanoic acid eicosane dodecyl acrylate | Awan et al. [109] |
| B. amyloliquefaciens JBC36 | Penicillium digitatum (green mold of citrus fruit) Penicillium italicum (blue mold of citrus fruit) | mixture of VOCs | Yu et al. [78] |
| B. amyloliquefaciens NJN-6 | Fusarium oxysporum f. sp. cubense | phenol 2,3,6-trimethyl-phenol 2-undecanone 2-dodecanone 2-tridecanone | Yuan et al., 2012 [44] |
| mixture of VOCs | Yuan et al. [110] | ||
| B. amyloliquefaciens W19 | Fusarium oxysporum f. sp. cubense (bannana Fusarium wilt) | o-xylene 2-heptanone benzene, 2-propenyl benzene,1,4-dichloro undecane, 1,2-methyl acetophenone 2-nonanone nonanane 1-(4-methylphenyl)ethanone 2- decanone naphthalene 2-undecanone tridecane 2- dodecanone tetradecane 2-tridecanone pentadecane hexadecane tetradecane | Wang et al. [111] |
| B. amyloliquefaciens NJZJSB3 | Sclerotinia sclerotiorum (canola stem rot) | toluene phenol benzothiazole | Wu et al. [99] |
| B. amyloliquefaciens CPA-8 | Monilinia laxa Monilinia fructicola Botrytis cinera (sweet cherry decay) | 1,3-pentadiene acetoin thiophene | Gotor-Vila et al. [30] |
| B. amyloliquefaciens DA12 | Fusarium asiaticum Fusarium graminearum Fusarium proliferatum Fusarium verticillioides Fusarium oxysporum f. sp. lycopersici (tomato wilt) Botrytis cinerea (cucumber grey mould) Colletotrichum coccodes (pepper anthracnose) Endothia parasitica (chestnut blight) Raffaelea quercus-mongolicae (oak wilt) Rhizoctonia solani (rice sheath blight) | 2-heptanone 5-methyl heptanone 6-methyl heptanone | Lee et al. [51] |
| B. amyloliquefaciens L3 | Fusarium oxysporum f. sp. niveum (watermellon Fusarium wilt) | 2-nonanone 2-heptanone | Wu et al. [96] |
| B. amylolicefaciens ALB629 B. amylolicefaciens UFLA285 | Colletotrichum lindemuthianum (common bean antrachnose) | 3-methylbutanoic 2-methylbutanoic acid | Martins et al. [41] |
| B. amyloliquefaciens BsA3MX B. amyloliquefaciens BsC11MX | Macrophomina phaseolina (cowpea charcoal rot) | mixture of VOCs | Rangel- Montoya et al. [112] |
| B. amyloliquefaciens D747 (Amylo-X®) B. amyloliquefaciens FZB24 (Taegro®) B. amyloliquefaciens MBI600 (Serifel®) B. amyloliquefaciens QST713 (Serenade®Aso) | Plenodomus tracheiphilus (Mal Secco disease of Citrus volkameriana) | mixture of VOCs | Aiello et al. [113] |
| B. velezensis 5YN8 B. velezensis DSN012 | Botrytis cinerea (pepper gray mold) | mixture of VOCs | Jiang et al. [91] |
| B. velezensis NKG-2 | Fusarium oxysporum Fusarium graminearum Botrytis cinerea Alternaria alternata Fulvia fulva Ustilaginoidea virens | mixture of VOCs | Myo et al. [55] |
| B. velezensis C2 | Verticillium dahliae (tomato wilt disease) | mixture of VOCs | Dhouib et al. [63] |
| B. velezensis OEE1 | Fusarium solani | mixture of VOCs | Cheffi et al. [114] |
| Verticillium dahliae | cyclo (Leu-Pro) | Cheffi Azabou et al. [86] | |
| B. velezensis XT1 | Verticillium dahliae (Verticillium wilt of olive tree) | mixture of VOCs | Castro et al. [85] |
| B. velezensis RDA1 | Rosellinia necatrix | mixture of VOCs | Sawant et al. [46] |
| B. velezensis JCK-1618 B. velezensis JCK-1696 | Epicoccum tobaicum Mycosphaerella cerasella | mixture of VOCs | Han et al. [25] |
| B. velezensis ZSY-1 | Alternaria solani Botrytis cinerea Valsa mali Monilinia fructicola Fusarium oxysporum f. sp. capsicum Colletotrichum lindemuthianum | pyrazine (2,5-dimethyl) benzothiazole 4-chloro-3-methyl phenol-2,4-bis (1,1-dimethylethyl) | Gao et al. [50] |
| B. velezensis G341 | Alternaria panax (ginseng blight) Botrytis cinerea (tomato gray mold) Colletotrichum coccodes (red pepper antrachnose) Fusarium oxysporum f. sp. lycopersici (tomato Fusarium wilt) Magnaporthe oryzae (rice blast) Phytophthora capsici Pythium infestans (tomato late blight) Pythium ultimum (cucumber damping-off) Rhizoctonia solani (rice sheath blight) Sclerotinia sclerotiorum (cucumber Sclerotinia rot) | dimethylsulfoxide 1-butanol acetoin | Lim et al. [54] |
| B. velezensis BUZ-14 B. velezensis I3 B. velezensis I5 | Monilinia fructicola Monilinia laxa Penicillium italicum Botrytis cinerea | benzaldehyde diacetyl | Calvo et al. [115] |
| B. velezensis CT32 | Verticillium dahliae Glomerella cingulata Thanatephorus cucumeris F. oxysporum f. sp. cucumerinum F. oxysporum f. sp. fragariae F. oxysporum f. sp. niveum Botryosphaeria dothidea Botrytis cinerea | decanal benzothiazole 3-undecanone 2-undecanone 2-undecanol undecanal 2,4-dimethyl-6-tert-butylphenol | Li et al. [116] |
| B. velezensis CE 100 | Colletotrichum gloeosporioides (walnut and jujube antrachnose) | 5-nonylamine 3-methylbutanoic acid | Choub et al. [75] |
| Colletotrichum acutatum Colletotrichum coccodes Colletotrichum dematium Colletotrichum gloeosporioides | mixture of VOCs | Kim et al. [74] | |
| B. velezensis JRX-YG39 | Botrytis cinerea Fusarium pernambucanum Alternaria alternata Colletotrichum gloeosporioides | dibutyl phthalate | Feng et al. [103] |
| B. velezensis L1 | Alternaria iridiaustralis Phytophthora capsici Colletotrichum capsici Fusarium oxysporum Fusarium graminearum Fusarium annulatum Fusarium arcuatisporum Botrytis cinerea Rhizoctonia solani Talaromyces tumuli Colletotrichum fioriniae | 2,3-butanedione | Ling et al. [73] |
| B. velezensis ZJ1 | Alternaria solani (tomato early blight) Botrytis cinerea (tomato gray mold) | isooctanol 2-nonanol | Ren et al. [70] |
| B. velezensis HY-3479 | Colletotrichum acutatum (pepper ripe rot) Cylindrocarpon destructans (ginseng root rot) Rhizoctonia solani (pepper damping-off) Sclerotinia sclerotiorum (pepper white mold) | 3-methyl-1-butanol (R, R)-2,3-butanediol acetoin benzoic acid | Song et al. [117] |
| B. velezensis SBB | Verticillium dahliae | 2-nonanol 2-heptanone 6-methyl-2-heptanone 2-nonanone | Wang et al. [80] |
| B. licheniformis BL350-2 | Aspergillus westerdijkiae Aspergillus carbonarius Aspergillus niger Aspergillus flavus Aspergillus parasiticus Aspergillus ochraceus Penicillium verrucosum | 3-methyl-1-butanol | Ul Hassan et al. [105] |
| B. pumilus TM-R | Alternaria alternata Cladosporium cladosporioides Curvularia lunata Fusarium oxysporum Penicillium italicum | methyl isobutyl ketone ethanol 5-methyl-2-heptanone S-()-2-methylbutylamine | Morita et al. [34] |
| B. megaterium BmBP17 | Phytophthora capsici Magnaporthe oryzae | pyrazine, 2-ethyl-3-methyl pyrazine, 2-ethyl- pyrazine, 2,5-dimethyl pyrazine, 2-methyl | Munjal et al. [27] |
| B. megaterium BM344-1 | Aspergillus flavus Aspergillus carbonarius Penicillium verrucosum Fusarium verticillioides | hexadecanoic acid methyl ester (palmitic acid) tetracosane | Saleh et al. [104] |
| B. mycoides | Rhizoctonia solani Kühn Pythium aphanidermatum Edson (cabbage damping-off) | dimethyl disulphide ammonia | Huang et al. [29] |
| B. mycoides BM02 | Fusarium oxysporum f. sp. lycopersici (tomato Fusarium wilt) | phenylacetic acid methylphenyl acetate | Wu et al. [81] |
| B. atrophaeus CAB-1 | Botrytis cinerea (tomato gray mold) Sphaerotheca fuliginea (cucumber powdery mildew) | O-anisaldehyde | Zhang et al. [60] |
| B. atrophaeus JZB120050 | Botrytis cinerea Fusarium oxysporum f. sp. conglutinans Fusarium oxysporum f. sp. niveum Fusarium oxysporum f. sp. vasinfectum Fusarium solani f. sp. pisi Fusarium oxysporum Schlecht Fusarium graminearum Rhizoctonia cereal Gaeumannomyces graminis Monilinia fructicola Botryosphaeria dothidea Colletotrichum gloeosporioides | mixture of VOCs | Ni et al. [28] |
| B. atrophaeus HAB-5 | Colletotrichum gloeosporioides | chloroacetic acid tetradecyl esters octadecane hexadecanoic acid, methyl ester | Rajaofera et al. [32] |
| B. cereus MH778713 | Fusarium oxysporum (tomato Fusarium wilt) | hentriacontane 2,4-di-tert-butylphenol | Ramírez et al. [97] |
| B. cereus CF4-51 | Sclerotinia sclerotiorum | 2-pentadecanone, 6,10,14-trimethyl- 1,2-benzenedicarboxylic acid bis(2-methylpropyl) ester dibutyl phthalate cyclododecane heptadecane | Hu et al. [66] |
| B. mojavensis I4 | Fusarium verticillioides Fusarium graminearum Rhizoctonia solani | mixture of VOCs | Ghazala et al. [76] |
| B. siamensis G-3 | Botrytis cinerea Rhizopus stolonifer | 2,6-di-tert-butyl-4-methylphenol 2,4-di-tert-butylphenol | Zhang et al. [39] |
| B. siamensis N-1 | Colletotrichum gloeosporioides Glomerella sp. Pestalotiopsis microspora Diaporthe phaseolorum Phomopsis sp. Diaporthe phaseolorum Geotrichum candidum Fusarium lateritium Fusarium oxysporm Fusarium equiseti Fusarium incarnatum Fusarium sp. Lasiodiplodia theobromae Phomopsis caricae-papayae Thielaviopsis paradoxa | 1-undecene 3-methyl-1-butanol 2-nonanone 1,3,5,7-cyclooctatetraene phenol | You et al. [79] |
| B. siamensis LZ88 | Alternaria alternata (tobacco brown spot) | mixture of VOCs | Xie et al. [92] |
| 2-methylbutanoic acid 3-methylbutanoic acid | Wang et al. [69] | ||
| B. safensis STJP | Alternaria alternata | phenol, 2,4-bis (1,1-dimethylethyl)-3-hexadecanol pyrrolo (1,2-a)pyrazine-1,4-dione hexahydro-3-(2-methyl-propyl)- 5,10-diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo(1,2-a:10,20-d)pyrazine hexadecanoic acid | Prakash and Arora [82] |
| Bacillus sp. B44 | Fusarium oxysporum f. sp. lycopersici | mixture of VOCs | Jangir et al. [40] |
| Bacillus sp. ACB-65 Bacillus sp. ACB-73 | Phyllosticta citricarpa (orange black spot) | mixture of VOCs | Fujimoto et al. [31] |
| Bacillus sp. T6 | Verticillium dahliae (cotton Verticillium wilt) | styrene | Zhang et al. [64] |
| Bacillus sp. LPPC170 | Fusarium kalimantanense (Panama disease of bannana) | acetic acid propanoic acid butanoic acid valeric acid isovaleric acid | de Ávila Santos et al. [67] |
| B. subtilis XF-1 B. amyloliquefaciens subsp. plantarum FZB42 B. amyloliquefaciens subsp. plantarum YAU B9601-Y2 B. subtilis 168 Bacillus spp. strains 033, 041, 355 and 285 | Fusarium solani | mixture of VOCs | Li et al. [97] |
| B. cereus KY094642 B. safensis KY094643 | Alternaria sp. (leaf spot and blight disease of lentils) | mixture of VOCs | Roy et al. [118] |
| B. amyloliquefaciens RS-25 B. licheniformis MG-4 B. subtilis Z-14 B. subtilis Pnf-4 | Botrytis cinerea (tomato, strawberry, and grapefruit gray mold) | mixture of VOCs | Chen et al. [119] |
| B. methylotrophicus BCN2 B. thuringiensis BCN10 | Fusarium oxysporum Botryosphaeria sp. Trichoderma atroviride Colletotrichum gloeosporioides Penicillium expansum | mixture of VOCs | He et al. [106] |
| B. velezensis BUZ-14 B. ginsengihumi S38 | Botrytis cinerea | mixture of VOCs | Calvo et al. [35] |
| B. mycoides B. subtilis B. thuringiensis | Aspergillus ochraceus Aspergillus westerdijkiae Aspergillus flavus Aspergillus parasiticus | mixture of VOCs | Hlebová et al. [53] |
| B. safensis RGM 2450 B. siamensis RGM | Botrytis cinerea Colletotrichum acutatum Fusarium oxysporum Phytophtora cinnamomi | mixture of VOCs | Altimira et al. [120] |
| B. subtilis PPCB001 B. amyloliquefaciens PPCB004 | Penicillium digitatum Sacc. Penicillium italicum Wehmer Penicillium crustosum Thom | acetoin | Arrebola et al. [42] |
| B. pumilus TB09 B. thuringiensis TB72 | Colletotrichum gloeosporioides (mango antrachnose) | 2-nonanone b-benzeneethanamine 2-decanone thymol 2-methylpyrazine | Zheng et al. [94] |
| B. subtilis B. amyloliquefaciens B. cereus | Aspergillus niger Aspergillus flavus Aspergillus parasiticus Aspergillus clavatus Fusarium oxysporum f.sp. lactucae Moniliophthora perniciosa | propanone 1-butanol 3-methyl-1-butanol acetic acid 2-methylpropanoic acid carbon disulphide 3-methylbutanoic acid ethyl acetate | Chaves-Lopez et al. [1] |
| B. vallismortis 12a B. altitudinis 14b | Monilinia fructicola (peach brown rot) | 6-methyl-2-heptanone 2-pentylfuran cedrol isodecyl methacrylate | Liu et al. [65] |
| B. velezensis VM11 B. velezensis VM10 B. amyloliquefaciens VM42 | Sclerotinia sclerotiorum | 2-undecanone 1,3-butadiene benzothiazole N,N-dimethyldodecylamine | Massawe et al. [57] |
| B. amyloliquefaciens HA B. stratosphericus SO B. acidiceler SJJ B. mycoides HB | Fusarium solani Fusarium sp. Colletotrichum gloeosporioides Phytophthora cinnamomi | 2,3,5-trimethylpyrazine 2-nonanone 2-decanone 2-dodecanone dimethyl disulfide dimethyl trisulfide | Guevara-Avendaño et al. [121] |
| B. nakamurai B. pseudomycoides B. proteolyticus B. thuringiensis | Botrytis cinerea | 2-heptanone dodecanal dimethyl disulfide dimethyl trisulfide 3-methylbutan-1-ol | Chaouachi et al. [122] |
| Paenibacillus polymyxa BMP-11 B. subtilis BL02 B. pumilus BSH-4 B. pumilus ZB13 | Sclerotinia sclerotiorum Botrytis cinerea Alternaria brassicae Alternaria solani Ascochyta citrullina Fusarium oxysporum Cercospora kikuchii Chupp Rhizoctonia solani Phoma arachnidicola Verticillium dahiae Fusarium graminerum | mixture of VOCs | Liu et al. [56] |
| Pseudomonas fluorescens Pf 9A-14 Pseudomonas sp. Psp. 8D-45 B. subtilis Bs 8B-1 | Pythium capsici (cucumber damping-off) Phytophthora capsici (cucumber root rot) Rhizoctonia solani (radish damping-off) | mixture of VOCs | Khabbaz et al. [45] |
| B. megaterium KU143 Pseudomonas protegens AS15 | Aspergillus flavus | mixture of VOCs | Mannaa et al. [52] |
| Pichia kudriavzevii Candida labiduridarum B. acidiceler B. macauenses B. amyloliquefaciens B. pumilus | Sclerotinia sclerotiorum | mixture of VOCs | Cavalcanti et al. [123] |
| B. amyloliquefaciens SF14 B. amyloliquefaciens SP10 Alcaligenes faecalis ACBC1 Pantoea agglomerans ACBP1 | Monilinia fructigena Monilinia laxa (apple brown rot) | mixture of VOCs | Lahlali et al. [101] |
| Pseudomonas brassicacearum Pseudomonas putida B. megaterium | Botrytis cinerea Phytophthora nicotianae Rhizoctonia solani Sclerotinia sclerotiorum Verticillium dahliae Fusarium oxysporum Macrophomina phaseolina | acetic acid 2-nonanone dimethyl trisulfide | Giorgio et al. [49] |
| Bacillus spp. Paenibacillus spp. | Rhizoctonia solani Fusarium graminearum Phytophthora capsici Pythium aphanidermatum Podosphaera fuliginea | acetoin diacetyl | Khalaf and Raizada [124] |
| B. amyloliquefaciens LI24 B. amyloliquefaciens PP19 B. licheniformis HS10 B. pumilus PI26 Exiguobacterium acetylicum SI17 | Peronophythora litchii (litchi downy blight) | 1-(2-aminophenyl)ethanone benzothiazole α-farnesene | Zheng et al. [38] |
| B. amyloliquefaciens UQ154 B. velezensis UQ156 Acinetobacter sp. UQ202 | Phytophthora capsici | isovaleraldehyde 2-ethylhexanol 2-heptanone benzyl alcohol 3-methylbutanol | Syed-Ab-Rahman et al. [87] |
| B. atrophaeus L193 B. velezensis XT1 Psychrobacillus vulpis Z8 | Alternaria alternata Botrytis cinerea Fusarium oxysporum Fusarium solani Monilinia fructicola Monilinia laxa Sclerotinia sclerotiorum | acetoin acetic acid 2,3-butanediol isopentanol dimethyl disulphide isopentyl isobutanoate | Toral et al. [62] |
4. Nematicidal Action of Bacillus-Based VOCs
4.1. Styrene as the Nematicidal Bacillus-Based VOC
4.2. Interference of Bacillus-Based VOCs with Nematodes’ Chemotaxis
4.3. Interference of Bacillus-Based VOCs with Nematodes’ Antioxidant Metabolism
4.4. Specific Bacillus-Based VOCs Exhibiting Nematicidal Action
| Bacillus Strain | Plant Pathogen | Nematicidal VOCs | Reference |
|---|---|---|---|
| B. mycoides R2 | Caenorhabditis elegans Meloidogyne incognita | styrene | Luo et al. [126] |
| B. subtilis Bs-1 | Meloidogyne incognita | CO2 acetic acid 2-heptanone pyrazine, 2,5-dimethyl- dimethyl disulfide | Cao et al. [125] |
| Bacillus sp. GBSC56 | Meloidogyne incognita | dimethyl disulfide methyl isovalerate 2-undecanone | Ayaz et al. [131] |
| B. cereus Bc-cm103 | Meloidogyne incognita | dimethyl disulfide S-methyl ester butanethioic acid | Yin et al. [130] |
| B. licheniformis JF-22 | Meloidogyne incognita | acetoin 2,3-butanediol hexamethyl cyclotrisiloxane | Du et al. [132] |
| B. altitudinis AMCC 1040 | Meloidogyne incognita | 2,3-butanedione acetic acid 2-isopropoxy ethylamine 2-methyl-butyric acid 3-methylbutyric acid octanoic acid | Ye et al. [133] |
| Bacillus megaterium YMF3.25 | Meloidogyne incognita | benzeneacetaldehyde decanal dimethyl disulfide 2-nonanone 2-undecanone | Huang et al. [129] |
| Bacillus aryabhattai MCCC 1K02966 | Meloidogyne incognita | dimethyl disulfide methyl thioacetate | Chen et al. [134] |
5. Future Outlook on Bacillus-Based VOCs Research and Application
5.1. The Necessity to Investigate the Effects of Microbial Communities on Bacillus-VOCs Synthesis and Vice-Versa
5.2. The Necessity to Better Understand the VOCs’ Mechanisms of Action against Broader Spectrum of Plant Pathogens and Hosts
5.3. Research Directions Related to VOCs Production by Bacillus spp.
5.4. VOCs Application-Related Remarks
5.5. Possible Risks of Bacillus-Based VOCs Application in Biocontrol of Plant Diseases and Pathogens
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chaves-López, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Paramasivan, M.; Sahayarayan, J.J. Microbial volatile organic compounds: An alternative for chemical fertilizers in sustainable agriculture development. Microorganisms 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Kai, M. Diversity and distribution of volatile secondary metabolites throughout Bacillus subtilis isolates. Front. Microbiol. 2020, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zang, H.; Jun Wu, H.; Uddin Rajer, F.; Gao, X. Antibacterial effects of volatiles produced by Bacillus Strain D13 against Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 2018, 19, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rajer, F.U.; Wu, H.; Xie, Y.; Xie, S.; Raza, W.; Tahir, H.A.S.; Gao, X. Volatile organic compounds produced by a soil-isolate, Bacillus subtilis fa26 induce adverse ultra-structural changes to the cells of Clavibacter michiganensis ssp. sepedonicus, the causal agent of bacterial ring rot of potato. Microbiology 2017, 163, 523–530. [Google Scholar] [CrossRef]
- Ajilogba, C.F.; Babalola, O.O. GC–MS analysis of volatile organic compounds from Bambara groundnut rhizobacteria and their antibacterial properties. World J. Microbiol. Biotechnol. 2019, 35, 83. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Jousset, A.; Friman, V.P.; Mei, X.; Wang, S.; Wei, Z.; Shen, Q. Bacterial community richness shifts the balance between volatile organic compound-mediated microbe–pathogen and microbe–plant interactions. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200403. [Google Scholar] [CrossRef]
- Lammers, A.; Lalk, M.; Garbeva, P. Air ambulance: Antimicrobial power of bacterial volatiles. Antibiotics 2022, 11, 109. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Safdar, A.; Huang, Z.; Rajer, F.U.; Gao, X. Effect of volatile compounds produced by Ralstonia solanacearum on plant growth promoting and systemic resistance inducing potential of Bacillus volatiles. BMC Plant Biol. 2017, 17, 133. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. MVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef]
- Thomas, G.; Withall, D.; Birkett, M. Harnessing microbial volatiles to replace pesticides and fertilizers. Microb. Biotechnol. 2020, 13, 1366–1376. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef]
- Rudrappa, T.; Biedrzycki, M.L.; Kunjeti, S.G.; Donofrio, N.M.; Czymmek, K.J.; Paré, P.W.; Bais, H.P. The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun. Integr. Biol. 2010, 3, 130–138. [Google Scholar] [CrossRef]
- Song, G.C.; Ryu, C.-M. Two volatile organic compounds trigger plant self-defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef]
- Song, G.C.; Riu, M.; Ryu, C.-M. Beyond the two compartments Petri-dish: Optimising growth promotion and induced resistance in cucumber exposed to gaseous bacterial volatiles in a miniature greenhouse system. Plant Methods 2019, 15, 9. [Google Scholar] [CrossRef]
- Song, G.C.; Choi, H.K.; Ryu, C.-M. Gaseous 3-pentanol primes plant immunity against a bacterial speck pathogen, Pseudomonas syringae pv. tomato via salicylic acid and jasmonic acid-dependent signaling pathways in Arabidopsis. Front. Plant Sci. 2015, 6, 821. [Google Scholar] [CrossRef]
- Choi, H.K.; Song, G.C.; Yi, H.-S.; Ryu, C.-M. Field evaluation of the bacterial volatile derivative 3-pentanol in priming for induced resistance in pepper. J. Chem. Ecol. 2014, 40, 882–892. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Wang, J.; Wu, Y.; Ling, N.; Wei, Z.; Huang, Q.; Shen, Q. Effects of volatile organic compounds produced by Bacillus amyloliquefaciens on the growth and virulence traits of tomato bacterial wilt pathogen Ralstonia solanacearum. Appl. Microbiol. Biotechnol. 2016, 100, 7639–7650. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Wei, Z.; Ling, N.; Huang, Q.; Shen, Q. Effect of organic fertilizers prepared from organic waste materials on the production of antibacterial volatile organic compounds by two biocontrol Bacillus amyloliquefaciens strains. J. Biotechnol. 2016, 227, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Niu, Y.; Huo, R.; Gao, X. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 2017, 7, 40481. [Google Scholar] [CrossRef] [PubMed]
- Han, V.-C.; Yu, N.H.; Yoon, H.; Ahn, N.-H.; Son, Y.K.; Lee, B.-H.; Kim, J.-C. Identification, characterization, and efficacy evaluation of Bacillus velezensis for shot-hole disease biocontrol in flowering cherry. Plant Pathol. J. 2022, 38, 115–130. [Google Scholar] [CrossRef]
- Jelušić, A.; Popović, T.; Dimkić, I.; Mitrović, P.; Peeters, K.; Miklavčić Višnjevec, A.; Tavzes, Č.; Stanković, S.; Berić, T. Changes in the winter oilseed rape microbiome affected by Xanthomonas campestris pv. campestris and biocontrol potential of the indigenous Bacillus and Pseudomonas isolates. Biol. Control 2021, 160, 104695. [Google Scholar] [CrossRef]
- Munjal, V.; Nadakkakath, A.V.; Sheoran, N.; Kundu, A.; Venugopal, V.; Subaharan, K.; Rajamma, S.; Eapen, S.J.; Kumar, A. Genotyping and identification of broad spectrum antimicrobial volatiles in black pepper root endophytic biocontrol agent, Bacillus megaterium BP17. Biol. Control 2015, 92, 66–76. [Google Scholar] [CrossRef]
- Ni, M.; Wu, Q.; Wang, J.; Liu, W.C.; Ren, J.H.; Zhang, D.P.; Zhao, J.; Liu, D.E.W.; Rao, Y.H.; Lu, C.G. Identification and comprehensive evaluation of a novel biocontrol agent Bacillus atrophaeus. J. Environ. Sci. Health Part B 2018, 53, 777–785. [Google Scholar] [CrossRef]
- Huang, J.-S.; Peng, Y.-H.; Chung, K.-R.; Huang, J.-W. Suppressive efficacy of volatile compounds produced by Bacillus mycoides on damping-off pathogens of cabbage seedlings. J. Agric. Sci. 2018, 156, 795–809. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Teixidó, N.; Di Francesco, A.; Usall, J.; Ugolini, L.; Torres, R.; Mari, M. Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 2017, 64, 219–225. [Google Scholar] [CrossRef]
- Fujimoto, A.; Augusto, F.; Fill, T.P.; Moretto, R.K.; Kupper, K.C. Biocontrol of Phyllosticta citricarpa by Bacillus spp.: Biological and chemical aspects of the microbial interaction. World J. Microbiol. Biotechnol. 2022, 38, 53. [Google Scholar] [CrossRef]
- Asari, S.; Matzén, S.; Petersen, M.A.; Bejai, S.; Meijer, J. Multiple effects of Bacillus amyloliquefaciens volatile compounds: Plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol. Ecol. 2016, 92, fiw070. [Google Scholar] [CrossRef]
- Rajaofera, M.J.N.; Wang, Y.; Dahar, G.Y.; Jin, P.; Fan, L.; Xu, L.; Liu, W.; Miao, W. Volatile Organic compounds of Bacillus atrophaeus HAB-5 inhibit the growth of Colletotrichum gloeosporioides. Pestic. Biochem. Physiol. 2019, 156, 170–176. [Google Scholar] [CrossRef]
- Morita, T.; Tanaka, I.; Ryuda, N.; Ikari, M.; Ueno, D.; Someya, T. Antifungal spectrum characterization and identification of strong volatile organic compounds produced by Bacillus pumilus TM-R. Heliyon 2019, 5, e01817. [Google Scholar] [CrossRef]
- Calvo, H.; Roudet, J.; Gracia, A.P.; Venturini, M.E.; Fermaud, M. Comparison of efficacy and modes of action of two high-potential biocontrol Bacillus strains and commercial biocontrol products against Botrytis cinerea in table grapes. OENO One 2021, 3, 229–243. [Google Scholar] [CrossRef]
- Gao, H.; Li, P.; Xu, X.; Zeng, Q.; Guan, W. Research on volatile organic compounds from Bacillus subtilis CF-3: Biocontrol effects on fruit fungal pathogens and dynamic changes during fermentation. Front. Microbiol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Gao, H.; Xu, X.; Zeng, Q.; Li, P. Optimization of headspace solid-phase microextraction for gc-ms analysis of volatile compounds produced by biocontrol strain Bacillus subtilis CF-3 using response surface methodology. Food Sci. Technol. Res. 2017, 23, 583–593. [Google Scholar] [CrossRef]
- Zheng, L.; Situ, J.; Zhu, Q.; Xi, P.; Zheng, Y.; Liu, H.; Zhou, X.; Jiang, Z. Identification of volatile organic compounds for the biocontrol of postharvest litchi fruit pathogen Peronophythora litchii. Postharvest Biol. Technol. 2019, 155, 37–46. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Z.; Zhang, X.; Bai, W.; Zhang, L.; Pei, H.; Zhang, Y. Control effects of Bacillus siamensis G-3 volatile compounds on raspberry postharvest diseases caused by Botrytis cinerea and Rhizopus stolonifer. Biol. Control 2020, 141, 104135. [Google Scholar] [CrossRef]
- Jangir, M.; Pathak, R.; Sharma, S.; Sharma, S. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol. Control 2018, 123, 60–70. [Google Scholar] [CrossRef]
- Martins, S.J.; Faria, A.F.; Pedroso, M.P.; Cunha, M.G.; Rocha, M.R.; Medeiros, F.H.V. Microbial volatiles organic compounds control anthracnose (Colletotrichum lindemuthianum) in common bean (Phaseolus vulgaris L.). Biol. Control 2019, 131, 36–42. [Google Scholar] [CrossRef]
- Arrebola, E.; Sivakumar, D.; Korsten, L. Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol. Control 2010, 53, 122–128. [Google Scholar] [CrossRef]
- Leelasuphakul, W.; Hemmanee, P.; Chuenchitt, S. Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol. Technol. 2008, 48, 113–121. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef] [PubMed]
- Khabbaz, S.E.; Zhang, L.; Cáceres, L.A.; Sumarah, M.; Wang, A.; Abbasi, P.A. Characterisation of antagonistic Bacillus and Pseudomonas strains for biocontrol potential and suppression of damping-off and root rot diseases. Ann. Appl. Biol. 2015, 166, 456–471. [Google Scholar] [CrossRef]
- Sawant, S.S.; Song, J.; Seo, H.J. Characterization of Bacillus velezensis RDA1 as a biological control agent against white root rot disease caused by Rosellinia necatrix. Plants 2022, 11, 2486. [Google Scholar] [CrossRef]
- Liu, W.; Mu, W.; Zhu, B.; Lui, F. Antifungal activities and components of VOCs produced by Bacillus subtilis G8. Curr. Res. Bacteriol. 2008, 1, 28–34. [Google Scholar] [CrossRef]
- Islam, M.R.; Jeong, Y.T.; Lee, Y.S.; Song, C.H. Isolation and identification of antifungal compounds from Bacillus subtilis C9 inhibiting the growth of plant pathogenic fungi. Mycobiology 2018, 40, 59–65. [Google Scholar] [CrossRef]
- Giorgio, A.; De Stradis, A.; Lo Cantore, P.; Iacobellis, N.S. Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 2015, 6, 1056. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis zsy-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Lee, T.; Park, D.; Kim, K.; Lim, S.M.; Yu, N.H.; Kim, S.; Kim, H.Y.; Jung, K.S.; Jang, J.Y.; Park, J.C.; et al. Characterization of Bacillus amyloliquefaciens DA12 showing potent antifungal activity against mycotoxigenic Fusarium species. Plant Pathol. J. 2017, 33, 499–507. [Google Scholar] [CrossRef]
- Mannaa, M.; Oh, J.Y.; Kim, K.D. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus flavus and aflatoxin production on stored rice grains. Mycobiology 2017, 45, 213–219. [Google Scholar] [CrossRef]
- Hlebová, M.; Uzsáková, V.; Charousová, I.; Hleba, L. The in vitro antagonistic effects of some Bacillus spp. on the growth and mycotoxin production of toxigenic aspergilli. J. Microbiol. Biotech. Food Sci. 2021, 11, e5412. [Google Scholar] [CrossRef]
- Lim, S.M.; Yoon, M.Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Shin, T.S.; Park, H.W.; Yu, N.H.; Kim, Y.H.; Kim, J.C. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol. J. 2017, 33, 488–498. [Google Scholar] [CrossRef]
- Myo, E.M.; Liu, B.; Ma, J.; Shi, L.; Jiang, M.; Zhang, K.; Ge, B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Liu, W.; Mu, W.; Zhu, B.; Du, Y.; Liu, F. Antagonistic activities of volatiles from four strains of Bacillus spp. and Paenibacillus spp. against soil-borne plant pathogens. Agric. Sci. China 2008, 7, 1104–1114. [Google Scholar] [CrossRef]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Tahir, H.A.S.; Gu, Q.; Wu, H.; Gao, X. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef]
- Monteiro, F.P.; Ferreira, L.C.; Pacheco, L.P.; Souza, P.E. Antagonism of Bacillus subtilis against Sclerotinia sclerotiorum on Lactuca sativa. J. Agric. Sci. 2013, 5, 214–223. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, T.; Shen, A.; Yang, X.; Yu, Y.; Gao, C.; Li, Z.; Cheng, Y.; Chen, J.; Guo, L.; et al. Biocontrol potential of Bacillus subtilis IBFCBF-4 against Fusarium wilt of watermelon. J. Plant. Pathol. 2020, 102, 433–441. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Wang, Y.; Guo, Q.; Lu, X.; Li, S.; Ma, P. Lipopeptides, a Novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Appl. Microbiol. Biotechnol. 2013, 97, 9525–9534. [Google Scholar] [CrossRef]
- Mu, J.; Li, X.; Jiao, J.; Ji, G.; Wu, J.; Hu, F.; Li, H. Biocontrol potential of vermicompost through antifungal volatiles produced by indigenous bacteria. Biol. Control 2017, 112, 49–54. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Martínez-Checa, F.; Montaño, A.; Cortés-Delgado, A.; Smolinska, A.; Llamas, I.; Sampedro, I. Identification of volatile organic compounds in extremophilic bacteria and their effective use in biocontrol of postharvest fungal phytopathogens. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Dhouib, H.; Zouari, I.; Abdallah, D.B.; Belbahri, L.; Taktak, W.; Triki, M.A.; Tounsi, S. Potential of a novel endophytic Bacillus velezensis in tomato growth promotion and protection against Verticillium wilt disease. Biol. Control 2019, 139, 104092. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Lei, S.; Zhang, H.; Liu, Z.; Yang, J.; Niu, Q. Effect of volatile compounds produced by the cotton endophytic bacterial strain Bacillus sp. T6 against Verticillium Wilt. BMC Microbiol. 2023, 23, 8. [Google Scholar] [CrossRef]
- Liu, C.; Yin, X.; Wang, Q.; Peng, Y.; Ma, Y.; Liu, P.; Shi, J. Antagonistic activities of volatiles produced by two Bacillus strains against Monilinia fructicola in peach fruit. J. Sci. Food Agric. 2018, 98, 5756–5763. [Google Scholar] [CrossRef]
- Hu, J.; Dong, B.; Wang, D.; Meng, H.; Li, X.; Zhou, H. Genomic and metabolic features of Bacillus cereus, inhibiting the growth of Sclerotinia sclerotiorum by synthesizing secondary metabolites. Arch. Microbiol. 2023, 205, 8. [Google Scholar] [CrossRef]
- de Ávila Santos, J.E.; de Brito, M.V.; Ávila Pimenta, A.T.Á.; da Silva, G.S.; Zocolo, G.J.; Muniz, C.R.; de Medeiros, S.C.; Grangeiro, T.B.; Lima, M.A.S.; da Silva, C.F.B. Antagonism of volatile organic compounds of the Bacillus sp. against Fusarium kalimantanense. World J. Microbiol. Biotechnol. 2023, 39, 60. [Google Scholar] [CrossRef]
- Zhang, D.; Qiang, R.; Zhao, J.; Zhang, J.; Cheng, J.; Zhao, D.; Fan, Y.; Yang, Z.; Zhu, J. Mechanism of a volatile organic compound (6-methyl-2-heptanone) emitted from Bacillus subtilis ZD01 against Alternaria solani in potato. Front. Microbiol. 2022, 12, 808337. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Yuan, Y.; Chu, D.; Cao, J.; Sun, G.; Ai, Y.; Cui, Z.; Zhang, Y.; Wang, F.; et al. Identification of non-volatile and volatile organic compounds produced by Bacillus siamensis LZ88 and their antifungal activity against Alternaria alternata. Biol. Control 2022, 169, 104901. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, J.; Yin, H.; Qin, N.; Yao, F.; Ma, D.; Zhao, X. Antifungal activity and control efficiency of endophytic Bacillus velezensis ZJ1 strain and its volatile compounds against Alternaria solani and Botrytis cinerea. J. Plant Pathol. 2022, 104, 575–589. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.; Yang, Y.; Zhang, J.; Zhao, D.; Pan, Y.; Fan, S.; Yang, Z.; Zhu, J. Antifungal effects of volatiles produced by Bacillus subtilis against Alternaria solani in potato. Front. Microbiol. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Ling, L.; Zhao, Y.; Tu, Y.; Yang, C.; Ma, W.; Feng, S.; Lu, L.; Zhang, J. The inhibitory effect of volatile organic compounds produced by Bacillus subtilis CL2 on pathogenic fungi of wolfberry. J. Basic Microbiol. 2021, 61, 110–121. [Google Scholar] [CrossRef]
- Ling, L.; Luo, H.; Yang, C.; Wang, Y.; Cheng, W.; Pang, M.; Jiang, K. Volatile organic compounds produced by Bacillus velezensis L1 as a potential biocontrol agent against postharvest diseases of wolfberry. Front. Microbiol. 2022, 13, 987844. [Google Scholar] [CrossRef]
- Kim, T.Y.; Hwang, S.H.; Noh, J.S.; Cho, J.Y.; Maung, C.E.H. Antifungal potential of Bacillus velezensis CE 100 for the control of different Colletotrichum species through isolation of active dipeptide, cyclo-(d-phenylalanyl-d-prolyl). Int. J. Mol. Sci. 2022, 23, 7786. [Google Scholar] [CrossRef]
- Choub, V.; Won, S.J.; Ajuna, H.B.; Moon, J.H.; Choi, S.I.; Lim, H.I.; Ahn, Y.S. Antifungal activity of volatile organic compounds from Bacillus velezensis CE 100 against Colletotrichum gloeosporioides. Horticulturae 2022, 8, 557. [Google Scholar] [CrossRef]
- Ghazala, I.; Chiab, N.; Saidi, M.N.; Gargouri-Bouzid, R. Volatile organic compounds from Bacillus mojavensis I4 promote plant growth and inhibit phytopathogens. Physiol. Mol. Plant Pathol. 2022, 121, 101887. [Google Scholar] [CrossRef]
- Zhou, M.; Li, P.; Wu, S.; Zhao, P.; Gao, H. Bacillus subtilis CF-3 volatile organic compounds inhibit Monilinia fructicola growth in peach fruit. Front. Microbiol. 2019, 10, 1804. [Google Scholar] [CrossRef]
- Yu, S.-M.; Oh, B.-T.; Lee, Y.H. Biocontrol of green and blue molds in postharvest satsuma mandarin using Bacillus amyloliquefaciens JBC36. Biocontrol Sci. Technol. 2012, 22, 1181–1197. [Google Scholar] [CrossRef]
- You, W.; Ge, C.; Jiang, Z.; Chen, M.; Li, W.; Shao, Y. Screening of a broad-spectrum antagonist-Bacillus siamensis, and its possible mechanisms to control postharvest disease in tropical fruits. Biol. Control 2021, 157, 104584. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Kong, W.-L.; Liao, Y.-C.-Z.; Zhu, L.-H. Identification of Bacillus velezensis SBB and its antifungal effects against Verticillium dahliae. J. Fungi 2022, 8, 1021. [Google Scholar] [CrossRef]
- Wu, J.J.; Huang, J.W.; Deng, W.L. Phenylacetic acid and methylphenyl acetate from the biocontrol bacterium Bacillus mycoides BM02 suppress spore germination in Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2020, 11, 569263. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Arora, N.K. Novel metabolites from Bacillus safensis and their antifungal property against Alternaria alternata. Int. J. Gen. Mol. Microbiol. 2021, 114, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, J.; Gu, S.; Chen, X.; Jiang, H.; Ding, T. Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Ann. Microbiol. 2020, 70, 2. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.M. Are bacterial volatile compounds poisonous odors to a fungal pathogen Botrytis cinerea, alarm signals to Arabidopsis seedlings for eliciting induced resistance, or both? Front. Microbiol. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.; Torres, M.; Sampedro, I.; Martínez-Checa, F.; Torres, B.; Béjar, V. Biological control of Verticillium wilt on olive trees by the salt-tolerant strain Bacillus velezensis XT1. Microorganisms 2020, 8, 1080. [Google Scholar] [CrossRef]
- Cheffi Azabou, M.; Gharbi, Y.; Medhioub, I.; Ennouri, K.; Barham, H.; Tounsi, S.; Triki, M.A. The endophytic strain Bacillus velezensis OEE1: An efficient biocontrol agent against Verticillium wilt of olive and a potential plant growth promoting bacteria. Biol. Control 2020, 142, 104168. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.T.; Chung, F.Y.; Moyle, P.M.; Eltanahy, E.G.; Schenk, P.M. Soil bacterial diffusible and volatile organic compounds inhibit Phytophthora capsici and promote plant growth. Sci. Total Environ. 2019, 692, 267–280. [Google Scholar] [CrossRef]
- Wang, K.; Qin, Z.; Wu, S.; Zhao, P.; Zhen, C.; Gao, H. Antifungal mechanism of volatile organic compounds produced by Bacillus subtilis CF-3 on Colletotrichum gloeosporioides assessed using omics technology. J. Agric. Food Chem. 2021, 69, 5267–5278. [Google Scholar] [CrossRef]
- Zhao, P.; Li, P.; Wu, S.; Zhou, M.; Zhi, R.; Gao, H. Volatile organic compounds (VOCs) from Bacillus subtilis CF-3 reduce anthracnose and elicit active defense responses in harvested litchi fruits. AMB Express 2019, 9, 119. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.; Li, X.; Ma, L.; Gu, Q.; Wu, H.; Liu, J.; Borriss, R.; Wu, Z.; Gao, X. Stomatal closure and SA-, JA/ET-signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Jiang, C.H.; Liao, M.J.; Wang, H.K.; Zheng, M.Z.; Xu, J.J.; Guo, J.H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Xie, Z.; Li, M.; Wang, D.; Wang, F.; Shen, H.; Sun, G.; Feng, C.; Wang, X.; Chen, D.; Sun, X. Biocontrol efficacy of Bacillus siamensis LZ88 against brown spot disease of tobacco caused by Alternaria alternata. Biol. Control 2021, 154, 104508. [Google Scholar] [CrossRef]
- Lanisnik, R.T.; Wheeler, M.H. Melanin biosynthesis in the fungus Curvularia lunata (teleomorph: Cochliobolus lunatus). Can. J. Microbiol. 2003, 49, 110–119. [Google Scholar] [CrossRef]
- Zheng, M.; Shi, J.; Shi, J.; Wang, Q.; Li, Y. Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biol. Control 2013, 65, 200–206. [Google Scholar] [CrossRef]
- Li, X.Y.; Mao, Z.C.; Wu, Y.X.; Ho, H.H.; He, Y.Q. Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocontrol Sci. Technol. 2015, 25, 132–143. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. MicrobiologyOpen 2019, 8, e00813. [Google Scholar] [CrossRef]
- Ramírez, V.; Martínez, J.; Bustillos-Cristales, M.D.R.; Catañeda-Antonio, D.; Munive, J.A.; Baez, A. Bacillus cereus MH778713 elicits tomato plant protection against Fusarium oxysporum. J. Appl. Microbiol. 2022, 132, 470–482. [Google Scholar] [CrossRef]
- Shifa, H.; Gopalakrishnan, C.; Velazhahan, R. Characterization of antifungal antibiotics produced by Bacillus subtilis G-1 antagonistic to Sclerotium rolfsii. Biochem. Cell. Arch. 2015, 15, 99–104. [Google Scholar]
- Wu, Y.; Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Biocontrol traits and antagonistic potential of Bacillus amyloliquefaciens strain NJZJSB3 against Sclerotinia sclerotiorum, a causal agent of canola stem rot. J. Microbiol. Biotechnol. 2014, 24, 1327–1336. [Google Scholar] [CrossRef]
- Zou, L.; Wang, Q.; Wu, R.; Zhang, Y.; Wu, Q.; Li, M.; Ye, K.; Dai, W.; Huang, J. Biocontrol and plant growth promotion potential of endophytic Bacillus subtilis JY-7-2L on Aconitum carmichaelii Debx. Front. Microbiol. 2023, 13, 1059549. [Google Scholar] [CrossRef]
- Lahlali, R.; Aksissou, W.; Lyousfi, N.; Ezrari, S.; Blenzar, A.; Tahiri, A.; Ennahli, S.; Hrustić, J.; MacLean, D.; Amiri, S. Biocontrol activity and putative mechanism of Bacillus amyloliquefaciens (SF14 and SP10), Alcaligenes faecalis ACBC1, and Pantoea agglomerans ACBP1 against brown rot disease of fruit. Microb. Pathog. 2020, 139, 103914. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhen, C.; Wang, K.; Gao, H. Effects of Bacillus subtilis CF-3 VOCs combined with heat treatment on the control of Monilinia fructicola in peaches and Colletotrichum gloeosporioides in litchi fruit. J. Food Sci. 2019, 84, 3418–3428. [Google Scholar] [CrossRef]
- Feng, B.; Chen, D.; Jin, R.; Li, E.; Li, P. Bioactivities evaluation of an endophytic bacterial strain Bacillus velezensis JRX-YG39 inhabiting wild grape. BMC Microbiol. 2022, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.E.; Ul-Hassan, Z.; Zeidan, R.; Al-Shamary, N.; Al-Yafei, T.; Alnaimi, H.; Higazy, N.S.; Migheli, Q.; Jaoua, S. Biocontrol activity of Bacillus megaterium BM344-1 against toxigenic fungi. ACS Omega 2021, 6, 10984–10990. [Google Scholar] [CrossRef] [PubMed]
- Ul Hassan, Z.; Al Thani, R.; Alnaimi, H.; Migheli, Q.; Jaoua, S. Investigation and application of Bacillus licheniformis volatile compounds for the biological control of toxigenic Aspergillus and Penicillium spp. ACS Omega 2019, 4, 17186–17193. [Google Scholar] [CrossRef]
- He, C.N.; Ye, W.Q.; Zhu, Y.Y.; Zhou, W.W. Antifungal activity of volatile organic compounds produced by Bacillus methylotrophicus and Bacillus thuringiensis against five common spoilage fungi on loquats. Molecules 2020, 25, 3360. [Google Scholar] [CrossRef]
- Kupper, K.C.; Moretto, R.K.; Fujimoto, A. Production of antifungal compounds by Bacillus spp. isolates and its capacity for controlling citrus black spot under field conditions. World J. Microbiol. Biotechnol. 2020, 36, 7. [Google Scholar] [CrossRef]
- Surya, M.; Thiruvudainambi, S.; Ebenezar, E.G.; Vanniarajan, C.; Kumutha, K. GC-MS Analysis of antimicrobial compounds produced by Bacillus spp. against rice sheath rot pathogen Sarocladium oryzae. J. Entomol. Zool. Stud. 2020, 8, 1417–1423. [Google Scholar]
- Awan, Z.A.; Shoaib, A.; Schenk, P.M.; Ahmad, A.; Alansi, S.; Paray, B.A. Antifungal potential of volatiles produced by Bacillus subtilis BS-01 against Alternaria solani in Solanum lycopersicum. Front. Plant Sci. 2023, 13, 1089562. [Google Scholar] [CrossRef]
- Yuan, J.; Ruan, Y.; Wang, B.; Zhang, J.; Waseem, R.; Huang, Q.; Shen, Q. Plant growth-promoting rhizobacteria strain Bacillus amyloliquefaciens Njn-6-enriched bio-organic fertilizer suppressed Fusarium wilt and promoted the growth of banana plants. J. Agric. Food Chem. 2013, 61, 3774–3780. [Google Scholar] [CrossRef]
- Wang, B.; Yuan, J.; Zhang, J.; Shen, Z.; Zhang, M.; Li, R.; Ruan, Y.; Shen, Q. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fertil. Soils 2013, 49, 435–446. [Google Scholar] [CrossRef]
- Rangel-Montoya, E.A.; Delgado-Ramírez, C.S.; Sepulveda, E.; Hernández-Martínez, R. Biocontrol of Macrophomina phaseolina using Bacillus amyloliquefaciens strains in cowpea (Vigna unguiculata L.). Agronomy 2022, 12, 676. [Google Scholar] [CrossRef]
- Aiello, D.; Leonardi, G.R.; Di Pietro, C.; Vitale, A.; Polizzi, G. A new strategy to improve management of citrus mal secco disease using bioformulates based on Bacillus amyloliquefaciens strains. Plants 2022, 11, 446. [Google Scholar] [CrossRef]
- Cheffi, M.; Bouket, A.C.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea l. root endophyte Bacillus velezensis Oee1 counteracts oomycete and fungal harmful pathogens and harbours a large repertoire of secreted and volatile metabolites and beneficial functional genes. Microorganisms 2019, 7, 314. [Google Scholar] [CrossRef]
- Calvo, H.; Mendiara, I.; Arias, E.; Gracia, A.P.; Blanco, D.; Venturini, M.E. Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biol. Technol. 2020, 166, 111208. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Shi, X.; Wang, B.; Li, M.; Wang, Q.; Zhang, S. Antifungal effect of volatile organic compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes 2020, 8, 1674. [Google Scholar] [CrossRef]
- Song, S.; Jeon, E.K.; Hwang, C.W. Characteristic analysis of soil-isolated Bacillus velezensis HY-3479 and its antifungal activity against phytopathogens. Curr. Microbiol. 2022, 79, 357. [Google Scholar] [CrossRef]
- Roy, T.; Bandopadhyay, A.; Sonawane, P.J.; Majumdar, S.; Mahapatra, N.R.; Alam, S.; Das, N. Bio-effective disease control and plant growth promotion in lentil by two pesticide degrading strains of Bacillus sp. Biol. Control 2018, 127, 55–63. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Gao, Y.; Gao, T.; Zhang, D. Inhibitory abilities of Bacillus isolates and their culture filtrates against the gray mold caused by Botrytis cinerea on postharvest fruit. Plant Pathol. J. 2019, 35, 425–436. [Google Scholar] [CrossRef]
- Altimira, F.; Godoy, S.; Arias-Aravena, M.; Araya, B.; Montes, C.; Castro, J.F.; Dardón, E.; Montenegro, E.; Pineda, W.; Viteri, I.; et al. Genomic and experimental analysis of the biostimulant and antagonistic properties of phytopathogens of Bacillus safensis and Bacillus siamensis. Microorganisms 2022, 10, 670. [Google Scholar] [CrossRef]
- Guevara-Avendaño, E.; Bejarano-Bolívar, A.A.; Kiel-Martínez, A.L.; Ramírez-Vázquez, M.; Méndez-Bravo, A.; von Wobeser, E.A.; Sánchez-Rangel, D.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiol. Res. 2019, 219, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Chaouachi, M.; Marzouk, T.; Jallouli, S.; Elkahoui, S.; Gentzbittel, L.; Ben, C.; Djébali, N. Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of Botrytis cinerea. Postharvest Biol. Technol. 2021, 172, 111389. [Google Scholar] [CrossRef]
- Cavalcanti, V.P.; Araújo, N.A.F.; Machado, N.B.; Costa Júnior, P.S.P.; Pasqual, M.; Alves, E.; Schwan-Estrada, K.R.F.; Dória, J. Yeasts and Bacillus spp. as potential biocontrol agents of Sclerotinia sclerotiorum in garlic. Sci. Hortic. 2020, 261, 108931. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Raizada, M.N. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol. 2018, 9, 42. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, Y.; Yin, N.; Li, Y.; Ling, J.; Mao, Z.; Yang, Y.; Xie, B. Analysis of the activity and biological control efficacy of the Bacillus subtilis strain Bs-1 against Meloidogyne incognita. Crop Prot. 2019, 122, 125–135. [Google Scholar] [CrossRef]
- Luo, T.; Hou, S.; Yang, L.; Qi, G.; Zhao, X. Nematodes avoid and are killed by Bacillus mycoides-produced styrene. J. Invertebr. Pathol. 2018, 159, 129–136. [Google Scholar] [CrossRef]
- Adam, M.; Heuer, H.; Hallmann, J. Bacterial antagonists of fungal pathogens also control root-knot nematodes by induced systemic resistance of tomato plants. PLoS ONE 2014, 9, e90402. [Google Scholar] [CrossRef]
- Oka, Y.; Shuker, S.; Tkachi, N. Nematicidal efficacy of MCW-2, a new nematicide of the fluoroalkenyl group, against the root-knot nematode Meloidogyne javanica. Pest Manag. Sci. 2009, 65, 1082–1089. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Ma, L.; Zhang, K.; Duan, C.; Mo, M. Characterisation of Volatiles produced from Bacillus megaterium YFM3.25 and their nematicidal activity against Meloidogyne incognita. Eur. J. Plant Pathol. 2010, 126, 417–422. [Google Scholar] [CrossRef]
- Yin, N.; Liu, R.; Zhao, J.L.; Khan, R.A.A.; Li, Y.; Ling, J.; Liu, W.; Yang, Y.H.; Xie, B.Y.; Mao, Z.C. Volatile organic compounds of Bacillus cereus strain Bc-Cm103 exhibit fumigation activity against Meloidogyne incognita. Plant Dis. 2021, 105, 904–911. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal volatiles from Bacillus atrophaeus Gbsc56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Du, J.; Gao, Q.; Ji, C.; Song, X.; Liu, Y.; Li, H.; Li, C.; Zhang, P.; Li, J.; Liu, X. Bacillus licheniformis JF-22 to control Meloidogyne incognita and its effect on tomato rhizosphere microbial community. Front. Microbiol. 2022, 13, 863341. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.Y.; Liu, X.F.; Guan, Q.; Dou, N.X.; Li, J.; Zhang, Q.; Gao, Y.M.; Wang, M.; Li, J.S.; et al. Nematicidal activity of volatile organic compounds produced by Bacillus altitudinis AMCC 1040 against Meloidogyne incognita. Arch. Microbiol. 2022, 204, 521. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Huang, D.; Cheng, W.; Shao, Z.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Volatile organic compounds from Bacillus aryabhattai MCCC 1K02966 with multiple modes against Meloidogyne incognita. Molecules 2021, 27, 103. [Google Scholar] [CrossRef]
- Audrain, B.; Farag, M.A.; Ryu, C.-M.; Ghigo, J.-M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef]
- Jousset, A.; Schmid, B.; Scheu, S.; Eisenhauer, N. Genotypic richness and dissimilarity opposingly affect ecosystem functioning. Ecol. Lett. 2011, 14, 537–545. [Google Scholar] [CrossRef]
- de Boer, W.; Li, X.; Meisner, A.; Garbeva, P. Pathogen suppression by microbial volatile organic compounds in soils. FEMS Microbiol. Ecol. 2019, 95, fiz105. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Gómez-Tenorio, M.A.; Zanón, M.J.; de Cara, M.; Lupión, B.; Tello, J.C. Efficacy of dimethyl disulfide (DMDS) against Meloidogyne sp. and three formae speciales of Fusarium oxysporum under controlled conditions. Crop Prot. 2015, 78, 263–269. [Google Scholar] [CrossRef]
- Papazlatani, C.; Rousidou, C.; Katsoula, A.; Kolyvas, M.; Genitsaris, S.; Papadopoulou, K.K.; Karpouzas, D.G. Assessment of the impact of the fumigant dimethyl disulfide on the dynamics of major fungal plant pathogens in greenhouse soils. Eur. J. Plant Pathol. 2016, 146, 391–400. [Google Scholar] [CrossRef]
- Mulay, P.R.; Cavicchia, P.; Watkins, S.M.; Tovar-Aguilar, A.; Wiese, M.; Calvert, G.M. Acute illness associated with exposure to a new soil fumigant containing dimethyl disulfide—Hillsborough County, Florida, 2014. J. Agromedicine 2016, 21, 373–379. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grahovac, J.; Pajčin, I.; Vlajkov, V. Bacillus VOCs in the Context of Biological Control. Antibiotics 2023, 12, 581. https://doi.org/10.3390/antibiotics12030581
Grahovac J, Pajčin I, Vlajkov V. Bacillus VOCs in the Context of Biological Control. Antibiotics. 2023; 12(3):581. https://doi.org/10.3390/antibiotics12030581
Chicago/Turabian StyleGrahovac, Jovana, Ivana Pajčin, and Vanja Vlajkov. 2023. "Bacillus VOCs in the Context of Biological Control" Antibiotics 12, no. 3: 581. https://doi.org/10.3390/antibiotics12030581
APA StyleGrahovac, J., Pajčin, I., & Vlajkov, V. (2023). Bacillus VOCs in the Context of Biological Control. Antibiotics, 12(3), 581. https://doi.org/10.3390/antibiotics12030581