Effects of Sulfamethoxazole and Florfenicol on Growth, Antioxidant Capacity, Immune Responses and Intestinal Microbiota in Pacific White Shrimp Litopenaeus vannamei at Low Salinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Growth Evaluation and Sampling
2.3. Biochemical Assays
2.4. Quantitative Real-Time PCR
2.5. Intestinal Microbiota Analyses
2.6. Statistical Analyses
3. Results
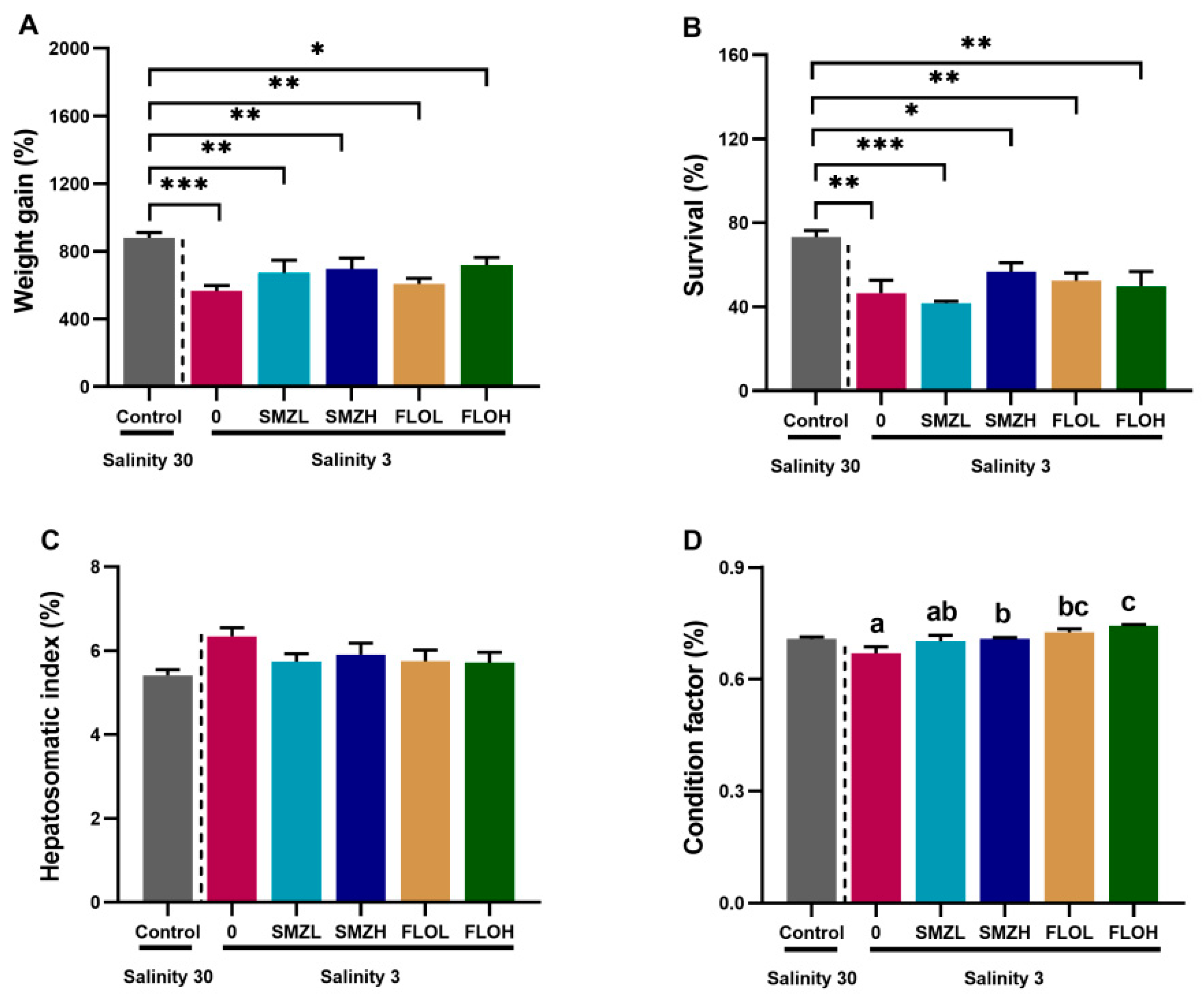
3.1. Growth Performance
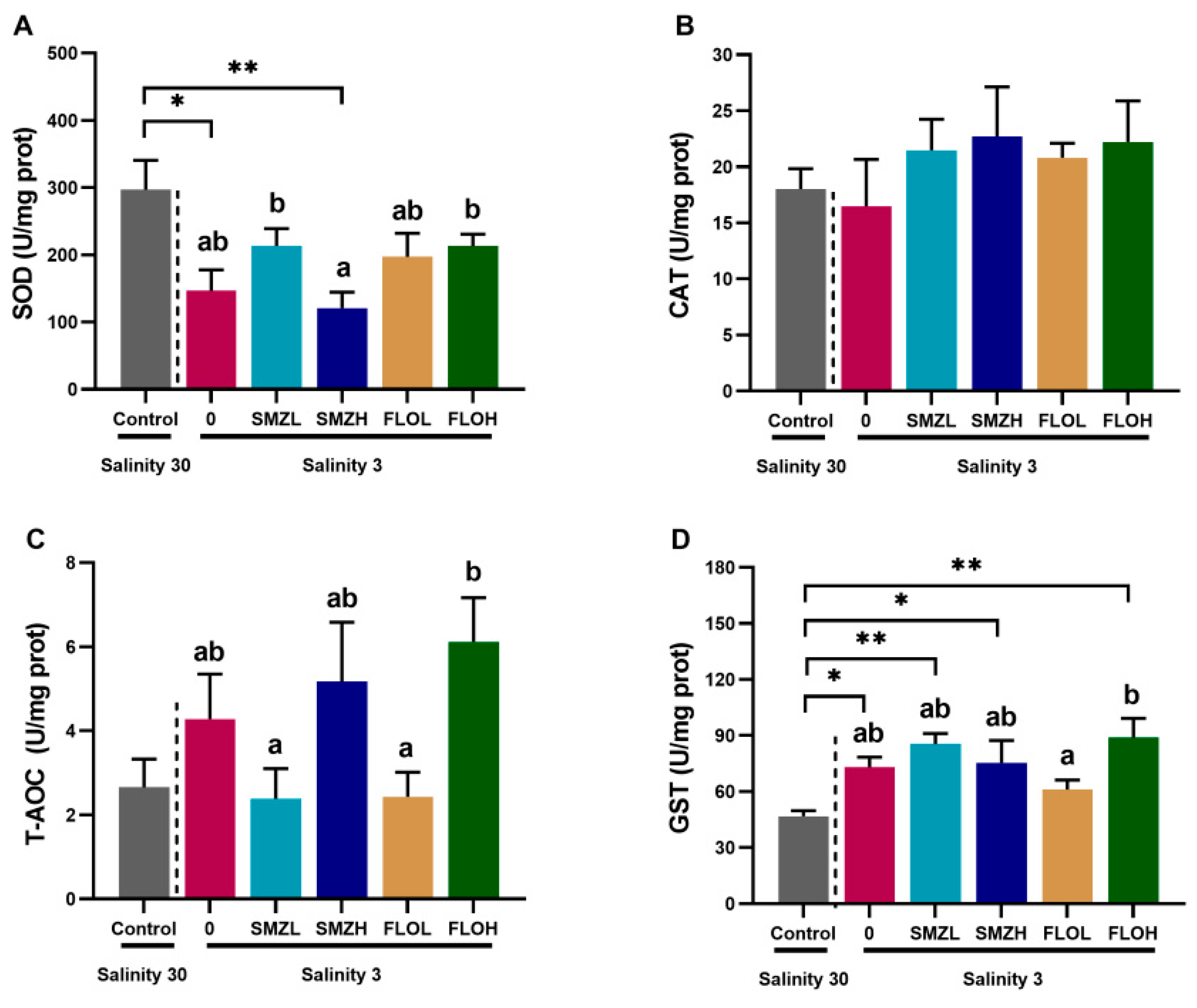
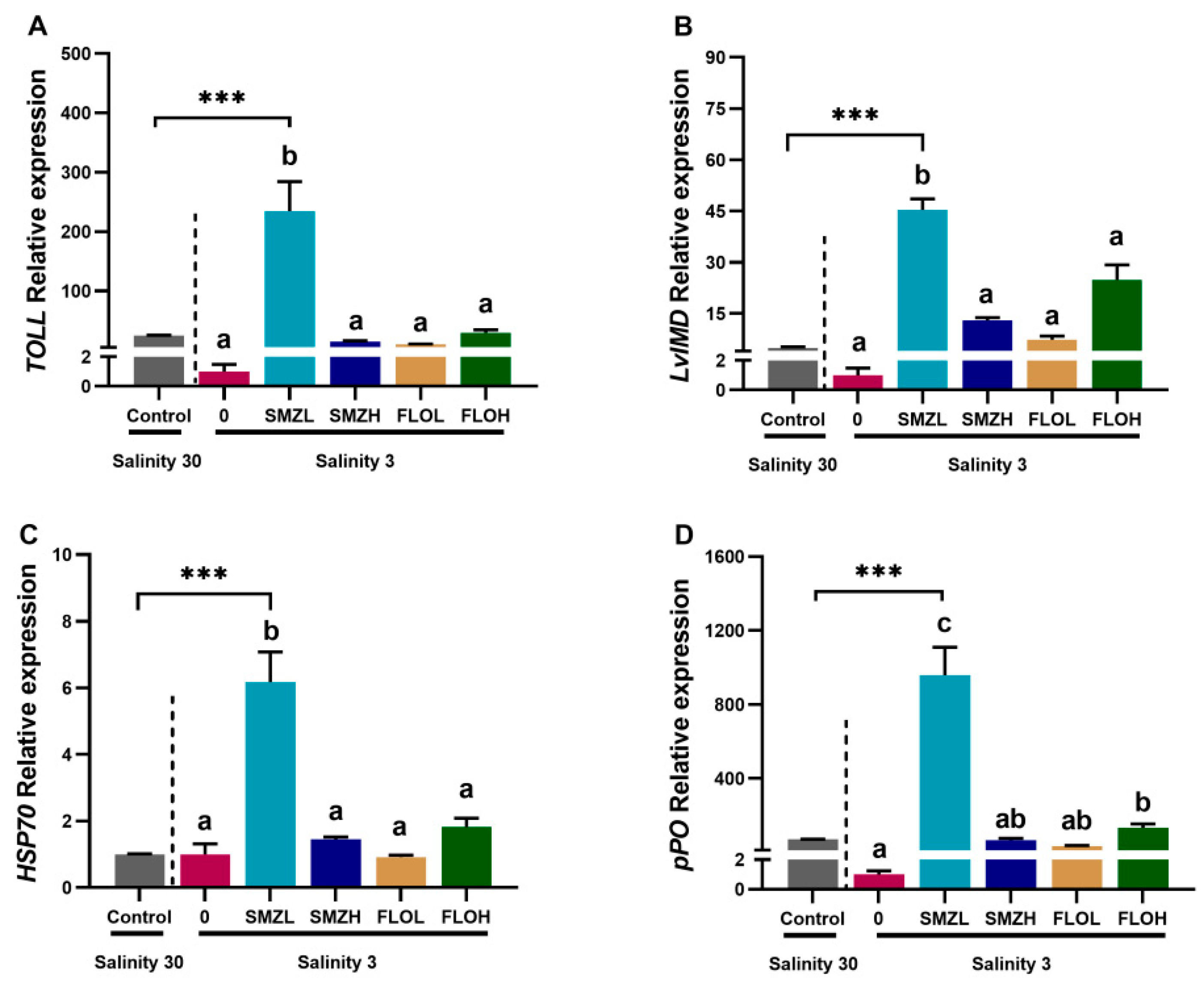
3.2. Antioxidant Capacity and Immune Responses
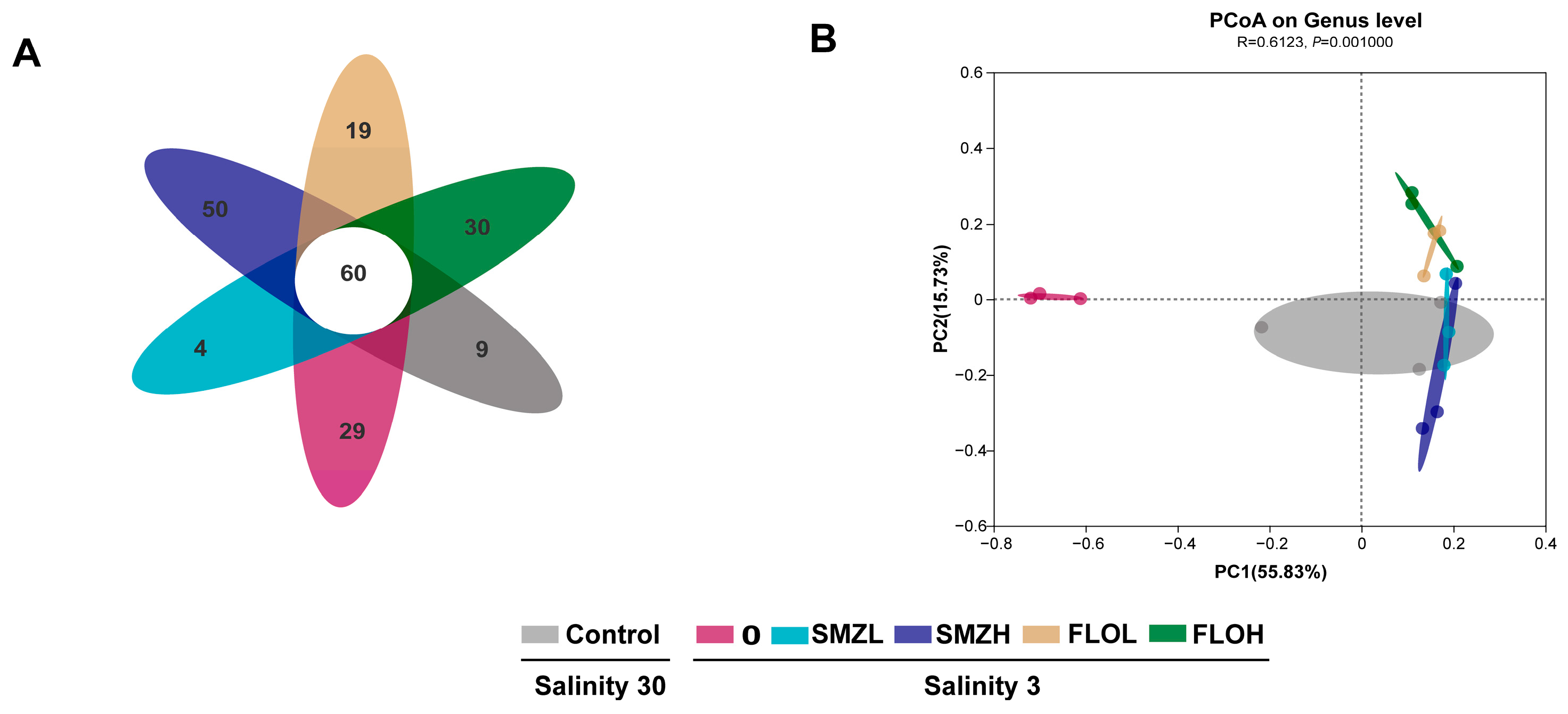
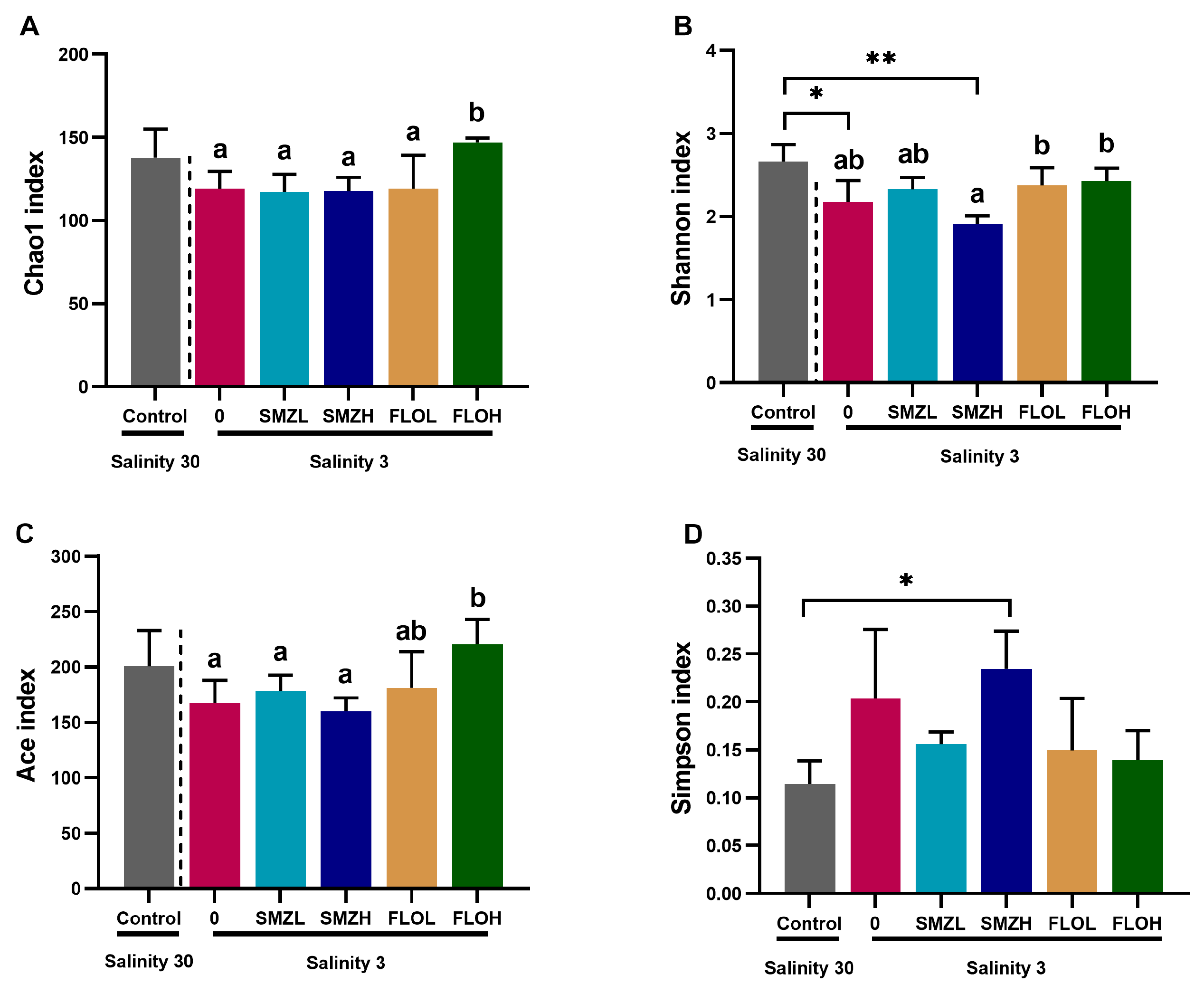
3.3. Richness and Diversity of Intestinal Microbiota
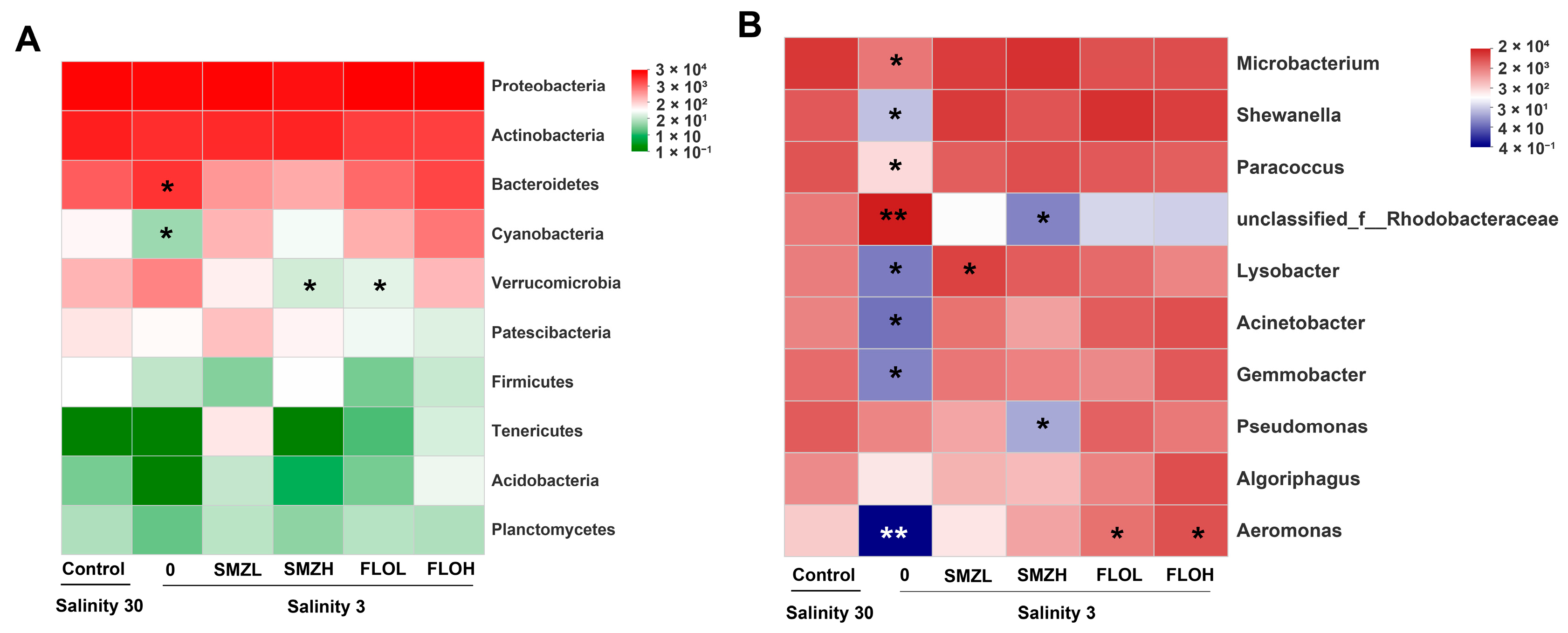
3.4. Community Composition Analysis of Intestinal Microbiota
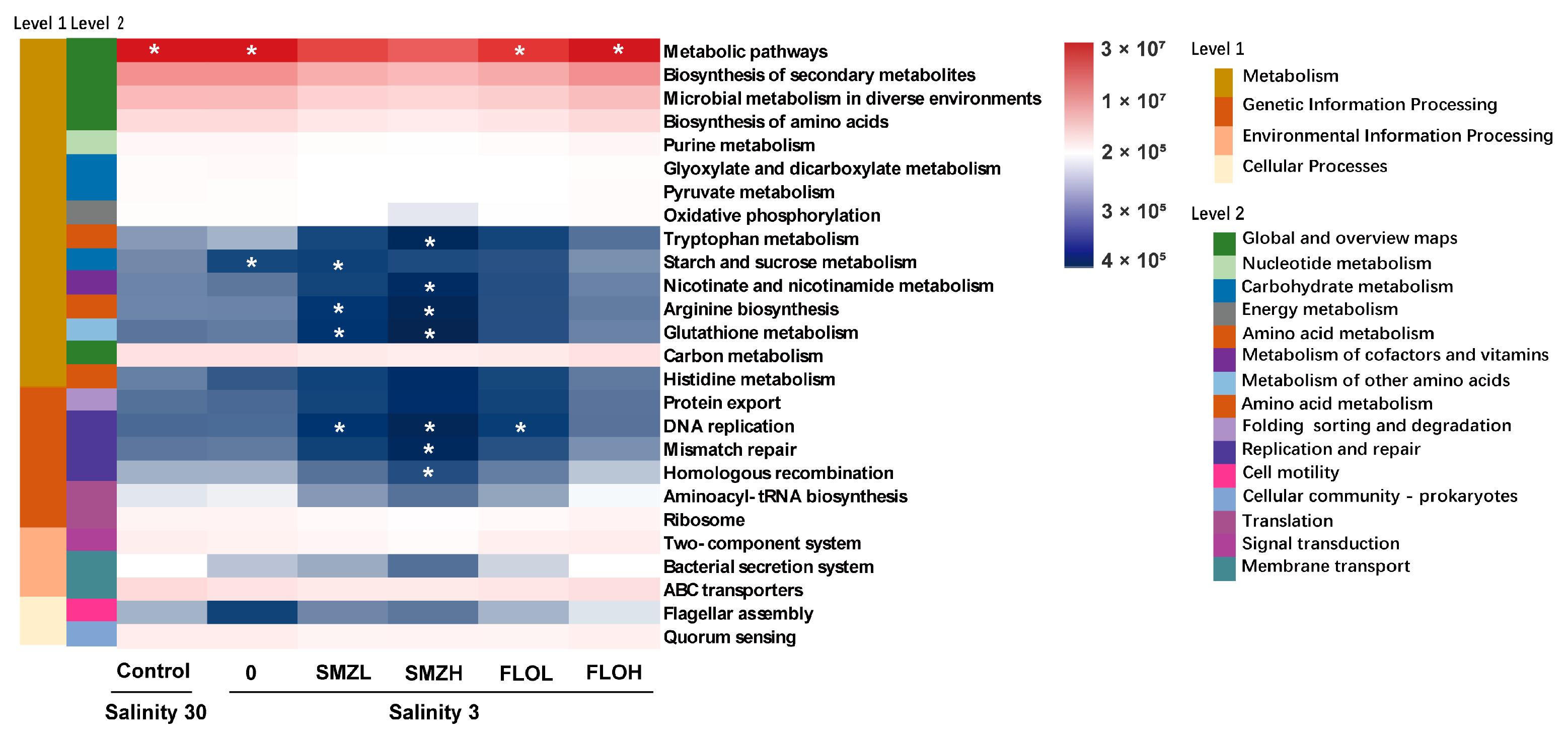
3.5. Functional Predictions of Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, J.; Li, J.; Cheng, Z.; Chaemfa, C.; Liu, D.; Zheng, Q.; Song, M.; Luo, C.; Zhang, G. Occurrence and risks of antibiotics in the coastal aquatic environment of the Yellow Sea, North China. Sci. Total Environ. 2013, 450–451, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.R.; Liu, S.S.; Zhou, G.J.; Sun, K.F.; Zhao, J.L.; Ying, G.G. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure. Mar. Pollut. Bull. 2015, 90, 181–187. [Google Scholar] [CrossRef]
- Chen, C.Q.; Zheng, L.; Zhou, J.L.; Zhao, H. Persistence and risk of antibiotic residues and antibiotic resistance genes in major mariculture sites in Southeast China. Sci. Total Environ. 2017, 580, 1175–1184. [Google Scholar] [CrossRef]
- Du, J.; Zhao, H.; Wang, Y.; Xie, H.; Zhu, M.; Chen, J. Presence and environmental risk assessment of selected antibiotics in coastal water adjacent to mariculture areas in the Bohai Sea. Ecotoxicol. Environ. Saf. 2019, 177, 117–123. [Google Scholar] [CrossRef]
- Uchida, K.; Konishi, Y.; Harada, K.; Okihashi, M.; Yamaguchi, T.; Do, M.H.N.; Thi Bui, L.; Duc Nguyen, T.; Do Nguyen, P.; Thi Khong, D.; et al. Monitoring of Antibiotic Residues in Aquatic Products in Urban and Rural Areas of Vietnam. J. Agric. Food Chem. 2016, 64, 6133–6138. [Google Scholar] [CrossRef]
- Kim, H.; Hong, Y.; Park, J.E.; Sharma, V.K.; Cho, S. Il Sulfonamides and tetracyclines in livestock wastewater. Chemosphere 2013, 91, 888–894. [Google Scholar] [CrossRef]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial resistance in aquaculture: A crisis for concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Rocha, C.P.; Cabral, H.N.; Marques, J.C.; Gonçalves, A.M.M. A Global Overview of Aquaculture Food Production with a Focus on the Activity’s Development in Transitional Systems—The Case Study of a South European Country (Portugal). J. Mar. Sci. Eng. 2022, 10, 417. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Guo, M.; Liu, Y.; Yu, H.; Xing, M. Environmentally relevant concentration of cypermethrin or/and sulfamethoxazole induce neurotoxicity of grass carp: Involvement of blood-brain barrier, oxidative stress and apoptosis. Sci. Total Environ. 2021, 762, 143054. [Google Scholar] [CrossRef]
- Liu, J.; Wei, T.; Wu, X.; Zhong, H.; Qiu, W.; Zheng, Y. Early exposure to environmental levels of sulfamethoxazole triggers immune and inflammatory response of healthy zebrafish larvae. Sci. Total Environ. 2020, 703, 134724. [Google Scholar] [CrossRef]
- Zhou, L.; Limbu, S.M.; Shen, M.; Zhai, W.; Qiao, F.; He, A.; Du, Z.Y.; Zhang, M. Environmental concentrations of antibiotics impair zebrafish gut health. Environ. Pollut. 2018, 235, 245–254. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Z.; Gao, B.; Liu, P.; Li, J. Effects of florfenicol on the antioxidant status, detoxification system and biomolecule damage in the swimming crab (Portunus trituberculatus). Ecotox. Environ. Saf. 2017, 143, 6–11. [Google Scholar] [CrossRef]
- Sun, S.; Korheina, D.K.A.; Fu, H.; Ge, X. Chronic exposure to dietary antibiotics affects intestinal health and antibiotic resistance gene abundance in oriental river prawn (Macrobrachium nipponense), and provokes human health risk. Sci. Total Environ. 2020, 720, 137478. [Google Scholar] [CrossRef]
- Castille, F.L.; Lawrence, A.L. The effect of salinity on the osmotic, sodium, and chloride concentrations in the hemolymph of the freshwater shrimps, Macrobrachium ohione smith and Macrobrachium rosenbergii de man. Comp. Biochem. Physiol. Part A Physiol. 1981, 70, 47–52. [Google Scholar] [CrossRef]
- Cheng, K.M.; Hu, C.Q.; Liu, Y.N.; Zheng, S.X.; Qi, X.J. Effects of dietary calcium, phosphorus and calcium/phosphorus ratio on the growth and tissue mineralization of Litopenaeus vannamei reared in low-salinity water. Aquaculture 2006, 251, 472–483. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C.; Li, C.C.; Morni, W.Z.; Suhaili, A.S.N.; Kuo, Y.H.; Chang, Y.H.; Chen, L.L.; Tsui, W.C.; Chen, Y.Y.; et al. Modulation of the innate immune system in white shrimp Litopenaeus vannamei following long-term low salinity exposure. Fish Shellfish Immunol. 2012, 33, 324–331. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Liu, Y.; Qiao, F.; Chen, L.; Liu, W.T.; Du, Z.; Li, E. Response of gut microbiota to salinity change in two euryhaline aquatic animals with reverse salinity preference. Aquaculture. 2016, 454, 72–80. [Google Scholar] [CrossRef]
- Hertzler, P.L.; Freas, W.R. Pleonal muscle development in the shrimp Penaeus (Litopenaeus) vannamei (Crustacea: Malacostraca: Decapoda: Dendrobranchiata). Arthropod Struct. Dev. 2009, 38, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, X.; Yu, Y.; Huang, H.; Li, F.; Xiang, J. Comparative transcriptomic characterization of the early development in Pacific white shrimp Litopenaeus vannamei. PLoS ONE 2014, 9, e106201. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, H.; Liu, S.; Xie, H.; Wang, Y.; Chen, J. Antibiotics in the coastal water of the South Yellow Sea in China: Occurrence, distribution and ecological risks. Sci. Total Environ. 2017, 595, 521–527. [Google Scholar] [CrossRef]
- Yu, Q.; Xie, J.; Huang, M.; Chen, C.; Qian, D.; Qin, J.G.; Chen, L.; Jia, Y.; Li, E. Growth and health responses to a long-term pH stress in Pacific white shrimp Litopenaeus vannamei. Aquac. Rep. 2020, 16, 100280. [Google Scholar] [CrossRef]
- Wang, W.N.; Zhou, J.; Wang, P.; Tian, T.T.; Zheng, Y.; Liu, Y.; Mai, W.; Wang, A.L. Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Biochem. Physiol.-C Toxicol. Pharmacol. 2009, 150, 428–435. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Chen, X.; Yu, N.; Lai, Q.; Qin, J.G. Growth, body composition, respiration and ambient ammonia nitrogen tolerance of the juvenile white shrimp, Litopenaeus vannamei, at different salinities. Aquaculture 2007, 265, 385–390. [Google Scholar] [CrossRef]
- Huang, D.J.; Hou, J.H.; Kuo, T.F.; Lai, H.T. Toxicity of the veterinary sulfonamide antibiotic sulfamonomethoxine to five aquatic organisms. Environ. Toxicol. Pharmacol. 2014, 38, 874–880. [Google Scholar] [CrossRef]
- Lin, T.; Chen, Y.Q.; Chen, W. Impact of toxicological properties of sulfonamides on the growth of zebrafish embryos in the water. Environ. Toxicol. Pharmacol. 2013, 36, 1068–1076. [Google Scholar] [CrossRef]
- Putra, D.F.; Muhammadar, A.A.; Muhammad, N.; Damora, A.; Waliul, A.; Abidin, M.Z.; Othman, N. Length-weight relationship and condition factor of white shrimp, Penaeus merguiensis in West Aceh waters, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 216, 012022. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Rico, D.; Martín-González, A.; Díaz, S.; de Lucas, P.; Gutiérrez, J.C. Heavy metals generate reactive oxygen species in terrestrial and aquatic ciliated protozoa. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 90–96. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Yu, N.; Xiong, Z.; Chen, X.; Qin, J.G. Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture 2008, 274, 80–86. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Q.; Wang, Y.; Zhang, J.; Xiong, D. Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 2018, 78, 279–288. [Google Scholar] [CrossRef]
- Ren, X.; Pan, L.; Wang, L. Effect of florfenicol on selected parameters of immune and antioxidant systems, and damage indexes of juvenile Litopenaeus vannamei following oral administration. Aquaculture 2014, 432, 106–113. [Google Scholar] [CrossRef]
- Tu, H.T.; Silvestre, F.; Bernard, A.; Douny, C.; Phuong, N.T.; Tao, C.T.; Maghuin-Rogister, G.; Kestemont, P. Oxidative stress response of black tiger shrimp (Penaeus monodon) to enrofloxacin and to culture system. Aquaculture 2008, 285, 244–248. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Kafatos, F.C.; Janeway, C.A., Jr.; Ezekowitz, R.A.B. Phylogenetic perspectives in innate immunity. Science 1999, 284, 1313–1318. [Google Scholar] [CrossRef]
- Chen, Y.H.; He, J.G. Effects of environmental stress on shrimp innate immunity and white spot syndrome virus infection. Fish Shellfish Immunol. 2019, 84, 744–755. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, C.; Xie, J.; Xu, C.; Zhao, Q.; Qin, J.G.; Chen, L.; Li, E. Intestinal bacterial signatures of the “cotton shrimp-like” disease explain the change of growth performance and immune responses in Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2019, 92, 629–636. [Google Scholar] [CrossRef]
- Arts, J.A.J.; Cornelissen, F.H.J.; Cijsouw, T.; Hermsen, T.; Savelkoul, H.F.J.; Stet, R.J.M. Molecular cloning and expression of a Toll receptor in the giant tiger shrimp, Penaeus monodon. Fish Shellfish Immunol. 2007, 23, 504–513. [Google Scholar] [CrossRef]
- De La Vega, E.; Hall, M.R.; Degnan, B.M.; Wilson, K.J. Short-term hyperthermic treatment of Penaeus monodon increases expression of heat shock protein 70 (HSP70) and reduces replication of gill associated virus (GAV). Aquaculture 2006, 253, 82–90. [Google Scholar] [CrossRef]
- Roy, S.; Kumar, V.; Bossier, P.; Norouzitallab, P.; Vanrompay, D. Phloroglucinol treatment induces transgenerational epigenetic inherited resistance against Vibrio infections and thermal stress in a brine shrimp (Artemia franciscana) model. Front. Immunol. 2019, 10, 2745. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, X.Q.; Tian, L.X.; Liu, Y.J.; Niu, J. Enhanced intestinal health, immune responses and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary hydrolyzed yeast (Rhodotorula mucilaginosa) and Bacillus licheniformis. Aquac. Rep. 2020, 17, 100385. [Google Scholar] [CrossRef]
- Huang, M.; Xie, J.; Yu, Q.; Xu, C.; Zhou, L.; Qin, J.G.; Chen, L.; Li, E. Toxic effect of chronic nitrite exposure on growth and health in Pacific white shrimp Litopenaeus vannamei. Aquaculture 2020, 529, 735664. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Liu, Q.; Xiong, D.; Zhang, J. Transcriptomic and microbiota response on Litopenaeus vannamei intestine subjected to acute sulfide exposure. Fish Shellfish Immunol. 2019, 88, 335–343. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Dong, H.; Zheng, X.; Wang, Y.; Li, H.; Liu, Q.; Zhang, J. Effect of dietary poly-β-hydroxybutyrate (PHB) on growth performance, intestinal health status and body composition of Pacific white shrimp Litopenaeus vannamei (Boone, 1931). Fish Shellfish Immunol. 2017, 60, 520–528. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhou, L.; Qu, Y.; Lu, K.; Han, F.; Li, E. Effects of Different Dietary β-Glucan Levels on Antioxidant Capacity and Immunity, Gut Microbiota and Transcriptome Responses of White Shrimp (Litopenaeus vannamei) under Low Salinity. Antioxidants 2022, 11, 2282. [Google Scholar] [CrossRef]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.G.; Chen, L. Gut Microbiota and its Modulation for Healthy Farming of Pacific White Shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Jia, J.; Chen, Q.; Lauridsen, T. A Systematic Investigation into the Environmental Fate of Microcystins and The Potential Risk: Study in Lake Taihu. Toxins 2016, 8, 170. [Google Scholar] [CrossRef]
- Cardman, Z.; Arnosti, C.; Durbin, A.; Ziervogel, K.; Cox, C.; Steen, A.D.; Teske, A. Verrucomicrobia are candidates for polysaccharide-degrading bacterioplankton in an Arctic fjord of Svalbard. Appl. Environ. Microbiol. 2014, 80, 3749–3756. [Google Scholar] [CrossRef]
- Suo, Y.; Li, E.; Li, T.; Jia, Y.; Qin, J.G.; Gu, Z.; Chen, L. Response of gut health and microbiota to sulfide exposure in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2017, 63, 87–96. [Google Scholar] [CrossRef]
- Hayward, A.C.; Fegan, N.; Fegan, M.; Stirling, G.R. Stenotrophomonas and Lysobacter: Ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. J. Appl. Microbiol. 2010, 108, 756–770. [Google Scholar] [CrossRef]
- Dogs, M.; Wemheuer, B.; Wolter, L.; Bergen, N.; Daniel, R.; Simon, M.; Brinkhoff, T. Rhodobacteraceae on the marine brown alga Fucus spiralis are abundant and show physiological adaptation to an epiphytic lifestyle. Syst. Appl. Microbiol. 2017, 40, 370–382. [Google Scholar] [CrossRef]
- Yoo, Y.; Lee, D.W.; Lee, H.; Kwon, B.-O.; Khim, J.S.; Yim, U.H.; Park, H.; Park, B.; Choi, I.-G.; Kim, B.S.; et al. Gemmobacter lutimaris sp. nov., a marine bacterium isolated from a tidal flat. Int. J. Syst. Evol. Microbiol. 2019, 69, 1676–1681. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Liu, Y.; Wang, X.; Qin, J.G.; Chen, L. Comparative proteome analysis of the hepatopancreas from the Pacific white shrimp Litopenaeus vannamei under long-term low salinity stress. J. Proteom. 2017, 162, 1–10. [Google Scholar] [CrossRef]
- Shinji, J.; Okutsu, T.; Jayasankar, V.; Jasmani, S.; Wilder, M.N. Metabolism of amino acids during hyposmotic adaptation in the whiteleg shrimp, Litopenaeus vannamei. Amino Acids 2012, 43, 1945–1954. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Xu, K.; Zhang, X.; Sun, H.; Fan, L.; Yan, M. White spot syndrome virus (WSSV) infection impacts intestinal microbiota composition and function in Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 84, 130–137. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Li, J.; Zou, J.; Fan, L. The immune defense response of Pacific white shrimp (Litopenaeus vannamei) to temperature fluctuation. Fish Shellfish Immunol. 2020, 103, 103–110. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′-3′) | GenBank Accession Number |
|---|---|---|
| Toll-F | GACCATCCCTTTTACACCAGACT | DQ923424 |
| Toll-R | CCTCGCACATCCAGGACTTTTA | |
| LvIMD-F | TGGGTCCGTGTCCAGTGAT | [26] |
| LvIMD-R | ACAAACAACCACACACAAGCAG | |
| HSP70-F | CAACGATTCTCAGCGTCAGG | XM_027369405 |
| HSP70-R | ACCTTCTTGTCGAGGCCGTA | |
| pPO-F | CAATGACCAGCAGCGTCTTC | AY723296 |
| pPO-R | CACGGAAGGAGGCGTATCAT | |
| β-actin-F | GCAGTCCAACCCGAGAGGAAG | AF300705 |
| β-actin-R | GTGCATCGTCACCAGCGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhou, L.; Yu, Q.; Li, E.; Xie, J. Effects of Sulfamethoxazole and Florfenicol on Growth, Antioxidant Capacity, Immune Responses and Intestinal Microbiota in Pacific White Shrimp Litopenaeus vannamei at Low Salinity. Antibiotics 2023, 12, 575. https://doi.org/10.3390/antibiotics12030575
Chen Y, Zhou L, Yu Q, Li E, Xie J. Effects of Sulfamethoxazole and Florfenicol on Growth, Antioxidant Capacity, Immune Responses and Intestinal Microbiota in Pacific White Shrimp Litopenaeus vannamei at Low Salinity. Antibiotics. 2023; 12(3):575. https://doi.org/10.3390/antibiotics12030575
Chicago/Turabian StyleChen, Yunsong, Li Zhou, Qiuran Yu, Erchao Li, and Jia Xie. 2023. "Effects of Sulfamethoxazole and Florfenicol on Growth, Antioxidant Capacity, Immune Responses and Intestinal Microbiota in Pacific White Shrimp Litopenaeus vannamei at Low Salinity" Antibiotics 12, no. 3: 575. https://doi.org/10.3390/antibiotics12030575
APA StyleChen, Y., Zhou, L., Yu, Q., Li, E., & Xie, J. (2023). Effects of Sulfamethoxazole and Florfenicol on Growth, Antioxidant Capacity, Immune Responses and Intestinal Microbiota in Pacific White Shrimp Litopenaeus vannamei at Low Salinity. Antibiotics, 12(3), 575. https://doi.org/10.3390/antibiotics12030575







