Antimicrobial and Antibiofilm Potential of Thymus vulgaris and Cymbopogon flexuosus Essential Oils against Pure and Mixed Cultures of Foodborne Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Essential Oils
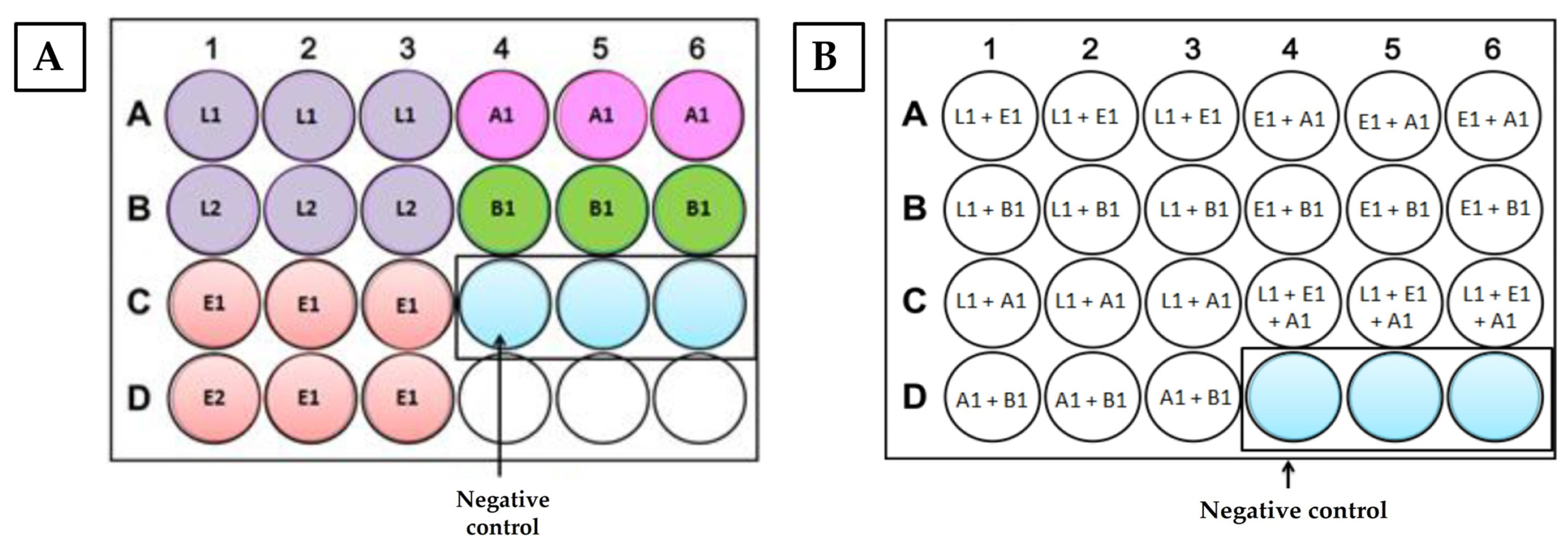
2.3. Biofilm Formation
2.3.1. Biofilm Formation in Polystyrene
2.3.2. Biofilm Formation in Stainless Steel Disks
2.4. Antimicrobial Activity
2.4.1. Initial Screening: Agar Diffusion Method
2.4.2. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.4.3. Determination of the Minimum Biofilm Eradication Concentration (MBEC)
2.5. Data Analysis
2.5.1. Statistical Analysis
2.5.2. In Silico Absorption and Toxicity Prediction
3. Results and Discussion
3.1. Biofilm Formation by Pure and Mixed Cultures on Different Surfaces—Polystyrene and Stainless Steel
3.2. Antimicrobial Activity
3.2.1. Initial Screening: Agar Diffusion Method
3.2.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
Pure Cultures
Mixed Cultures
3.2.3. Determination of the Minimum Biofilm Eradication Concentration (MBEC)
Pure Cultures
Mixed Cultures
3.3. In Silico Absorption and Toxicity Prediction
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding Spoilage Microbial Community and Spoilage Mechanisms in Foods of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Posgay, M.; Greff, B.; Kapcsándi, V.; Lakatos, E. Effect of Thymus vulgaris L. Essential Oil and Thymol on the Microbiological Properties of Meat and Meat Products: A Review. Heliyon 2022, 8, e10812. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Langsrud, S. Residential Bacteria on Surfaces in the Food Industry and Their Implications for Food Safety and Quality. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1022–1041. [Google Scholar] [CrossRef]
- Somrani, M.; Debbabi, H.; Palop, A. Antibacterial and Antibiofilm Activity of Essential Oil of Clove against Listeria Monocytogenes and Salmonella Enteritidis. Food Sci. Technol. Int. 2022, 28, 331–339. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- van Houdt, R.; Michiels, C.W. Biofilm Formation and the Food Industry, a Focus on the Bacterial Outer Surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef]
- Hascoët, A.S.; Ripolles-Avila, C.; Guerrero-Navarro, A.E.; Rodríguez-Jerez, J.J. Microbial Ecology Evaluation of an Iberian Pig Processing Plant through Implementing Sch Sensors and the Influence of the Resident Microbiota on Listeria Monocytogenes. Appl. Sci. 2019, 9, 4611. [Google Scholar] [CrossRef]
- Sharan, M.; Vijay, D.; Dhaka, P.; Bedi, J.S.; Gill, J.P.S. Biofilms as a Microbial Hazard in the Food Industry: A Scoping Review. J. Appl. Microbiol. 2022, 133, 2210–2234. [Google Scholar] [CrossRef]
- Azeredo, J.; García, P.; Drulis-Kawa, Z. Targeting Biofilms Using Phages and Their Enzymes. Curr. Opin. Biotechnol. 2021, 68, 251–261. [Google Scholar] [CrossRef]
- Augustin, M.; Ali-Vehmas, T.; Atroshi, F. Assessment of Enzymatic Cleaning Agents and Disinfectants against Bacterial Biofilms. J. Pharm. Pharm. Sci. 2004, 7, 55–64. [Google Scholar] [PubMed]
- Ashraf, M.A.; Ullah, S.; Ahmad, I.; Qureshi, A.K.; Balkhair, K.S.; Abdur Rehman, M. Green Biocides, a Promising Technology: Current and Future Applications to Industry and Industrial Processes. J. Sci. Food Agric. 2014, 94, 388–403. [Google Scholar] [CrossRef]
- Tong, C.; Hu, H.; Chen, G.; Li, Z.; Li, A.; Zhang, J. Disinfectant Resistance in Bacteria: Mechanisms, Spread, and Resolution Strategies. Environ. Res. 2021, 195, 110897. [Google Scholar] [CrossRef] [PubMed]
- Salanță, L.C.; Cropotova, J. An Update on Effectiveness and Practicability of Plant Essential Oils in the Food Industry. Plants 2022, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Sharma, M.M.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanilla Modulates the Activity of Antibiotics and Inhibits Efflux Pumps in Drug-Resistant Pseudomonas aeruginosa. Biologia 2020, 76, 781–791. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, F.; Li, Q.; Huang, J.; Ju, J. Antifungal Mechanism of Essential Oil against Foodborne Fungi and Its Application in the Preservation of Baked Food. Crit. Rev. Food Sci. Nutr. 2022, 1–13. [Google Scholar] [CrossRef]
- Iseppi, R.; Camellini, S.; Sabia, C.; Messi, P. Combined Antimicrobial Use of Essential Oils and Bacteriocin BacLP17 as Seafood Biopreservative to Control Listeria Monocytogenes Both in Planktonic and in Sessile Forms. Res. Microbiol. 2020, 171, 351–356. [Google Scholar] [CrossRef]
- Liu, F.; Jin, P.; Gong, H.; Sun, Z.; Du, L.; Wang, D. Antibacterial and Antibiofilm Activities of Thyme Oil against Foodborne Multiple Antibiotics-Resistant Enterococcus faecalis. Poult. Sci. 2020, 99, 5127. [Google Scholar] [CrossRef]
- Felix e Silva, A.; Pires, I.C.; da Costa, M.M.; Melo, J.F.B.; Lorenzo, V.P.; de Melo, F.V.S.T.; Copatti, C.E. Antibacterial and Antibiofilm Activities and Synergism with Florfenicol from the Essential Oils of Lippia Sidoides and Cymbopogon Citratus against Aeromonas hydrophila. J. Appl. Microbiol. 2022, 132, 1802–1812. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Girigoswami, A.; Girigoswami, K. Antimicrobial, Pesticidal and Food Preservative Applications of Lemongrass Oil Nanoemulsion: A Mini-Review. Recent Adv. Food Nutr. Agric. 2022, 13, 51–58. [Google Scholar] [CrossRef]
- Carlos, A.R.; Semedo-Lemsaddek, T.; Barreto-Crespo, M.T.; Tenreiro, R. Transcriptional Analysis of Virulence-Related Genes in Enterococci from Distinct Origins. J. Appl. Microbiol. 2010, 108, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Hancock, L.E.; Perego, M. The Enterococcus faecalis Fsr Two-Component System Controls Biofilm Development through Production of Gelatinase. J. Bacteriol. 2004, 186, 5629–5639. [Google Scholar] [CrossRef]
- Andrade, J.C.; Bernardo, R.; Barreto, A.S.; Nunes, T.; Henriques, A.R. Temperature Effect on Listeria Monocytogenes Planktonic Growth and Biofilm-Forming Ability. J. Vet. Med. Anim. Sci. 2020, 3, 1044. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, T.; Cervantes-Huamán, B.R.H.; Turitich, L.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Removal of Listeria Monocytogenes Biofilms on Stainless Steel Surfaces through Conventional and Alternative Cleaning Solutions. Int. J. Food Microbiol. 2022, 381, 109888. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, L.; Khodaei, N.; Salmieri, S.; Karboune, S.; Lacroix, M. Correlation between Chemical Composition and Antimicrobial Properties of Essential Oils against Most Common Food Pathogens and Spoilers: In-Vitro Efficacy and Predictive Modelling. Microb. Pathog. 2020, 147, 104212. [Google Scholar] [CrossRef] [PubMed]
- Moran, U.; Phillips, R.; Milo, R. SnapShot: Key Numbers in Biology. Cell 2010, 141, 1262.e1. [Google Scholar] [CrossRef] [PubMed]
- Quendera, A.P.; Barreto, A.S.; Semedo-Lemsaddek, T. Antimicrobial Activity of Essential Oils against Foodborne Multidrug-Resistant Enterococci and Aeromonads in Planktonic and Biofilm State. Food Sci. Technol. Int. 2019, 25, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Stremick, C.A.; Turner, R.J.; Allan, N.D.; Olson, M.E.; Ceri, H. Microtiter Susceptibility Testing of Microbes Growing on Peg Lids: A Miniaturized Biofilm Model for High-Throughput Screening. Nat. Protoc. 2010, 5, 1236–1254. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P.; Patel, J.B.; Burnhman, C.-A.; ZImmer, B.L. Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Standard, Approval CDM-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume 38. [Google Scholar]
- Oulkheir, S.; Aghrouch, M.; el Mourabit, F.; Dalha, F.; Graich, H.; Amouch, F.; Ouzaid, K.; Moukale, A.; Chadli, S.; Chadli Antibacterial, S. Antibacterial Activity of Essential Oils Extracts from Cinnamon, Thyme, Clove and Geranium Against a Gram Negative and Gram Positive Pathogenic Bacteria. J. Dis. Med. Plants 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Macià, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial Susceptibility Testing in Biofilm-Growing Bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R. Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128110317. [Google Scholar]
- Yuan, L.; Sadiq, F.A.; Wang, N.; Yang, Z.; He, G. Recent Advances in Understanding the Control of Disinfectant-Resistant Biofilms by Hurdle Technology in the Food Industry. Crit. Rev. Food Sci. Nutr. 2021, 61, 3876–3891. [Google Scholar] [CrossRef]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm Formation and Control in Food Processing Facilities. Compr. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef] [PubMed]
- González-Rivas, F.; Ripolles-Avila, C.; Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Rodríguez-Jerez, J.J. Biofilms in the Spotlight: Detection, Quantification, and Removal Methods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1261–1276. [Google Scholar] [CrossRef]
- Garvey, M.; Curran, D.; Savage, M. Efficacy Testing of Teat Dip Solutions Used as Disinfectants for the Dairy Industry: Antimicrobial Properties. Int. J. Dairy Technol. 2017, 70, 179–187. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Guo, A.; Zhang, X.; Liu, W.; Ruan, Y. Formation of Multispecies Biofilms and Their Resistance to Disinfectants in Food Processing Environments: A Review. J. Food Prot. 2021, 84, 2071–2083. [Google Scholar] [CrossRef]
- Shak, J.R.; Whitaker, J.A.; Ribner, B.S.; Burd, E.M. Aminoglycoside-Resistant Aeromonas hydrophila as Part of a Polymicrobial Infection Following a Traumatic Fall into Freshwater. J. Clin. Microbiol. 2011, 49, 1169–1170. [Google Scholar] [CrossRef]
- Chao, C.M.; Lai, C.C.; Tang, H.J.; Ko, W.C.; Hsueh, P.R. Skin and Soft-Tissue Infections Caused by Aeromonas Species. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 543–547. [Google Scholar] [CrossRef]
- Rosado de Castro, M.; da Silva Fernandes, M.; Kabuki, D.Y.; Kuaye, A.Y. Biofilm Formation on Stainless Steel as a Function of Time and Temperature and Control through Sanitizers. Int. Dairy J. 2017, 68, 9–16. [Google Scholar] [CrossRef]
- Craveiro, S.; Alves-Barroco, C.; Barreto Crespo, M.T.; Salvador Barreto, A.; Semedo-Lemsaddek, T. Aeromonas Biofilm on Stainless Steel: Efficiency of Commonly Used Disinfectants. Int. J. Food Sci. Technol. 2015, 50, 851–856. [Google Scholar] [CrossRef]
- Nagar, V.; Pansare Godambe, L.; Bandekar, J.R.; Shashidhar, R. Biofilm Formation by Aeromonas Strains under Food-Related Environmental Stress Conditions. J. Food Process. Preserv. 2017, 41, 6–11. [Google Scholar] [CrossRef]
- Wagner, E.M.; Pracser, N.; Thalguter, S.; Fischel, K.; Rammer, N.; Pospíšilová, L.; Alispahic, M.; Wagner, M.; Rychli, K. Identification of Biofilm Hotspots in a Meat Processing Environment: Detection of Spoilage Bacteria in Multi-Species Biofilms. Int. J. Food Microbiol. 2020, 328, 108668. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.M.; Martinez-Garcia, E.; Xavier, J.; Durham, W.M.; Kolter, R.; Kim, W.; Foster, K.R. Biofilm Formation As a Response to Ecological Competition. PLoS Biol. 2015, 13, e1002191. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, J.J.; Hong, B.; Tan, L.; Yan, J.; Zhang, Z.; Liu, H.; Pan, Y.; Zhao, Y. Characterization of Mixed-Species Biofilm Formed by Vibrio parahaemolyticus and Listeria monocytogenes. Front. Microbiol. 2019, 10, 2543. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Synergistic Antimicrobial Activities of Plant Essential Oils against Listeria Monocytogenes in Organic Tomato Juice. Food Control 2021, 125, 108000. [Google Scholar] [CrossRef]
- de Oliveira, M.M.M.; Brugnera, D.F.; do Nascimento, J.A.; Piccoli, R.H. Control of Planktonic and Sessile Bacterial Cells by Essential Oils. Food Bioprod. Process. 2012, 90, 809–818. [Google Scholar] [CrossRef]
- Ozogul, Y.; Kuley Boğa, E.; Akyol, I.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Köşker, A.R. Antimicrobial Activity of Thyme Essential Oil Nanoemulsions on Spoilage Bacteria of Fish and Food-Borne Pathogens. Food Biosci. 2020, 36, 100635. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical Composition and in Vitro Antibacterial Properties of Essential Oils of Four Thymus Species from Organic Growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M.A. Evaluation of Common Antibacterial Screening Methods Utilized in Essential Oil Research. J. Essent. Oil Res. 2003, 15, 428–433. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- al Laham, S.A.; al Fadel, F.M. Antibacterial Activity of Various Plants Extracts against Antibiotic-Resistant Aeromonas hydrophila. Jundishapur. J. Microbiol. 2014, 7, e11370. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.; Rüben, C.; Upmann, M. Antimicrobial Activity of Essential Oil Components against Potential Food Spoilage Microorganisms. Curr. Microbiol. 2013, 67, 200–208. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro Antibacterial Activity of Some Plant Essential Oils. BMC Complement. Med. Ther. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono- and Polymicrobic Bacterial Cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A Review of Current and Emergent Biofilm Control Strategies. LWT 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Fuentes, C.; Fuentes, A.; Barat, J.M.; Ruiz, M.J. Relevant Essential Oil Components: A Minireview on Increasing Applications and Potential Toxicity. Toxicol. Mech. Methods 2021, 31, 559–565. [Google Scholar] [CrossRef]
- Wu, X.S.; Xie, T.; Lin, J.; Fan, H.Z.; Huang-Fu, H.J.; Ni, L.F.; Yan, H.F. An Investigation of the Ability of Elemene to Pass through the Blood-Brain Barrier and Its Effect on Brain Carcinomas. J. Pharm. Pharmacol. 2009, 61, 1653–1656. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]



| Bacterial Strains | Codes | Growth Conditions |
|---|---|---|
| E. faecalis QSE123 | E1 | 37 °C, for 24 h |
| E. faecalis V583 | E2 | |
| L. monocytogenes CECT 937 (serotype 3b) | L1 | |
| L. monocytogenes CECT 935 (serotype 4b) | L2 | |
| A. hydrophila (A259) | A1 | |
| B. thermosphacta ATCC 11509T | B1 | 25 °C, for 24 h |
| Botanic Name | Common Name | Plant Part | Origin | Product Code | Major Components |
|---|---|---|---|---|---|
| Thymus vulgaris | Thyme red (natural blend) essential oil | Leaves | India | 90015-A01 | Thymol 36.3%; p-cymene 18.5%; γ-terpinene 10.9%; Linalool 7.1%; Carvacrol 5.2%; β-caryophyllene 4.5%; β-myrcene 2.2%; α-pinene 2% |
| Cymbopogon flexuosus | Lemongrass organic essential oil | Leaves | India | 50032-A18 | Geranial 41.3%; Neral 32%; Geraniol 6.7%; Geranyl acetate 3.2% |
| Contact Surface | Stainless Steel | Polystyrene | |||
|---|---|---|---|---|---|
| Temperature | 4 °C | 25 °C | 4 °C | 25 °C | |
| Combinations Mixed cultures | Microorganism Pure cultures | Statistical significance (p-values) | |||
| B1 + L1 + E1 + A1 | B1 | p < 0.001 | N.S. | N.S. | N.S. |
| L1 | N.S. | N.S. | N.S. | p < 0.001 | |
| E1 | p = 0.005 | N.S. | N.S. | N.S. | |
| A1 | p < 0.001 | N.S. | p = 0.032 | p = 0.003 | |
| B1 + E1 | B1 | p < 0.001 | N.S. | N.S. | N.S. |
| E1 | p = 0.002 | N.S. | N.S. | N.S. | |
| B1 + A1 | B1 | p = 0.002 | N.S. | N.S. | N.S. |
| A1 | p < 0.001 | N.S. | N.S. | p < 0.001 | |
| B1 + L1 | B1 | p < 0.001 | N.S. | N.S. | N.S. |
| L1 | N.S. | N.S. | N.S. | p < 0.001 | |
| L1 + E1 + A1 | L1 | N.S. | N.S. | N.S. | p = 0.001 |
| E1 | N.S. | N.S. | N.S. | N.S. | |
| A1 | p < 0.001 | N.S. | N.S. | N.S. | |
| E1 + A1 | E1 | N.S. | N.S. | N.S. | N.S. |
| A1 | p = 0.001 | N.S. | N.S. | N.S. | |
| L1 + A1 | L1 | N.S. | N.S. | N.S. | p = 0.003 |
| A1 | p < 0.001 | N.S. | N.S. | N.S. | |
| L1 + E1 | L1 | N.S. | N.S. | N.S. | p < 0.001 |
| E1 | p < 0.001 | N.S. | N.S. | N.S. | |
| Antimicrobial Compound/EO | Growth Inhibition Zone (mm) per Microorganism | |||||
|---|---|---|---|---|---|---|
| L1 | L2 | E1 | E2 | A1 | B1 | |
| EOT | 5.5 ± 0.4 | 5.2 ± 0.2 | 5.5 ± 0.5 | 5.0 ± 0.0 | 6.2 ± 0.2 | 5.7 ± 0.5 |
| EOL | 5.0 ± 0.4 | 4.8 ± 0.6 | Ø | Ø | 4.0 ± 0.4 | Ø |
| Microorganisms (Pure Cultures) | EOT | EOL | ||||
|---|---|---|---|---|---|---|
| MIC (µg/µL) | MBC (µg/µL) | MBC/MIC Ratio | MIC (µg/µL) | MBC (µg/µL) | MBC/MIC Ratio | |
| L1—L. monocytogenes CECT 937 | 0.24 | 0.49 | 2.04 | 0.49 | 0.98 | 2 |
| L2—L. monocytogenes CECT 935 | 0.24 | 0.49 | 2.04 | 7.80 | 15.60 | 2 |
| E1—E. faecalis QSE123 | 0.24 | 0.49 | 2.04 | 1.95 | 3.90 | 2 |
| E2—E. faecalis V583 | 0.24 | 0.97 | 4.04 | 0.98 | 1.95 | 1.99 |
| A1—A. hydrophila A259 | 0.06 | 0.12 | 2.00 | >15.60 | >15.60 | N.D. |
| B1—B. thermosphacta ATCC 11509Τ | 0.24 | 0.97 | 4.04 | 0.98 | 1.95 | 1.99 |
| Mixed Cultures | EOT | EOL | ||||
|---|---|---|---|---|---|---|
| MIC (µg/µL) | MBC (µg/µL) | MBC/MIC Ratio | MIC (µg/µL) | MBC (µg/µL) | MBC/MIC Ratio | |
| L1 + E1 | 0.97 | 1.94 | 2.00 | 1.95 | 3.90 | 2 |
| L1 + B1 | 0.12 | 0.97 | 8.08 | 0.49 | 1.95 | 3.98 |
| L1 + A1 | 0.49 | 0.97 | 1.98 | 0.98 | 1.95 | 1.99 |
| A1 + B1 | 0.49 | 0.97 | 1.98 | 0.98 | 1.95 | 1.99 |
| E1+ A1 | 0.49 | 0.97 | 1.98 | 1.95 | 3.90 | 2 |
| E1 + B1 | 0.24 | 0.97 | 4.04 | 1.95 | 3.90 | 2 |
| L1 + E1 + A1 | 0.97 | 1.94 | 2.00 | 1.95 | 3.90 | 2 |
| L1 + E1 + A1 + B1 | 0.49 | 1.94 | 3.96 | 0.98 | 3.90 | 3.98 |
| Microorganisms (Pure Cultures) | MBEC (µg/µL) | |
|---|---|---|
| EOT | EOL | |
| L1—L. monocytogenes CECT 937 | 443.14 | 443.14 |
| L2—L. monocytogenes CECT 935 | 110.79 | 443.14 |
| E1—E. faecalis QSE123 | 221.57 | >886.28 |
| E2—E. faecalis V583 | 886.28 | 886.28 |
| A1—A. hydrophila A259 | 443.14 | 886.28 |
| B1—B. thermosphacta ATCC 11509Τ | 886.28 | 886.28 |
| Mixed Culture | MBEC (µg/µL) | |
|---|---|---|
| EOT | EOL | |
| L1 + E1 | >886.28 | >886.28 |
| L1 + B1 | 443.14 | >886.28 |
| L1 + A1 | >886.28 | 886.28 |
| A1 + B1 | 443.14 | >886.28 |
| E1 + A1 | >886.28 | >886.28 |
| E1 + B1 | 886.28 | >886.28 |
| L1 + E1 + A1 | >886.28 | >886.28 |
| L1 + E1 + A1 + B1 | 886.28 | >886.28 |
| Molecular Structure | Pharmacokinetics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Essential Oil | Constituent | Intestinal Absorption (%) | Skin Permeability (logKp) | BBB * Permeability (logBB) | AMES Toxicity | Hepatotoxicity | Skin Sensitization | Max. Tolerated Dose (mg/kg/day) | |
| Thyme | Thymol (31.2%) PubChem C10H14O | 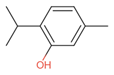 | 90.84 | −1.62 | 0.41 | No | Yes | Yes | 1.00 |
| P-cymene (18.5%) PubChem C10H14 |  | 93.54 | −1.19 | 0.48 | No | No | Yes | 0.90 | |
| γ-terpinene (10.9%) PubChem C10H16 |  | 96.22 | −1.49 | 0.75 | No | No | No | 0.76 | |
| Linalool (7.1%) PubChem C10H18O | 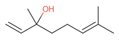 | 93.16 | −1.74 | 0.60 | No | No | Yes | 0.78 | |
| Carvacrol (5.2%) PubChem C10H14O |  | 90.84 | −1.62 | 0.41 | No | Yes | Yes | 1.00 | |
| β-caryophyllene (4.5%) PubChem C15H24 | 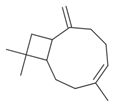 | 94.85 | −1.58 | 0.73 | No | No | Yes | 0.35 | |
| β-myrcene (2.2%) PubChem C10H16 |  | 94.70 | −1.04 | 0.78 | No | No | No | 0.62 | |
| α-pinene (2%) PubChem C10H16 | 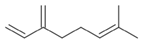 | 94.70 | −1.04 | 0.78 | No | No | No | 0.62 | |
| Lemongrass | Geranial (41.3%) PubChem C10H16O |  | 95.92 | −2.41 | 0.63 | No | No | Yes | 0.54 |
| Neral (32%) PubChem C10H16O | 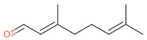 | 95.32 | −2.413 | 0.63 | No | No | Yes | 0.54 | |
| Geraniol (6.7%) PubChem C10H18O | 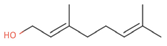 | 92.80 | −1.51 | 0.61 | No | No | Yes | 0.65 | |
| Geranyl acetate (3.2%) PubChem C12H20O2 |  | 94.90 | −1.67 | 0.57 | No | No | Yes | 0.47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro Marques, J.; Serrano, S.; Selmi, H.; Giesteira Cotovio, P.; Semedo-Lemsaddek, T. Antimicrobial and Antibiofilm Potential of Thymus vulgaris and Cymbopogon flexuosus Essential Oils against Pure and Mixed Cultures of Foodborne Bacteria. Antibiotics 2023, 12, 565. https://doi.org/10.3390/antibiotics12030565
Monteiro Marques J, Serrano S, Selmi H, Giesteira Cotovio P, Semedo-Lemsaddek T. Antimicrobial and Antibiofilm Potential of Thymus vulgaris and Cymbopogon flexuosus Essential Oils against Pure and Mixed Cultures of Foodborne Bacteria. Antibiotics. 2023; 12(3):565. https://doi.org/10.3390/antibiotics12030565
Chicago/Turabian StyleMonteiro Marques, Joana, Susana Serrano, Hiba Selmi, Pedro Giesteira Cotovio, and Teresa Semedo-Lemsaddek. 2023. "Antimicrobial and Antibiofilm Potential of Thymus vulgaris and Cymbopogon flexuosus Essential Oils against Pure and Mixed Cultures of Foodborne Bacteria" Antibiotics 12, no. 3: 565. https://doi.org/10.3390/antibiotics12030565
APA StyleMonteiro Marques, J., Serrano, S., Selmi, H., Giesteira Cotovio, P., & Semedo-Lemsaddek, T. (2023). Antimicrobial and Antibiofilm Potential of Thymus vulgaris and Cymbopogon flexuosus Essential Oils against Pure and Mixed Cultures of Foodborne Bacteria. Antibiotics, 12(3), 565. https://doi.org/10.3390/antibiotics12030565







