Abstract
Staphylococci is an opportunistic bacterial population that is permanent in the normal flora of milk and poses a serious threat to animal and human health with some virulence factors and antibiotic-resistance genes. This study was aimed at identifying staphylococcal species isolated from raw milk and to determine hemolysis, biofilm, coagulase activities, and beta-lactam resistance. The raw milk samples were collected from the Düzce (Türkiye) region, and the study data represent a first for this region. The characterization of the bacteria was performed with MALDI-TOF MS and 16S rRNA sequence analysis. The presence of coa, icaB, blaZ, and mecA was investigated with PCR. A nitrocefin chromogenic assay was used for beta-lactamase screening. In this context, 84 staphylococci were isolated from 10 different species, and the dominant species was determined as S. aureus (32.14%). Although 32.14% of all staphylococci were positive for beta hemolysis, the icaB gene was found in 57.14%, coa in 46.42%, mecA in 15.47%, and blaZ in 8.33%. As a result, Staphylococcus spp. strains that were isolated from raw milk in this study contained some virulence factors at a high level, but also contained a relatively low level of beta-lactam resistance genes. However, considering the animal–environment–human interaction, it is considered that the current situation must be monitored constantly in terms of resistance concerns. It must not be forgotten that the development of resistance is in constant change among bacteria.
1. Introduction
The presence of pathogens in foods of animal origin is a common healthcare issue. Some virulence factors of pathogens and resistance to antimicrobial agents constitute the basis of this problem. Also, the fact that the antimicrobial resistance characteristics of these pathogens, which are mostly opportunistic, are constantly changing, makes it difficult to monitor and solve the problem [1,2].
Although the microbial load of milk is low when it is first obtained, the variety of microorganisms it contains is important because it may contain mastitis factors that affect animal health and the milk quality. Mastitis is an inflammation of the mammary gland and the majority of infections are caused by Staphylococcus spp., Streptococcus spp., and Gram-negative bacteria [3,4]. Some of these factors are contagious and some are environmental. Knowing the microbial load obtained from raw milk and identifying microorganisms are important in terms of determining the subchronic potential. Despite many studies conducted on mastitis in Türkiye and around the world, it is still common in dairy cattle breeding. Therefore, antibiotics are used for its treatment [5,6].
The most common isolated bacterium from milk is Staphylococcus aureus. S. aureus, which is persistent in the normal flora of milk, sometimes causes toxic syndrome and staphylococcal food poisoning in animals and humans. Previous studies reported coagulase-positive (CoP) and coagulase-negative (CoN) S. aureus in milk and dairy products [7,8]. Coagulase characterization is considered a virulence factor for Staphylococcus spp. [9]. Staphylococcus spp., whose other virulence characteristic is biofilm formation, can sustain its existence in milk in a more stable way by forming a biofilm [3].
Another important issue is the microbiome aside from the opportunistic pathogen content in milk. Resistance genes in the microbiome pose a risk to animal and human health. Antibiotic resistance is already a growing crisis. This reduces the effectiveness of drugs and poses problems in the treatment of animals and humans. In recent years, resistance to beta-lactams has especially increased [10,11]. Although bacteria such as Enterobacteriaceae are reported to be the main source of beta-lactamases, they are also frequently found in Gram-positive bacteria. This resistance is acquired by exogenous genes or chromosomal mutations. However, bacteria can spread these genes horizontally and vertically among species [12,13]. For this reason, exposure to antibiotics is not a necessary condition for the development of resistance. Also, the transfer and spread of genes providing antimicrobial resistance are not only associated with environmental pathogens, but bacterial natural ecosystems are also determinant in this respect [14]. However, it was shown in previous studies that cow’s milk plays a role in the spread of methicillin-resistant S. aureus (MRSA), which is an extremely clinically important strain [15]. For this reason, mobile genetic elements can be faced in many different bacterial species. However, although the genes transferred to probiotic or commensal microorganisms and their resistance to antibiotics appear to be a positive condition, specific antibiotic resistance markers carried on mobile genetic elements pose a safety issue for health [16]. It is considered that overcoming this safety problem is possible with the “One Health Approach”, which focuses on the fact that infectious diseases and antimicrobial agent resistance are interconnected in terms of animal and human healthcare and must be continuously monitored together [17]. This study is the first report for Düzce, Türkiye. Thus, the first data about the state of the region are provided. Healthy-looking animals were selected as subjects. In the present study, the purpose was to identify staphylococcal species obtained from raw milk and to investigate some virulence characteristics.
2. Results
2.1. MALDI-TOF MS and Sequence Analysis
A total of 84 Staphylococcus-suspected isolates were obtained from 117 milk samples that were collected in the study. After some biochemical tests, the Staphylococcus spp. species were determined with MALDI-TOF MS in the isolates. The percentage dispersion of the isolates according to species is given in Figure 1. No weak spectrum was detected in the analysis and all of the isolates (100%) could be identified based on species. The score values of the isolates were determined between 1743 and 2786, which was found above 2000 for 79% of the isolates (Figure 2). However, no finding was detected with a score below 1700 (the cut-off value of the MALDI-TOF MS). The isolates were sent for post-PCR sequencing and 16rRNA characterization, and the results were found to be the same as for MALDI-TOF MS. In this respect, 84 bacteria including 10 different Staphylococcus species were obtained and the most isolated species was S. aureus (32.14%), followed by S. borealis (14.28%), S. chromogenes (10.71%), S. warneri (10.71%), and S. epidermidis (10.71%). S. haemolyticus, S. hominis, S. xylosus, and S. vitulinus species were detected at a rate of at least 3.57%.
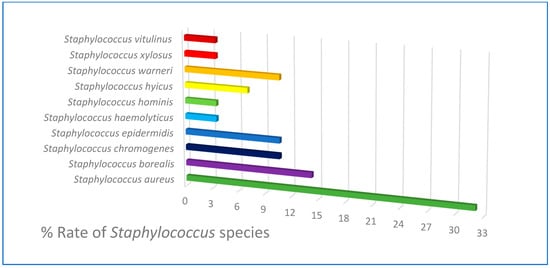
Figure 1.
Staphylococcus species isolated from raw milk.
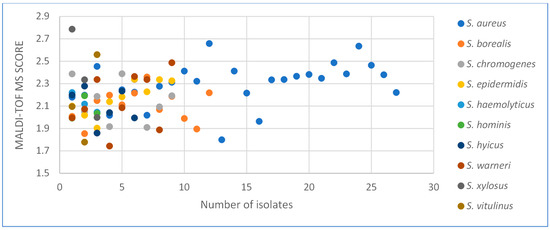
Figure 2.
Dispersion of MALDI-TOF MS Scores.
2.2. Evaluation of Hemolysis, Biofilm, and Coagulase Ability
Although β-hemolysis was detected in 32.14% of the total isolates, α-hemolysis was not detected. The remaining isolates were evaluated as non-hemolytic. Although coagulase positivity was detected in 66.6% of S. aureus (n = 27) isolates in the species, strong biofilm formation was detected in 55.5% and hemolysis in 22.2%. Coagulase positivity was detected in some strains of S. borealis, S. chromogenes, S. epidermidis, and S. hyicus, and hemolysis was detected in some strains of S. vitulinus, S. warneri, S. hyicus, S. chromogenes, and S. borealis. In general, coagulase was detected in 46.42% of the total isolates (n = 84). Strong biofilm formation was detected in 57.14% of the Staphylococcus spp. isolates and 14.28% of them formed weak biofilms. The results determined by the slime-forming ability of the isolates on Congo red agar (Figure 3) showed parallelism with the biofilm formation that was determined by the microplate method. Some characteristics of the isolates according to the species are given in Table 1.
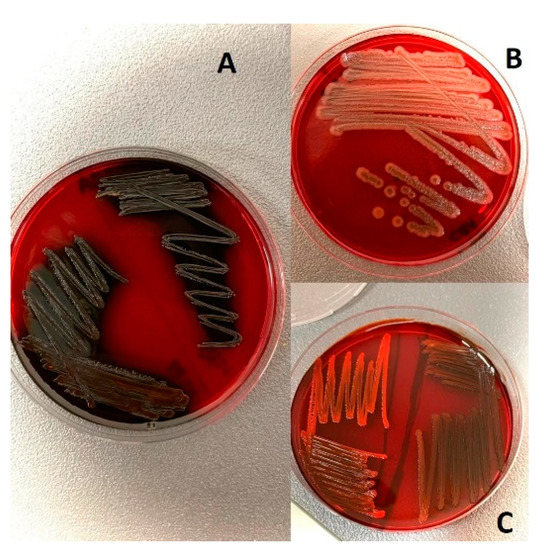
Figure 3.
Staphylococcus spp. on CRA: (A) Strong slime-forming strain. (B) Non-producing slime. (C) Weak slime-forming strains.

Table 1.
Number of antibiotic-resistant isolates.
2.3. Antibiotic Resistance Profiles
The antibiotic susceptibility of the isolates was determined by bacterial growth inhibition zones (Figure 4). The antibiotic resistance of the isolates was observed at a relatively low level in the study. The highest resistance was found for kanamycin 21.42% and penicillin 19.04%, respectively. The least resistance was detected against 1.19% vancomycin and 3.57% ciprofloxacin. Two of the isolates were found resistant to at least four different classes, and 15 isolates were found resistant to least three different classes. The highest resistance was observed against the aminoglycoside class 40.54%. In addition, resistance to antibiotics in the beta-lactam group was observed in 39.63%. Multidrug resistance was observed in 20.23% of the isolates. The resistance profiles of the isolates are given in Table 1.
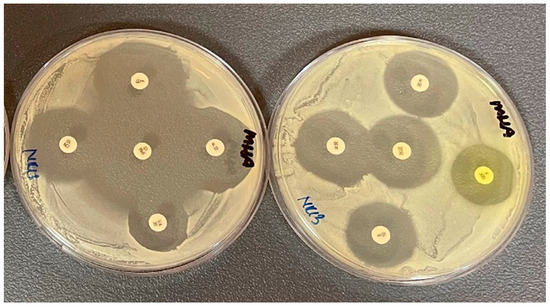
Figure 4.
The images of disk diffusion assay on S. aureus.
2.4. Beta-Lactamase Screening
The beta-lactamase presence of all the isolates were tested by chromogenic disk assay and positivity was detected in only seven isolates, four of which were S. aureus and three were S. epidermidis isolates. The color change in the positive isolates was from yellow to pink (Figure 5).
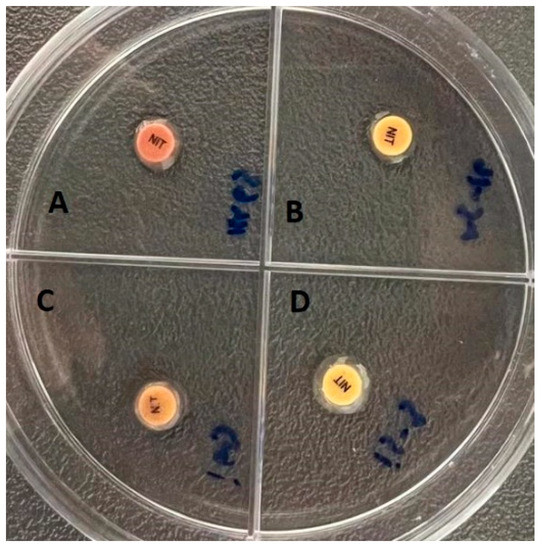
Figure 5.
Chromogenic disk assay: (A,C) Beta-lactamase positive strains. (B,D) Beta-lactamase negative strains.
2.5. PCR Results
The presence of the icaB gene (57.14%) was detected in all strains of S. aureus, S. borealis, S. chromogenes, S. hyicus, S. warneri, and S. epidermidis, which formed strong biofilms by Congo red agar assay. No ica gene was detected in any of the S. warneri, S. xylosus, or S. haemolyticus strains, which were found to form weak biofilms in the microplate assay. The coagulase gene coa was was detected in 46.42% of all the isolates. The total ratio in the species was as follows: S. aureus (21.42%), S. borealis, S. hyicus, and S. chromogenes (7.14%), S. epidermidis (3.57%). The resistance gene mecA was detected in three of the S. borealis isolates, and both the mecA and blaZ genes were detected in three S. epidermidis isolates. Although seven isolates were found to be mecA-positive in the S. aureus strains, four blaZ-positive isolates were detected, including the blaZ gene in three mecA-positive isolates and the blaZ gene in one mecA-negative isolate. Also, no other strains that carried mecA and blaZ genes were detected, which means that 15.47% of 84 staphylococci were mecA-positive and 8.33% blaZ-positive. The virulence factors and PCR results of the isolates according to the species are given in Table 2 and Figure 6. The percentage evaluation of the virulence genes in each species is given in Figure 7.

Table 2.
Number of isolates with positive test results.
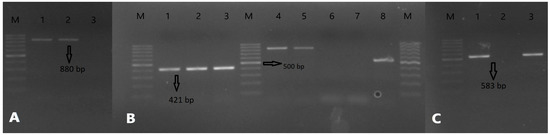
Figure 6.
PCR results: (A) PCR bands for icaB (880 bp), 1,2: Positive sample, 3: Negative sample. (B) PCR bands for blaZ (421 bp) and coa (variable), 1: Positive control for blaZ, 2,3: Positive samples for blaZ, which is from the S. aureus strains, 4,5: Positive result for coa is almost 850 bp, 6,7: Negative samples, 8: Positive result for coa is almost 550 bp. (C) PCR bands for mecA (583 bp), 1,3: Positive samples for mecA, which is from the S. epidermidis strains, 2: Negative sample. M: Marker, 100–1000 bp DNA ladder is used.
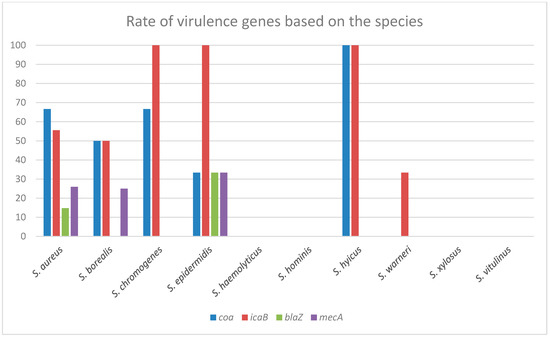
Figure 7.
Distribution of virulence genes within the species: This table does not give the general distribution of virulence genes. In this table, information is given about how many of a species detected in the study have which gene. For example, while all S. epidermidis strains were icaB positive, more than 30% of them were positive for coa, mecA, and blaZ. At least six different species in the study contain one or more virulence genes. In the remaining four species, the virulence genes investigated in this study could not be detected.
2.6. Statistical Analysis
It was concluded that there is a complete correlation between biofilm forming and slime forming in all bacteria. Also, the data show that there is a strong correlation between biofilm-forming, slime forming, and the icaB gene. When all the virulence genes were evaluated collectively, a correlation was found between them. The statistical data are given in Table 3.

Table 3.
Correlations between virulence features.
3. Discussion
Bovine milk is an important food source for humans and calves with its nutritive contents and probiotic microorganisms. However, it can also be a source of transmission for some foodborne diseases [18,19]. When first obtained, milk always has a microbial flora whose importance in terms of health is determined by the diversity of species and some characteristics of the species it includes [20,21]. Because of this, the purpose was to investigate and characterize the staphylococcal community in raw milk in the present study. There was no previous study in which flora was screened and the virulence characteristics were investigated in raw cow milk in the region where the milk samples were collected (Düzce, Türkiye). The only previous study was the screening of Mycobacterium bovis in raw milk samples [22]. Hereby, the present study is the first in terms of microbial screening in dairy cattle in this area. On the other hand, it can be considered that the number of samples collected in the study was limited. However, the results are considered important and will contribute to future studies because this is the first survey in the area. Microbial diversity and resistance development in bacteria must be constantly monitored in the environment–human–animal triangle, in both the wide and the narrow environments [23,24]. Especially considering that mastitis is the most important factor that causes economic losses in dairy cattle breeding in Türkiye, it would be useful to follow up on every farm and village-type farming in every area. Also, the identification of bacteria in milk shows the potential for subclinical mastitis [25,26,27]. The data obtained in this study are the first for the city of Düzce in Türkiye.
In general, the population of staphylococci is similar to that found in raw milk collected from healthy cows [3]. The identification findings in our study were similar to previous studies. The findings of the sequence analysis attest to MALDI-TOF MS identification. Therefore, it can be considered that MALDI-TOF MS is a highly accurate method for bacterial identification. MALDI-TOF MS has been used in microbiology practice for over 10 years and has almost replaced biochemical tests in terms of accuracy and costs, especially in the last 5 years. It is also an FDA-approved (Food and Drug Administration) method for microbial identification [28,29].
Some virulence factors determine the pathogenic importance of staphylococci opportunistic microorganism, which are persistent in the normal flora of milk [30]. Coagulase is one of the most important virulence factors for staphylococci. The coa gene was observed in two different sizes (approximately 850 bp and 550 bp) in this study. Because the coa gene encodes the coagulase protein, is highly polymorphic because of the variable sequences. With the repetitive sequence numbers, PCR products may have different lengths, even within the same species [31]. Javid et al. [31] have identified two different sizes of 595 bp and 802 bp for coa in their study, and these are so similar to our study. Six different genotypes of coa, 440 bp, 510 bp, 547 bp, 680 bp, 740 bp, and 820 bp, were found in another study [32]. According to another assessment, not only does S. aureus have a CoP characteristic, but other staphylococci also have a coagulase characteristic. There are studies in which coa-positive was detected even in strains known as CoN among Staphylococcus species. Unfortunately, other coagulase-positive staphylococci are ignored in veterinary medicine [33,34]. In this study, the coa gene was also detected in some of the S. chromogenes, S. borealis, and S. epidermidis strains, which are generally known as CoN. In the study of Javid [31], the coa gene was detected in 25% of S. aureus that was isolated from nasal swabs in cattle and in 86.3% of S. aureus isolated from milk with mastitis. In another study, it was reported that coa-positive and negative S. hyicus, S. haemolyticus, S. epidermidis, S. chromogenes, and S. aureus isolates were obtained in strains isolated from goat and sheep milk [34]. Eventually, the prevalence of CoPS (coagulase-positive staphylococci) was considered too high in this study because CoPS is usually pathogenic [32]. This is also an indicator of the genetic exchange between bacteria in natural environments. Some studies emphasize that commensal Staphylococcus spp. species is a good reservoir for virulence genes [35,36,37]. Gene transfer experiments were not performed in this study; however, they may be needed future studies.
Another virulence factor in staphylococci is biofilm formation. Biofilm-producing bacteria adhere to living/inanimate surfaces with the polymer structures they produce and imprison themselves in a matrix [38]. The presence of biofilm-forming strains in milk is clinically important because biofilm formation facilitates attachment to mammalian epithelial cells. Biofilms can protect bacteria from antibiotic stress and phagocytosis [3]. One study reported that 53.6% of S. epidermidis isolates were able to produce a biofilm [31]. Rudenko et al. [39] reported that high proportion of microorganisms isolated from cows with mastitis have the ability to form biofilms. Therefore, the presence of biofilm-forming strains in this study may be a potential hazard for mastitis formation. Researchers characterize biofilm as an important factor in terms of disease pathogenicity and drug resistance [40]. The icaB gene could not be detected in 12 strains that were found to form weak biofilms in phenotype tests. This may be because of other related genes. Previous studies show that Staphylococcus species can form biofilms independent of ica genes. The expression of other possible genes may be causing poor biofilm formation. There is no reason for the formation of a weak biofilm. Moreover, it may be a defect in the existing gene. Thus, there may also be deficiencies at the gene expression level. In general, biofilm formation takes place in two critical steps, attachment and accumulation. The adhesive proteins that provide this are encoded by many different genes. Additionally, in the transformative step, the bacterial cell surface province is complete, as it does not come into contact, but long-distance interactions occur between the bacterial cell and the surface. These are electrostatic forces, hydrophobic interactions, and van der Waals forces, which are weak interactions. Besides, the biofilm may not be passing through the maturation stage in these strains because biofilm formation is a multi-stage process [40,41,42,43,44]. Apart from this, another important virulence factor in staphylococci is the content of hemolysin. The strains damage the host cell membrane with their exotoxins, such as hemolysin, and transparent zones are formed in blood agar with the lysis of erythrocytes. Previous studies also reported that there are positive strains of hemolysis in staphylococci isolated from milk similar to our study [43,44,45,46].
Another issue that makes the bacteria in milk important for healthcare is the potential to carry antimicrobial resistance genes, because antibiotic consumption in animal husbandry can be unconscious in most developing countries [47,48]. Antibiotic resistance is not only a major crisis for non-healthy animals (with mastitis), but also a potential problem for healthy animals. Acute mastitis and chronic mastitis, which cannot be treated because of antibiotic resistance, cause loss of productivity in animals, as well as economic losses [6,49]. Hence, it is necessary to determine and monitor the resistance profile of bacteria in milk. In this study, the beta-lactamase content of staphylococci was investigated with the nitrocefin chromogenic test. Nitrocefin is a chromogenic cephalosporin, and the nitrocefin substrate is hydrolyzed by the β-lactamase producing culture upon reaction with the β-lactamase in bacteria, producing a colored compound. The nitrocefin test results were confirmed with PCR because the bacteria are genotype positive in some cases, although the chromogenic nitrocefin test is negative. For example, Bidya and Suman [50] investigated the phenotypic beta-lactamase with three different methods and reported that the highest accuracy belonged to the chromogenic nitrocefin test. In another study, the nitrocefin test yielded correct results for S. aureus and S. epidermidis, but was false-negative for S. lugdunensis [51]. In our study, both test results were compatible with each other.
Based on another perspective, the contents of the mecA and blaZ genes were investigated in the determination of the beta-lactam antibiotic resistance of the isolates in the present study. The blaZ encodes a protein (penicillinase) that inactivates penicillin by hydrolyzing the beta-lactam ring and appears to be the gene responsible for beta-lactam resistance in staphylococci [51]. The mecA encodes a penicillin-binding protein in methicillin-resistant Staphylococcus spp. For this reason, the presence of these two genes in bacteria is very important. However, the occurrence of these genes in natural ecosystems poses a threat to public and animal health [52]. The drug resistance profile of bacteria is constantly changing [12,13]. Different prevalences can be detected in studies conducted in different regions or in studies conducted in the same region at different times. In their study, Zhang et al. [53] detected 92.95% of the blaZ gene in S. aureus isolated from milk samples. In another study, 54% of the blaZ gene was detected in Enterococcus spp., which was isolated from sheep and goat milk, and attention was drawn to subclinical mastitis [54]. In this study, some isolates that contained both blaZ and mecA were evaluated as more critical because these two genes are very important virulence genes. The presence of the mecA gene is a common and growing crisis, especially in staphylococci. In his study, Abdeen reported that more than half of the isolates contained mecA [55]. Similarly, other studies are reporting that more than 50% mecA was detected in bacteria isolated from bovine milk [56,57]. The prevalence of mecA is a relatively low rate. However, as mentioned earlier, it poses a potential danger because resistance genes in bacteria are mostly mobile genetic elements and resistance development is in constant exchange among bacteria [58]. The multidrug resistance levels of the isolates in this study are moderately risky. On the other hand, some experts predict that there will be 10 million deaths each year associated with antibiotic resistance by the year 2050, and one of the major pathogens causing it will be S. aureus [59,60]. Based on a different aspect, more than 30% of S. epidermidis contained mecA and blaZ genes. This rate is relatively high when evaluated within the S. epidermidis population because the bacteria that contain blaZ are insensitive to beta-lactam antibiotics. The beta-lactam antibiotic group covers a large class and is a serious danger [61]. Also, although mecA is generally associated with methicillin resistance, studies show that mecA also facilitates resistance to other beta-lactam antibiotics [15,62]. Additionally, a correlation was observed between the presence of coagulase, mecA, and blaZ in biofilm-forming species. This shows that the strains contain more than one virulence factor.
The potential risks of antimicrobial agent resistance have focused on human health and concerns in the food sector. The fact is that antimicrobial agent resistance is a burden for many sectors throughout the world. Antimicrobial agent resistance screening should be performed in the field. The results of our study are the first for this region. However, the present situation must be monitored in the human–environment–animal triangle and resolved with the “One Health Approach”.
4. Materials and Methods
4.1. Sample Collection
The milk samples (117) were freshly collected directly from healthy animals (cattle) in 8 different villages from Düzce, Türkiye between March and May 2022 in 50 mL sterile falcon tubes. The animals were selected from village-type breeding barns in Türkiye, not from industrial dairy farms. They were not under any special care and reinforcement. There was only routine maintenance and feeding conditions. The history of illness and antibiotics use dated back at least 2.5 or 3 months for each of them. The milk samples were taken directly from the udder by hand milking method. Before the milking process, the udder was first washed with normal water, then wiped with sterile water and 70% alcohol. Disposable gloves were used during milking. The samples were taken from individual animals separately and were inoculated directly on nutrient agar (NA) (Condalab, Madrid, Spain) supplemented with 10% defibrinated sheep blood. After 24 h of incubation at 37 °C, different colonies were selected and included in the study. Tryptic soy agar (TSA) (Merck, Darmstadt, Germany) and tryptic soy broth (TSB) (Condalab, Spain) media were used for culture.
4.2. Isolation of Bacteria and MALDI-TOF MS Identificaiton
Following the incubation on blood agar, the colonies were purified into TSA medium according to their characteristics, such as colony morphology and pigmentation. The following tests were performed on each colony: Gram-staining, catalase (3.0% (w/v) H2O2), oxidase (1.0% tetramethyl-phenylenediamine dihydrochloride) OF (oxidation-fermentation) test results, growth abilities in the MacConkey agar (MAC) (Condalab, Spain) medium and the medium that contained 6.5% NaCl were recorded, respectively. The identification of the isolates that were found to be Gram-positive was performed with the matrix-mediated laser desorption ionization time-of-flight mMass spectrometry (MALDI-TOF MS) method. The MALDI-TOF MS device (Bruker Microflex LT, Bremen, Germany) and Flex Control 3.0 software were used for the microbial biomass analysis. In line with the manufacturer’s instructions, the scores between 2000 and 3000 were evaluated as “possible species identification”, scores between 1700 and 1999 were considered as “probable genus identification”, and scores below 1699 were evaluated as “unreliable genus identification”.
4.3. Molecular Characterization
The DNA extraction was performed with the TE (10 mM Tris-HCl and 1mM EDTA, pH:8.0) boiling method in all isolates. Molecular confirmation of identification was made with 16S rRNA gene sequence analysis of the isolates F- ATT CTA GAG TTT GAT CAT GGC TCA and with PCR R-ATG GTA CCG TGT GAC GGG CGG TGT GTA primers [63]. PCR master mix (K0171 Thermo Scientific, USA) 10 pmol reverse and forward primers and RNAse DNAse free PCR water were used in the reaction. Thermocycle initial denaturation was performed at 95 °C for 2 min, 1 min at 94 °C for 35 cycles, 1 min at 50 °C, 2 min at 72 °C, and 5 min at 72 °C after the last cycle. The resulting PCR products were sent to the Macrogen Company (Wageningen, The Netherlands) for sequence analysis. The comparison of the results was made with the GenBank Database by using the BLAST program.
4.4. Hemolysis Ability of Isolates
The prepared fresh cultures were inoculated on blood agar and the zone diameters around the colonies were evaluated after 24 h of incubation at 37 °C. Bright zone beta hemolysis, green zones alpha hemolysis, and no zone were considered non-hemolytic.
4.5. Congo Red Agar Assay
Congo red agar medium was used to determine the slime forming of the isolates. The Congo red agar (CRA) was prepared by using 0.4 g Congo red, 18 g sucrose, and 500 mL brain heart infusion agar (BHI) (all chemicals were obtained from Merck). The agar plates containing the inoculum of the bacteria were incubated at 37 °C for 24 h and then overnight at room temperature. Red colonies were defined as non-biofilm-forming strains, dark-colored colonies were defined as forming weak biofilms, and black colony-forming strains were defined as strong biofilm-forming bacteria [64].
4.6. Biofilm Assay
The biofilm formation was confirmed by microplate assay. A hundred µL bacterial culture (106 CFU/mL) in TSB supplemented with 1% sucrose (Sigma-Aldrich, St. Louis, MO, USA) was added into each well of a sterile 96-well flat-bottom microplate. The microplate was left for incubation at 37 °C for 24 h. After the incubation, the absorbance was measured at OD630 nm using a microplate ELISA reader (Biotek BT 800, Winooski, VT, USA). Then planktonic cells were removed and each well was washed three times with sterile phosphate-buffered saline (PBS) (Sigma-Aldrich). The adherent bacterial cells were fixed at 60 °C for 45 min. After this fixing period, the cells were stained with 100 µL of 0.1% crystal violet (Himedia) for 15 min at room temperature (RT). Then the contents of each well were removed and washed 3 times with PBS again. After the 96-well plate was left to dry at RT for 15 min, 100 µL of 95% ethanol was added into each well to dissolve the bacteria on the bottom. The absorbance was measured at OD490 nm. The absorbance values were placed in the formula B = A490/A630. The results were evaluated as non-biofilm-forming microorganisms (B < 0.1), weak (0.1 < B < 0.5), moderate (0.5 < B < 1), and strong biofilm-forming microorganisms (B ≥ 1) [65].
4.7. Phenotypic Presence of Coagulase Enzyme
Rabbit plasma with EDTA was used to determine the coagulase properties of Staphylococcus spp. For the tube coagulase test, 0.5 mL of plasma was placed in each tube. A few colonies were inoculated from the cultures that were prepared the day before in TSA and incubated at 37 °C for 24 h. At the end of the incubation period, clot formation was considered positive. S. aureus ATCC 25923 was used as the control.
4.8. Multiple Antibiotic Resistance
The antibiotic resistance profiles of all the isolates were determined by a disk diffusion test recommended by the Clinical and Laboratory Standards Institute (CLSI) [66]. In this case, 15 different antibiotic discs from 7 different classes (Bioanalyse, Türkiye) were used in the study. These were vancomycin (30 µg), penicillin-G (10U), streptomycin (10 µg), tetracycline (30 µg), kanamycin (30 µg), neomycin (30 µg), nitrofurantoin (300 µg), erythromycin (15 µg), imipenem (10 µg), gentamicin (10 µg), amoxicillin-clavulanic acid (30 µg), ampicillin-sulbactam (20 µg), ciprofloxacin (5 µg), cefoxitin (30 µg), and oxacillin (1 µg). The test results were evaluated as sensitive (S), moderately sensitive (I), and resistant®. The S. aureus ATCC 25923 strain was used as a control. Multiple antibiotic resistance was considered as resistance to at least three different antimicrobial classes [67].
4.9. Chromogenic Nitrocefin Disk Method
The disk was wetted with 10 µL of distilled water. Suspicious colonies were applied directly on the disk in line with the manufacturer’s instructions and a color change (from light yellow to pink) within 15 min was evaluated as positive. A nitrocefin disk (Bioanalyse, Ankara, Türkiye) was used in the study.
4.10. Determination of coa, blaZ, mecA, and icaB genes
The primers used for coa (for the genotypic presence of coagulase enzyme), icaB (intercellular adhesion gene), and blaZ and mecA (resistance genes) in the isolates are given in Table 4. Sterile PCR water, PCR master mix, and 10 pmol reverse and forward primers were used for PCR mix. Each PCR reaction was run as 35 cycles with a total volume of 25 µL. In the reaction, the steps were pre-denaturation at 95 °C for 5 min, denaturation at 95 °C for 30 s, denaturation at 47 °C for coa, blaZ at 50 °C, mecA at 46 °C, icaB at 52 °C for 30-s primary bonding temperature, 30-s synthesis at 72 °C, and finally at 72 °C 5 min as the final synthesis. S. aureus ATCC 25923, S. aureus ATCC 29213, and S. epidermidis ATCC 35,984 were used as the positive control. The negative control was conducted with the PCR master mix and PCR water.

Table 4.
Primers used in the study.
4.11. Statistical Analysis
The data from this study have been analysed by SPSS 17.0 software. The correlation between the biofilm and slime abilities of bacteria and the icaB, coa, mecA, and blaZ content was determined by a Pearson correlation test.
5. Conclusions
In conclusion, staphylococcal species were characterized in this study and S. aureus is the most isolated bacteria from milk samples. Furthermore, it was observed that all staphylococci contain various virulence factors. When evaluating the characteristics of all the bacteria, it can be seen that they have a high amount of coagulase enzyme and biofilm forming ability. Moreover, there are a lot of bacteria that contain a high level of other virulence factors, while the presence of antibiotic resistance genes remains relatively low in bacteria because the multidrug resistance rates are around 20% and the presence of mecA and blaZ is not very high. It may be useful to determine the prevalence of other genes responsible for resistance with future studies. Likewise, it may be more useful to increase the number of samples. This study is a preliminary screening because there have been no previous studies in the study region. These results do not only benefit the formation of a strategic plan in terms of subclinical mastitis and animal welfare, they may encourage further research. Knowing the presence of potential factors for mastitis can be useful for taking precautions. In addition, it must not be forgotten that humans and animals are in constant contact. Antibiotic resistance and health risk management caused by pathogens are only possible with a holistic health approach. In this respect, it is considered that the data on animal origin are very important because they constitute the data for the whole public health. Addedly, it should be remembered that antibiotic resistance is in a constant state of change between bacteria. Therefore, further studies are planned.
Author Contributions
Conceptualization, N.S.; methodology, N.S., E.K., C.Ç. and O.P.; resources, N.S., E.K., C.Ç. and O.P.; data curation, N.S., E.K., C.Ç. and O.P.; investigation, N.S.; visualization E.K.; writing—original draft preparation, N.S.; writing—review and editing, N.S., E.K., C.Ç. and O.P.; project administration, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In this study, the cows were milked as per the normal routine. Therefore, ethical approval was not required for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data in this study are presented in the submitted manuscript and in the figures.
Acknowledgments
In the study, the MALDI-TOF MS analyses were performed in the Republic of Türkiye, Ministry of Health General Directorate of Public Health Department of Microbiology Reference Laboratory and Biological Products Ankara, Türkiye. I would like to thank Yasemin Numanoğlu Çevik for contributing to the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pérez-Rodríguez, F.; Mercanoglu Taban, B. A state-of-art review on multi-drug resistant pathogens in foods of animal origin: Risk factors and mitigation strategies. Front. Microbiol. 2019, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Loayza, F.; Graham, J.P.; Trueba, G. Factors obscuring the role of E. coli from domestic animals in the global antimicrobial resistance crisis: An evidence-based review. Int. J. Environ. Res. Public Health 2020, 17, 3061. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The role of Streptococcus spp. in bovine mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef]
- Sezener, M.G.; Fındık, A.; Ergüden, V.E.; Akgöz, S.; Gülhan, T.; Çiftci, A. The determination of antibiotic resistances and some virulence genes of Staphylococcus aureus isolated from bovine mastitis. JAES 2019, 4, 182–187. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Yatoo, M.I.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Pekana, A.; Green, E. Antimicrobial resistance profiles of Staphylococcus aureus isolated from meat carcasses and bovine milk in abattoirs and dairy farms of the Eastern Cape, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 2223. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, N.; Ceniti, C.; Santoro, A.; Clausi, M.T.; Casalinuovo, F. Foodborne pathogen assessment in raw milk cheeses. Int. J. Food Sci. 2020, 2020, 3616713. [Google Scholar] [CrossRef]
- González-Martín, M.; Corbera, J.A.; Suárez-Bonnet, A.; Tejedor-Junco, M.T. Virulence factors in coagulase-positive staphylococci of veterinary interest other than Staphylococcus aureus. Vet. Q. 2020, 40, 118–131. [Google Scholar] [CrossRef]
- Skočková, A.; Bogdanovıčová, K.; Koláčková, I.; Karpíšková, R. Antimicrobial-resistant and extended-spectrum β-Lactamase–producing Escherichia coli in raw cow’s milk. J. Food Prot. 2015, 78, 72–77. [Google Scholar] [CrossRef]
- Ahmed, I.M. Detection of CTX-M gene in extended spectrum β-lactamases producing Enterobacteriaceae isolated from bovine milk. Iraqi J. Vet. Sci. 2021, 35, 397–402. [Google Scholar] [CrossRef]
- Read, A.F.; Woods, R.J. Antibiotic resistance management. Evol. Med. Public Health 2014, 2014, 147. [Google Scholar] [CrossRef]
- Andersson, D.I.; Balaban, N.Q.; Baquero, F. Antibiotic resistance: Turning evolutionary principles into clinical reality. FEMS Microbiol. Rev. 2020, 44, 171–188. [Google Scholar] [CrossRef]
- Sipahi, N.; Karakaya, E.; Ikiz, S. Phenotypic and genotypic investigation of the heavy metal resistance in Escherichia coli isolates recovered from cattle stool samples. Turk. J. Vet. Anim. Sci. 2019, 43, 684–691. [Google Scholar] [CrossRef]
- Melo, D.A.D.; Coelho, I.D.S.; Motta, C.C.D.; Rojas, A.C.C.M.; Dubenczuk, F.C.; Coelho, S.D.M.D.O.; Souza, M.M.S.D. Impairments of mecA gene detection in bovine Staphylococcus spp. Braz. J. Microbiol. 2014, 45, 1075–1082. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; G de los Reyes-Gavilán, C.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Sedky, D.; Ghazy, A.A.; Soliman, N.A.; Shaapan, R.M. Comparative diagnosis of infectious bacteria in bovine milk. J. Anim. Health Prod. 2020, 8, 171–182. [Google Scholar] [CrossRef]
- Taye, Y.; Degu, T.; Fesseha, H.; Mathewos, M. Isolation and identification of lactic acid bacteria from cow milk and milk products. Sci. World J. 2021, 2021, 4697445. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Hur, Y.K.; Kim, E.J.; Ahn, Y.T.; Kim, J.G.; Choi, Y.J.; Huh, C.S. Comparative analysis of the microbial communities in raw milk produced in different regions of Korea. Asian-Australas J. Anim. Sci. 2017, 30, 1643. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; In Lee, S.; Rackerby, B.; Frojen, R.; Goddik, L.; Ha, S.D.; Park, S.H. Assessment of overall microbial community shift during Cheddar cheese production from raw milk to aging. Appl. Microbiol. Biotechnol. 2020, 104, 6249–6260. [Google Scholar] [CrossRef] [PubMed]
- Kılınçel, Ö.; Çelik, O.E.; Aytan, A.; Alkan, İ.; Ayvaz, İ.B.; Öztürk, C.E. Düzce İlinde çiğ süt örneklerinde Mycobacterium bovis aranması. Düzce Üniversitesi Sağlık Bilim. Enstitüsü Derg. 2018, 8, 112–114. [Google Scholar]
- Wang, H.; Shen, J.; Zhu, C.; Ma, K.; Fang, M.; Li, B.; Wang, W.; Xue, T. Antibiotics Resistance and Virulence of Staphylococcus aureus Isolates Isolated from Raw Milk from Handmade Dairy Retail Stores in Hefei City, China. Foods 2022, 11, 2185. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.A.J.; Dierikx, C.M.; van Essen-Zandbergen, A.; Mevius, D.; Stegeman, A.; Velkers, F.C.; Klinkenberg, D. Competition between Escherichia coli Populations with and without Plasmids Carrying a Gene Encoding Extended-Spectrum Beta-Lactamase in the Broiler Chicken Gut. Appl. Environ. Microbiol. 2019, 85, e00892-19. [Google Scholar] [CrossRef]
- Rusenova, N.; Gebreyes, W.; Koleva, M.; Mitev, J.; Penev, T.; Vasilev, N.; Miteva, T. Comparison of three methods for routine detection of Staphylococcus aureus isolated from bovine mastitis. Kafkas Univ. Vet. Fak. Derg. 2013, 19, 709–712. [Google Scholar] [CrossRef]
- Savaşan, S.; Kırkan, Ş.; Erbaş, G.; Parın, U.; Ciftci, A. Determination of virulence factors of staphylococci isolated from bovine mastitis. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 947–952. [Google Scholar] [CrossRef]
- Özenç, E.; Şeker, E.; Yılmaz, M. Abattoir-Based survey of mastitis in cattle in Afyonkarahisar Province. Kocatepe Vet. J. 2019, 12, 437–442. [Google Scholar] [CrossRef]
- Clark, C.M.; Costa, M.S.; Sanchez, L.M.; Murphy, B.T. Coupling MALDI-TOF mass spectrometry protein and specialized metabolite analyses to rapidly discriminate bacterial function. Proc. Natl. Acad. Sci. USA 2018, 115, 4981–4986. [Google Scholar] [CrossRef]
- Cheng, K.; Chui, H.; Domish, L.; Hernandez, D.; Wang, G. Recent development of mass spectrometry and proteomics applications in identification and typing of bacteria. Proteom-Clin. Appl. 2016, 10, 346–357. [Google Scholar] [CrossRef]
- Javed, M.U.; Ijaz, M.; Fatima, Z.; Anjum, A.A.; Aqib, A.I.; Ali, M.M.; Rehman, A.; Ahmed, A.; Ghaffar, A. Frequency and antimicrobial susceptibility of methicillin and vancomycin-resistant Staphylococcus aureus from bovine milk. Pak. Vet. J. 2021, 41, 463–468. [Google Scholar] [CrossRef]
- Javid, F.; Taku, A.; Bhat, M.A.; Badroo, G.A.; Mudasir, M.; Sofi, T.A. Molecular typing of Staphylococcus aureus based on coagulase gene. Vet. World 2018, 11, 423–430. [Google Scholar] [CrossRef]
- Effendi, M.H.; Hisyam, M.A.M.; Hastutiek, P.; Tyasningsih, W. Detection of coagulase gene in Staphylococcus aureus from several dairy farms in East Java, Indonesia, by polymerase chain reaction. Vet. World. 2019, 12, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Hiramatsu, K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef]
- Pashangeh, S.; Shekarforoush, S.S.; Aminlari, M.; Hosseinzadeh, S.; Nizet, V.; Dahesh, S.; Rahmdel, S. Inhibition of histamine accumulation by novel histamine-degrading species of Staphylococcus sp. isolated from goats and sheep milk. Food Sci. Nutr. 2022, 10, 354–362. [Google Scholar] [CrossRef]
- Otto, M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: Staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. Bioessays 2013, 35, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Rosalino, L.M.; Palmeira, J.D.; Torres, R.T.; Cunha, M.V. Antimicrobial resistance in commensal Staphylococcus aureus from wild ungulates is driven by agricultural land cover and livestock farming. Environ. Pollut. 2022, 303, 119116. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.; Alatfeehy, N.; Marouf, S. Isolation and antibiogram profiles of Staphylococcus aureus isolates from cow milk and dog samples. J. Appl. Vet. Sci. 2022, 8, 38–44. [Google Scholar] [CrossRef]
- Meroni, G.; Soares Filipe, J.F.; Drago, L.; Martino, P.A. Investigation on antibiotic-resistance, biofilm formation and virulence factors in multi drug resistant and non multi drug resistant Staphylococcus pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef]
- Rudenko, P.; Sachivkina, N.; Vatnikov, Y.; Shabunin, S.; Engashev, S.; Kontsevaya, S.; Karamyan, A.; Bokov, D.; Kuznetsova, O.; Vasilieva, E. Role of microorganisms isolated from cows with mastitis in Moscow region in biofilm formation. Vet. World 2021, 14, 40–48. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Azara, E.; Longheu, C.; Sanna, G.; Tola, S. Biofilm formation and virulence factor analysis of Staphylococcus aureus isolates collected from ovine mastitis. J. Appl. Microbiol. 2017, 123, 372–379. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, S.; Lou, X.; Cheng, S.; Liu, X.; Zheng, W.; Wang, H. Biofilm formation and prevalence of adhesion genes among Staphylococcus aureus isolates from different food sources. Microbiologyopen 2020, 9, e00946. [Google Scholar] [CrossRef]
- Poulsen, L.V. Microbial biofilm in food processing. LWT-Food Sci. Technol. 1999, 32, 321–326. [Google Scholar] [CrossRef]
- Gün, İ.; Ekİncİ, F.Y. Biofilms: Microbial life on surfaces. J. Food 2009, 34, 165–173. [Google Scholar]
- Wu, Y.; Li, J.; Qiao, M.; Meng, D.; Meng, Q.; Qiao, J.; Cai, X. Characteristic profiles of biofilm, enterotoxins and virulence of Staphylococcus aureus isolates from dairy cows in Xinjiang Province, China. J. Vet. Med. 2019, 20, e74. [Google Scholar] [CrossRef]
- Zheng, J.; Shang, Y.; Wu, Y.; Wu, J.; Chen, J.; Wang, Z.; Yu, Z. Diclazuril inhibits biofilm formation and hemolysis of Staphylococcus aureus. ACS Infect. Dis. 2021, 7, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.T.; da Silva, J.G.; Aragão, B.B.; Peixoto, R.M.; Mota, R.A. Occurrence of β-lactam-resistant Staphylococcus aureus in milk from primiparous dairy cows in the northeastern region of Brazil. Trop. Anim. Health Prod. 2020, 52, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, P.; Severgnini, M.; Romanò, A.; Sala, L.; Luini, M.; Castiglioni, B. Bovine milk microbiota: Comparison among three different DNA extraction protocols to identify a better approach for bacterial analysis. Microbiol. Spectr. 2021, 9, e00374-21. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Meng, L.; Liu, H.; Wu, H.; Schroyen, M.; Zheng, N.; Wang, J. Effect of cephalosporin treatment on the microbiota and antibiotic resistance genes in feces of dairy cows with clinical mastitis. Antibiotics 2022, 11, 117. [Google Scholar] [CrossRef]
- Bidya, S.; Suman, R.S. Comparative study of three β lactamase test methods in Staphylococcus aureus isolated from two Nepalese hospitals. Open J. Clin. Diag. 2014, 4, 44037. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Martins, K.B.; Silva, V.R.D.; Mondelli, A.L.; Cunha, M.D.L.R.D.S.D. Correlation of phenotypic tests with the presence of the blaZ gene for detection of beta-lactamase. Braz. J. Microbiol. 2017, 48, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.G.; Csabai, I.; Krikó, E.; Tőzsér, D.; Maróti, G.; Patai, Á.V.; Solymosi, N. Antimicrobial resistance genes in raw milk for human consumption. Sci. Rep. 2020, 10, 7464. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, X.; Wang, X.; Li, H. Detection of antibiotic resistance, virulence gene, and drug resistance gene of Staphylococcus aureus ısolates from bovine mastitis. Microbiol. Spectr. 2022, 10, e00471-22. [Google Scholar] [CrossRef] [PubMed]
- El-Zamkan, M.A.; Mohamed, H.M. Antimicrobial resistance, virulence genes and biofilm formation in Enterococcus species isolated from milk of sheep and goat with subclinical mastitis. PloS ONE 2021, 16, e0259584. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, E.E.; Mousa, W.S.; Abdelsalam, S.Y.; Heikal, H.S.; Shawish, R.R.; Nooruzzaman, M.; Abdeen, A. Prevalence and characterization of coagulase positive Staphylococci from food products and human specimens in Egypt. Antibiotics 2021, 10, 75. [Google Scholar] [CrossRef]
- Haran, K.P.; Godden, S.M.; Boxrud, D.; Jawahir, S.; Bender, J.B.; Sreevatsan, S. Prevalence and characterization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, isolated from bulk tank milk from Minnesota dairy farms. J. Clin. Microbiol. 2012, 50, 688–695. [Google Scholar] [CrossRef]
- Tang, Y.; Larsen, J.; Kjeldgaard, J.; Andersen, P.S.; Skov, R.; Ingmer, H. Methicillin-resistant and -susceptible Staphylococcus aureus from retail meat in Denmark. Int. J. Food Microbiol. 2017, 249, 72–76. [Google Scholar] [CrossRef]
- Delesalle, L.; Sadoine, M.L.; Mediouni, S.; Denis-Robichaud, J.; Zinszer, K.; Zarowsky, C.; Aenishaenslin, C.; Carabin, H. How are large-scale One Health initiatives targeting infectious diseases and antimicrobial resistance evaluated? A scoping review. One Health 2022, 14, 100380. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; van Eerde, A. Pollution by Antibiotics and Antimicrobial Resistance in LiveStock and Poultry Manure in China, and Countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef]
- Romandini, A.; Pani, A.; Schenardi, P.A.; Pattarino, G.A.C.; De Giacomo, C.; Scaglione, F. Antibiotic Resistance in Pediatric Infections: Global Emerging Threats, Predicting the Near Future. Antibiotics 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef] [PubMed]
- Brosius, J.; Palmer, M.L.; Kennedy, P.J.; Noller, H.F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 1978, 75, 4801–4805. [Google Scholar] [CrossRef]
- Mariana, N.S.; Salman, S.A.; Neela, V.; Zamberi, S. Evaluation of modified Congo red agar for detection of biofilm produced by clinical isolates of methicillinresistance Staphylococcus aureus. Afr. J. Microbiol. Res. 2009, 3, 330–338. [Google Scholar]
- Zhang, H.; Xie, L.; Zhang, W.; Zhou, W.; Su, J.; Liu, J. The association of biofilm formation with antibiotic resistance in lactic acid bacteria from fermented foods. J. Food Saf. 2013, 33, 114–120. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals. In Approved Standard Document, 5th ed.; CLSI supplement VET01S; CLSI: Malvern, PA, USA, 2020. [Google Scholar]
- De Jong, A.; Simjee, S.; El Garch, F. Antimicrobial susceptibility of enterococci recovered from healthy cattle, pigs and chickens in nine EU countries (EASSA Study) to critically important antibiotics. Vet. Microbiol. 2018, 216, 168–175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).