Antimicrobial Therapy Duration for Bloodstream Infections Caused by Pseudomonas aeruginosa or Acinetobacter baumannii-calcoaceticus complex: A Retrospective Cohort Study
Abstract
1. Introduction
2. Results
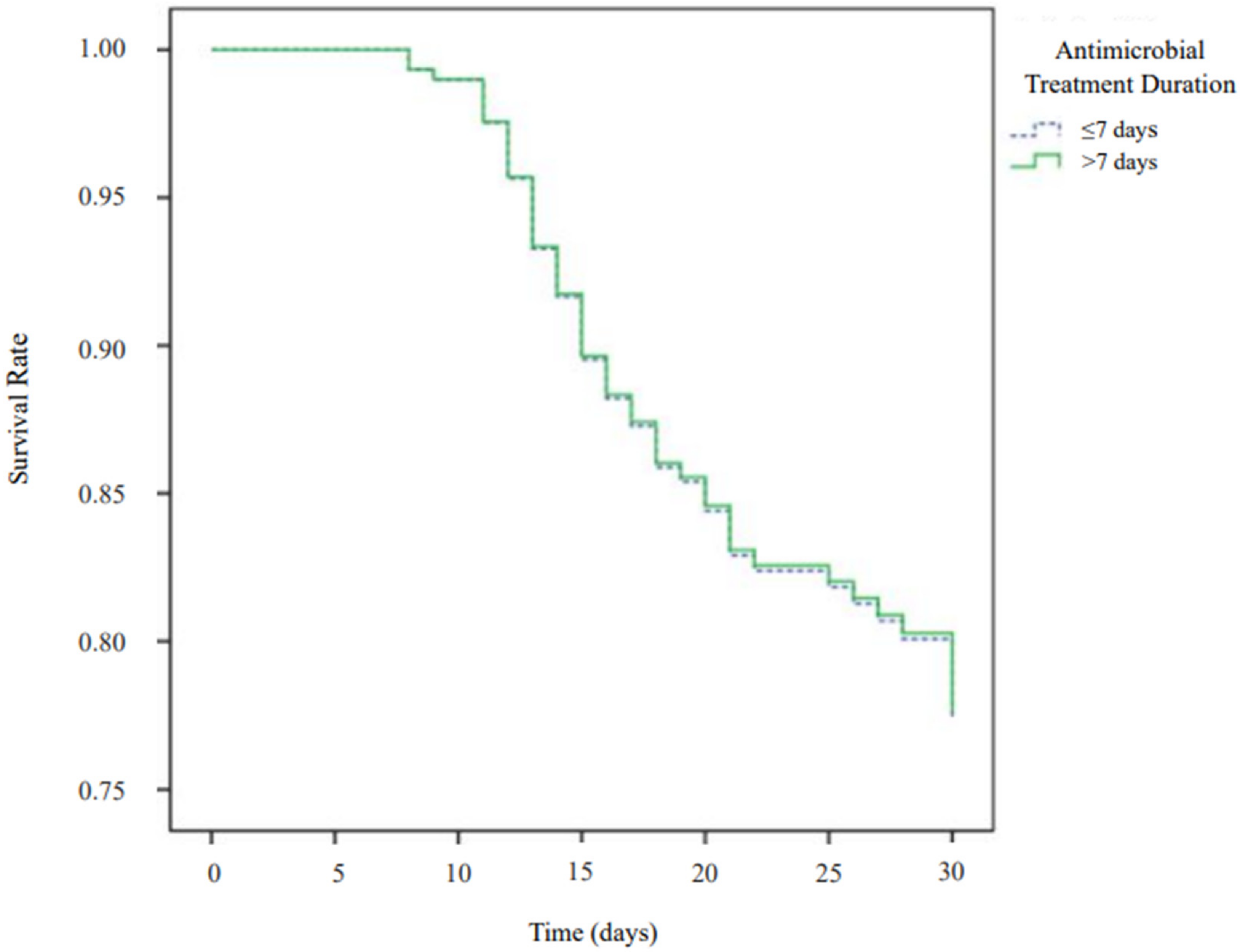
2.1. Primary Outcome
2.2. Secondary Outcomes
2.3. Subgroup Analyses
3. Discussion
4. Materials and Methods
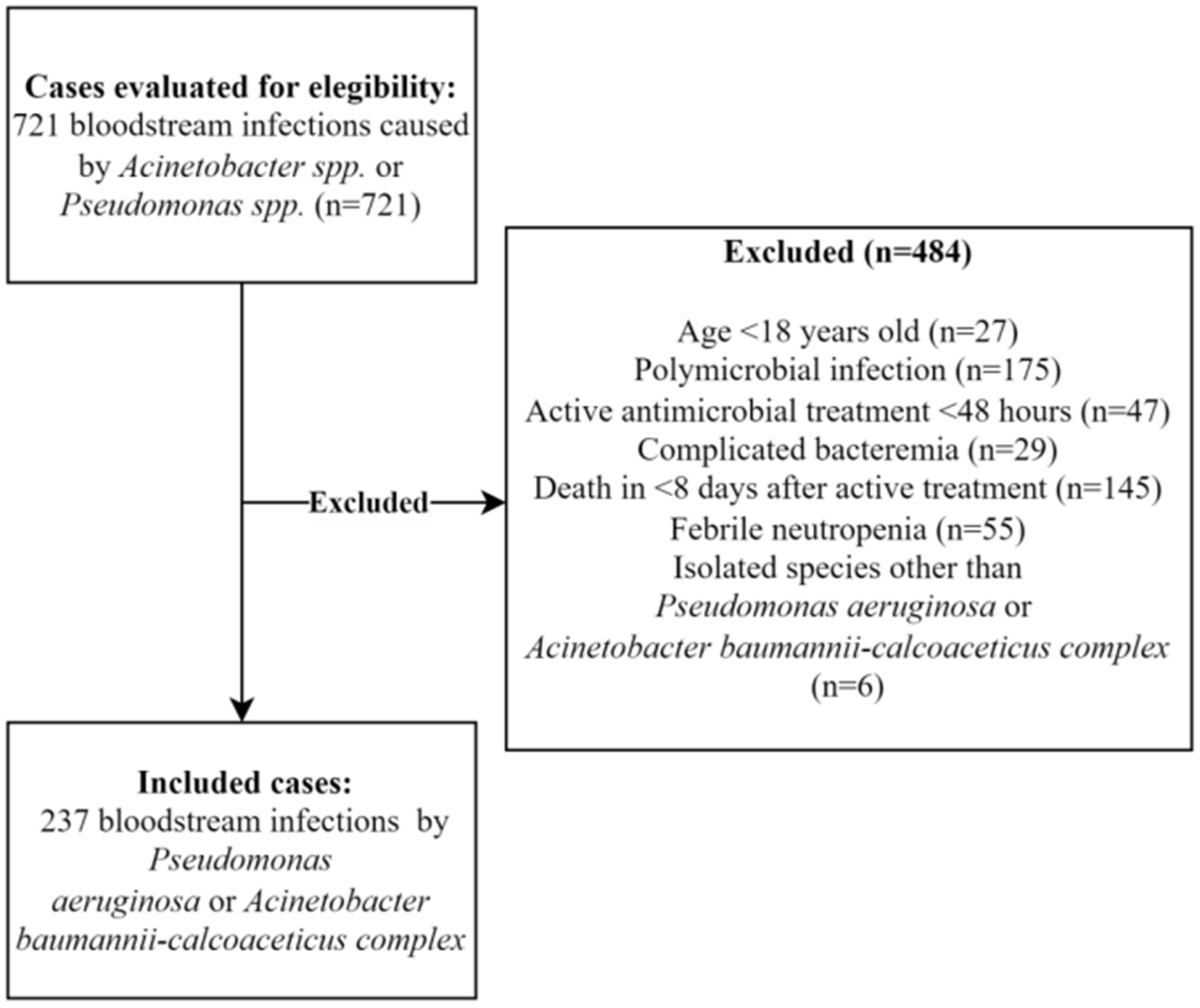
4.1. Study Designs and Settings
4.2. Inclusion and Exclusion Criteria
4.3. Variables and Definitions
4.4. Sample Size
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannella, M.; Bartoletti, M.; Gatti, M.; Viale, P. Advances in the therapy of bacterial bloodstream infections. Clin. Microbiol. Infect. 2020, 26, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.J.; Kim, S.H.; Kim, H.T.; Moon, C.; Wi, Y.M. The epidemiology of bloodstream infection contributing to mortality: The difference between community-acquired, healthcare-associated, and hospital-acquired infections. BMC Infect. Dis. 2022, 22, 336. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Hsueh, P.-R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.; Jones, R.N. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemother. 2019, 63, e00355-19. [Google Scholar] [CrossRef] [PubMed]
- Almeida Junior, E.R.; Braga, I.A.; Filho, P.P.G.; Ribas, R.M. Multicentre surveillance of epidemiologically important pathogens causing nosocomial bloodstream infections and pneumonia trials in Brazilian adult intensive care units. J. Med. Microbiol. 2023, 72, 001654. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruigómez, M.; Aguado, J.M. Duration of antibiotic therapy in central venous catheter-related bloodstream infection due to Gram-negative bacilli. Curr. Opin. Infect. Dis. 2021, 34, 681–685. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; Van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, 1109–1116. [Google Scholar]
- Heil, E.L.; Bork, J.T.; Abbo, L.M.; Barlam, T.F.; Cosgrove, S.E.; Davis, A.; Ha, D.R.; Jenkins, T.C.; Kaye, K.S.; Lewis, J.S.; et al. Optimizing the Management of Uncomplicated Gram-Negative Bloodstream Infections: Consensus Guidance Using a Modified Delphi Process. Open Forum Infect. Dis. 2021, 8, ofab434. [Google Scholar] [CrossRef]
- Fabre, V.; Amoah, J.; Cosgrove, S.E.; Tamma, P.D. Antibiotic Therapy for Pseudomonas aeruginosa bloodstream Infections: How Long Is Long Enough? Clin. Infect. Dis. 2019, 69, 2011–2014. [Google Scholar] [CrossRef]
- Bae, M.; Jeong, Y.; Bae, S.; Kim, M.J.; Chong, Y.P.; Kim, S.H.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Jung, J. Short versus prolonged courses of antimicrobial therapy for patients with uncomplicated Pseudomonas aeruginosa bloodstream infection: A retrospective study. J. Antimicrob. Chemother. 2021, 77, 223–228. [Google Scholar] [CrossRef]
- Babich, T.; Naucler, P.; Valik, J.K.; Giske, C.G.; Benito, N.; Cardona, R.; Rivera, A.; Pulcini, C.; Fattah, M.A.; Haquin, J.; et al. Duration of Treatment for Pseudomonas aeruginosa Bacteremia: A Retrospective Study. Infect. Dis. Ther. 2022, 11, 1505–1519. [Google Scholar] [CrossRef]
- Katip, W.; Uitrakul, S.; Oberdorfer, P. Short-Course Versus Long-Course Colistin for Treatment of Carbapenem-Resistant A. baumannii in Cancer Patient. Antibiotics 2021, 10, 484. [Google Scholar] [CrossRef]
- Yahav, D.; Franceschini, E.; Koppel, F.; Turjeman, A.; Babich, T.; Bitterman, R.; Neuberger, A.; Ghanem-Zoubi, N.; Santoro, A.; Eliakim-Raz, N.; et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-negative Bacteremia: A Non inferiority Randomized Controlled Trial. Clin. Infect. Dis. 2019, 69, 1091–1098. [Google Scholar] [CrossRef]
- Molina, J.; Montero-Mateos, E.; Praena-Segovia, J.; León-Jiménez, E.; Natera, C.; López-Cortés, L.E.; Valiente, L.; Rosso-Fernández, C.M.; Herrero, M.; Aller-García, A.I.; et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by Enterobacterales: A randomized, controlled trial. Clin. Microbiol. Infect. 2022, 28, 550–557. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, Q.; Liu, L.; Ma, R.; Chen, J.; Shen, Y.; Zhu, G.; Jiang, E.; Mi, Y.; Han, M.; et al. Risk Factors and Outcomes of Antibiotic-resistant Pseudomonas aeruginosa Bloodstream Infection in Adult Patients With Acute Leukemia. Clin. Infect. Dis. 2020, 71, S386–S393. [Google Scholar] [CrossRef]
- Amanati, A.; Sajedianfard, S.; Khajeh, S.; Ghasempour, S.; Mehrangiz, S.; Nematolahi, S.; Shahhosein, Z. Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance. BMC Infect. Dis. 2021, 21, 636. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Carvalhaes, C.G.; Picao, R.C.; Gales, A.C. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: Resistance mechanisms and implications for therapy. Expert Rev. Anti-Infect. Ther. 2010, 8, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.L.; Anderson, M.T.; Mobley, H.L.T.; Bachman, M.A. Pathogenesis of Gram-Negative Bacteremia. Clin. Microbiol. Rev. 2021, 34, e00234-20. [Google Scholar] [CrossRef]
- Lee, C.M.; Kim, Y.-J.; Jung, S.-I.; Kim, S.E.; Park, W.B.; Choe, P.G.; Kim, E.S.; Kim, C.-J.; Choi, H.J.; Lee, S.; et al. Different clinical characteristics and impact of carbapenem-resistance on outcomes between Acinetobacter baumannii and Pseudomonas aeruginosa bacteraemia: A prospective observational study. Sci. Rep. 2022, 12, 8527. [Google Scholar] [CrossRef]
- McDonald, E.G.; Prosty, C.; Hanula, R.; NClin, É.B.C.; Albuquerque, A.M.; Tong, S.Y.; Hamilton, F.; Lee, T.C. Observational vs. Randomized Controlled Trials to Inform Antibiotic Treatment Durations: A Narrative Review. Clin. Microbiol. Infect. 2022, 29, 165–170. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards Antimicrobial Susceptibility Testing. In Twenty-Six Informational Supplement M100S; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.Y.; Kwon, K.T.; Ki, H.K.; Shin, S.Y.; Jung, D.S.; Chung, D.R.; Ha, B.C.; Peck, K.R.; Song, J.H. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: A comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock 2009, 31, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.B.; Mancuso, A.C.B.; Camey, S.A.; Leotti, V.B.; Hirakata, V.N.; Azambuja, G.S.; Castro, S.M.D.J. Power and Sample Size for Health Researchers: Uma ferramenta para cálculo de tamanho amostral e poder do teste voltado a pesquisadores da área da saúde. Clin. Biomed. Res. 2021, 40, 247–253. [Google Scholar] [CrossRef]
| Characteristic | Total Cohort | Therapy Duration | ||
|---|---|---|---|---|
| N = 237 | ≤7 Days, N = 67 | >7 Days, N = 170 | p Value | |
| Demographic | ||||
| Age | 61.6 ± 16.0 | 60.5 ± 15.8 | 62.0 ± 16.1 | 0.50 |
| Gender (masculine) | 122 (51.5) | 34 (50.7) | 88 (51.8) | 0.99 |
| Comorbidity | ||||
| Cardiovascular | 152 (64.1) | 46 (68.7) | 106 (62.4) | 0.45 |
| Pulmonary | 66 (27.8) | 21 (31.3) | 45 (26.5) | 0.52 |
| Neurological | 90 (38.0) | 31 (46.3) | 59 (34.7) | 0.11 |
| Hepatic | 13 (5.5) | 0 (0) | 13 (7.6) | 0.02 |
| Chronic kidney disease | 70 (29.5) | 19 (28.4) | 51 (30.0) | 0.88 |
| Gastrointestinal | 41 (17.3) | 11 (16.4) | 30 (17.6) | 0.99 |
| Diabetes | 64 (27.0) | 14 (20.9) | 50 (29.4) | 0.20 |
| HIV | 16 (6.8) | 5 (7.5) | 11 (6.5) | 0.78 |
| Rheumatic | 8 (3.4) | 3 (4.5) | 5 (2.9) | 0.69 |
| Solid organ cancer | 55 (23.2) | 11 (16.4) | 44 (25.9) | 0.13 |
| Haematological cancer | 8 (3.4) | 4 (6.0) | 4 (2.4) | 0.23 |
| Charlson Comorbidity Index | 5 (3–7) | 5 (2–7) | 5 (3–8) | 0.09 |
| Hospital of Admission | ||||
| Hospital A | 215(90.7) | 57(85.1) | 158(92.9) | 0.08 |
| Disease severity | ||||
| ICU | 110 (46.4) | 22 (32.8) | 88(51.8) | <0.01 |
| Mechanical ventilation | 64 (27.0) | 10 (14.9) | 54 (31.8) | <0.01 |
| Pitt bacteraemia score | 1 (0–4) | 1 (0–2) | 2 (0–6) | 0.10 |
| Septic shock | 46 (19.4) | 5 (7.5) | 41 (24.1) | <0.01 |
| Microbiological data | ||||
| ABC infections | 122 (51.5) | 33 (49.3) | 89 (52.4) | 0.77 |
| Pseudomonas aeruginosa infections | 115 (48.5) | 34 (50.7) | 81 (47.6) | 0.77 |
| Multidrug resistance | 115 (48.5) | 26 (38.8) | 89 (52.4) | 0.06 |
| Carbapenem resistance | 118 (49.8) | 27 (40.3) | 91 (53.5) | 0.08 |
| Time from hospitalization to bacteraemia (days) | 13 (3.0–29.8) | 16 (4–27) | 12 (2–30.5) | 0.76 |
| Infection site | ||||
| Pulmonary | 97 (40.9) | 19 (28.4) | 78 (45.9) | 0.02 |
| Urinary | 36 (15.2) | 12 (17.9) | 24 (14.1) | 0.55 |
| Abdominal | 19 (8.0) | 5 (7.5) | 14 (8.2) | 0.99 |
| Central venous catheter | 50 (21.1) | 20 (29.9) | 30 (17.6) | 0.05 |
| Removal of CVC (up to 48 h) | 34 (68.0) | 12 (60.0) | 22 (73.3) | 0.35 |
| Skin and soft tissues | 19 (8.0) | 8 (11.9) | 11 (6.5) | 0.19 |
| Undefined site | 33 (13.9) | 10 (14.9) | 23 (13.5) | 0.84 |
| Antimicrobial treatment | ||||
| Ampicillin-sulbactam * | 21 (8.9) | 7 (10.4) | 14 (8.2) | 0.62 |
| Ceftazidime | 26 (11.0) | 7 (10.4) | 19 (11.2) | 0.99 |
| Cefepime | 15 (6.3) | 5 (7.5) | 10 (5.9) | 0.77 |
| Piperacillin-tazobactam | 52 (21.9) | 17 (28.4) | 33 (19.4) | 0.16 |
| Ciprofloxacin | 35 (14.8) | 13 (19.4) | 22 (12.9) | 0.23 |
| Amikacin | 3 (1.3) | 1(1.5) | 2 (1.2) | 0.99 |
| Meropenem | 52 (21.9) | 10 (14.9) | 42 (24.7) | 0.19 |
| Polymyxin B | 115 (48.5) | 22 (32.8) | 93 (54.7) | <0.01 |
| Colistin | 4 (1.7) | 2 (3.0) | 2 (1.2) | 0.32 |
| Time to start active antimicrobial therapy (days) | 1 (0–2) | 1 (0–2) | 0 (0–2) | 0.05 |
| Combination therapy | 86 (36.2) | 16 (23.9) | 70 (41.2) | 0.01 |
| Polymyxin B + meropenem | 73 (30.8) | 13 (19.4) | 60 (35.3) | |
| Polymyxin B + amikacin | 4 (1.7) | 1 (1.5) | 3 (1.8) | |
| Polymyxin B + ampicillin-sulbactam | 2 (0.84) | 0 | 2 (1.2) | |
| Polymyxin B + other antibiotics | 3 (1.3) | 0 | 3 (1.8) | |
| Colistin+ other antibiotics | 4 (1.7) | 2 (3.0) | 2 (1.2) | |
| Variables | 30-Day Mortality | In-Hospital Mortality | ||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | p | aHR | 95% CI | p Value | |
| Short-term therapy (vs. long-term) ** | 1.01 | 0.47–2.20 | 0.98 | 0.70 | 0.37–1.34 | 0.70 |
| Pitt bacteraemia score | 1.10 | 1.02–1.19 | 0.02 | 1.08 | 1.01–1.15 | 0.03 |
| Charlson Comorbidity Index | 1.18 | 1.07–1.30 | <0.01 | 1.13 | 1.04–1.22 | <0.01 |
| Carbapenem resistance | 2.65 | 1.28–5.48 | <0.01 | 1.91 | 1.09–3.36 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.D.; Garcia, R.C.L.; Bittencourt, G.A.; Waichel, V.B.; Garcia, E.C.L.; Rigatto, M.H. Antimicrobial Therapy Duration for Bloodstream Infections Caused by Pseudomonas aeruginosa or Acinetobacter baumannii-calcoaceticus complex: A Retrospective Cohort Study. Antibiotics 2023, 12, 538. https://doi.org/10.3390/antibiotics12030538
Rodrigues RD, Garcia RCL, Bittencourt GA, Waichel VB, Garcia ECL, Rigatto MH. Antimicrobial Therapy Duration for Bloodstream Infections Caused by Pseudomonas aeruginosa or Acinetobacter baumannii-calcoaceticus complex: A Retrospective Cohort Study. Antibiotics. 2023; 12(3):538. https://doi.org/10.3390/antibiotics12030538
Chicago/Turabian StyleRodrigues, Rodrigo Douglas, Rebeca Carvalho Lacerda Garcia, Gabriel Almeida Bittencourt, Vicente Bouchet Waichel, Ester Carvalho Lacerda Garcia, and Maria Helena Rigatto. 2023. "Antimicrobial Therapy Duration for Bloodstream Infections Caused by Pseudomonas aeruginosa or Acinetobacter baumannii-calcoaceticus complex: A Retrospective Cohort Study" Antibiotics 12, no. 3: 538. https://doi.org/10.3390/antibiotics12030538
APA StyleRodrigues, R. D., Garcia, R. C. L., Bittencourt, G. A., Waichel, V. B., Garcia, E. C. L., & Rigatto, M. H. (2023). Antimicrobial Therapy Duration for Bloodstream Infections Caused by Pseudomonas aeruginosa or Acinetobacter baumannii-calcoaceticus complex: A Retrospective Cohort Study. Antibiotics, 12(3), 538. https://doi.org/10.3390/antibiotics12030538







