Antibacterial Properties of Eucalyptus globulus Essential Oil against MRSA: A Systematic Review
Abstract
1. Introduction
2. Results
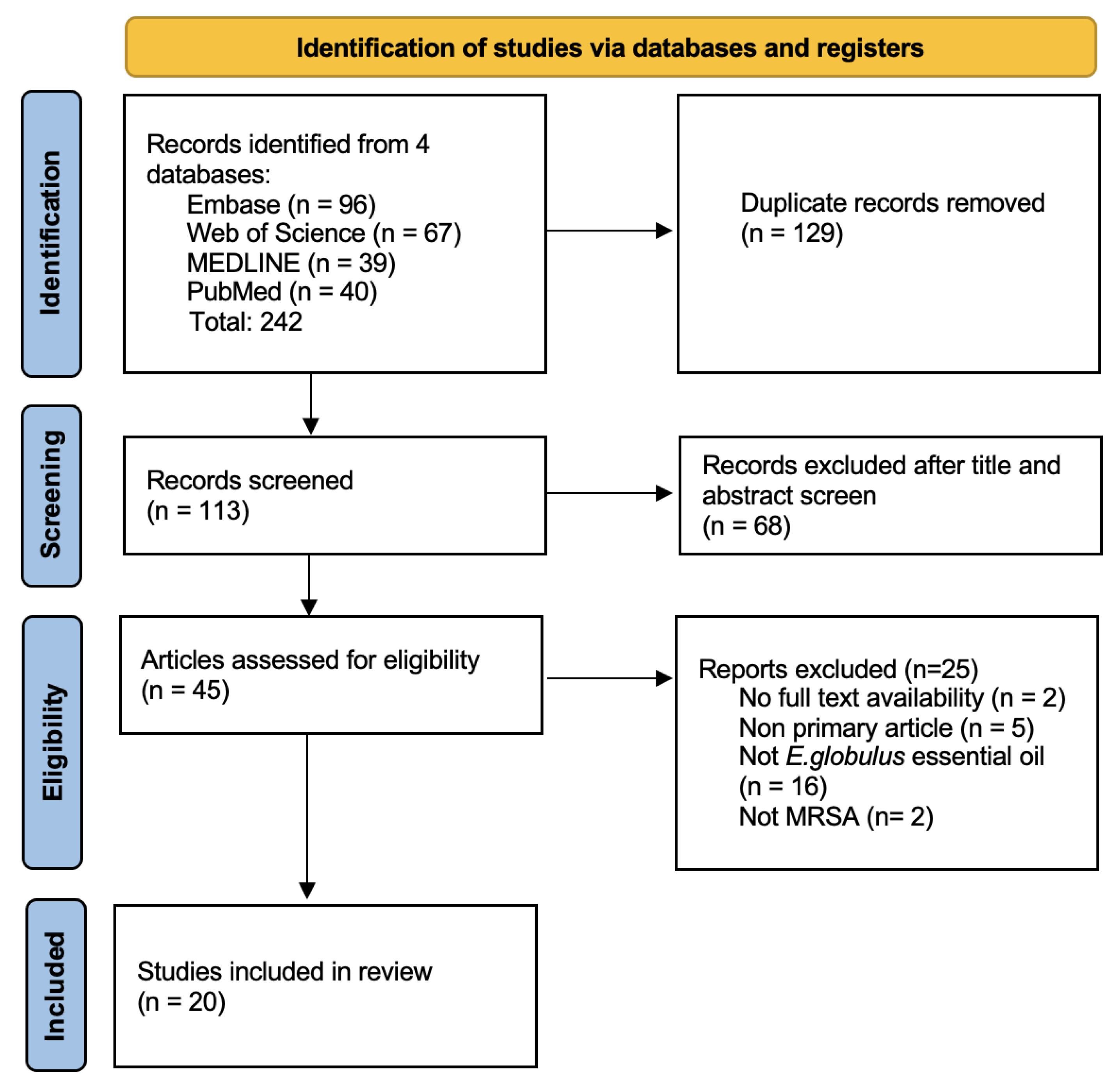
2.1. Search Results
2.2. Effectiveness of Essential Oil against MRSA Alone as Per Different Methodology
2.2.1. Chemical Composition—GC/MS
2.2.2. MIC
2.2.3. Biofilm
2.2.4. Zones of Inhibition
2.2.5. Vapour Phase
2.2.6. Time-Kill Assays
2.3. Effectiveness of E. globulus Essential Oil in Combination with Other Agents
3. Discussion
3.1. Composition of E. globulus Essential Oil
3.2. MIC of E. globulus Essential Oil against MRSA Strains
3.3. Zones of Inhibition Produced by E. globulus Essential Oil
3.4. Effectiveness of Combination of Agents against MRSA Strains
3.5. Biofilm Inhibition by E. globulus Essential Oil
3.6. Vapour Phase of E. globulus Essential Oil
3.7. Time–Kill Test to Determine Bactericidal Potential of E. globulus
3.8. Strengths and Limitations of Study
4. Materials and Methods
4.1. Focused Question
4.2. PICO Question
4.3. Search Strategy
4.4. Eligibility Criteria
4.4.1. Inclusion Criteria
4.4.2. Exclusion Criteria
4.5. Study Selection
4.6. Study Quality and Risk of Bias
4.7. Data Extraction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habboush, Y.; Guzman, N. Antibiotic Resistance; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Richardson, L.A. Understanding and overcoming antibiotic resistance. PLoS Biol. 2017, 15, e2003775. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- WHO. WHO World Antimicrobial Awareness Week-WAAW from 18 to 24 November 2020. Available online: https://www.who.int/campaigns/world-antimicrobial-awareness-week/2020 (accessed on 9 April 2021).
- Siddiqui, A.H.; Koirala, J. Methicillin Resistant Staphylococcus Aureus; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Mariani, M.; Condo, C.; Sabia, C.; Messi, P. Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Boren, K.; Crown, A.; Carlson, R. Multidrug and Pan-Antibiotic Resistance—The Role of Antimicrobial and Synergistic Essential Oils: A Review. Nat. Prod. Commun. 2020, 15, 1934578X2096259. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Immaroh, N.Z.; Kuliahsari, D.E.; Nugraheni, S.D. Review: Eucalyptus globulus essential oil extraction method. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012103. [Google Scholar] [CrossRef]
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. A systematic and comprehensive review on current understanding of the pharmacological actions, molecular mechanisms, and clinical implications of the genus Eucalyptus. Phytomed. Plus 2021, 1, 100089. [Google Scholar] [CrossRef]
- Acs, K.; Bencsik, T.; Boszormenyi, A.; Kocsis, B.; Horvath, G. Essential Oils and Their Vapors as Potential Antibacterial Agents against Respiratory Tract Pathogens. Nat. Prod. Commun. 2016, 11, 1709–1712. [Google Scholar]
- Ali, T.; Anjum, A.A.; Sattar MM, K.; Ali, M.A.; Kamran, M.; Tariq, M.; Manzoor, R. Antibacterial activity of plant essential oils against indigenously characterized methicillin-resistant Staphylococcus aureus (MRSA). Trop. Biomed. 2022, 39, 17–25. [Google Scholar]
- Bouras, M.; Abbaci, N.B.; Bennadja, S. Antibacterial activity of essential oil and aqueous extract of Eucalyptus globulus against methicillin resistance Staphylococcus aureus and methicillin sensitive Staphylococcus aureus. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1717–1721. [Google Scholar]
- Chao, S.; Young, G.; Oberg, C.; Nakaoka, K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour Fragr. J. 2008, 23, 444–449. [Google Scholar] [CrossRef]
- Cui, Z.H.; He, H.L.; Wu, S.B.; Dong, C.L.; Lu, S.Y.; Shan, T.J.; Fang, L.-X.; Liao, X.P.; Liu, Y.H.; Sun, J. Rapid screening of essential oils as substances which enhance antibiotic activity using a modifiedwell diffusion method. Antibiotics 2021, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Farsi, R.; Alaidaroos, B. Antibacterial Activity of Eucalyptus Essential Oil loaded on Silica Dioxide Nanoparticles (SiNPs) Against Some Pathogenic Bacteria. Int. J. Pharm. Phytopharm. Res. 2022, 12, 29–37. [Google Scholar] [CrossRef]
- Hamoud, R.; Sporer, F.; Reichling, J.; Wink, M. Antimicrobial activity of a traditionally used complex essential oil distillate (Olbas(®) Tropfen) in comparison to its individual essential oil ingredients. Phytomedicine 2012, 19, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.; Conway, B.; Worthington, T. Antimicrobial Efficacy of a Novel Eucalyptus Oil, Chlorhexidine Digluconate and Isopropyl Alcohol Biocide Formulation. Int. J. Mol. Sci. 2012, 13, 14016–14025. [Google Scholar] [CrossRef]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef]
- Horváth, G.; Jámbor, N.; Kocsis, E.; Böszörményi, A.; Lemberkovics, É.; Héthelyi, É.; Kovács, K.; Kocsis, B. Role of Direct Bioautographic Method for Detection of Antistaphylococcal Activity of Essential Oils. Nat. Prod. Commun. 2011, 6, 1379–1384. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Wojciuk, B.; Dołęgowska, B.; Wajs-Bonikowska, A.; Sienkiewicz, M.; Mężyńska, M.; Łopusiewicz, Ł. The Influence of Essential Oil Compounds on Antibacterial Activity of Mupirocin-Susceptible and Induced Low-Level Mupirocin-Resistant MRSA Strains. Molecules 2019, 24, 3105. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Punitha, T.; Moorthy, K.; Vijayalakshmi, P.; Vinodhini, R.; Saranya, S.; Bhuvaneshwari, M.; Kanimozhi, C. In vitro antibacterial activity of essential plant oils against biofilm forming methicillin resistant staphylococcus aureus. Asian J. Pharm. Clin. Res. 2014, 7 (Suppl. S1), 220–225. [Google Scholar]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Hammami, M.; Khammassi, S.; Hrigua, I.; Nefisi, S.; et al. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crop. Prod. 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Simsek, M.; Duman, R. Investigation of Effect of 1,8-cineole on Antimicrobial Activity of Chlorhexidine Gluconate. Pharmacogn. Res. 2017, 9, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Tohidpour, A.; Sattari, M.; Omidbaigi, R.; Yadegar, A.; Nazemi, J. Antibacterial effect of essential oils from two medicinal plants against Methicillin-resistant Staphylococcus aureus (MRSA). Phytomedicine 2010, 17, 142–145. [Google Scholar] [CrossRef]
- Warnke, P.H.; Becker, S.T.; Podschun, R.; Sivananthan, S.; Springer, I.N.; Russo, P.A.; Wiltfang, J.; Fickenscher, H.; Sherry, E. The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J. Craniomaxillofac Surg. 2009, 37, 392–397. [Google Scholar] [CrossRef]
- Fokou, J.B.H.; Dongmo, P.M.J.; Boyom, F.F. Essential Oil’s Chemical Composition and Pharmacological Properties. In Essential Oils-Oils of Nature; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/68008 (accessed on 19 July 2022).
- Eichert, T.; Fernández, V. Chapter 4-Uptake and Release of Elements by Leaves and Other Aerial Plant Parts. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 71–84. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid Based Complement Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial Activity of Selected Essential Oil Compounds Alone and in Combination with β-Lactam Antibiotics Against MRSA Strains. Int. J. Mol. Sci. 2020, 21, 7106. [Google Scholar] [CrossRef]
- Cernohorska, L.; Votava, M. Biofilms and their significance in medical microbiology. Epidemiol. Mikrobiol. Imunol. 2002, 51, 161–164. [Google Scholar]
- Laird, K.; Phillips, C. Vapour phase: A potential future use for essential oils as antimicrobials? Lett. Appl. Microbiol. 2012, 54, 169–174. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. In situ SEM, TEM and AFM studies of the antimicrobial activity of lemon grass oil in liquid and vapour phase against Candida albicans. Micron 2010, 41, 797–805. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
| Author, Year, Location | Methodology | Objective | Intervention | Findings |
|---|---|---|---|---|
| Acs et al., 2016 [13]; Hungary | Chemical analyses of Eos—GC-FID and GC-MS Antimicrobial susceptibility—Disc diffusion In vitro tube dilution and vapour phase technique | To evaluate the antibacterial effect of various EOs against pathogens responsible for nosocomial respiratory tract infections | EOs: cinnamon bark, citronella, clove, eucalyptus, peppermint, Scots pine, thyme |
|
| Ali et al. (2022) [14]; Pakistan | Antibiotic sensitivity testing—CLSI 2020 manual Antibacterial activity of EO—Well diffusion MIC | To identify the therapeutic potential of various plant EOs against MRSA | EOs: Syzygium aromaticum, Eucalyptus globulus, Cinnamomum verum, Ferula assafoetida |
|
| Bouras et al. (2016) [15]; Algeria | Sensitivity test of EO and aqueous extract-Agar disc diffusion MIC and MBC—Agar dilution | To evaluate the antibacterial activity of EO and aqueous extract derived from E. globulus leaves | EO and aqueous extract of E. globulus leaves | The antibacterial activity of EO significant, but was lower than that of aqueous extract
|
| Chao et al. (2008) [16]; USA | Zone of inhibition—Disc diffusion assay in accordance with the Manual of Clinical Microbiology of the American Society for Microbiology | To screen EOs for inhibitory activity against MRSA to determine their potential for use as disinfectants, antiseptics or topical treatments | 91 EOs alone, including E. globulus oil | Zone of inhibition eucalyptus oil—0 mm |
| Cui et al. (2021) [17]; UK | MIC as per CLSI Synergistic effects of oils and antibiotic—Modified Well Diffusion Time-kill assay | To determine various synergistic combinations for antimicrobial therapies as a potential strategy for treatment of multidrug resistant infection | 29 plant EOs alone and EO—antibiotic combinations | MIC eucalyptus oil alone—0.313 v/v%, optimum concentration—1.56 v/v% Low level of synergy between eucalyptus oil and various antibiotics such as vancomycin, streptomycin, gentamicin, and tetracycline |
| Farsi and Alaidaroos, (2022) [18]; Saudi Arabia | Synthesis of silica nanoparticles (SiNPs) and oil encapsulation Evaluation of distribution of size, shape, and aggregation state SiNP—Transmission Electron Microscopes Determination of antimicrobial activity of eucalyptus EO with and without SiNPs—Agar well diffusion | To evaluate antibacterial efficacy using eucalyptus EO loaded on SiNPs against various pathogenic bacteria. | Eucalyptus oil alone, Silica dioxide nanoparticles alone and combination of both |
|
| Hamoud et al. (2012) [19]; Germany | GLC-MS analysis MIC and MMC—determined by micro dilution method according to the German DIN regulation 58940-8 Time-kill assay | To investigate the antimicrobial activities of Olbas® Tropfen (a complex EO distillate) in comparison to its isolated EO ingredients | Olbas (10 g) consists of peppermint oil (5.3 g), eucalyptus oil (2.1 g), cajuput oil (2.1 g) juniper berry oil (0.3 g) and wintergreen oil (0.2 g) + EOs individually |
|
| Hendry et al. (2012) [20]; UK | Establishment of microbial biofilms on stainless steel discs Determination of antimicrobial efficacy of the wipes—Agar diffusion assay Removal of microbial surface contamination by wipes and potential to promote cross-contamination | To investigate the antimicrobial efficacy of 5% eucalyptus oil and 2% eucalyptus oil containing wipes, specifically, its ability to remove microorganisms from hard surfaces, induce cross contamination and potential to eliminate bacterial biofilms | Wipes containing 5% and 2% EO, 2% CHG and 70% isopropyl alcohol (IPA) |
|
| Hendry et al. (2009) [21]; UK | MIC of aqueous CHG E. globulus EO and 1,8-cineole as per CLSI Chequerboard assays Biofilm: Chequerboard assay to assess the antimicrobial activity of CHG in combination with eucalyptus oil and 1,8-cineole against microorganisms in biofilm | To compare the antimicrobial efficacy of crude eucalyptus oil with its major constituent 1,8-cineole alone and in combination with CHG, against various pathogens when grown in planktonic and biofilm modes of growth | Aqueous CHG, eucalyptus oil and 1,8-cineole alone and CHG in combination with eucalyptus oil and 1,8-cineole |
|
| Horvath et al. (2011) [22]; Hungary | GLC/MS analysis—to determine antibacterial properties of oils and their components Direct bioautography assay—zone of inhibition | To chemically characterise the EOs of thyme, clove, eucalyptus, tea tree and cinnamon bark by TLC and identify the antibacterial activity of the oils and their main components against MRSA strains | EOs of thyme, clove, eucalyptus, tea tree and cinnamon bark and their isolated main compounds |
|
| Iseppi et al. (2021) [7]; Italy | Agar disk diffusion assay as per CLSI MIC FIC was determined Time-kill studies EO activity on mature biofilm—The effects of EOs, antibiotics, and the EO–EO and EO–antibiotic combinations on 24 h formed biofilm | To investigate if certain plant products can produce antibacterial effects against antibiotic-resistant pathogens, both alone and in combination with traditional antibiotics to which the bacterial strains were resistant. | Eucalyptus oil alone, eucalyptus oil in combination with other oils, eucalyptus oil in combination with oxacillin |
|
| Kwiatkowski et al. (2019) [23]; Poland | MIC as per CLSI Checkerboard method to identify synergy between 1,8-cineole and mupirocin | To investigate the antibacterial activity of EO compounds on mupirocin-susceptible and induced low-level mupirocin-resistant MRSA strains. | Isolated EO compounds (1,8-cineole, eugenol, carvacrol, linalool, (-)-menthone, linalyl acetate, and trans-anethole) in combination with mupirocin |
|
| Merghni et al. (2018) [24]; Tunisia | Agar disk diffusion assay to determine antibacterial activity MIC and MBC—broth dilution method Inhibition of cell attachment—anti-adhesion properties tested following a microplate biofilm assay Antiquorum sensing | To identify antibacterial, antibiofilm and antiquorum sensing potential of E. globulus EO and its main component, 1,8-cineole against MRSA strains | E.globulus EO and its main component 1,8 cineole |
|
| Mulyaningsih et al. (2011) [25]; Germany | The MIC of the samples was determined by broth microdilution methods The chemical composition of the fruit oil of E. globulus (EGF) was determined by GLC-MS, in comparison to the leaf oils from E. globulus (EGL) | To examine the antimicrobial activity of the fruit and leaf oil of E. globulus, E. radiata (ERL) and E. citriodora (ECL) against multidrug resistant bacteria. The major components of the oils were also isolated to identify a relationship between their chemical composition and antimicrobial properties. | E. globulus EO from fruits and leaves, individual components of the oil—aromadendrene, 1,8-cineole, citronellal, and citronellol |
|
| Mulyaningsih et al. (2010) [26]; Germany | GLC/MS analysis MIC/MBC—Broth microdilution assays as per CLSI Checkerboard method for synergistic, additive or antagonistic effects of combinations of individual compounds at different concentrations. Fractional inhibitory concentration indexes Time-kill experiments as per results of the checkerboard assay | To investigate E. globulus fruit (EGF) oil and its three major components (aromadendrene, 1,8-cineole, and globulol) against antibiotic-susceptible and antibiotic-resistant microorganisms and test their synergistic effects when applied in combination | EGF alone, the main constituents alone (aromadendrene, 1,8-cineole, and globulol) and main constituents in combinations at different concentrations | Aromadendrene was the most abundant compound of EGF (31.17%) followed by 1,8-cineole (14.55%), globulol (10.69%), and ledene (7.13%)
|
| Punitha et al. (2014) [27]; India | Antibiotic susceptibility test—Disc Diffusion method of Kirby Bauer on Muller-Hinton agar as per CLSI Biofilm production assay—Congo red agar method Antibacterial screening—agar well diffusion method | To evaluate whether essential oils could inhibit the growth of S. aureus biofilm forming isolates | EOs (Eucalyptus, Mint, Turpentine, Neem and Amla) |
|
| Salem et al. (2018) [28]; Tunisia | Gas chromatography analysis Antibacterial activity—disk diffusion method MIC—microdilution method Synergistic interaction by determining fractional inhibitory concentration index (FICI)—2D checkerboard method | To evaluate the antioxidant, antimicrobial, and cytotoxicity properties of E. globulus EOs and assess synergy between the EOs and conventional antimicrobials | E.globulus EOs at vegetative, full flowering and fructifications stages + synergy between E. globulus EOs and conventional antimicrobials | 67 volatile compounds were identified as part of the oil. The chemical composition differed depending on the plant stage. 1,8-cineole was the major compound at vegetative and full flowering (32.19%), p- cymene was the major compound at fructification stages (37.82%)
|
| Simsek and Duman, (2017) [29]; Turkey | MIC determined FIC determined | To compare the antimicrobial efficacy of 1,8-cineole, alone and in combination with CHG against various microorganisms | 1,8-cineole isolated from E. globulus alone and in combination CHG |
|
| Tohidpour et al. (2010) [30]; Iran | Antibacterial susceptibility—disc diffusion GC-MS—chemical composition MIC—agar dilution method as approved by NCCLS with minor modification | To test the antibacterial effect of EOs from T. vulgaris and E. globulus against MRSA and analyse the biochemical composition of the oils | Eucalyptus oil EO and Thymus vulgaris EO alone |
|
| Warnke et al. (2009) [31]; Germany, Australia, UK | Antibacterial activity—disk diffusion method | To identify the antibacterial efficacy of various essential oils on frequently isolated and hospital-acquired MRSA | 13 EOs alone |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elangovan, S.; Mudgil, P. Antibacterial Properties of Eucalyptus globulus Essential Oil against MRSA: A Systematic Review. Antibiotics 2023, 12, 474. https://doi.org/10.3390/antibiotics12030474
Elangovan S, Mudgil P. Antibacterial Properties of Eucalyptus globulus Essential Oil against MRSA: A Systematic Review. Antibiotics. 2023; 12(3):474. https://doi.org/10.3390/antibiotics12030474
Chicago/Turabian StyleElangovan, Shakthi, and Poonam Mudgil. 2023. "Antibacterial Properties of Eucalyptus globulus Essential Oil against MRSA: A Systematic Review" Antibiotics 12, no. 3: 474. https://doi.org/10.3390/antibiotics12030474
APA StyleElangovan, S., & Mudgil, P. (2023). Antibacterial Properties of Eucalyptus globulus Essential Oil against MRSA: A Systematic Review. Antibiotics, 12(3), 474. https://doi.org/10.3390/antibiotics12030474






