Diverticular Disease and Rifaximin: An Evidence-Based Review
Abstract
1. Background
1.1. Epidemiology and Risk Factors
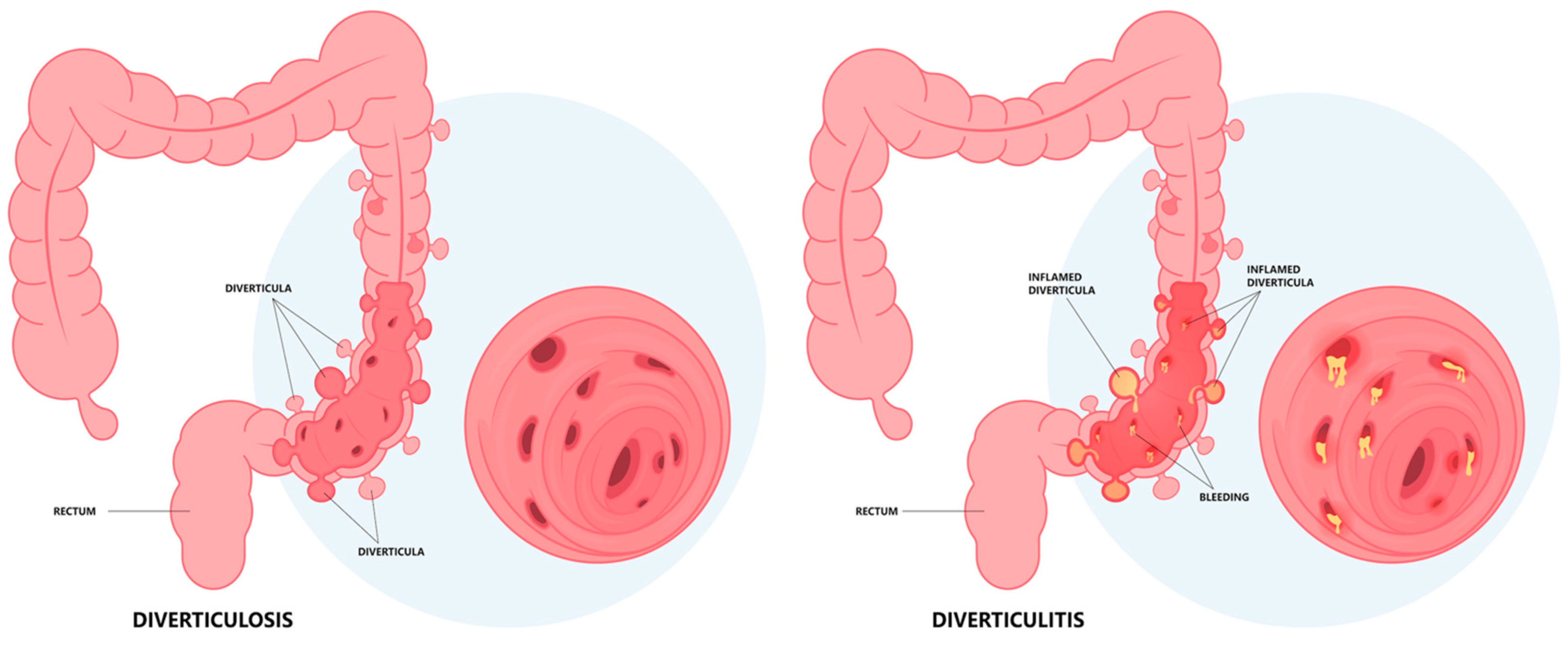
1.2. Pathogenesis
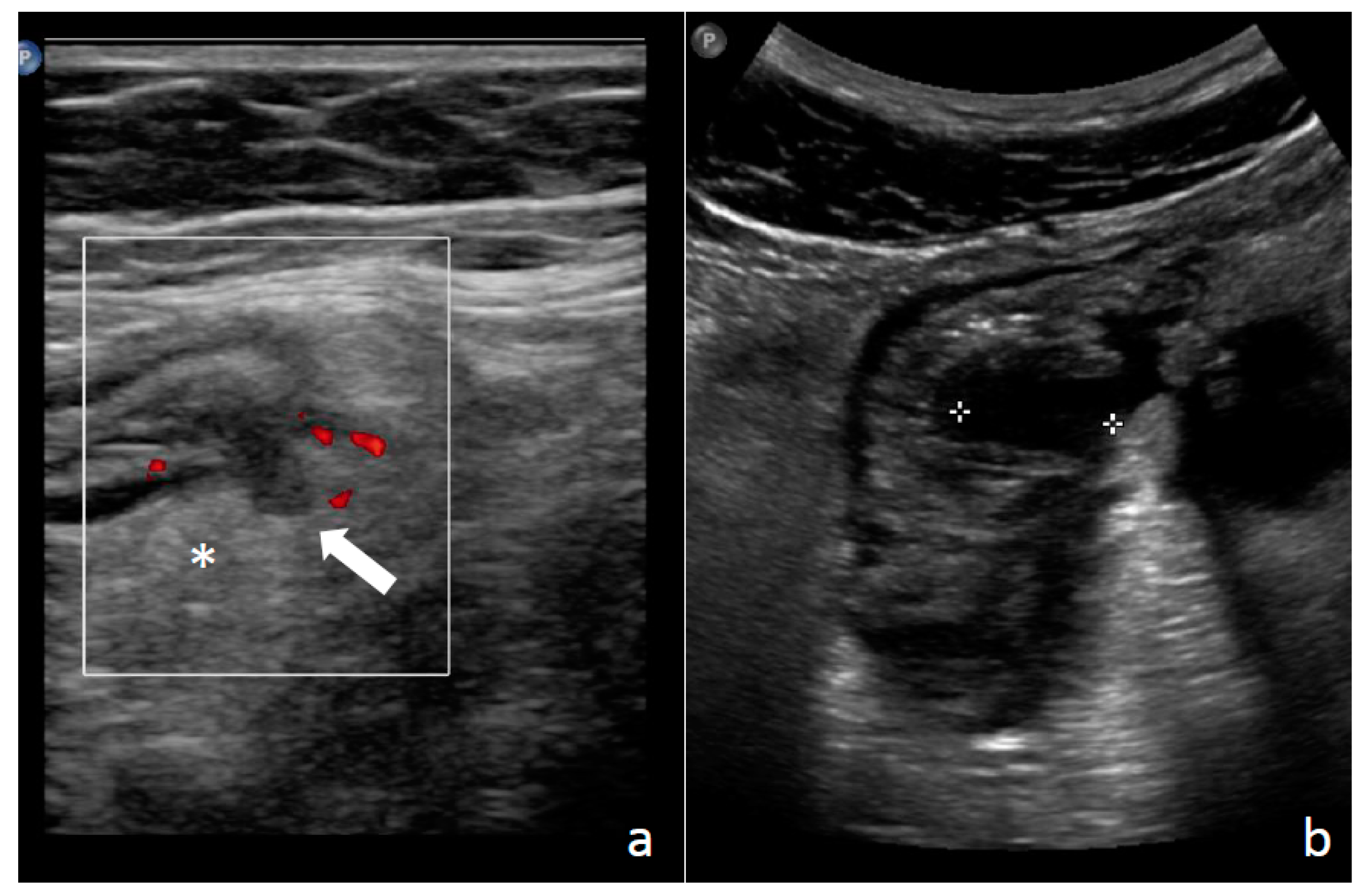
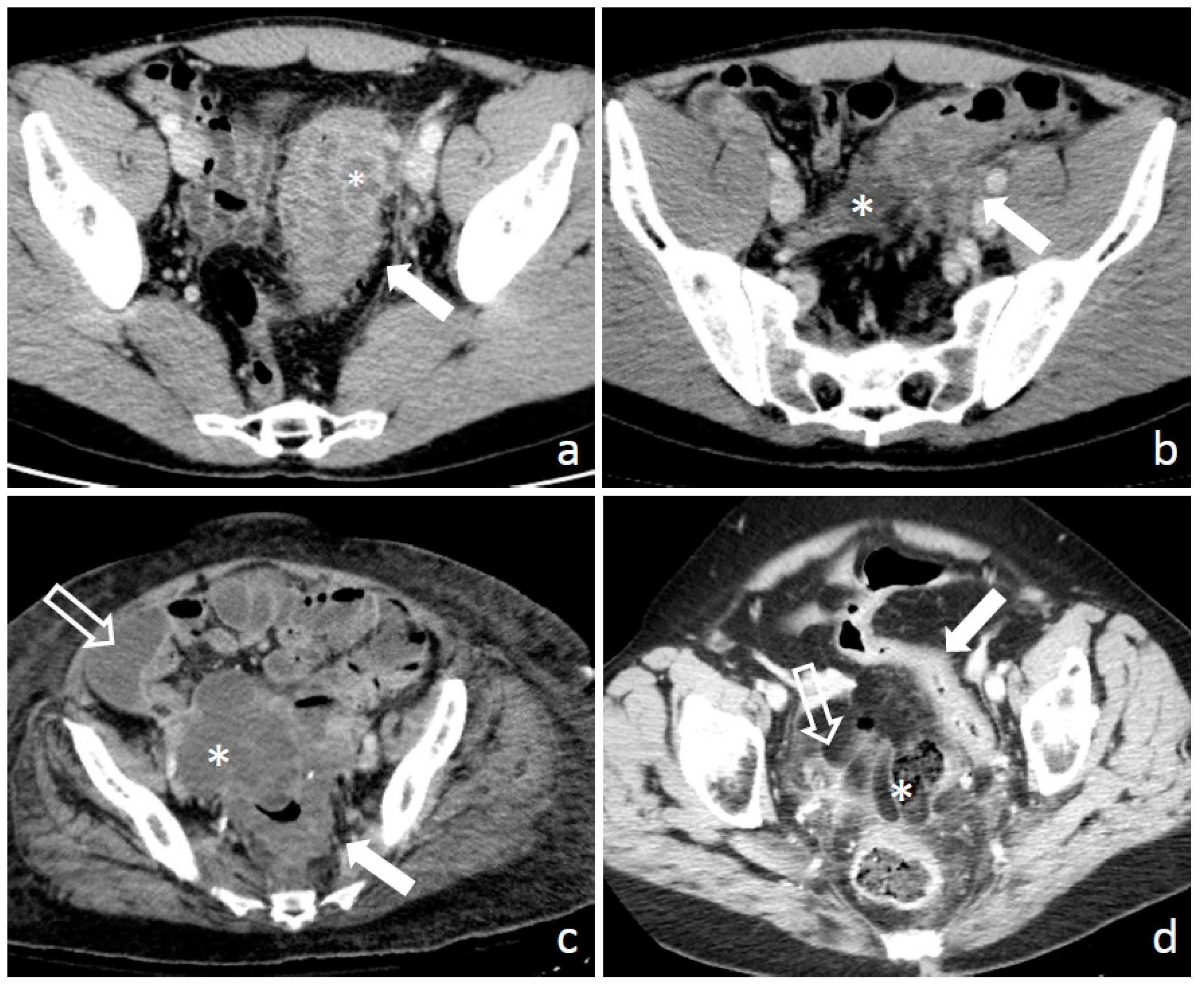
1.3. Diagnosis: Clinical Findings, Laboratory, Imaging, and Endoscopy
1.4. Principles of Management Strategies in Diverticular Disease
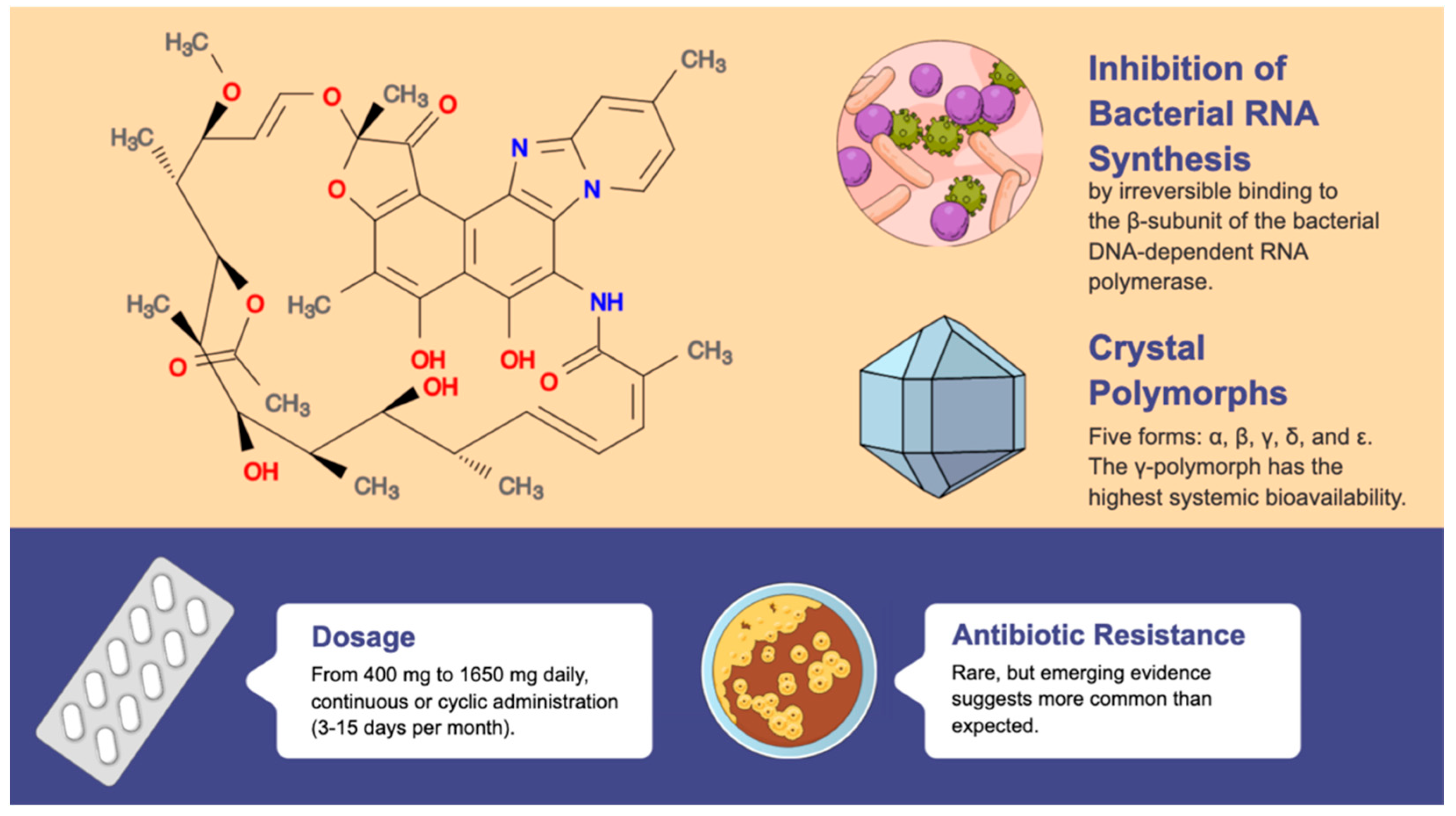
2. Rifaximin
2.1. Indications, Effects, and Therapeutic Strategies
2.2. Long-Term Use and Antimicrobial Resistance
2.3. Should Rifaximin Be Administered in Diverticulosis?
2.4. Is Rifaximin Effective at Relieving Symptoms in Individuals with SUDD?
2.5. Is rifaximin Useful in Primary Prevention of Diverticulitis in Individuals with SUDD?
2.6. Is Rifaximin Useful in Secondary Prevention in Patients with Previous Diverticulitis Episodes?
2.7. Can Rifaximin Be Used in the Treatment of Uncomplicated Acute Diverticulitis?
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tursi, A.; Scarpignato, C.; Strate, L.L.; Lanas, A.; Kruis, W.; Lahat, A.; Danese, S. Colonic diverticular disease. Nat. Rev. Dis. Prim. 2020, 6, 20. [Google Scholar] [CrossRef]
- Schieffer, K.M.; Kline, B.P.; Yochum, G.S.; Koltun, W.A. Pathophysiology of diverticular disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 683–692. [Google Scholar] [CrossRef]
- Tursi, A.; Papa, A.; Danese, S. Review article: The pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment. Pharmacol. Ther. 2015, 42, 664–684. [Google Scholar] [CrossRef]
- Maconi, G. Diagnosis of symptomatic uncomplicated diverticular disease and the role of Rifaximin in management. Acta Bio Med. Atenei Parm. 2017, 88, 25–32. [Google Scholar]
- Everhart, J.E.; Ruhl, C.E. Burden of Digestive Diseases in the United States Part II: Lower Gastrointestinal Diseases. Gastroenterology 2009, 136, 741–754. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015, 149, 1731–1741. [Google Scholar] [CrossRef]
- Yamamichi, N.; Shimamoto, T.; Takahashi, Y.; Sakaguchi, Y.; Kakimoto, H.; Matsuda, R.; Kataoka, Y.; Saito, I.; Tsuji, Y.; Yakabi, S.; et al. Trend and Risk Factors of Diverticulosis in Japan: Age, Gender, and Lifestyle/Metabolic-Related Factors May Cooperatively Affect on the Colorectal Diverticula Formation. PLoS ONE 2015, 10, e0123688. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.S.; Lee, J.H.; Ok, K.S.; Ryu, S.H.; Lee, J.H.; Moon, J.S. Clinical Characteristics of Colonic Diverticulosis in Korea: A Prospective Study. Korean J. Intern. Med. 2010, 25, 140–146. [Google Scholar] [CrossRef]
- Shahedi, K.; Fuller, G.; Bolus, R.; Cohen, E.; Vu, M.; Shah, R.; Agarwal, N.; Kaneshiro, M.; Atia, M.; Sheen, V.; et al. Long-term Risk of Acute Diverticulitis Among Patients with Incidental Diverticulosis Found During Colonoscopy. Clin. Gastroenterol. Hepatol. 2013, 11, 1609–1613. [Google Scholar] [CrossRef]
- Peery, A.F.; Sandler, R.S. Diverticular Disease: Reconsidering Conventional Wisdom. Clin. Gastroenterol. Hepatol. 2013, 11, 1532–1537. [Google Scholar] [CrossRef]
- Schultz, J.K.; Azhar, N.; Binda, G.A.; Barbara, G.; Biondo, S.; Boermeester, M.A.; Chabok, A.; Consten, E.C.J.; van Dijk, S.T.; Johanssen, A.; et al. European Society of Coloproctology: Guidelines for the management of diverticular disease of the colon. Color. Dis. 2020, 22, 5–28. [Google Scholar] [CrossRef]
- Böhm, S.K.; Kruis, W. Lifestyle and other risk factors for diverticulitis. Minerva Gastroenterol. Dietol. 2017, 63, 110–118. [Google Scholar] [CrossRef]
- Strate, L.L.; Morris, A.M. Epidemiology, Pathophysiology, and Treatment of Diverticulitis. Gastroenterology 2019, 156, 1282–1298. [Google Scholar] [CrossRef]
- Jovani, M.; Ma, W.; Joshi, A.D.; Liu, P.-H.; Nguyen, L.H.; Cao, Y.; Tam, I.; Wu, K.; Giovannucci, E.L.; Chan, A.T.; et al. Menopausal Hormone Therapy and Risk of Diverticulitis. Am. J. Gastroenterol. 2019, 114, 315–321. [Google Scholar] [CrossRef]
- Wedel, T.; Barrenschee, M.; Lange, C.; Cossais, F.; Böttner, M. Morphologic Basis for Developing Diverticular Disease, Diverticulitis, and Diverticular Bleeding. Viszeralmedizin. 2015, 31, 76–82. [Google Scholar] [CrossRef]
- Feldman, M.; Friedman, L.S.; Brandt, L.J. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Daniels, L.; Budding, A.E.; De Korte, N.; Eck, A.; Bogaards, J.A.; Stockmann, H.B.; Consten, E.C.; Savelkoul, P.H.; Boermeester, M.A. Fecal microbiome analysis as a diagnostic test for diverticulitis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1927–1936. [Google Scholar] [CrossRef]
- Hullar, M.A.; Sandstrom, R.S.; Stamatoyannopoulos, J.A.; Lampe, J.W.; Strate, L.L. The fecal microbiome in diverticulitis and asymptomatic diverticulosis: A case-control study in the US. medRxiv 2019, 19001404. [Google Scholar] [CrossRef]
- Barbara, G.; Scaioli, E.; Barbaro, M.R.; Biagi, E.; Laghi, L.; Cremon, C.; Marasco, G.; Colecchia, A.; Picone, G.; Salfi, N.; et al. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut 2017, 66, 1252–1261. [Google Scholar] [CrossRef]
- Van Rossen, T.M.; Ooijevaar, R.E.; Kuyvenhoven, J.P.; Eck, A.; Bril, H.; Buijsman, R.; Boermeester, M.A.; Stockmann, H.B.A.C.; de Korte, N.; Budding, A.E. Microbiota composition and mucosal immunity in patients with asymptomatic diverticulosis and controls. PLoS ONE 2021, 16, e0256657. [Google Scholar] [CrossRef]
- Kechagias, A.; Sofianidis, A.; Zografos, G.; Leandros, E.; Alexakis, N.; Dervenis, C. Index C-reactive protein predicts increased severity in acute sigmoid diverticulitis. Ther. Clin. Risk Manag. 2018, 14, 1847–1853. [Google Scholar] [CrossRef]
- Talabani, A.J.; Endreseth, B.H.; Lydersen, S.; Edna, T.-H. Clinical diagnostic accuracy of acute colonic diverticulitis in patients admitted with acute abdominal pain, a receiver operating characteristic curve analysis. Int. J. Color. Dis. 2017, 32, 41–47. [Google Scholar] [CrossRef]
- Mäkelä, J.T.; Klintrup, K.; Takala, H.; Rautio, T. The role of C-reactive protein in prediction of the severity of acute diverticulitis in an emergency unit. Scand. J. Gastroenterol. 2015, 50, 536–541. [Google Scholar] [CrossRef]
- Dehghan, A.; Kardys, I.; de Maat, M.P.; Uitterlinden, A.G.; Sijbrands, E.J.; Bootsma, A.H.; Stijnen, T.; Hofman, A.; Schram, M.T.; Witteman, J.C. Genetic Variation, C-Reactive Protein Levels, and Incidence of Diabetes. Diabetes 2007, 56, 872–878. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J. Am. Med. Assoc. 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Schwerk, W.B.; Schwarz, S.; Rothmund, M. Sonography in acute colonic diverticulitis. A prospective study. Dis. Colon Rectum 1992, 35, 1077–1084. [Google Scholar] [CrossRef]
- Puylaert, J.B. Ultrasound of Colon Diverticulitis. Dig. Dis. 2012, 30, 56–59. [Google Scholar] [CrossRef]
- Hollerweger, A.; Macheiner, P.; Rettenbacher, T.; Brunner, W.; Gritzmann, N. Colonic diverticulitis: Diagnostic value and appearance of inflamed diverticula–sonographic evaluation. Eur. Radiol. 2001, 11, 1956–1963. [Google Scholar] [CrossRef]
- Liljegren, G.; Chabok, A.; Wickbom, M.; Smedh, K.; Nilsson, K. Acute colonic diverticulitis: A systematic review of diagnostic accuracy. Color. Dis. 2007, 9, 480–488. [Google Scholar] [CrossRef]
- Dickerson, E.C.; Chong, S.T.; Ellis, J.H.; Watcharotone, K.; Nan, B.; Davenport, M.S.; Al-Hawary, M.; Mazza, M.B.; Rizk, R.; Morris, A.M.; et al. Recurrence of Colonic Diverticulitis: Identifying Predictive CT Findings—Retrospective Cohort Study. Radiology 2017, 285, 850–858. [Google Scholar] [CrossRef]
- Hinchey, E.J.; Schaal, P.G.; Richards, G.K. Treatment of perforated diverticular disease of the colon. Adv. Surg. 1978, 12, 85–109. [Google Scholar]
- Sartelli, M.; A Moore, F.; Ansaloni, L.; Di Saverio, S.; Coccolini, F.; A Griffiths, E.; Coimbra, R.; Agresta, F.; Sakakushev, B.; A Ordoñez, C.; et al. A proposal for a CT driven classification of left colon acute diverticulitis. World J. Emerg. Surg. 2015, 10, 3. [Google Scholar] [CrossRef]
- Klarenbeek, B.R.; de Korte, N.; van der Peet, D.L.; Cuesta, M.A. Review of current classifications for diverticular disease and a translation into clinical practice. Int. J. Color. Dis. 2012, 27, 207–214. [Google Scholar] [CrossRef]
- Sher, M.E.; Agachan, F.; Bortul, M.; Nogueras, J.J.; Weiss, E.G.; Wexner, S.D. Laparoscopic surgery for diverticulitis. Surg. Endosc. 1997, 11, 264–267. [Google Scholar] [CrossRef]
- Wasvary, H.; Turfah, F.; Kadro, O.; Beauregard, W. Same Hospitalization Resection for Acute Diverticulitis. Am. Surg. 1999, 65, 632–636. [Google Scholar] [CrossRef]
- Kaiser, A.M.; Jiang, J.-K.; Lake, J.P.; Ault, G.; Artinyan, A.; Gonzalez-Ruiz, C.; Essani, R.; Beart, R.W. The Management of Complicated Diverticulitis and the Role of Computed Tomography. Am. J. Gastroenterol. 2005, 100, 910–917. [Google Scholar] [CrossRef]
- Feingold, D.; Steele, S.R.; Lee, S.; Kaiser, A.; Boushey, R.; Buie, W.D.; Rafferty, J.F. Practice Parameters for the Treatment of Sigmoid Diverticulitis. Dis. Colon Rectum 2014, 57, 284–294. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Di Mario, F.; Andreoli, A.; Annunziata, M.L.; Astegiano, M.; Bianco, M.A.; Buri, L.; Cammarota, G.; Capezzuto, E.; et al. Development and Validation of an Endoscopic Classification of Diverticular Disease of the Colon: The DICA Classification. Dig. Dis. 2015, 33, 68–76. [Google Scholar] [CrossRef]
- Scarpignato, C.; Barbara, G.; Lanas, A.; Strate, L.L. Management of colonic diverticular disease in the third millennium: Highlights from a symposium held during the United European Gastroenterology Week 2017. Ther. Adv. Gastroenterol. 2018, 11, 1756284818771305. [Google Scholar] [CrossRef]
- Strate, L.L.; Modi, R.; Cohen, E.; Spiegel, B.M.R. Diverticular Disease as a Chronic Illness: Evolving Epidemiologic and Clinical Insights. Am. J. Gastroenterol. 2012, 107, 1486–1493. [Google Scholar] [CrossRef]
- Comparato, G.; Fanigliulo, L.; Aragona, G.; Cavestro, G.M.; Cavallaro, L.G.; Leandro, G.; Pilotto, A.; Nervi, G.; Soliani, P.; Sianesi, M.; et al. Quality of Life in Uncomplicated Symptomatic Diverticular Disease: Is It Another Good Reason for Treatment? Dig. Dis. 2007, 25, 252–259. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Barbaro, M.R.; Bellacosa, L.; Stanghellini, V. Treatment of Diverticular Disease with Aminosalicylates: The Evidence. J. Clin. Gastroenterol. 2016, 50, S60–S63. [Google Scholar] [CrossRef]
- Kruis, W.; Meier, E.; Schumacher, M.; Mickisch, O.; Greinwald, R.; Mueller, R. Randomised clinical trial: Mesalazine (Salofalk granules) for uncomplicated diverticular disease of the colon—A placebo-controlled study. Aliment. Pharmacol. Ther. 2013, 37, 680–690. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Elisei, W.; Picchio, M.; Forti, G.; Pianese, G.; Rodino, S.; D’Amico, T.; Sacca, N.; Portincasa, P.; et al. Randomised clinical trial: Mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease—A double-blind, randomised, placebo-controlled study. Aliment. Pharmacol. Ther. 2013, 38, 741–751. [Google Scholar] [CrossRef]
- Picchio, M.; Elisei, W.; Brandimarte, G.; Di Mario, F.; Malfertheiner, P.; Scarpignato, C.; Tursi, A. Mesalazine for the Treatment of Symptomatic Uncomplicated Diverticular Disease of the Colon and for Primary Prevention of Diverticulitis: A Systematic Review of Randomized Clinical Trials. J. Clin. Gastroenterol. 2016, 50, S64–S69. [Google Scholar] [CrossRef]
- Slim, K.; Joris, J.; Beyer-Berjot, L. The end of antibiotics in the management of uncomplicated acute diverticulitis. J. Visc. Surg. 2019, 156, 373–375. [Google Scholar] [CrossRef]
- Rottier, S.J.; van Dijk, S.T.; Ünlü, Ç.; van Geloven, A.A.W.; Schreurs, W.H.; Boermeester, M.A. Complicated Disease Course in Initially Computed Tomography-Proven Uncomplicated Acute Diverticulitis. Surg. Infect. 2019, 20, 453–459. [Google Scholar] [CrossRef]
- Bolkenstein, H.E.; Consten, E.C.J.; van der Palen, J.; van de Wall, B.J.; Broeders, I.A.; Bemelman, W.A.; Lange, J.F.; Boermeester, M.A.; Draaisma, W.A.; Dutch Diverticular Disease (3D) Collaborative Study Group. Long-term Outcome of Surgery Versus Conservative Management for Recurrent and Ongoing Complaints After an Episode of Diverticulitis: 5-year Follow-up Results of a Multicenter Randomized Controlled Trial (DIRECT-Trial). Ann. Surg. 2019, 269, 612–620. [Google Scholar] [CrossRef]
- Jaung, R.; Kularatna, M.; Robertson, J.P.; Vather, R.; Rowbotham, D.; MacCormick, A.D.; Bissett, I.P. Uncomplicated Acute Diverticulitis: Identifying Risk Factors for Severe Outcomes. World J. Surg. 2017, 41, 2258–2265. [Google Scholar] [CrossRef]
- Van De Wall, B.J.M.; Draaisma, W.A.; Van Iersel, J.J.; Van Der Kaaij, R.; Consten, E.C.J.; Broeders, I.A.M.J. Dietary restrictions for acute diverticulitis: Evidence-based or expert opinion? Int. J. Color. Dis. 2013, 28, 1287–1293. [Google Scholar] [CrossRef]
- Van Dijk, S.T.; Rottier, S.J.; Van Geloven, A.A.W.; Boermeester, M.A. Conservative Treatment of Acute Colonic Diverticulitis. Curr. Infect. Dis. Rep. 2017, 19, 44. [Google Scholar] [CrossRef]
- Rezapour, M.; Stollman, N. Antibiotics in Uncomplicated Acute Diverticulitis: To Give or Not to Give? Inflamm. Intest. Dis. 2018, 3, 75–79. [Google Scholar] [CrossRef]
- Mocanu, V.; Dang, J.T.; Switzer, N.; Tavakoli, I.; Tian, C.; de Gara, C.; Birch, D.W.; Karmali, S. The role of antibiotics in acute uncomplicated diverticulitis: A systematic review and meta-analysis. Am. J. Surg. 2018, 216, 604–609. [Google Scholar] [CrossRef]
- Emile, S.H.; Elfeki, H.; Sakr, A.; Shalaby, M. Management of acute uncomplicated diverticulitis without antibiotics: A systematic review, meta-analysis, and meta-regression of predictors of treatment failure. Tech. Coloproctol. 2018, 22, 499–509. [Google Scholar] [CrossRef]
- Evans, J. Does a 48-Hour Rule Predict Outcomes in Patients with Acute Sigmoid Diverticulitis? J. Gastrointest. Surg. 2008, 12, 577–582. [Google Scholar] [CrossRef]
- Gregersen, R.; Mortensen, L.Q.; Burcharth, J.; Pommergaard, H.-C.; Rosenberg, J. Treatment of patients with acute colonic diverticulitis complicated by abscess formation: A systematic review. Int. J. Surg. 2016, 35, 201–208. [Google Scholar] [CrossRef]
- Young-Fadok, T.M. Diverticulitis. N. Engl. J. Med. 2018, 379, 1635–1642. [Google Scholar] [CrossRef]
- Jacobs, D.O. Clinical practice. Diverticulitis. N. Engl. J. Med. 2007, 357, 2057–2066. [Google Scholar] [CrossRef]
- Biondo, S. The diminishing role of surgery for acute diverticulitis. Br. J. Surg. 2019, 106, 308–309. [Google Scholar] [CrossRef]
- Papi, C.; Ciaco, A.; Koch, M.; Capurso, L. Efficacy of rifaximin in the treatment of symptomatic diverticular disease of the colon. A multicentre double-blind placebo-controlled trial. Aliment. Pharmacol. Ther. 1995, 9, 33–39. [Google Scholar] [CrossRef]
- Schieffer, K.M.; Sabey, K.; Wright, J.R.; Toole, D.R.; Drucker, R.; Tokarev, V.; Harris, L.R.; Deiling, S.; Eshelman, M.A.; Hegarty, J.P.; et al. The Microbial Ecosystem Distinguishes Chronically Diseased Tissue from Adjacent Tissue in the Sigmoid Colon of Chronic, Recurrent Diverticulitis Patients. Sci. Rep. 2017, 7, 8467. [Google Scholar] [CrossRef]
- Tursi, A.; Mastromarino, P.; Capobianco, D.; Elisei, W.; Miccheli, A.; Capuani, G.; Tomassini, A.; Campagna, G.; Picchio, M.; Giorgetti, G.; et al. Assessment of Fecal Microbiota and Fecal Metabolome in Symptomatic Uncomplicated Diverticular Disease of the Colon. J. Clin. Gastroenterol. 2016, 50, S9–S12. [Google Scholar] [CrossRef]
- Corazza, G.R.; Di Stefano, M.; Scarpignato, C. Treatment of Functional Bowel Disorders: Is There Room for Antibiotics? Digestion 2006, 73, 38–46. [Google Scholar] [CrossRef]
- Frieri, G.; Pimpo, M.T.; Scarpignato, C. Management of Colonic Diverticular Disease. Digestion 2006, 73, 58–66. [Google Scholar] [CrossRef]
- Rothstein, D.M. Rifamycins, Alone and in Combination. Cold Spring Harb. Perspect. Med. 2016, 6, a027011. [Google Scholar] [CrossRef]
- Dupont, H.L. Review article: The antimicrobial effects of rifaximin on the gut microbiota. Aliment. Pharmacol. Ther. 2016, 43, 3–10. [Google Scholar] [CrossRef]
- Descombe, J.J.; Dubourg, D.; Picard, M. Palazzini, E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int. J. Clin. Pharmacol. Res. 1994, 14, 51–56. Available online: https://europepmc.org/article/med/7836025 (accessed on 5 January 2023).
- Schoenfeld, P.; Pimentel, M.; Chang, L.; Lembo, A.; Chey, W.D.; Yu, J.; Paterson, C.; Bortey, E.; Forbes, W.P. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: A pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment. Pharmacol. Ther. 2014, 39, 1161–1168. [Google Scholar] [CrossRef]
- Jiang, Z.-D.; Ke, S.; Palazzini, E.; Riopel, L.; Dupont, H. In Vitro Activity and Fecal Concentration of Rifaximin after Oral Administration. Antimicrob. Agents Chemother. 2000, 44, 2205–2206. [Google Scholar] [CrossRef]
- Viscomi, G.C.; Campana, M.; Barbanti, M.; Grepioni, F.; Polito, M.; Confortini, D.; Rosini, G.; Righi, P.; Cannata, V.; Braga, D. Crystal forms of rifaximin and their effect on pharmaceutical properties. CrystEngComm 2008, 10, 1074–1081. [Google Scholar] [CrossRef]
- Blandizzi, C.; Viscomi, G.C.; Marzo, A.; Scarpignato, C. Is generic rifaximin still a poorly absorbed antibiotic? A comparison of branded and generic formulations in healthy volunteers. Pharmacol. Res. 2014, 85, 39–44. [Google Scholar] [CrossRef]
- Shayto, R.H.; Abou Mrad, R.; Sharara, A.I. Use of rifaximin in gastrointestinal and liver diseases. World J. Gastroenterol. 2016, 22, 6638–6651. [Google Scholar] [CrossRef]
- Umezawa, H.; Mizuno, S.; Yamazaki, H.; Nitta, K. Inhibition of DNA-dependent RNA synthesis by rifamycins. J. Antibiot. 1968, 21, 234–236. [Google Scholar] [CrossRef]
- Adachi, J.A.; Dupont, H.L. Rifaximin: A Novel Nonabsorbed Rifamycin for Gastrointestinal Disorders. Clin. Infect. Dis. 2006, 42, 541–547. [Google Scholar] [CrossRef]
- Gomi, H.; Jiang, Z.-D.; Adachi, J.A.; Ashley, D.; Lowe, B.; Verenkar, M.P.; Steffen, R.; DuPont, H.L. In Vitro Antimicrobial Susceptibility Testing of Bacterial Enteropathogens Causing Traveler’s Diarrhea in Four Geographic Regions. Antimicrob. Agents Chemother. 2001, 45, 212–216. [Google Scholar] [CrossRef]
- Hoover, W.W.; Gerlach, E.; Hoban, D.J.; Eliopoulos, G.M.; Pfaller, M.A.; Jones, R.N. Antimicrobial activity and spectrum of rifaximin, a new topical rifamycin derivative. Diagn. Microbiol. Infect. Dis. 1993, 16, 111–118. [Google Scholar] [CrossRef]
- Sierra, J.M.; Ruiz, J.; Navia, M.M.; Vargas, M.; Gascon, J.; Vila, J. In Vitro Activity of Rifaximin against Enteropathogens Producing Traveler’s Diarrhea. Antimicrob. Agents Chemother. 2001, 45, 643–644. [Google Scholar] [CrossRef]
- Scrascia, M.; Forcillo, M.; Maimone, F.; Pazzani, C. Susceptibility to rifaximin of Vibrio cholerae strains from different geographical areas. J. Antimicrob. Chemother. 2003, 52, 303–305. [Google Scholar] [CrossRef]
- Wang, F.D.; Liao, C.-H.; Lin, Y.T.; Sheng, W.H.; Hsueh, P.-R. Trends in the susceptibility of commonly encountered clinically significant anaerobes and susceptibilities of blood isolates of anaerobes to 16 antimicrobial agents, including fidaxomicin and rifaximin, 2008–2012, northern Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2041–2052. [Google Scholar] [CrossRef]
- Holton, J.; Vaira, D.; Menegatti, M.; Barbara, L. The susceptibility of Helicobacter pylori to the rifamycin, rifaximin. J. Antimicrob. Chemother. 1995, 35, 545–549. [Google Scholar] [CrossRef]
- Jiang, Z.-D.; DuPont, H.L.; La Rocco, M.; Garey, K.W. In vitro susceptibility of Clostridium difficile to rifaximin and rifampin in 359 consecutive isolates at a university hospital in Houston, Texas. J. Clin. Pathol. 2010, 63, 355–358. [Google Scholar] [CrossRef]
- Gobernado, M.; Ponce, J.A. Sociedad Española de Quimioterapia Revisión Rifaximina. Junio 2004, 17, 141–153. Available online: http://www.seq.es/seq/0214-3429/17/2/141.pdf (accessed on 31 January 2023).
- Amenta, M.; Nogare, E.D.; Colomba, C.; Prestileo, T.; Di Lorenzo, F.; Fundarò, S.; Ferrieri, A. Intestinal Protozoa in HIV-Infected Patients: Effect of Rifaximin in Cryptosporidium parvum and Blastocystis hominis Infections. J. Chemother. 1999, 11, 391–395. [Google Scholar] [CrossRef]
- Marchese, A.; Salerno, A.; Pesce, A.; Debbia, E.A.; Schito, G.C. In vitro Activity of Rifaximin, Metronidazole and Vancomycin against Clostridium difficile and the Rate of Selection of Spontaneously Resistant Mutants against Representative Anaerobic and Aerobic Bacteria, Including Ammonia-Producing Species. Chemotherapy 2000, 46, 253–266. [Google Scholar] [CrossRef]
- Kim, M.-S.; Morales, W.; Hani, A.A.; Kim, S.; Kim, G.; Weitsman, S.; Chang, C.; Pimentel, M. The Effect of Rifaximin on Gut Flora and Staphylococcus Resistance. Dig. Dis. Sci. 2013, 58, 1676–1682. [Google Scholar] [CrossRef]
- Maccaferri, S.; Vitali, B.; Klinder, A.; Kolida, S.; Ndagijimana, M.; Laghi, L.; Calanni, F.; Brigidi, P.; Gibson, G.R.; Costabile, A. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: An in vitro approach using a continuous culture colonic model system. J. Antimicrob. Chemother. 2010, 65, 2556–2565. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Sanyal, A.J.; Hylemon, P.B.; Sterling, R.K.; Stravitz, R.T.; Fuchs, M.; Ridlon, J.M.; Daita, K.; Monteith, P.; et al. Modulation of the Metabiome by Rifaximin in Patients with Cirrhosis and Minimal Hepatic Encephalopathy. PLoS ONE 2013, 8, e60042. [Google Scholar] [CrossRef]
- Calanni, F.; Renzulli, C.; Barbanti, M.; Viscomi, G.C. Rifaximin: Beyond the traditional antibiotic activity. J. Antibiot. 2014, 67, 667–670. [Google Scholar] [CrossRef]
- Brown, E.L.; Xue, Q.; Jiang, Z.-D.; Xu, Y.; DuPont, H.L. Pretreatment of Epithelial Cells with Rifaximin Alters Bacterial Attachment and Internalization Profiles. Antimicrob. Agents Chemother. 2010, 54, 388–396. [Google Scholar] [CrossRef]
- Ma, X.; Shah, Y.M.; Guo, G.L.; Wang, T.; Krausz, K.W.; Idle, J.R.; Gonzalez, F.J. Rifaximin Is a Gut-Specific Human Pregnane X Receptor Activator. Experiment 2007, 322, 391–398. [Google Scholar] [CrossRef]
- Gillis, J.C.; Brogden, R.N. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs 1995, 49, 467–484. [Google Scholar] [CrossRef]
- De Leo, C.; Eftimiadi, C.; Schito, G.C. Rapid disappearance from the intestinal tract of bacteria resistant to rifaximin. Drugs Exp. Clin. Res. 1986, 12, 979–981. Available online: https://europepmc.org/article/med/3569008 (accessed on 5 January 2023).
- Brigidi, P.; Swennen, E.; Rizzello, F.; Bozzolasco, M.; Matteuzzi, D. Effects of Rifaximin Administration on the Intestinal Microbiota in Patients with Ulcerative Colitis. J. Chemother. 2002, 14, 290–295. [Google Scholar] [CrossRef]
- Colecchia, A. Efficacy of long term cyclic administration of the poorly absorbed antibiotic Rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J. Gastroenterol. 2007, 13, 264–269. [Google Scholar] [CrossRef]
- Cianci, R.; Frosali, S.; Pagliari, D.; Cesaro, P.; Petruzziello, L.; Casciano, F.; Landolfi, R.; Costamagna, G.; Pandolfi, F. Uncomplicated Diverticular Disease: Innate and Adaptive Immunity in Human Gut Mucosa before and after Rifaximin. J. Immunol. Res. 2014, 2014, 696812. [Google Scholar] [CrossRef]
- Latella, G.; Pimpo, M.; Sottili, S.; Zippi, M.; Viscido, A.; Chiaramonte, M.; Frieri, G. Rifaximin improves symptoms of acquired uncomplicated diverticular disease of the colon. Int. J. Color. Dis. 2003, 18, 55–62. [Google Scholar] [CrossRef]
- Cole, K.A.; Rivard, K.R.; Dumkow, L.E. Antimicrobial Stewardship Interventions to Combat Antibiotic Resistance: An Update on Targeted Strategies. Curr. Infect. Dis. Rep. 2019, 21, 33. [Google Scholar] [CrossRef]
- Zuccaro, V.; Columpsi, P.; Sacchi, P.; Lucà, M.G.; Fagiuoli, S.; Bruno, R. Antibiotic stewardship and empirical antibiotic treatment: How can they get along? Dig. Liver Dis. 2017, 49, 579–584. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Pecere, S.; Lopetuso, L.; Scaldaferri, F.; Cammarota, G.; Gasbarrini, A. Rifaximin for the treatment of irritable bowel syndrome—A drug safety evaluation. Expert Opin. Drug Saf. 2016, 15, 983–991. [Google Scholar] [CrossRef]
- Reigadas, E.; Alcalá, L.; Gómez, J.; Marín, M.; Martin, A.; Onori, R.; Muñoz, P.; Bouza, E. Breakthrough Clostridium difficile Infection in Cirrhotic Patients Receiving Rifaximin. Clin. Infect. Dis. 2018, 66, 1086–1091. [Google Scholar] [CrossRef]
- Padilla, E.; Oms, L.; Espejo, E.; Gómez, L.; Pagespetit, L.; Boada, N.; Bella, F.; Pérez, J. Rifampin Resistance in Staphylococci after Rifaximin Intake for Surgical Prophylaxis in Elective Colorectal Surgery. Antimicrob. Agents Chemother. 2018, 62, e01353-18. [Google Scholar] [CrossRef]
- Baumert, P.M.; Camp, J.; Gölz, H.; Vavra, M.; Schuster, S.; Kern, W.V.; Mischnik, A.; Armean, S.; Boldt, A.C.; Bui, M.T.; et al. Detection of High-Level Rifaximin Resistance in Enteric Bacteria by Agar Screen. Microb. Drug Resist. 2020, 26, 545–549. [Google Scholar] [CrossRef]
- Cuomo, R.; Barbara, G.; Annibale, B. Rifaximin and diverticular disease: Position paper of the Italian Society of Gastroenterology (SIGE). Dig. Liver Dis. 2017, 49, 595–603. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Francuzik, W.; Bobkiewicz, A.; Krokowicz, Ł.; Borejsza-Wysocki, M.; Paszkowski, J.; Studniarek, A.; Krokowicz, P.; Grochowalski, M.; Zastawna, K.; et al. The influence of rifaximin on diverticulitis rate and quality of life in patients with diverticulosis. Pol. J. Surg. 2017, 89, 22–31. [Google Scholar] [CrossRef]
- De Bastiani, R.; Sanna, G.; Fracasso, P.; D’Urso, M.; Benedetto, E.; Tursi, A. The Management of Patients with Diverticulosis and Diverticular Disease in Primary Care. J. Clin. Gastroenterol. 2016, 50, S89–S92. [Google Scholar] [CrossRef]
- De Bastiani, R.; Sanna, G.; Bertolusso, L.; Casella, G.; De Polo, M.; Zamparella, M.; Cottone, C.; Tosetti, C.; Mancuso, M.; Pirrotta, E.; et al. General practitioners’ management of symptomatic uncomplicated diverticular disease of the colon by using rifaximin, a non-adsorbable antibiotic. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 423–430. [Google Scholar] [CrossRef]
- Bianchi, M.; Festa, V.; Moretti, A.; Ciaco, A.; Mangone, M.; Tornatore, V.; Dezi, A.; Luchetti, R.; De Pascalis, B.; Papi, C.; et al. Meta-analysis: Long-term therapy with rifaximin in the management of uncomplicated diverticular disease. Aliment. Pharmacol. Ther. 2011, 33, 902–910. [Google Scholar] [CrossRef]
- Stallinger, S.; Eller, N.; Högenauer, C. Non-interventional study evaluating efficacy and tolerability of rifaximin for treatment of uncomplicated diverticular disease. Wien. Klin. Wochenschr. 2014, 126, 9–14. [Google Scholar] [CrossRef]
- Moniuszko, A.; Rydzewska, G. The effect of cyclic rifaximin therapy on symptoms of diverticular disease from the perspective of the gastroenterology outpatient clinic: A ‘real-life’ study. Gastroenterol. Rev./Przegląd Gastroenterol. 2017, 12, 145–151. [Google Scholar] [CrossRef]
- Di Mario, F.; Miraglia, C.; Cambiè, G.; Violi, A.; Nouvenne, A.; Franceschi, M.; Brandimarte, G.; Elisei, W.; Picchio, M.; Tursi, A. Long-term efficacy of rifaximin to manage the symptomatic uncomplicated diverticular disease of the colon. J. Investig. Med. 2019, 67, 767–770. [Google Scholar] [CrossRef]
- Cuomo, R.; Barbara, G.; Pace, F.; Annese, V.; Bassotti, G.; Binda, G.A.; Casetti, T.; Colecchia, A.; Festi, D.; Fiocca, R.; et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United Eur. Gastroenterol. J. 2014, 2, 413–442. [Google Scholar] [CrossRef]
- Binda, G.A.; Cuomo, R.; Laghi, A.; Nascimbeni, R.; Serventi, A.; Bellini, D.; Gervaz, P.; Annibale, B. Practice parameters for the treatment of colonic diverticular disease: Italian Society of Colon and Rectal Surgery (SICCR) guidelines. Tech. Coloproctol. 2015, 19, 615–626. [Google Scholar] [CrossRef]
- Copaci, I.; Constantinescu, G.; Mihăilă, M.; Micu, L.; Franculescu-Bertea, A. Efficacy of Rifaximin-α vs Dietary Fiber on the evolution of uncomplicated colonic diverticular disease. Surg. Gastroenterol. Oncol. 2019, 24, 233–240. [Google Scholar] [CrossRef]
- Salem, T.A.; Molloy, R.G.; O’Dwyer, P.J. Prospective, Five-Year Follow-up Study of Patients with Symptomatic Uncomplicated Diverticular Disease. Dis. Colon Rectum 2007, 50, 1460–1464. [Google Scholar] [CrossRef]
- Papi, C.; Ciaco, A.; Koch, M.; Capurso, L. Efficacy of rifaximin on symptoms of uncomplicated diverticular disease of the colon. A pilot multicentre open trial. Diverticular Disease Study Group. Ital. J. Gastroent. 1992, 24, 452–546. Available online: https://pubmed.ncbi.nlm.nih.gov/1330083/ (accessed on 5 January 2023).
- Hall, J.F.; Roberts, P.L.; Ricciardi, R.; Read, T.; Scheirey, C.; Wald, C.; Marcello, P.W.; Schoetz, D.J. Long-Term Follow-up After an Initial Episode of Diverticulitis: What Are the Predictors of Recurrence? Dis. Colon Rectum 2011, 54, 283–288. [Google Scholar] [CrossRef]
- Festa, V.; Alegiani, S.S.; Chiesara, F.; Moretti, A.; Bianchi, M.; Dezi, A.; Traversa, G.; Koch, M. Retrospective comparison of long-term ten-day/month rifaximin or mesalazine in prevention of relapse in acute diverticulitis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1397–1404. [Google Scholar]
- Lanas, A.; Ponce, J.; Bignamini, A.; Mearin, F. One year intermittent rifaximin plus fibre supplementation vs. fibre supplementation alone to prevent diverticulitis recurrence: A proof-of-concept study. Dig. Liver Dis. 2013, 45, 104–109. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Daffinà, R. Long-term treatment with mesalazine and rifaximin versus rifaximin alone for patients with recurrent attacks of acute diverticulitis of colon. Dig. Liver Dis. 2002, 34, 510–515. [Google Scholar] [CrossRef]
- Stollman, N.; Smalley, W.; Hirano, I.; Adams, M.A.; Dorn, S.D.; Dudley-Brown, S.L.; Flamm, S.L.; Gellad, Z.F.; Gruss, C.B.; Kosinski, L.R.; et al. American Gastroenterological Association Institute Guideline on the Management of Acute Diverticulitis. Gastroenterology 2015, 149, 1944–1949. [Google Scholar] [CrossRef]
- Poola, S.; Ritchie, M. Antibiotics for uncomplicated diverticulitis. Am. Fam. Physician 2020, 11. Available online: https://www.aafp.org/pubs/afp/issues/2020/1201/od2.html (accessed on 5 January 2023).
- Morris, A.M.; Regenbogen, S.E.; Hardiman, K.M.; Hendren, S. Sigmoid diverticulitis: A systematic review. JAMA 2014, 311, 287–297. [Google Scholar] [CrossRef]
- Ünlü, Ç.; Daniels, L.; Vrouenraets, B.C.; Boermeester, M.A. Systematic review of medical therapy to prevent recurrent diverticulitis. Int. J. Colorectal. Dis. 2012, 27, 1131–1136. [Google Scholar] [CrossRef]
- Chabok, A.; Påhlman, L.; Hjern, F.; Haapaniemi, S.; Smedh, K.; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br. J. Surg. 2012, 99, 532–539. [Google Scholar] [CrossRef]
- Daniels, L.; Ünlü, Ç.; de Korte, N.; van Dieren, S.; Stockmann, H.B.; Vrouenraets, B.C.; Consten, E.C.; van der Hoeven, J.A.; Eijsbouts, Q.A.; Faneyte, I.F.; et al. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br. J. Surg. 2017, 104, 52–61. [Google Scholar] [CrossRef]
| Diverticular Disease Severity | Recommendation | Therapeutic Regimen |
|---|---|---|
| Diverticulosis | Absence of supporting strong evidence | None |
| Symptomatic uncomplicated diverticular disease | Yes, in addition to fiber supplementation (e.g., glucomannan 2–4 g/day) | 400 mg twice daily for 12–24 months, administered cyclically for 7–10 days each month. |
| Acute diverticulitis (primary prophylaxis) | Yes, in addition to fiber supplementation (e.g., glucomannan 2–4 g/day) | 400 mg twice daily for 12–24 months, administered cyclically for 7–10 days each month. |
| Acute diverticulitis (secondary prophylaxis) | Yes, absence of supporting strong evidence for fiber supplementation. | 400 mg twice daily, administered cyclically for 7–10 days each month. |
| Uncomplicated acute diverticulitis | Absence of supporting strong evidence | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccin, A.; Gulotta, M.; di Bella, S.; Martingano, P.; Crocè, L.S.; Giuffrè, M. Diverticular Disease and Rifaximin: An Evidence-Based Review. Antibiotics 2023, 12, 443. https://doi.org/10.3390/antibiotics12030443
Piccin A, Gulotta M, di Bella S, Martingano P, Crocè LS, Giuffrè M. Diverticular Disease and Rifaximin: An Evidence-Based Review. Antibiotics. 2023; 12(3):443. https://doi.org/10.3390/antibiotics12030443
Chicago/Turabian StylePiccin, Anna, Marco Gulotta, Stefano di Bella, Paola Martingano, Lory Saveria Crocè, and Mauro Giuffrè. 2023. "Diverticular Disease and Rifaximin: An Evidence-Based Review" Antibiotics 12, no. 3: 443. https://doi.org/10.3390/antibiotics12030443
APA StylePiccin, A., Gulotta, M., di Bella, S., Martingano, P., Crocè, L. S., & Giuffrè, M. (2023). Diverticular Disease and Rifaximin: An Evidence-Based Review. Antibiotics, 12(3), 443. https://doi.org/10.3390/antibiotics12030443







