Effects of Clostridium butyricum as an Antibiotic Alternative on Growth Performance, Intestinal Morphology, Serum Biochemical Response, and Immunity of Broilers
Abstract
1. Introduction
2. Results
2.1. Growth Performance
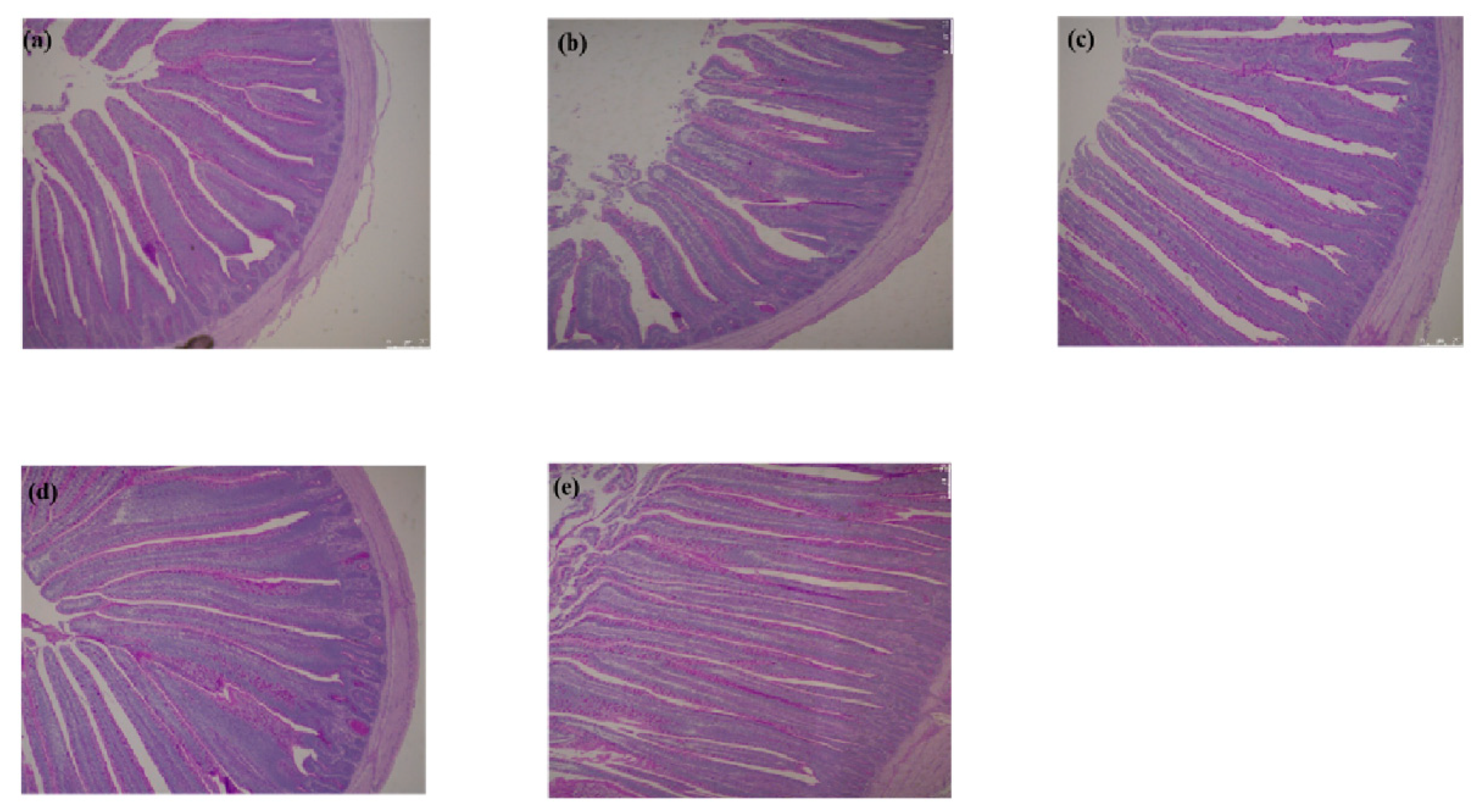
2.2. Intestinal Morphology
2.3. Biochemical Indices in Serum
2.4. Immunity
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Culture Conditions, and Preparation
4.2. Experimental Design, Animals, and Housing
4.3. Growth Performance
4.4. Sample Collection
4.5. Intestinal Morphology Analysis
4.6. Immunological and Biochemical Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hardy, B. The issue of antibiotic use in the livestock industry: What have we learned? Anim. Biotechnol. 2002, 13, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2014, 8, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.S.; Finch, R.; Wegener, H.C.; Bywater, R.; Walters, J.; Lipsitch, M. Antibiotic resistance—The interplay between antibiotic use in animals and human beings. Lancet Infect. Dis. 2003, 3, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Cogliani, C.; Goossens, H.; Greko, C. Restricting Antimicrobial Use in Food Animals: Lessons from Europe. Microbe 2011, 6, 274–279. [Google Scholar] [CrossRef]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef]
- Lee, K.W.; Lillehoj, H.S. An update on direct-fed microbials in broiler chickens in post-antibiotic era. Anim. Prod. Sci. 2017, 57, 1575–1581. [Google Scholar] [CrossRef]
- Wang, K.; Cao, G.; Zhang, H.; Li, Q.; Yang, C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef]
- Dong, Y.; Li, R.; Liu, Y.; Ma, L.; Zha, J.; Qiao, X.; Chai, T.; Wu, B. Benefit of Dietary Supplementation with Bacillus subtilis BYS2 on Growth Performance, Immune Response, and Disease Resistance of Broilers. Probiotics Antimicrob. Proteins 2020, 12, 1385–1397. [Google Scholar] [CrossRef]
- Liang, J.; Raza, S.H.A.; Kou, S.S.; Chen, C.; Yao, M.; Wu, Y.Y.; Wang, S.H.; Ma, X.; Zhang, W.J.; Nie, C.X. Effect of Clostridium butyricum on Plasma Immune Function, Antioxidant Activity and Metabolomics of Weaned Piglets. Livest. Sci. 2020, 241, 104267. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Honma, K.; Oshima, K.; Takami, S.; Goda, T. Regulation of hepatic genes related to lipid metabolism and antioxidant enzymes by sodium butyrate supplementation. Metabol. Open 2020, 7, 100043. [Google Scholar] [CrossRef]
- Wang, T.; Fu, J.; Xiao, X.; Lu, Z.; Wang, F.; Jin, M.; Wang, Y.; Zong, X. CBP22, a Novel Bacteriocin Isolated from Clostridium butyricum ZJU-F1, Protects against LPS-Induced Intestinal Injury through Maintaining the Tight Junction Complex. Mediat. Inflamm. 2021, 2021, 8032125. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, B.; Wang, L.; Sun, Q.; Deng, W.; Wei, F.; Ma, H.; Fu, C.; Wang, G.; Li, S. Effects of Clostridium butyricum on Growth Performance, Gut Microbiota and Intestinal Barrier Function of Broilers. Front. Microbiol. 2021, 12, 777456. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Wang, J.; Zhang, H.J.; Wu, S.G.; Qi, G.H. Supplemental Clostridium butyricum Modulates Lipid Metabolism Through Shaping Gut Microbiota and Bile Acid Profile of Aged Laying Hens. Front. Microbiol. 2020, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.; Yang, C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Yang, C.M.; Cao, G.T.; Ferket, P.R.; Liu, T.T.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Chen, A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012, 91, 2121–2129. [Google Scholar] [CrossRef]
- Liao, X.D.; Ma, G.; Cai, J.; Fu, Y.; Yan, X.Y.; Wei, X.B.; Zhang, R.J. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 2015, 94, 662–667. [Google Scholar] [CrossRef]
- Krysiak, K.; Konkol, D.; Korczynski, M. Overview of the Use of Probiotics in Poultry Production. Animals 2021, 11, 1620. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef]
- Zhang, J.; Su, H.; Li, Q.; Wu, H.; Liu, M.; Huang, J.; Zeng, M.; Zheng, Y.; Sun, X. Oral administration of Clostridium butyricum CGMCC0313-1 inhibits beta-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut Pathog. 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.X.; Qi, L.L.; Wu, T.X.; Xia, T.T.; Wang, J.B. Immunomodulatory effects of Clostridium butyricumon human enterocyte-like HT-29 cells. Anim. Cells Syst. 2013, 17, 121–126. [Google Scholar]
- Xu, X.; Yang, S.L.; Olajide, J.S.; Qu, Z.G.; Gong, Z.X.; Wang, J.; Zhang, Y.B.; Wang, H.; Xiong, L.; Zhang, K.; et al. Clostridium butyricum Supplement can Ameliorate the Intestinal Barrier Roles in Broiler Chickens Experimentally Infected with Clostridium perfringens. Front. Physiol. 2021, 12, 737481. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Du, M.S.; Wang, X.B.; Wang, J.Y.; Li, J.Z.; Jiang, X.H.; Zhang, R.J.; Si, D.Y. Effects of Dietary Clostridium butyricum on Carcass Traits, Antioxidant Capacity, Meat Quality, and Fatty Acid Composition of Broilers. Agriculture 2022, 12, 1607. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, X.; Yang, Z.; Shen, Y.; Zhang, S.; Shi, S. Effects of Clostridium butyricum on breast muscle lipid metabolism of broilers. Ital. J. Anim. Sci. 2018, 17, 1010–1020. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Song, D.; Lu, Z.; Dong, Z.; Miao, H.; Wang, W.; He, J.; Li, A. Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food Agric. Immunol. 2018, 29, 797–807. [Google Scholar] [CrossRef]
- Xiao, X.; Fu, Z.; Li, N.; Yang, H.; Wang, W.; Lyu, W. Modulation of the Intestinal Microbiota by the Early Intervention with Clostridium Butyricum in Muscovy Ducks. Antibiotics 2021, 10, 826. [Google Scholar] [CrossRef]
- Han, Y.; Tang, C.; Li, Y.; Yu, Y.; Zhan, T.; Zhao, Q.; Zhang, J. Effects of Dietary Supplementation with Clostridium butyricum on Growth Performance, Serum Immunity, Intestinal Morphology, and Microbiota as an Antibiotic Alternative in Weaned Piglets. Animals 2020, 10, 2287. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Shang, Z.; Qiao, J. Clostridium butyricum Helps to Alleviate Inflammation in Weaned Piglets Challenged with Enterotoxigenic Escherichia coli K88. Front. Vet. Sci. 2021, 8, 683863. [Google Scholar] [CrossRef]
- Lu, J.; Yao, J.; Xu, Q.; Zheng, Y.; Dong, X. Clostridium butyricum relieves diarrhea by enhancing digestive function, maintaining intestinal barrier integrity, and relieving intestinal inflammation in weaned piglets. Livest. Sci. 2020, 239, 104112. [Google Scholar] [CrossRef]
- Mohamed, T.M.; Sun, W.; Bumbie, G.Z.; Dosoky, W.M.; Rao, Z.; Hu, P.; Wu, L.; Tang, Z. Effect of Dietary Supplementation of Bacillus subtilis on Growth Performance, Organ Weight, Digestive Enzyme Activities, and Serum Biochemical Indices in Broiler. Animals 2022, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ni, X.; Qing, X.; Zeng, D.; Luo, M.; Liu, L.; Li, G.; Pan, K.; Jing, B. Live Probiotic Lactobacillus johnsonii BS15 Promotes Growth Performance and Lowers Fat Deposition by Improving Lipid Metabolism, Intestinal Development, and Gut Microflora in Broilers. Front. Microbiol. 2017, 8, 1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, L.; Zhou, Y.; Tang, L.; Zeng, Z.; Wang, Q.; Zou, P.; Yu, D.; Li, W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021, 7, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Niu, J.; Liu, H.; Jiao, N.; Huang, L.; Jiang, S.; Yan, L.; Yang, W. Effects of Dietary Macleaya cordata Extract Containing Isoquinoline Alkaloids Supplementation as an Alternative to Antibiotics in the Diets on Growth Performance and Liver Health of Broiler Chickens. Front. Vet. Sci. 2022, 9, 950174. [Google Scholar] [CrossRef]
- Song, D.; Li, A.; Wang, Y.; Song, G.; Cheng, J.; Wang, L.; Liu, K.; Min, Y.; Wang, W. Effects of synbiotic on growth, digestibility, immune and antioxidant performance in broilers. Animal 2022, 16, 100497. [Google Scholar] [CrossRef]
- Wan, F.; Deng, F.L.; Chen, L.; Zhong, R.Q.; Wang, M.Y.; Yi, B.; Liu, L.; Zhao, H.B.; Zhang, H.F. Long-term chemically protected sodium butyrate supplementation in broilers as an antibiotic alternative to dynamically modulate gut microbiota. Poult. Sci. 2022, 101, 102221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; An, K.; Gong, X.; Xia, Z. Effect of dietary Clostridium butyricum supplementation on growth performance, intestinal barrier function, immune function, and microbiota diversity of Pekin ducks. Animals 2021, 11, 2514. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Q.; Zhang, H.R.; Wu, Y.P.; Zhang, R.Q.; Yue, M.; Yang, C.M.; Cao, G.T. Clostridium butyricum alone or combined with 1, 25-dihydroxyvitamin D3 improved early-stage broiler health by modulating intestinal flora. J. Appl. Microbiol. 2021, 132, 155–166. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A.; Cocolin, L.; et al. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet. Res. 2018, 14, 383. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, C.; Zhang, H.; Lai, W.; Wei, H.; Peng, J. Effects of Different Probiotics on Laying Performance, Egg Quality, Oxidative Status, and Gut Health in Laying Hens. Animals 2019, 9, 1110. [Google Scholar] [CrossRef]
- Zeng, X.; Li, Q.; Yang, C.; Yu, Y.; Fu, Z.; Wang, H.; Fan, X.; Yue, M.; Xu, Y. Effects of Clostridium butyricum- and Bacillus spp.-Based Potential Probiotics on the Growth Performance, Intestinal Morphology, Immune Responses, and Caecal Microbiota in Broilers. Antibiotics 2021, 10, 624. [Google Scholar] [CrossRef]
- Casas, G.A.; Blavi, L.; Cross, T.L.; Lee, A.H.; Swanson, K.S.; Stein, H.H. Inclusion of the direct-fed microbial Clostridium butyricum in diets for weanling pigs increases growth performance and tends to increase villus height and crypt depth, but does not change intestinal microbial abundance. J. Anim. Sci. 2020, 98, skz372. [Google Scholar] [CrossRef]
- Long, M.; Yang, S.; Li, P.; Song, X.; Pan, J.; He, J.; Zhang, Y.; Wu, R. Combined Use of C. butyricum Sx-01 and L. salivarius C-1-3 Improves Intestinal Health and Reduces the Amount of Lipids in Serum via Modulation of Gut Microbiota in Mice. Nutrients 2018, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Saarela, M.H. Safety aspects of next generation probiotics. Curr. Opin. Food Sci. 2019, 30, 8–13. [Google Scholar] [CrossRef]
- Xue, F.; Shi, L.; Li, Y.; Ni, A.; Ma, H.; Sun, Y.; Chen, J. Effects of replacing dietary Aureomycin with a combination of plant essential oils on production performance and gastrointestinal health of broilers. Poult. Sci. 2020, 99, 4521–4529. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Bohm, J. Effect of addition of a probiotic micro-organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. J. Anim. Physiol. Anim. Nutr. 2010, 94, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, B.; Zeng, Z.; Liu, R.; Tang, L.; Gong, L.; Li, W. Effects of probiotics Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 on intestinal barrier function, antioxidative capacity, apoptosis, immune response, and biochemical parameters in broilers. Poult. Sci. 2019, 98, 5028–5039. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, B.; Mei, X.; Xu, H.; Qin, Y.; Li, W.; Zhou, Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018, 89, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, J.; Liu, L.; Zhang, S. Effects of Clostridium butyricum and corn bran supplementation on growth performance, nutrient digestibility, faecal volatile fatty acids and microbiota in weaned pigs. J. Appl. Anim. Res. 2020, 48, 313–319. [Google Scholar] [CrossRef]
- Hussein, E.; Selim, S. Efficacy of yeast and multi-strain probiotic alone or in combination on growth performance, carcass traits, blood biochemical constituents, and meat quality of broiler chickens. Livest. Sci. 2018, 216, 153–159. [Google Scholar] [CrossRef]
- Ahmat, M.; Cheng, J.; Abbas, Z.; Cheng, Q.; Fan, Z.; Ahmad, B.; Hou, M.; Osman, G.; Guo, H.; Wang, J.; et al. Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens. Antibiotics 2021, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Alkhalf, A.; Alhaj, M.; Al-Homidan, I. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi. J. Biol. Sci. 2010, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed to Salmonella typhimurium challenge during the starter phase. J. Appl. Anim. Res. 2016, 45, 538–542. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Meimandipour, A.; Alami, F.; Mahdavi, A.; Mohiti-Asli, M.; Lotfollahian, H.; Cross, D. Effects of Ground Thyme and Probiotic Supplements in Diets on Broiler Performance, Blood Biochemistry and Immunological Response to Sheep Red Blood Cells. Ital. Anim. Sci. 2016, 12, e19. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Zhang, H.; Xie, Y.; Xiong, L.; Liu, H.; Wang, F. Effects of a probiotic on the growth performance, intestinal flora, and immune function of chicks infected with Salmonella pullorum. Poult. Sci. 2020, 99, 5316–5323. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Jia, H.; Zhu, Z.; Li, H.; Ma, Y. Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens. Open Life. Sci. 2021, 16, 311–322. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.P.; Yan, L.; Huang, Y.Q. Evaluation of probiotics in diets with different nutrient densities on growth performance, blood characteristics, relative organ weight and breast meat characteristics in broilers. Br. Poult. Sci. 2013, 54, 635–641. [Google Scholar] [CrossRef]
- Zhan, H.Q.; Dong, X.Y.; Li, L.L.; Zheng, Y.X.; Gong, Y.J.; Zou, X.T. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 2019, 98, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, C.; Zhou, Y. Dietary synbiotic incorporation as an alternative to antibiotic improves growth performance, intestinal morphology, immunity and antioxidant capacity of broilers. J. Sci. Food Agric. 2018, 98, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.R.; Gong, J.; Gyles, C.L.; Hayes, M.A.; Zhou, H.; Sanei, B.; Chambers, J.R.; Sharif, S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006, 13, 975–980. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Qing, X.; Liu, L.; Xin, J.; Luo, M.; Khalique, A.; Dan, Y.; Pan, K.; Jing, B.; et al. Probiotic Lactobacillus johnsonii BS15 Improves Blood Parameters Related to Immunity in Broilers Experimentally Infected with Subclinical Necrotic Enteritis. Front. Microbiol. 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Al-Homidan, I.; Al-Dokhail, A.; Ebeid, T.; Abou-Emera, O.; Alsagan, A. Effects of dietary probiotic (Bacillus subtilis) supplementation on productive performance, immune response and egg quality characteristics in laying hens under high ambient temperature. Ital. J. Anim. Sci. 2018, 17, 804–814. [Google Scholar] [CrossRef]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
| Item | Treatment 2 | SEM 3 | p-Value | Response to CB | |||||
|---|---|---|---|---|---|---|---|---|---|
| CON | AM | CBL | CBM | CBH | Linear | Quadratic | |||
| Initial BW (1 day, kg) | 47.67 | 47.35 | 47.53 | 47.27 | 47.77 | 0.089 | 0.369 | 0.973 | 0.161 |
| Final BW (39 day, kg) | 1620.17 b | 1662.37 ab | 1667.30 ab | 1733.02 a | 1693.98 ab | 12.157 | 0.038 | 0.005 | 0.047 |
| 1 to 21 days | |||||||||
| ADG (g/day) | 23.79 | 23.53 | 24.01 | 23.90 | 24.33 | 0.226 | 0.873 | 0.545 | 0.848 |
| ADFI (g/day) | 39.15 | 36.43 | 39.68 | 39.45 | 40.73 | 0.492 | 0.063 | 0.370 | 0.736 |
| FCR (g/g) | 1.65 | 1.55 | 1.65 | 1.65 | 1.68 | 0.018 | 0.218 | 0.648 | 0.755 |
| 22 to 39 days | |||||||||
| ADG (g/day) | 59.61 b | 62.27 ab | 61.97 ab | 65.78 a | 63.08 ab | 0.660 | 0.045 | 0.014 | 0.044 |
| ADFI (g/day) | 107.34 | 111.78 | 110.97 | 111.98 | 111.20 | 1.559 | 0.897 | 0.402 | 0.510 |
| FCR (g/g) | 1.80 | 1.79 | 1.79 | 1.70 | 1.76 | 0.017 | 0.402 | 0.231 | 0.351 |
| 1 to 39 days | |||||||||
| ADG (g/day) | 40.32 b | 41.41 ab | 41.53 ab | 43.23 a | 42.21 ab | 0.312 | 0.037 | 0.005 | 0.045 |
| ADFI (g/day) | 74.68 | 73.27 | 75.66 | 78.06 | 77.65 | 1.125 | 0.663 | 0.359 | 0.799 |
| FCR (g/g) | 1.84 | 1.77 | 1.82 | 1.80 | 1.84 | 0.022 | 0.819 | 0.868 | 0.583 |
| Item | Treatment 2 | SEM 3 | p Value | Response to CB | |||||
|---|---|---|---|---|---|---|---|---|---|
| CON | AM | CBL | CBM | CBH | Linear | Quadratic | |||
| 21 days | |||||||||
| Villus height (μm) | 1067.87 | 1201.50 | 1215.06 | 1361.43 | 1339.02 | 44.471 | 0.225 | 0.050 | 0.420 |
| Crypt depth (μm) | 188.15 ab | 210.80 a | 117.94 c | 151.12 bc | 151.61 bc | 7.406 | <0.001 | 0.100 | 0.002 |
| V/C (μm/μm) | 5.76 b | 5.80 b | 10.30 a | 8.98 a | 9.01 a | 0.430 | <0.001 | 0.010 | 0.003 |
| 39 days | |||||||||
| Villus height (μm) | 1070.10 b | 1182.72 b | 1367.54 b | 1335.96 b | 1689.48 a | 50.000 | <0.001 | <0.001 | 0.739 |
| Crypt depth (μm) | 165.52 abc | 183.78 ab | 146.12 bc | 138.52 c | 204.65 a | 6.258 | <0.001 | 0.025 | <0.001 |
| V/C (μm/μm) | 6.50 b | 6.55 b | 9.37 a | 9.77 a | 8.27 a | 0.304 | <0.001 | 0.004 | <0.001 |
| Item | Treatment 2 | SEM 3 | p Value | Response to CB | |||||
|---|---|---|---|---|---|---|---|---|---|
| CON | AM | CBL | CBM | CBH | Linear | Quadratic | |||
| 21 days | |||||||||
| GLU (mmol/L) | 10.67 | 10.98 | 10.50 | 10.50 | 9.45 | 0.175 | 0.055 | 0.041 | 0.255 |
| TC (mmol/L) | 3.34 | 4.12 | 3.53 | 3.24 | 3.63 | 0.120 | 0.156 | 0.658 | 0.725 |
| TG (mmol/L) | 0.32 ab | 0.39 a | 0.30 b | 0.35 ab | 0.35 ab | 0.010 | 0.048 | 0.174 | 0.737 |
| TP (g/L) | 26.50 | 23.12 | 24.80 | 26.08 | 24.63 | 0.565 | 0.361 | 0.470 | 0.925 |
| ALB (g/L) | 12.00 | 10.65 | 12.28 | 12.15 | 11.33 | 0.257 | 0.229 | 0.418 | 0.351 |
| UREA (mmol/L) | 0.53 ab | 0.59 a | 0.41 b | 0.49 ab | 0.43 b | 0.020 | 0.011 | 0.303 | 0.440 |
| CREA (μmol/L) | 9.05 b | 11.92 a | 11.15 ab | 11.03 ab | 10.45 ab | 0.297 | 0.022 | 0.142 | 0.036 |
| 39 days | |||||||||
| GLU (mmol/L) | 10.50 | 11.09 | 10.83 | 10.86 | 9.59 | 0.182 | 0.066 | 0.107 | 0.036 |
| TC (mmol/L) | 3.57 ab | 2.95 b | 3.32 ab | 4.24 a | 3.43 ab | 0.122 | 0.008 | 0.607 | 0.223 |
| TG (mmol/L) | 0.37 | 0.45 | 0.42 | 0.38 | 0.39 | 0.014 | 0.401 | 0.814 | 0.471 |
| TP (g/L) | 31.85 | 27.15 | 35.73 | 32.05 | 32.87 | 0.966 | 0.071 | 0.948 | 0.486 |
| ALB (g/L) | 11.38 a | 9.48 b | 12.38 a | 11.73 a | 11.83 a | 0.252 | 0.001 | 0.721 | 0.311 |
| UREA (mmol/L) | 0.44 | 0.59 | 0.55 | 0.42 | 0.47 | 0.022 | 0.069 | 0.798 | 0.523 |
| CREA (μmol/L) | 9.75 | 11.43 | 12.17 | 10.57 | 10.35 | 0.288 | 0.055 | 0.935 | 0.025 |
| Item | Treatment 1 | SEM 2 | p-Value | Response to CB | |||||
|---|---|---|---|---|---|---|---|---|---|
| CON | AM | CBL | CBM | CBH | Linear | Quadratic | |||
| 21 days | |||||||||
| Thymus (g/kg) | 2.41 b | 2.75 ab | 2.37 b | 3.22 a | 3.23 a | 0.112 | 0.012 | 0.003 | 0.928 |
| Spleen (g/kg) | 1.00 | 1.26 | 1.06 | 1.07 | 1.08 | 0.034 | 0.139 | 0.441 | 0.761 |
| Bursa of Fabricius (g/kg) | 2.50 | 2.67 | 2.39 | 2.33 | 2.57 | 0.067 | 0.532 | 0.882 | 0.277 |
| 39 days | |||||||||
| Thymus (g/kg) | 2.17 b | 2.55 ab | 2.17 b | 2.61 ab | 3.10 a | 0.099 | 0.008 | 0.001 | 0.197 |
| Spleen (g/kg) | 2.19 | 1.96 | 1.90 | 1.82 | 2.34 | 0.073 | 0.124 | 0.631 | 0.023 |
| Bursa of Fabricius (g/kg) | 1.11 ab | 1.31 ab | 1.02 b | 1.05 b | 1.40 a | 0.051 | 0.048 | 0.072 | 0.049 |
| Item | Treatment 1 | SEM 2 | p Value | Response to CB | |||||
|---|---|---|---|---|---|---|---|---|---|
| CON | AM | CBL | CBM | CBH | Linear | Quadratic | |||
| 21 days | |||||||||
| IgA | 1.02 | 0.89 | 0.94 | 0.99 | 0.99 | 0.026 | 0.619 | 0.882 | 0.610 |
| IgG | 6.61 ab | 5.91 b | 7.27 a | 6.91 ab | 7.65 a | 0.172 | 0.009 | 0.089 | 0.911 |
| IgM | 0.90 | 0.76 | 0.90 | 0.99 | 0.95 | 0.027 | 0.069 | 0.360 | 0.803 |
| 39 days | |||||||||
| IgA | 1.25 a | 0.86 b | 1.05 ab | 1.09 ab | 0.96 ab | 0.040 | 0.019 | 0. 035 | 0.621 |
| IgG | 7.65 ab | 6.42 b | 7.63 ab | 8.34 a | 7.54 ab | 0.186 | 0.015 | 0.808 | 0.303 |
| IgM | 0.95 | 0.85 | 0.98 | 0.86 | 0.82 | 0.022 | 0.091 | 0.031 | 0.462 |
| Item (%) | Starter (Day 1–21) | Finisher (Day 22–39) |
|---|---|---|
| Ingredients | ||
| Corn | 54.70 | 56.70 |
| High protein soybean meal | 34.70 | 25.90 |
| CaHPO4 | 1.50 | 1.20 |
| Limestone | 1.20 | 1.20 |
| Soybean oil | 0.90 | 3.50 |
| Wheat | 5.00 | 7.50 |
| Chicken bone meal | 0.00 | 2.00 |
| Premix 1 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 |
| Chemical composition analyzed 2 | ||
| ME, Mcal/kg | 2.95 | 3.10 |
| Dry matter | 87.35 | 87.70 |
| Crude protein | 22.00 | 20.50 |
| Calcium | 0.90 | 0.87 |
| Total phosphorus | 0.61 | 0.57 |
| Non-phytate phosphorus | 0.45 | 0.45 |
| Lysine | 1.37 | 1.20 |
| Methionine | 0.67 | 0.60 |
| Methionine + Cysteine | 0.96 | 0.82 |
| Threonine | 0.95 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Du, M.; Zhang, J.; Ahmad, B.; Cheng, Q.; Wang, X.; Abbas, Z.; Tong, Y.; Li, J.; Zhou, Y.; et al. Effects of Clostridium butyricum as an Antibiotic Alternative on Growth Performance, Intestinal Morphology, Serum Biochemical Response, and Immunity of Broilers. Antibiotics 2023, 12, 433. https://doi.org/10.3390/antibiotics12030433
Yang T, Du M, Zhang J, Ahmad B, Cheng Q, Wang X, Abbas Z, Tong Y, Li J, Zhou Y, et al. Effects of Clostridium butyricum as an Antibiotic Alternative on Growth Performance, Intestinal Morphology, Serum Biochemical Response, and Immunity of Broilers. Antibiotics. 2023; 12(3):433. https://doi.org/10.3390/antibiotics12030433
Chicago/Turabian StyleYang, Tiantian, Mengsi Du, Jing Zhang, Baseer Ahmad, Qiang Cheng, Xiaobing Wang, Zaheer Abbas, Yucui Tong, Jinzhuan Li, Yichen Zhou, and et al. 2023. "Effects of Clostridium butyricum as an Antibiotic Alternative on Growth Performance, Intestinal Morphology, Serum Biochemical Response, and Immunity of Broilers" Antibiotics 12, no. 3: 433. https://doi.org/10.3390/antibiotics12030433
APA StyleYang, T., Du, M., Zhang, J., Ahmad, B., Cheng, Q., Wang, X., Abbas, Z., Tong, Y., Li, J., Zhou, Y., Zhang, R., & Si, D. (2023). Effects of Clostridium butyricum as an Antibiotic Alternative on Growth Performance, Intestinal Morphology, Serum Biochemical Response, and Immunity of Broilers. Antibiotics, 12(3), 433. https://doi.org/10.3390/antibiotics12030433







