Impact of Bacterial Infections on COVID-19 Patients: Is Timing Important?
Abstract
:1. Introduction
2. Methods
2.1. Setting
2.2. Participants
2.3. Treatment
2.4. Definitions
2.5. Data Extraction
2.6. Statistical Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Marzio, M.A.L.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2022, 15, e0241955, Erratum in PLoS ONE 2022, 17, e0269291. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; Merchant, H.A.; Zaidi, S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Lyngdoh, T.; Kakkar, A.K. Deciphering the COVID-19 cytokine storm: Systematic review and meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13429. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; An, M.H.; Kim, W.J.; Hwang, T.-H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 2020, 17, e1003501. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.J.; Shiau, S.; Brunetti, L.; Xie, Y.; Solanki, K.; Khalid, S.; Mohayya, S.; Au, P.H.; Pham, C.; Uprety, P.; et al. Risk Factors and Outcomes of Hospitalized Patients With Severe Coronavirus Disease 2019 (COVID-19) and Secondary Bloodstream Infections: A Multicenter Case-Control Study. Clin. Infect. Dis. 2021, 72, e995–e1003. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Alamer, E.; Mir, M.; Alasmari, A.; Alshahrani, M.M.; Asiri, M.; Ahmad, I.; Alhazmi, A.; Algaissi, A. Bacterial Coinfections Increase Mortality of Severely Ill COVID-19 Patients in Saudi Arabia. Int. J. Environ. Res. Public Heal. 2022, 19, 2424. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Herkel, T.; Uvizl, R.; Doubravská, L.; Adamus, M.; Gabrhelik, T.; Sedlakova, M.H.; Kolar, M.; Hanulik, V.; Pudova, V.; Langova, K.; et al. Epidemiology of hospital-acquired pneumonia: Results of a Central European multicenter, prospective, observational study compared with data from the European region. Biomed. Pap. 2016, 160, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Malhotra, P.; Izard, S.; Kim, A.; Oppenheim, M.; Gautam-Goyal, P.; Chen, T.; Doan, T.-L.; Berlinrut, I.; Niknam, N.; et al. Hospital-Acquired Bloodstream Infections in Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 Infection (Coronavirus Disease 2019): Association With Immunosuppressive Therapies. Open Forum Infect. Dis. 2021, 8, ofab339. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, V.; Lawrence, H.; Lansbury, L.E.; Webb, K.; Safavi, S.; Zainuddin, N.I.; Huq, T.; Eggleston, C.; Ellis, J.; Thakker, C.; et al. Co-infection in critically ill patients with COVID-19: An observational cohort study from England. J. Med. Microbiol. 2021, 70, 001350. [Google Scholar] [CrossRef] [PubMed]
- Fehér, .; Szarvas, Z.; Lehoczki, A.; Fekete, M.; Fazekas-Pongor, V. Co-infections in COVID-19 patients and correlation with mortality rate. Minireview. Physiol. Int. 2022, 109, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buehler, P.K.; Zinkernagel, A.S.; Hofmaenner, D.A.; Garcia, P.D.W.; Acevedo, C.T.; Gómez-Mejia, A.; Shambat, S.M.; Andreoni, F.; Maibach, M.A.; Bartussek, J.; et al. Bacterial pulmonary superinfections are associated with longer duration of ventilation in critically ill COVID-19 patients. Cell Rep. Med. 2021, 2, 100229. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 370, m3339, Erratum in BMJ 2020, 371, m4334. [Google Scholar] [CrossRef] [PubMed]
- Coșeriu, R.L.; Vintilă, C.; Mare, A.D.; Ciurea, C.N.; Togănel, R.O.; Cighir, A.; Simion, A.; Man, A. Epidemiology, Evolution of Antimicrobial Profile and Genomic Fingerprints of Pseudomonas aeruginosa before and during COVID-19: Transition from Resistance to Susceptibility. Life 2022, 12, 2049. [Google Scholar] [CrossRef] [PubMed]
- Adalbert, J.R.; Varshney, K.; Tobin, R.; Pajaro, R. Clinical outcomes in patients co-infected with COVID-19 and Staphylococcus aureus: A scoping review. BMC Infect. Dis. 2021, 21, 985. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, R.M.; Julien, D.A.; Jelinski, D.C.; Larose, S.L.; Rennert-May, E.; Conly, J.M.; Dingle, T.C.; Chen, J.Z.; Tyrrell, G.J.; Ronksley, P.E.; et al. Antimicrobial resistance (AMR) in COVID-19 patients: A systematic review and meta-analysis (November 2019–June 2021). Antimicrob. Resist. Infect. Control. 2022, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Cruz, M.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Marquez-Valdelamar, L.M.; Bravata-Alcantara, J.C.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Ibáñez-Cervantes, G.; Fernández-Sánchez, V.; Castro-Escarpulli, G.; et al. ESKAPE bacteria characterization reveals the presence of Acinetobacter baumannii and Pseudomonas aeruginosa outbreaks in COVID-19/VAP patients. Am. J. Infect. Control. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Saha, B.K.; Ramani, A.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Polly, M.; de Almeida, B.L.; Lennon, R.P.; Cortês, M.F.; Costa, S.F.; Guimarães, T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am. J. Infect. Control. 2021, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]

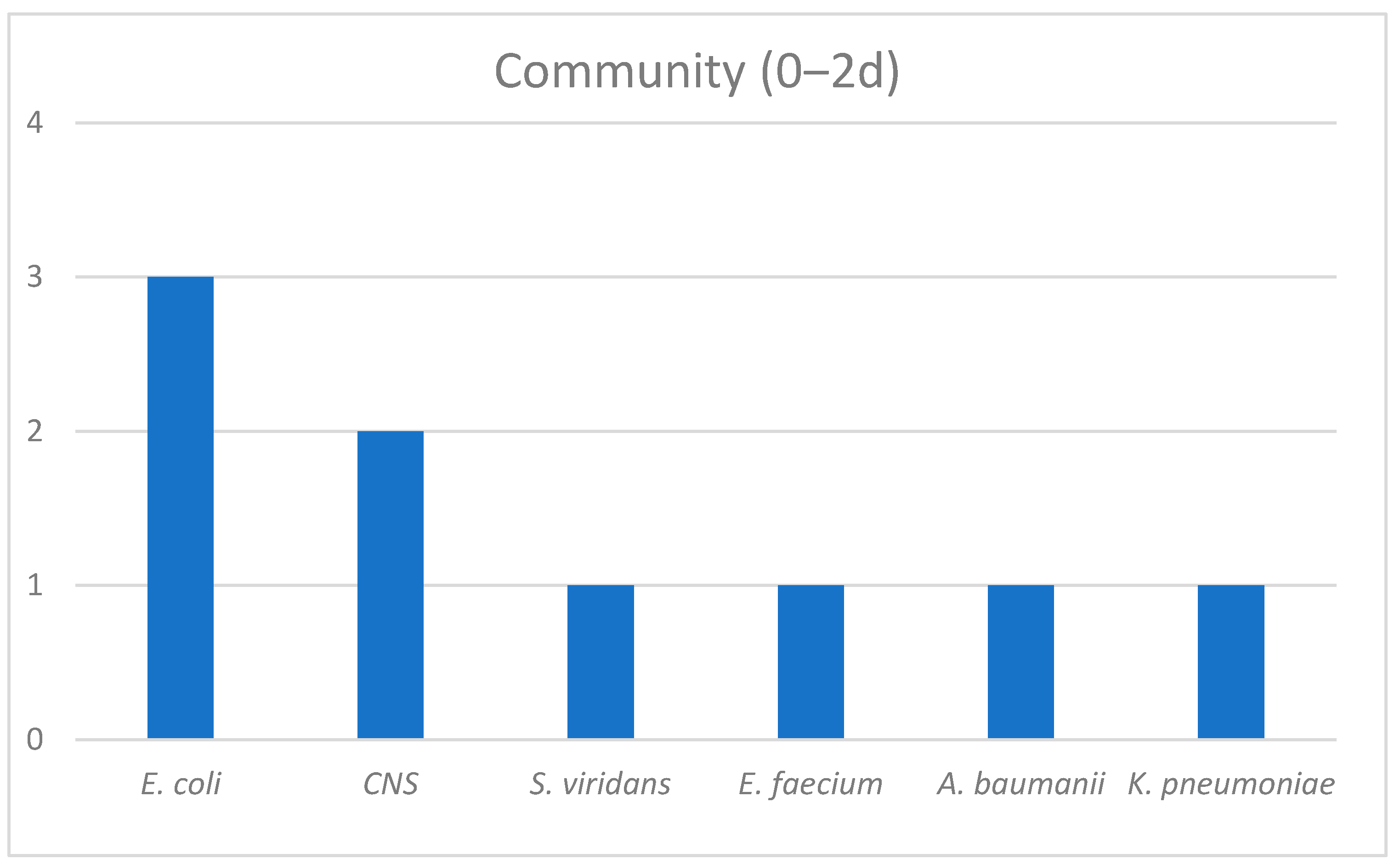
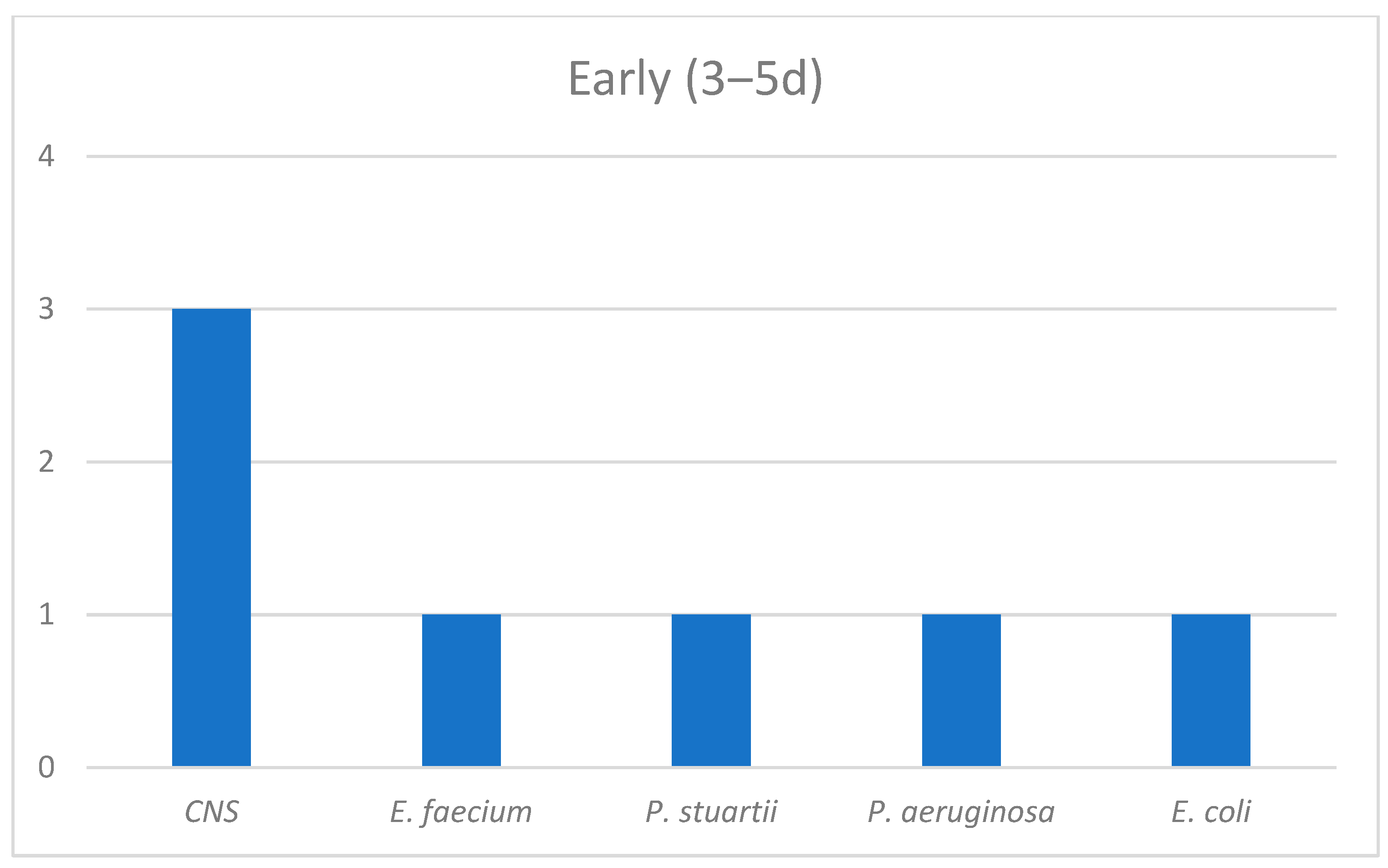
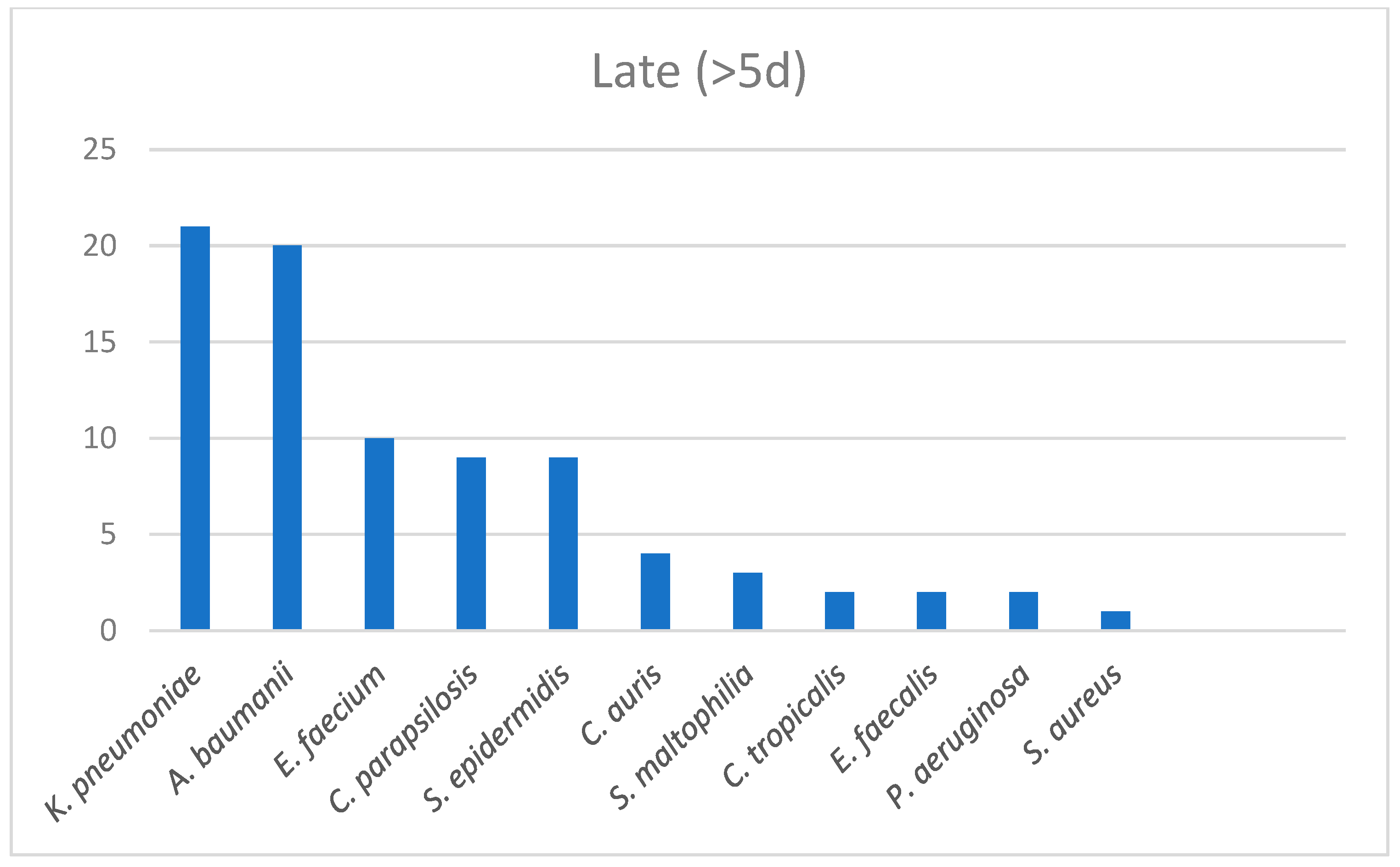
| Survivors (n = 124) | Non-Survivors (n = 53) | p-Value | |
|---|---|---|---|
| Age (Years) | 64.7 ± 32.2 | 75.2 ± 27.24 | <0.001 |
| Male (%) | 68 (54.8) | 29 (54.7) | 0.95 |
| Vaccinated (%) | 50 (40) | 13 (24.5) | 0.035 |
| 4C Mortality Score | 7.87 ± 8.4 | 13.1 ± 7.36 | <0.001 |
| PO2 (mmHg) | 74.1 (21.8) | 66.5 (45.5) | 0.021 |
| Lactate (mmol/L) | 1.0 (0.6) | 1.3 (1.08) | <0.001 |
| WBC (K/μL) | 6.05 (3.86) | 8.22 (6.34) | 0.005 |
| Neutrophils (K/μL) | 4.7 (4.2) | 6.95 (6.55) | 0.004 |
| Lymphocytes(K/μL) | 0.99 (0.75) | 0.66 (0.48) | <0.001 |
| Fibrinogen (mg/dL) | 543 (183.75) | 544 (178.5) | 0.96 |
| D-Dimers (μg/dL) | 0.835 (1.14) | 1.08 (1.41) | 0.024 |
| CRP (mg/dL) | 4.15 (8.09) | 9.36 (12.08) | <0.001 |
| HS-TnI (pg/mL) | 6.05 (12.35) | 22.85 (37.13) | <0.001 |
| Ferritin (ng/mL) | 386 (818.23) | 822 (1382.5) | <0.001 |
| Mortality % | Median Length of Hospital Stay (IQR) | |
|---|---|---|
| No Infection (n = 130) | 22.3% | 7 (6.25) |
| Community (n = 9) | 22.2% | 6 (9) |
| Early Infection (n = 6) | 33.3% | 14.5 (14.5) |
| Late Infection (n = 32) | 62.5% | 14 (21.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailides, C.; Paraskevas, T.; Karalis, I.; Koniari, I.; Pierrakos, C.; Karamouzos, V.; Marangos, M.; Velissaris, D. Impact of Bacterial Infections on COVID-19 Patients: Is Timing Important? Antibiotics 2023, 12, 379. https://doi.org/10.3390/antibiotics12020379
Michailides C, Paraskevas T, Karalis I, Koniari I, Pierrakos C, Karamouzos V, Marangos M, Velissaris D. Impact of Bacterial Infections on COVID-19 Patients: Is Timing Important? Antibiotics. 2023; 12(2):379. https://doi.org/10.3390/antibiotics12020379
Chicago/Turabian StyleMichailides, Christos, Themistoklis Paraskevas, Iosif Karalis, Ioanna Koniari, Charalampos Pierrakos, Vasilios Karamouzos, Markos Marangos, and Dimitrios Velissaris. 2023. "Impact of Bacterial Infections on COVID-19 Patients: Is Timing Important?" Antibiotics 12, no. 2: 379. https://doi.org/10.3390/antibiotics12020379
APA StyleMichailides, C., Paraskevas, T., Karalis, I., Koniari, I., Pierrakos, C., Karamouzos, V., Marangos, M., & Velissaris, D. (2023). Impact of Bacterial Infections on COVID-19 Patients: Is Timing Important? Antibiotics, 12(2), 379. https://doi.org/10.3390/antibiotics12020379





