Penetration through Outer Membrane and Efflux Potential in Pseudomonas aeruginosa of Bulgecin A as an Adjuvant to β-Lactam Antibiotics
Abstract
1. Introduction
2. Results and Discussion
3. Concluding Remarks
4. Materials and Methods
4.1. Bacteria, Media, Growth Conditions and Antibiotics
4.2. Antimicrobial Susceptibility and Potentiation Tests
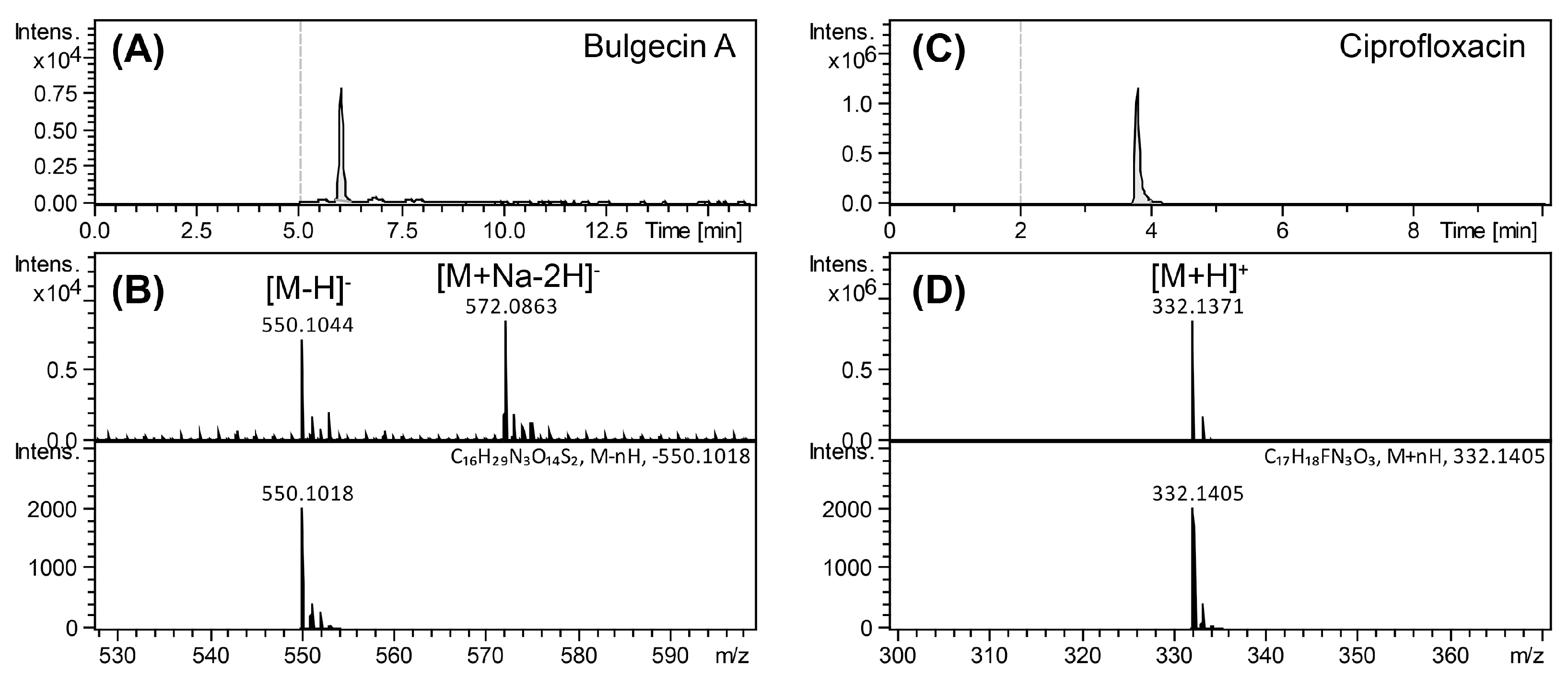
4.3. Accumulation of Bulgecin A
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Strain | MIC (µg/mL) | Strain | MIC (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CAZ | MEM | CAZ | MEM | ||||||
| Bulgecin A a | − | + | − | + | Bulgecin A a | − | + | − | + |
| MPAO1 b | 2 | 1 | 2 | 1 | PW1427 (opdF) | 2 | 1 | 1 | 1 |
| PW1778 (mexA) | 1 | 1 | 0.5 | 0.125 | PW1873 (oprM) | 0.5 | 0.5 | 0.25 | 0.25 |
| PW1781 (mexB) | 1 | 0.5 | 0.5 | 0.125 | PW2374 (opdH) | 2 | 1 | 1 | 1 |
| PW1265 (triA) | 2 | 1 | 1 | 0.5 | PW2855 (opdD) | 1 | 1 | 1 | 0.5 |
| PW1267 (triB) | 2 | 1 | 1 | 0.5 | PW3127 (oprH) | 2 | 1 | 1 | 1 |
| PW1271 (triC) | 2 | 1 | 1 | 0.5 | PW4134 (oprF) | 2 | 1 | 2 | 2 |
| PW3229 (PA1237) | 2 | 1 | 1 | 0.5 | PW4636 (opdO) | 2 | 1 | 1 | 2 |
| PW3231 (PA1238) | 2 | 1 | 1 | 0.5 | PW4767 (opdG) | 2 | 1 | 1 | 1 |
| PW3608 (mexM) | 2 | 1 | 1 | 0.5 | PW5076 (opdJ) | 2 | 1 | 1 | 0.5 |
| PW3611 (mexN) | 2 | 1 | 1 | 0.5 | PW5187 (oprN) | 2 | 1 | 2 | 1 |
| PW5222 (czcA) | 2 | 1 | 4 | 2 | PW5196 (opdT) | 2 | 1 | 1 | 0.5 |
| PW5224 (czcB) | 2 | 1 | 1 | 0.5 | PW5232 (opmB) | 2 | 1 | 1 | 0.5 |
| PW5226 (czcC) | 2 | 1 | 1 | 0.5 | PW5526 (opdB) | 2 | 1 | 1 | 0.5 |
| PW5233 (muxC) | 2 | 1 | 2 | 2 | PW5621 (oprQ) | 2 | 1 | 1 | 0.5 |
| PW5235 (muxB) | 2 | 1 | 1 | 0.5 | PW6095 (opdQ) | 2 | 1 | 1 | 0.5 |
| PW5237 (muxA) | 2 | 1 | 1 | 0.5 | PW6333 (oprB) | 2 | 1 | 1 | 0.5 |
| PW6963 (mexQ) | 2 | 1 | 1 | 0.5 | PW6504 (oprP) | 2 | 1 | 1 | 0.5 |
| PW6965 (mexP) | 2 | 1 | 1 | 0.5 | PW6506 (oprO) | 2 | 1 | 1 | 0.5 |
| PW7218 (mexK) | 2 | 1 | 2 | 2 | PW7089 (opdR) | 2 | 1 | 1 | 0.5 |
| PW7220 (mexJ) | 2 | 1 | 1 | 1 | PW7416 (oprC) | 2 | 1 | 1 | 0.5 |
| PW8135 (mexH) | 2 | 1 | 1 | 0.5 | PW7874 (oprG) | 2 | 1 | 1 | 0.5 |
| PW8137 (mexI) | 2 | 1 | 1 | 0.5 | PW8010 (opdL) | 2 | 1 | 1 | 0.5 |
| PW8385 (mexV) | 1 | 1 | 1 | 0.5 | PW8084 (opdN) | 2 | 1 | 1 | 0.5 |
| PW8390 (mexW) | 1 | 1 | 1 | 0.5 | PW8139 (opmD) | 2 | 1 | 2 | 0.5 |
| PW8750 (mexD) | 2 | 1 | 1 | 0.5 | PW8575 (opdP) | 2 | 1 | 1 | 0.5 |
| PW8751 (mexC) | 2 | 1 | 1 | 1 | PW8748 (oprJ) | 2 | 1 | 1 | 0.5 |
| PW9677 (PA5159) | 2 | 1 | 1 | 0.5 | PW9244 (opdK) | 2 | 1 | 1 | 0.5 |
| PW9679 (PA5160) | 1 | 1 | 1 | 0.5 | PW9369 (opmH) | 2 | 1 | 2 | 1 |
| PW1276 (opdC) | 2 | 1 | 2 | 1 | ΔoprD c | 2 | 1 | 2 | 2 |
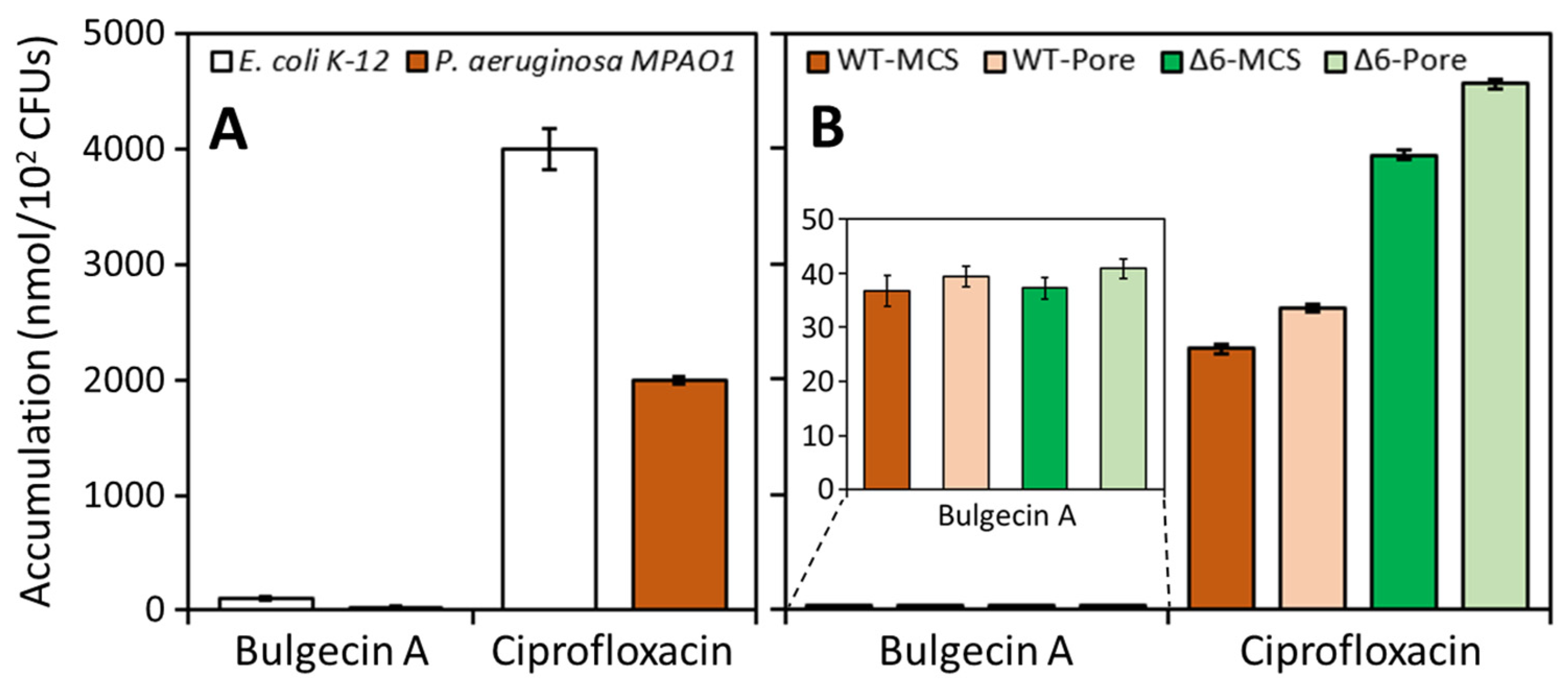
| Strain | Accumulation (nmol/1012 CFUs) a | |
|---|---|---|
| Bulgecin A | Ciprofloxacin b | |
| E. coli K-12 | 100 ± 17 | 4000 ± 180 |
| P. aeruginosa MPAO1 | 30 ± 3 | 2000 ± 32 |
| P. aeruginosa PAO1 | ||
| PAO-MCS | 37 ± 3 | 2300 ± 44 |
| PAO-Pore | 39 ± 2 | 2600 ± 35 |
| Δ6-MCS | 37 ± 2 | 4000 ± 33 |
| Δ6-Pore | 41 ± 2 | 4600 ± 38 |
References
- Imada, A.; Kintaka, K.; Nakao, M.; Shinagawa, S. Bulgecin, a bacterial metabolite which in concert with β-lactam antibiotics causes bulge formation. J. Antibiot. 1982, 35, 1400–1403. [Google Scholar] [CrossRef]
- Shinagawa, S.; Kasahara, F.; Wada, Y.; Harada, S.; Asai, M. Structures of bulgecins, bacterial metabolites with bulge-inducing activity. Tetrahedron 1984, 40, 3465–3470. [Google Scholar] [CrossRef]
- Dik, D.A.; Madukoma, C.S.; Tomoshige, S.; Kim, C.; Lastochkin, E.; Boggess, W.C.; Fisher, J.F.; Shrout, J.D.; Mobashery, S. Slt, MltD, and MltG of Pseudomonas aeruginosa as targets of bulgecin A in potentiation of β-lactam antibiotics. ACS Chem. Biol. 2019, 14, 296–303. [Google Scholar] [CrossRef]
- Tomoshige, S.; Dik, D.A.; Akabane-Nakata, M.; Madukoma, C.S.; Fisher, J.F.; Shrout, J.D.; Mobashery, S. Total syntheses of bulgecins A, B, and C and their bactericidal potentiation of the β-lactam antibiotics. ACS Infect. Dis. 2018, 4, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Johnson, N.V.; Kreamer, N.N.K.; Barnes, S.W.; Walker, J.R.; Woods, A.L.; Six, D.A.; Dean, C.R. Defects in efflux (oprM), β-lactamase (ampC), and lipopolysaccharide transport (lptE) genes mediate antibiotic hypersusceptibility of Pseudomonas aeruginosa strain Z61. Antimicrob. Agents Chemother. 2019, 63, e00784-19. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Ramage, E.; Jacobs, M.; Gallagher, L.; Manoil, C. Sequence-verified two-allele transposon mutant library for Pseudomonas aeruginosa PAO1. J. Bacteriol. 2012, 194, 6387–6389. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Ohya, S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1992, 36, 1847–1851. [Google Scholar] [CrossRef]
- Köhler, T.; Michea-Hamzehpour, M.; Epp, S.F.; Pechere, J.-C. Carbapenem activities against Pseudomonas aeruginosa: Respective contributions of OprD and efflux systems. Antimicrob. Agents Chemother. 1999, 43, 424–427. [Google Scholar] [CrossRef]
- Tam, V.H.; Schilling, A.N.; Neshat, S.; Poole, K.; Melnick, D.A.; Coyle, E.A. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 4920–4927. [Google Scholar] [CrossRef]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Rybenkov, V.V. Permeability barriers of Gram-negative pathogens. Ann. N. Y. Acad. Sci. 2020, 1459, 5–18. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic adjuvants: Rescuing antibiotics from resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef]
- González-Bello, C. Antibiotic adjuvants—A strategy to unlock bacterial resistance to antibiotics. Bioorg. Med. Chem. Lett. 2017, 27, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Hubble, V.B.; Hubbard, B.A.; Minrovic, B.M.; Melander, R.J.; Melander, C. Using small-molecule adjuvants to repurpose azithromycin for use against Pseudomonas aeruginosa. ACS Infect. Dis. 2018, 5, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Klobucar, K.; Brown, E.D. New potentiators of ineffective antibiotics: Targeting the Gram-negative outer membrane to overcome intrinsic resistance. Curr. Opin. Chem. Biol. 2022, 66, 102099. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Bös, C.; Braun, M.; Killmann, H. Outer membrane channels and active transporters for the uptake of antibiotics. J. Infect. Dis. 2001, 183, S12–S16. [Google Scholar] [CrossRef]
- Braun, V. FhuA (TonA), the career of a protein. J. Bacteriol. 2009, 191, 3431–3436. [Google Scholar] [CrossRef]
- Richter, M.F.; Drown, B.S.; Riley, A.P.; Garcia, A.; Shirai, T.; Svec, R.L.; Hergenrother, P.J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. [Google Scholar] [CrossRef]
- Cai, H.; Rose, K.; Liang, L.-H.; Dunham, S.; Stover, C. Development of a liquid chromatography/mass spectrometry-based drug accumulation assay in Pseudomonas aeruginosa. Anal. Biochem. 2009, 385, 321–325. [Google Scholar] [CrossRef]
- Iyer, R.; Ye, Z.; Ferrari, A.; Duncan, L.; Tanudra, M.A.; Tsao, H.; Wang, T.; Gao, H.; Brummel, C.L.; Erwin, A.L. Evaluating LC–MS/MS to measure accumulation of compounds within bacteria. ACS Infect. Dis. 2018, 4, 1336–1345. [Google Scholar] [CrossRef]
- Geddes, E.J.; Li, Z.; Hergenrother, P.J. An LC-MS/MS assay and complementary web-based tool to quantify and predict compound accumulation in E. coli. Nat. Protoc. 2021, 16, 4833–4854. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Standard/Guideline/Report/Supplement [M100]; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Choi, Y.; Park, J.S.; Kim, J.; Min, K.; Mahasenan, K.; Kim, C.; Yoon, H.-J.; Lim, S.; Cheon, D.H.; Lee, Y.; et al. Structure-based inhibitor design for reshaping bacterial morphology. Commun. Biol. 2022, 5, 395. [Google Scholar] [CrossRef] [PubMed]
| MIC (μg/mL) (Fold Change) a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic b | Bulgecin A | CAZ | MEM | AMP | CAR | FOX | |||||
| Bulgecin A c | − | + | − | + | − | + | − | + | − | + | |
| ATCC 27853 d | >256 | 2 | 0.5 (4) | 0.25 | 0.125 (2) | 2048 | 512 (4) | 64 | 32 (2) | 2048 | 1024 (2) |
| K799/WT | >256 | 1 | 0.5 (2) | 0.5 | 0.25 (2) | 2048 | 256 (8) | 128 | 32 (4) | 2048 | 512 (4) |
| K799/Z61 c | >256 | 0.5 | 0.25 (2) | 0.5 | 0.25 (2) | 0.25 | 0.06 (4) | 0.125 | 0.03 (4) | 0.5 | 0.25 (2) |
| MIC (µg/mL) (Fold Change) | ||||
|---|---|---|---|---|
| β-Lactam | CAZ | MEM | ||
| Bulgecin A a | − | + | − | + |
| PAO1 | 2 | 1 (2) | 1 | 0.5 (2) |
| Δ3-MCS b | 0.5 | 0.5 (1) | 0.125 | 0.06 (2) |
| Δ6-MCS b | 1 | 0.5 (2) | 0.25 | 0.125 (2) |
| MIC (μg/mL) (Fold Change) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β-Lactam | CAZ | MEM | AMP | CAR | FOX | |||||
| Bulgecin A b | − | + | − | + | − | + | − | + | − | + |
| P. aeruginosa PAO1 | ||||||||||
| PAO-MCS c | 2 | 1 (2) | 0.5 | 0.25 (2) | 2048 | 1024 (2) | 64 | 32 (2) | 2048 | 1024 (2) |
| PAO-Pore c | 0.06 | 0.03 (2) | 0.25 | 0.06 (4) | 128 | 64 (2) | 8 | 4 (2) | 256 | 64 (4) |
| Δ6-MCS c | 1 | 0.5 (2) | 0.25 | 0.125 (2) | 512 | 256 (2) | 2 | 1 (2) | 1024 | 512 (2) |
| Δ6-Pore c | 0.06 | 0.03 (2) | 0.125 | 0.03 (4) | 16 | 4 (4) | 0.25 | 0.125 (2) | 64 | 32 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Tomoshige, S.; Lee, M.; Zgurskaya, H.I.; Mobashery, S. Penetration through Outer Membrane and Efflux Potential in Pseudomonas aeruginosa of Bulgecin A as an Adjuvant to β-Lactam Antibiotics. Antibiotics 2023, 12, 358. https://doi.org/10.3390/antibiotics12020358
Kim C, Tomoshige S, Lee M, Zgurskaya HI, Mobashery S. Penetration through Outer Membrane and Efflux Potential in Pseudomonas aeruginosa of Bulgecin A as an Adjuvant to β-Lactam Antibiotics. Antibiotics. 2023; 12(2):358. https://doi.org/10.3390/antibiotics12020358
Chicago/Turabian StyleKim, Choon, Shusuke Tomoshige, Mijoon Lee, Helen I. Zgurskaya, and Shahriar Mobashery. 2023. "Penetration through Outer Membrane and Efflux Potential in Pseudomonas aeruginosa of Bulgecin A as an Adjuvant to β-Lactam Antibiotics" Antibiotics 12, no. 2: 358. https://doi.org/10.3390/antibiotics12020358
APA StyleKim, C., Tomoshige, S., Lee, M., Zgurskaya, H. I., & Mobashery, S. (2023). Penetration through Outer Membrane and Efflux Potential in Pseudomonas aeruginosa of Bulgecin A as an Adjuvant to β-Lactam Antibiotics. Antibiotics, 12(2), 358. https://doi.org/10.3390/antibiotics12020358






