Profiling the Immune Response to Periprosthetic Joint Infection and Non-Infectious Arthroplasty Failure
Abstract
1. Total Joint Arthroplasty Failure
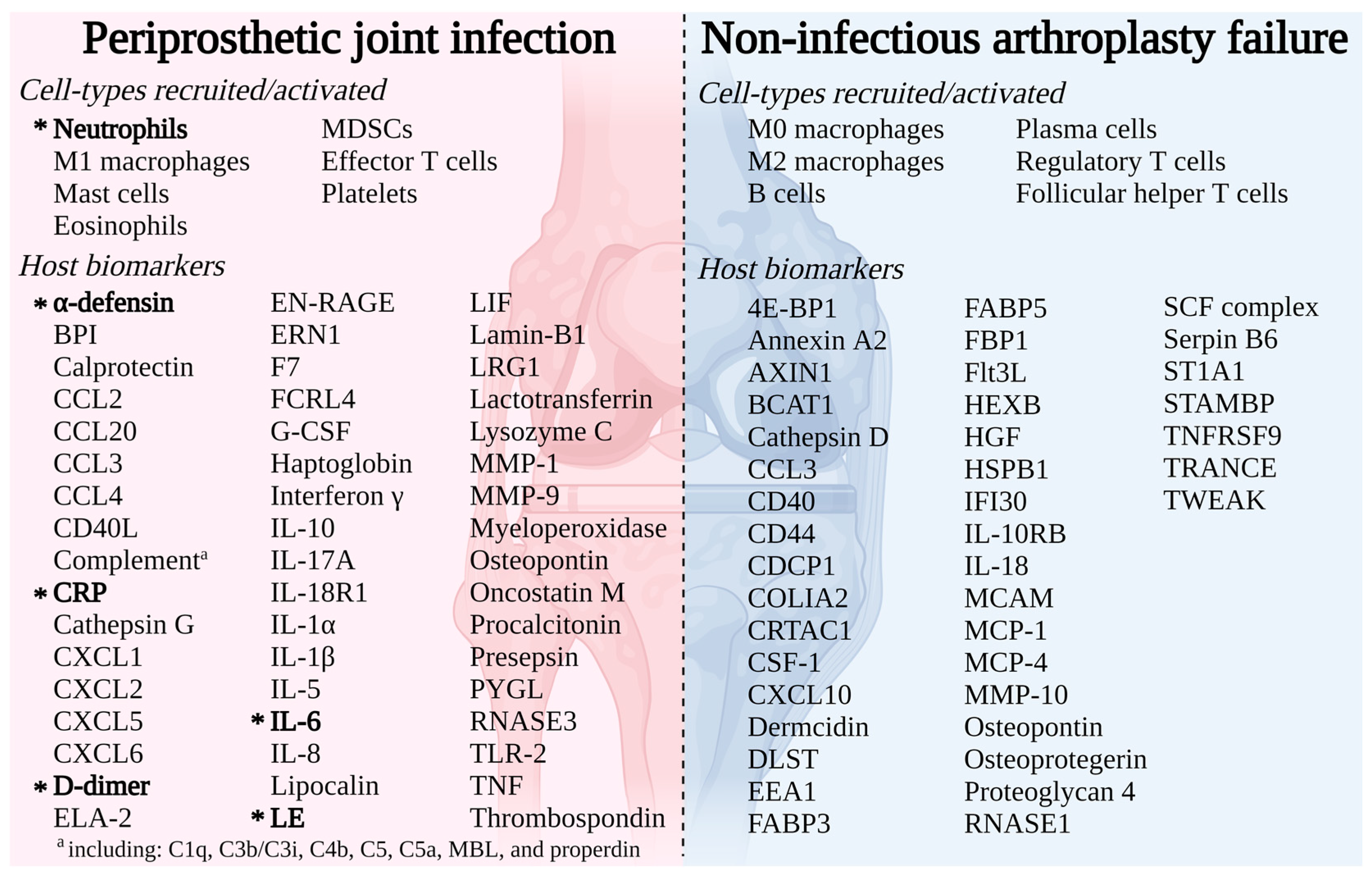
1.1. Periprosthetic Joint Infection
1.2. Non-Infectious Arthroplasty Failure
2. Current Arthroplasty Failure Diagnostic Techniques
2.1. Microbial-Based Diagnostic Techniques
2.2. Host-Based Diagnostic Techniques
2.3. Importance of Fast and Accurate Arthroplasty Failure Diagnosis
3. Detailed Immune Response Profiling for Arthroplasty Failure Diagnosis
3.1. Transcriptomic Immune Profiling
3.2. Proteomic Immune Profiling
3.3. Cellular Immune Profiling
3.4. Limitations of Immune Profiling for Arthroplasty Failure Diagnosis
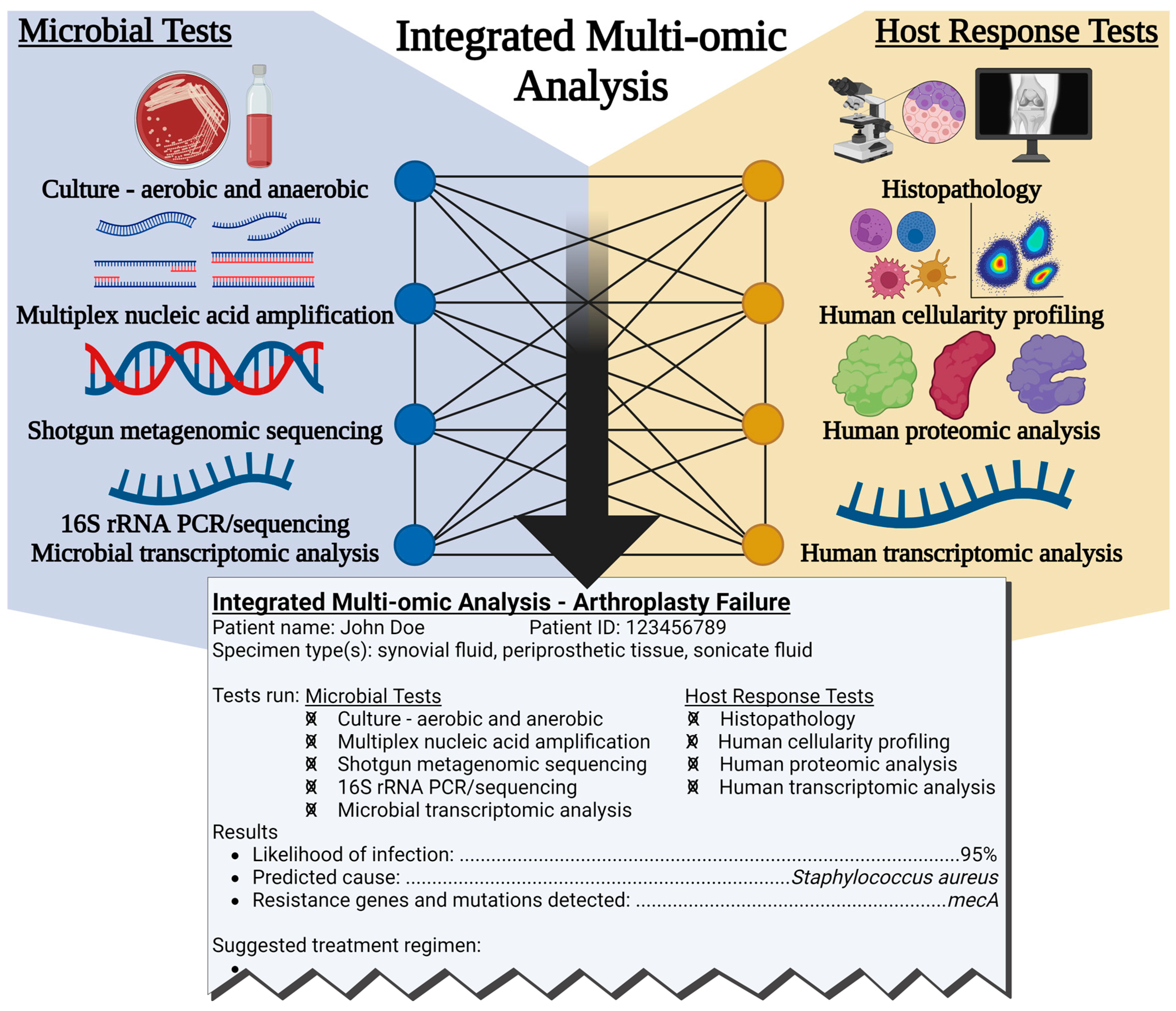
4. The Future of PJI and NIAF Diagnostics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, R. Periprosthetic joint infection. N. Engl. J. Med. 2023, 388, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e1483. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of total joint replacement in the United States: Future projections to 2020–2040 using the national inpatient sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Hilibrand, A.S.; Rubin, L.E.; Grauer, J.N. Geographic variations and trends in primary and revision knee and total hip arthroplasties in the United States. J. Bone Surg. 2020, 5, e0051. [Google Scholar] [CrossRef] [PubMed]
- Pivec, R.; Johnson, A.J.; Mears, S.C.; Mont, M.A. Hip arthroplasty. Lancet 2012, 380, 1768–1777. [Google Scholar] [CrossRef]
- Nilsdotter, A.K.; Toksvig-Larsen, S.; Roos, E.M. A 5 year prospective study of patient-relevant outcomes after total knee replacement. Osteoarthr. Cartil. 2009, 17, 601–606. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Murray, D.G. Total hip replacement. J. Am. Med. Assoc. 1995, 273, 1950–1956. [Google Scholar] [CrossRef]
- Gausden, E.B.; Cross, W.W., 3rd; Mabry, T.M.; Pagnano, M.W.; Berry, D.J.; Abdel, M.P. Total hip arthroplasty for femoral neck fracture: What are the contemporary reasons for failure? J. Arthroplast. 2021, 36, S272–S276. [Google Scholar] [CrossRef]
- Ledford, C.K.; Perry, K.I.; Hanssen, A.D.; Abdel, M.P. What are the contemporary etiologies for revision surgery and revision after primary, noncemented total hip arthroplasty? J. Am. Acad. Orthop. Surg. 2019, 27, 933–938. [Google Scholar] [CrossRef]
- Kenney, C.; Dick, S.; Lea, J.; Liu, J.; Ebraheim, N.A. A systematic review of the causes of failure of revision total hip arthroplasty. J. Orthop. 2019, 16, 393–395. [Google Scholar] [CrossRef]
- Mathis, D.T.; Lohrer, L.; Amsler, F.; Hirschmann, M.T. Reasons for failure in primary total knee arthroplasty—An analysis of prospectively collected registry data. J. Orthop. 2021, 23, 60–66. [Google Scholar] [CrossRef]
- Kelmer, G.; Stone, A.H.; Turcotte, J.; King, P.J. Reasons for revision: Primary total hip arthroplasty mechanisms of failure. J. Am. Acad. Orthop. Surg. 2021, 29, 78–87. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Farley, K.X.; Guild, G.N.; Bradbury, T.L., Jr. Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J. Arthroplast. 2020, 35, S79–S85. [Google Scholar] [CrossRef]
- Meehan, J.P.; Danielsen, B.; Kim, S.H.; Jamali, A.A.; White, R.H. Younger age is associated with a higher risk of early periprosthetic joint infection and aseptic mechanical failure after total knee arthroplasty. J. Bone Jt. Surg. Am. 2014, 96, 529–535. [Google Scholar] [CrossRef]
- Zimmerli, W. Infection and musculoskeletal conditions: Prosthetic-joint-associated infections. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1045–1063. [Google Scholar] [CrossRef]
- Trampuz, A.; Widmer, A.F. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 2006, 19, 349–356. [Google Scholar] [CrossRef]
- Kim, K.T.; Lee, S.; Ko, D.O.; Seo, B.S.; Jung, W.S.; Chang, B.K. Causes of failure after total knee arthroplasty in osteoarthritis patients 55 years of age or younger. Knee Surg. Relat. Res. 2014, 26, 13–19. [Google Scholar] [CrossRef]
- Postler, A.; Lutzner, C.; Beyer, F.; Tille, E.; Lutzner, J. Analysis of total knee arthroplasty revision causes. BMC Musculoskelet. Disord. 2018, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Dowsey, M.M.; Buising, K.L.; Liew, D.; Choong, P.F. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin. Microbiol. Infect. 2013, 19, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, P.R.; Dhotar, H.S.; Sternheim, A.; Gross, A.E.; Safir, O.; Backstein, D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: Techniques, controversies, and outcomes. J. Am. Acad. Orthop. Surg. 2014, 22, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Renz, N.; Trampuz, A. Management of periprosthetic joint infection. Hip. Pelvis. 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef]
- Wixted, C.M.; Charalambous, L.T.; Kim, B.I.; Cochrane, N.H.; Belay, E.S.; Joseph, H.L.; Seyler, T.M. Direct costs vary by outcome in two-stage revision arthroplasty for the treatment of hip periprosthetic joint infection. Arthroplast. Today 2023, 19, 101061. [Google Scholar] [CrossRef]
- Bozic, K.J.; Ries, M.D. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J. Bone Jt. Surg. Am. 2005, 87, 1746–1751. [Google Scholar] [CrossRef]
- Bozic, K.J.; Katz, P.; Cisternas, M.; Ono, L.; Ries, M.D.; Showstack, J. Hospital resource utilization for primary and revision total hip arthroplasty. J. Bone Jt. Surg. Am. 2005, 87, 570–576. [Google Scholar] [CrossRef]
- Akindolire, J.; Morcos, M.W.; Marsh, J.D.; Howard, J.L.; Lanting, B.A.; Vasarhelyi, E.M. The economic impact of periprosthetic infection in total hip arthroplasty. Can. J. Surg. 2020, 63, E52–E56. [Google Scholar] [CrossRef]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef]
- Namba, R.S.; Inacio, M.C.; Paxton, E.W. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J. Bone Jt. Surg. Br. 2012, 94, 1330–1338. [Google Scholar] [CrossRef]
- Namba, R.S.; Inacio, M.C.; Paxton, E.W. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: An analysis of 56,216 knees. J. Bone Jt. Surg. Am. 2013, 95, 775–782. [Google Scholar] [CrossRef]
- Malinzak, R.A.; Ritter, M.A.; Berend, M.E.; Meding, J.B.; Olberding, E.M.; Davis, K.E. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J. Arthroplast. 2009, 24, 84–88. [Google Scholar] [CrossRef]
- Dowsey, M.M.; Choong, P.F.M. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin. Orthop. Relat. R 2009, 467, 1577–1581. [Google Scholar] [CrossRef]
- Peersman, G.; Laskin, R.; Davis, J.; Peterson, M. Infection in total knee replacement: A retrospective review of 6489 total knee replacements. Clin. Orthop. Relat. Res. 2001, 392, 15–23. [Google Scholar] [CrossRef]
- Peel, T.N.; Dowsey, M.M.; Daffy, J.R.; Stanley, P.A.; Choong, P.F.; Buising, K.L. Risk factors for prosthetic hip and knee infections according to arthroplasty site. J. Hosp. Infect. 2011, 79, 129–133. [Google Scholar] [CrossRef]
- Dowsey, M.M.; Choong, P.F.M. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin. Orthop. Relat. R 2008, 466, 153–158. [Google Scholar] [CrossRef]
- Berbari, E.F.; Hanssen, A.D.; Duffy, M.C.; Steckelberg, J.M.; Ilstrup, D.M.; Harmsen, W.S.; Osmon, D.R. Risk factors for prosthetic joint infection: Case-control study. Clin. Infect. Dis. 1998, 27, 1247–1254. [Google Scholar] [CrossRef]
- Berbari, E.F.; Osmon, D.R.; Lahr, B.; Eckel-Passow, J.E.; Tsaras, G.; Hanssen, A.D.; Mabry, T.; Steckelberg, J.; Thompson, R. The Mayo prosthetic joint infection risk score: Implication for surgical site infection reporting and risk stratification. Infect. Control. Hosp. Epidemiol. 2012, 33, 774–781. [Google Scholar] [CrossRef]
- Bongartz, T.; Halligan, C.S.; Osmon, D.R.; Reinalda, M.S.; Bamlet, W.R.; Crowson, C.S.; Hanssen, A.D.; Matteson, E.L. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008, 59, 1713–1720. [Google Scholar] [CrossRef]
- Jamsen, E.; Huhtala, H.; Puolakka, T.; Moilanen, T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J. Bone Jt. Surg. Am. 2009, 91, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal biofilm development: Structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-00019. [Google Scholar] [CrossRef] [PubMed]
- Gries, C.M.; Kielian, T. Staphylococcal biofilms and immune polarization during prosthetic joint infection. J. Am. Acad. Orthop. Surg. 2017, 25 (Suppl. 1), S20–S24. [Google Scholar] [CrossRef]
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus aureus evasion of host immunity in the setting of prosthetic joint infection: Biofilm and beyond. Curr. Rev. Musculoskelet. Med. 2018, 11, 389–400. [Google Scholar] [CrossRef]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Patel, R. Infection associated with prosthetic joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef]
- Gross, C.E.; Della Valle, C.J.; Rex, J.C.; Traven, S.A.; Durante, E.C. Fungal periprosthetic joint infection: A review of demographics and management. J. Arthroplast. 2021, 36, 1758–1764. [Google Scholar] [CrossRef]
- Nace, J.; Siddiqi, A.; Talmo, C.T.; Chen, A.F. Diagnosis and management of fungal periprosthetic joint infections. J. Am. Acad. Orthop. Surg. 2019, 27, e804–e818. [Google Scholar] [CrossRef]
- Tai, D.B.G.; Patel, R.; Abdel, M.P.; Berbari, E.F.; Tande, A.J. Microbiology of hip and knee periprosthetic joint infections: A database study. Clin. Microbiol. Infect. 2022, 28, 255–259. [Google Scholar] [CrossRef]
- Marculescu, C.E.; Berbari, E.F.; Cockerill, F.R., 3rd; Osmon, D.R. Unusual aerobic and anaerobic bacteria associated with prosthetic joint infections. Clin. Orthop. Relat. Res. 2006, 451, 55–63. [Google Scholar] [CrossRef]
- Flurin, L.; Greenwood-Quaintance, K.E.; Patel, R. Microbiology of polymicrobial prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2019, 94, 255–259. [Google Scholar] [CrossRef]
- Palan, J.; Nolan, C.; Sarantos, K.; Westerman, R.; King, R.; Foguet, P. Culture-negative periprosthetic joint infections. EFORT Open Rev. 2019, 4, 585–594. [Google Scholar] [CrossRef]
- Berbari, E.F.; Marculescu, C.; Sia, I.; Lahr, B.D.; Hanssen, A.D.; Steckelberg, J.M.; Gullerud, R.; Osmon, D.R. Culture-negative prosthetic joint infection. Clin. Infect Dis. 2007, 45, 1113–1119. [Google Scholar] [CrossRef]
- Hersh, B.L.; Shah, N.B.; Rothenberger, S.D.; Zlotnicki, J.P.; Klatt, B.A.; Urish, K.L. Do culture negative periprosthetic joint infections remain culture negative? J. Arthroplast. 2019, 34, 2757–2762. [Google Scholar] [CrossRef]
- Okafor, C.; Hodgkinson, B.; Nghiem, S.; Vertullo, C.; Byrnes, J. Cost of septic and aseptic revision total knee arthroplasty: A systematic review. BMC Musculoskelet. Disord. 2021, 22, 706. [Google Scholar] [CrossRef]
- Lavernia, C.; Lee, D.J.; Hernandez, V.H. The increasing financial burden of knee revision surgery in the United States. Clin. Orthop. Relat. Res. 2006, 446, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Klouche, S.; Sariali, E.; Mamoudy, P. Total hip arthroplasty revision due to infection: A cost analysis approach. Orthop. Traumatol.-Sur. 2010, 96, 124–132. [Google Scholar] [CrossRef]
- Salmons, H.I.; Fruth, K.M.; Lewallen, D.G.; Trousdale, R.T.; Berry, D.J.; Abdel, M.P. Revision total hip arthroplasty for aseptically failed metal-on-metal hip resurfacing arthroplasty. J. Arthroplast. 2022, 37, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Cottino, U.; Sculco, P.K.; Sierra, R.J.; Abdel, M.P. Instability after total knee arthroplasty. Orthop. Clin. N. Am. 2016, 47, 311–316. [Google Scholar] [CrossRef]
- Owen, A.R.; Tibbo, M.E.; van Wijnen, A.J.; Pagnano, M.W.; Berry, D.J.; Abdel, M.P. Acquired idiopathic stiffness after contemporary total knee arthroplasty: Incidence, risk factors, and results over 25 years. J. Arthroplast. 2021, 36, 2980–2985. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, M.; Deschamps, G.; Neyret, P.; Chambat, P. Revision in non-infected total knee arthroplasty: An analysis of 69 consecutive cases. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2000, 86, 694–706. [Google Scholar]
- Athanasou, N.A. The pathobiology and pathology of aseptic implant failure. Bone Jt. Res. 2016, 5, 162–168. [Google Scholar] [CrossRef]
- Abdel, M.P.; Ledford, C.K.; Kobic, A.; Taunton, M.J.; Hanssen, A.D. Contemporary failure aetiologies of the primary, posterior-stabilised total knee arthroplasty. Bone Jt. J. 2017, 99, 647–652. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. J. Arthroplast. 2018, 33, 1309–1314.e1302. [Google Scholar] [CrossRef]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebse, R. The EBJIS definition of periprosthetic joint infection. Bone Jt. J. 2021, 103, 18–25. [Google Scholar] [CrossRef]
- Parvizi, J.; Gehrke, T.; Infection, I.C.G.o.P.J. Definition of periprosthetic joint infection. J. Arthroplast. 2014, 29, 1331. [Google Scholar] [CrossRef]
- Shohat, N.; Bauer, T.; Buttaro, M.; Budhiparama, N.; Cashman, J.; Della Valle, C.J.; Drago, L.; Gehrke, T.; Gomes, L.S.M.; Goswami, K. Hip and knee section, what is the definition of a periprosthetic joint infection (PJI) of the knee and the hip? Can the same criteria be used for both joints?: Proceedings of international consensus on orthopedic infections. J. Arthroplast. 2019, 34, S325–S327. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, I.K.; Luger, M.; Windhager, R.; McNally, M.A. Diagnosing periprosthetic joint infections: A comparison of infection definitions: EBJIS 2021, ICM 2018, and IDSA 2013. Bone Jt. Res. 2022, 11, 608–618. [Google Scholar] [CrossRef]
- Deirmengian, C.; McLaren, A.; Higuera, C.; Levine, B.R. Physician use of multiple criteria to diagnose periprosthetic joint infection may be less accurate than the use of an individual test. Cureus 2022, 14, e31418. [Google Scholar] [CrossRef]
- Higgins, E.; Suh, G.A.; Tande, A.J. Enhancing diagnostics in orthopedic infections. J. Clin. Microbiol. 2022, 60, e0219621. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Dylla, B.L.; Hughes, J.G.; Lynch, D.T.; Greenwood-Quaintance, K.E.; Cheng, A.C.; Mandrekar, J.N.; Patel, R. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. mBio 2016, 7, e01776-01715. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef]
- Yin, H.; Xu, D.; Wang, D. Diagnostic value of next-generation sequencing to detect periprosthetic joint infection. BMC Musculoskelet. Disord. 2021, 22, 252. [Google Scholar] [CrossRef]
- Tarabichi, S.; Goh, G.S.; Zanna, L.; Qadiri, Q.S.; Baker, C.M.; Gehrke, T.; Citak, M.; Parvizi, J. Time to positivity of cultures obtained for periprosthetic joint infection. J. Bone Jt. Surg. 2023, 105, 107–112. [Google Scholar] [CrossRef]
- Gomez, E.; Cazanave, C.; Cunningham, S.A.; Greenwood-Quaintance, K.E.; Steckelberg, J.M.; Uhl, J.R.; Hanssen, A.D.; Karau, M.J.; Schmidt, S.M.; Osmon, D.R.; et al. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J. Clin. Microbiol. 2012, 50, 3501–3508. [Google Scholar] [CrossRef]
- Melendez, D.P.; Uhl, J.R.; Greenwood-Quaintance, K.E.; Hanssen, A.D.; Sampath, R.; Patel, R. Detection of prosthetic joint infection by use of PCR-electrospray ionization mass spectrometry applied to synovial fluid. J. Clin. Microbiol. 2014, 52, 2202–2205. [Google Scholar] [CrossRef]
- Melendez, D.P.; Greenwood-Quaintance, K.E.; Berbari, E.F.; Osmon, D.R.; Mandrekar, J.N.; Hanssen, A.D.; Patel, R. Evaluation of a genus- and group-specific rapid PCR assay panel on synovial fluid for diagnosis of prosthetic knee infection. J. Clin. Microbiol. 2016, 54, 120–126. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Greenwood-Quaintance, K.E.; Hanssen, A.D.; Mandrekar, J.N.; Patel, R. Low sensitivity of periprosthetic tissue PCR for prosthetic knee infection diagnosis. Diagn. Microbiol. Infect. Dis. 2014, 79, 448–453. [Google Scholar] [CrossRef]
- Ivy, M.I.; Thoendel, M.J.; Jeraldo, P.R.; Greenwood-Quaintance, K.E.; Hanssen, A.D.; Abdel, M.P.; Chia, N.; Yao, J.Z.; Tande, A.J.; Mandrekar, J.N.; et al. Direct detection and identification of prosthetic joint infection pathogens in synovial fluid by metagenomic shotgun sequencing. J. Clin. Microbiol. 2018, 56, e00402-00418. [Google Scholar] [CrossRef]
- Thoendel, M.J.; Jeraldo, P.R.; Greenwood-Quaintance, K.E.; Yao, J.Z.; Chia, N.; Hanssen, A.D.; Abdel, M.P.; Patel, R. Identification of prosthetic joint infection pathogens using a shotgun metagenomics approach. Clin. Infect. Dis. 2018, 67, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Wolf, M.J.; Strasburg, A.P.; Daniels, M.L.; Starkey, J.C.; Donadio, A.D.; Abdel, M.P.; Greenwood-Quaintance, K.E.; Patel, R. Comparison of the BioFire joint infection panel to 16S ribosomal RNA gene-based targeted metagenomic sequencing for testing synovial fluid from patients with knee arthroplasty failure. J. Clin. Microbiol. 2022, 1, e0112622. [Google Scholar] [CrossRef] [PubMed]
- Flurin, L.; Hemenway, J.J.; Fisher, C.R.; Vaillant, J.J.; Azad, M.; Wolf, M.J.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Patel, R. Clinical use of a 16S ribosomal RNA gene-based Sanger and/or next generation sequencing assay to test preoperative synovial fluid for periprosthetic joint infection diagnosis. mBio 2022, 1, e0132222. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B.; Fehring, T.K.; Odum, S.M.; Griffin, W.L.; Nussman, D.S. The value of white blood cell counts before revision total knee arthroplasty. J. Arthroplast. 2003, 18, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Hanssen, A.D.; Osmon, D.R.; Mandrekar, J.; Steckelberg, J.M.; Patel, R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am. J. Med. 2004, 117, 556–562. [Google Scholar] [CrossRef]
- Zmistowski, B.; Restrepo, C.; Huang, R.; Hozack, W.J.; Parvizi, J. Periprosthetic joint infection diagnosis: A complete understanding of white blood cell count and differential. J. Arthroplast. 2012, 27, 1589–1593. [Google Scholar] [CrossRef]
- Dinneen, A.; Guyot, A.; Clements, J.; Bradley, N. Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection. Bone Jt. J. 2013, 95, 554–557. [Google Scholar] [CrossRef]
- Wyles, C.C.; Larson, D.R.; Houdek, M.T.; Sierra, R.J.; Trousdale, R.T. Utility of synovial fluid aspirations in failed metal-on-metal total hip arthroplasty. J. Arthroplast. 2013, 28, 818–823. [Google Scholar] [CrossRef]
- Gallo, J.; Juranova, J.; Svoboda, M.; Zapletalova, J. Excellent AUC for joint fluid cytology in the detection/exclusion of hip and knee prosthetic joint infection. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2017, 161, 310–319. [Google Scholar] [CrossRef]
- Higuera, C.A.; Zmistowski, B.; Malcom, T.; Barsoum, W.K.; Sporer, S.M.; Mommsen, P.; Kendoff, D.; Della Valle, C.J.; Parvizi, J. Synovial fluid cell count for diagnosis of chronic periprosthetic hip infection. J. Bone Jt. Surg. Am. 2017, 99, 753–759. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.G.; Jang, K.M.; Han, S.B.; Lim, H.C.; Bae, J.H. Diagnostic value of synovial white blood cell count and serum C-reactive protein for acute periprosthetic joint infection after knee arthroplasty. J. Arthroplast. 2017, 32, 3724–3728. [Google Scholar] [CrossRef]
- Lee, Y.S.; Koo, K.H.; Kim, H.J.; Tian, S.; Kim, T.Y.; Maltenfort, M.G.; Chen, A.F. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: A systematic review and meta-analysis. J. Bone Jt. Surg. Am. 2017, 99, 2077–2084. [Google Scholar] [CrossRef]
- Shahi, A.; Parvizi, J. The role of biomarkers in the diagnosis of periprosthetic joint infection. EFORT Open Rev. 2017, 1, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Serrano, P.; Gomes Dias, J.; Oliveira, J.C.; Oliveira, A. Improving the accuracy of synovial fluid analysis in the diagnosis of prosthetic joint infection with simple and inexpensive biomarkers: C-reactive protein and adenosine deaminase. Bone Jt. J. 2017, 99, 351–357. [Google Scholar] [CrossRef]
- Balato, G.; Franceschini, V.; Ascione, T.; Lamberti, A.; Balboni, F.; Baldini, A. Diagnostic accuracy of synovial fluid, blood markers, and microbiological testing in chronic knee prosthetic infections. Arch. Orthop. Trauma. Surg. 2018, 138, 165–171. [Google Scholar] [CrossRef]
- De Vecchi, E.; Romano, C.L.; De Grandi, R.; Cappelletti, L.; Villa, F.; Drago, L. Alpha defensin, leukocyte esterase, C-reactive protein, and leukocyte count in synovial fluid for pre-operative diagnosis of periprosthetic infection. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418806072. [Google Scholar] [CrossRef]
- Kuo, F.C.; Lu, Y.D.; Wu, C.T.; You, H.L.; Lee, G.B.; Lee, M.S. Comparison of molecular diagnosis with serum markers and synovial fluid analysis in patients with prosthetic joint infection. Bone Jt. J. 2018, 100, 1345–1351. [Google Scholar] [CrossRef]
- Tahta, M.; Simsek, M.E.; Isik, C.; Akkaya, M.; Gursoy, S.; Bozkurt, M. Does inflammatory joint diseases affect the accuracy of infection biomarkers in patients with periprosthetic joint infections? A prospective comparative reliability study. J. Orthop. Sci. 2019, 24, 286–289. [Google Scholar] [CrossRef]
- Carli, A.V.; Abdelbary, H.; Ahmadzai, N.; Cheng, W.; Shea, B.; Hutton, B.; Sniderman, J.; Philip Sanders, B.S.; Esmaeilisaraji, L.; Skidmore, B.; et al. Diagnostic accuracy of serum, synovial, and tissue testing for chronic periprosthetic joint infection after hip and knee replacements: A systematic review. J. Bone Jt. Surg. Am. 2019, 101, 635–649. [Google Scholar] [CrossRef]
- Dijkman, C.; Thomas, A.R.; Koenraadt, K.L.M.; Ermens, A.A.M.; van Geenen, R.C.I. Synovial neutrophilic gelatinase-associated lipocalin in the diagnosis of periprosthetic joint infection after total knee arthroplasty. Arch. Orthop. Trauma Surg. 2020, 140, 941–947. [Google Scholar] [CrossRef]
- Mihalic, R.; Zdovc, J.; Brumat, P.; Trebse, R. Synovial fluid interleukin-6 is not superior to cell count and differential in the detection of periprosthetic joint infection. Bone Jt Open 2020, 1, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Ivy, M.; Block, D.R.; Abdel, M.P.; Hanssen, A.D.; Beauchamp, C.; Perry, K.I.; Rosemark, C.L.; Greenwood-Quaintance, K.E.; Mandrekar, J.; et al. Comparative analysis of 23 synovial fluid biomarkers for hip and knee periprosthetic joint infection detection. J. Orthop. Res. 2020, 38, 2664–2674. [Google Scholar] [CrossRef] [PubMed]
- Ivy, M.I.; Sharma, K.; Greenwood-Quaintance, K.E.; Tande, A.J.; Osmon, D.R.; Berbari, E.F.; Mandrekar, J.; Beauchamp, C.P.; Hanssen, A.D.; Abdel, M.P.; et al. Synovial fluid alpha defensin has comparable accuracy to synovial fluid white blood cell count and polymorphonuclear percentage for periprosthetic joint infection diagnosis. Bone Jt. J. 2021, 103, 1119–1126. [Google Scholar] [CrossRef]
- Levent, A.; Neufeld, M.E.; Piakong, P.; Lausmann, C.; Gehrke, T.; Citak, M. Which International Consensus Meeting preoperative minor criteria is the most accurate marker for the diagnosis of periprosthetic joint infection in hip and knee arthroplasty? J. Arthroplast. 2021, 36, 3728–3733. [Google Scholar] [CrossRef] [PubMed]
- Van den Kieboom, J.; Tirumala, V.; Xiong, L.; Klemt, C.; Kwon, Y.M. Concomitant hip and knee periprosthetic joint infection in periprosthetic fracture: Diagnostic utility of serum and synovial fluid markers. J. Arthroplast. 2021, 36, 722–727. [Google Scholar] [CrossRef]
- Baker, C.M.; Goh, G.S.; Tarabichi, S.; Shohat, N.; Parvizi, J. Synovial c-reactive protein is a useful adjunct for diagnosis of periprosthetic joint infection. J. Arthroplast. 2022, 37, 2437–2443.e2431. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Li, M.; Li, W.; Fang, X.; Zhang, W. Synovial fluid neutrophil gelatinase-associated lipocalin can be used to accurately diagnose prosthetic joint infection. Int. J. Infect. Dis. 2022, 123, 170–175. [Google Scholar] [CrossRef]
- Lazic, I.; Burdach, A.; Pohlig, F.; von Eisenhart-Rothe, R.; Suren, C. Utility of synovial calprotectin lateral flow test to exclude chronic prosthetic joint infection in periprosthetic fractures: A prospective cohort study. Sci. Rep. 2022, 12, 18385. [Google Scholar] [CrossRef]
- Dilley, J.E.; Seetharam, A.; Meneghini, R.M.; Kheir, M.M. Synovial fluid absolute neutrophil count and neutrophil-to-lymphocyte ratio are not superior to polymorphonuclear percentage in detecting periprosthetic joint infection. J. Arthroplast. 2023, 38, 146–151. [Google Scholar] [CrossRef]
- Qin, L.; Hu, N.; Li, X.; Chen, Y.; Wang, J.; Huang, W. Evaluation of synovial fluid neutrophil CD64 index as a screening biomarker of prosthetic joint infection. Bone Jt. J. 2020, 102, 463–469. [Google Scholar] [CrossRef]
- Qin, L.; Li, X.; Wang, J.; Gong, X.; Hu, N.; Huang, W. Improved diagnosis of chronic hip and knee prosthetic joint infection using combined serum and synovial IL-6 tests. Bone Jt. Res. 2020, 9, 587–592. [Google Scholar] [CrossRef]
- Wang, H.; Qin, L.; Wang, J.; Huang, W. Synovial fluid IL-1beta appears useful for the diagnosis of chronic periprosthetic joint infection. J. Orthop. Surg. Res. 2021, 16, 144. [Google Scholar] [CrossRef]
- Qin, L.; Wang, H.; Zhao, C.; Chen, C.; Chen, H.; Li, X.; Wang, J.; Hu, N.; Huang, W. Serum and synovial biomarkers for distinguishing between chronic periprosthetic joint infections and rheumatoid arthritis: A prospective cohort study. J. Arthroplast. 2022, 37, 342–346. [Google Scholar] [CrossRef]
- Christensen, C.P.; Bedair, H.; Della Valle, C.J.; Parvizi, J.; Schurko, B.; Jacobs, C.A. The natural progression of synovial fluid white blood-cell counts and the percentage of polymorphonuclear cells after primary total knee arthroplasty: A multicenter study. J. Bone Jt. Surg. Am. 2013, 95, 2081–2087. [Google Scholar] [CrossRef]
- Deirmengian, C.; Madigan, J.; Kallur Mallikarjuna, S.; Conway, J.; Higuera, C.; Patel, R. Validation of the alpha defensin lateral flow test for periprosthetic joint infection. J. Bone Jt. Surg. Am. 2021, 103, 115–122. [Google Scholar] [CrossRef]
- Pupaibool, J.; Fulnecky, E.J.; Swords, R.L., Jr.; Sistrunk, W.W.; Haddow, A.D. Alpha-defensin-novel synovial fluid biomarker for the diagnosis of periprosthetic joint infection. Int. Orthop. 2016, 40, 2447–2452. [Google Scholar] [CrossRef]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 1995, 5, 521–527. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A double-edged sword in host immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Huard, M.; Detrembleur, C.; Poilvache, H.; Pastor, Y.G.I.; Van Cauter, M.; Driesen, R.; Yombi, J.C.; Neyt, J.; Cornu, O. Alpha defensin: A diagnostic accuracy depending on the infection definition used. J. Arthroplast. 2020, 35, 1355–1360. [Google Scholar] [CrossRef]
- Renz, N.; Yermak, K.; Perka, C.; Trampuz, A. Alpha defensin lateral flow test for diagnosis of periprosthetic joint infection: Not a screening but a confirmatory test. J. Bone Jt. Surg. Am. 2018, 100, 742–750. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Yermak, K.; Perka, C.; Trampuz, A.; Renz, N. Is the enzyme-linked immunosorbent assay more accurate than the lateral flow alpha defensin test for diagnosing periprosthetic joint infection? Clin. Orthop. Relat. R 2018, 476, 1645. [Google Scholar] [CrossRef] [PubMed]
- Unter Ecker, N.; Koniker, A.; Gehrke, T.; Salber, J.; Zahar, A.; Hentschke, M.; Citak, M. What is the diagnostic accuracy of alpha-defensin and leukocyte esterase test in periprosthetic shoulder infection? Clin. Orthop. Relat. R 2019, 477, 1712. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, L.; Plate, A.; Stadler, L.; Sutter, R.; Frustaci, D.; Zbinden, R.; Zingg, P.O.; Gerber, C.; Achermann, Y. Alpha-defensin lateral flow test does not appear to be useful in predicting shoulder periprosthetic joint infections. Int. Orthop. 2020, 44, 1023–1029. [Google Scholar] [CrossRef]
- Balato, G.; Dall'Anese, R.; Balboni, F.; Ascione, T.; Pezzati, P.; Bartolini, G.; Quercioli, M.; Baldini, A. Synovial fluid alpha-defensin in periprosthetic knee infection workup: Liquid chromatography-mass spectrometry detection of alpha-defensin in synovial fluid. Bone Jt. J. 2022, 104, 1047–1051. [Google Scholar] [CrossRef]
- Suen, K.; Keeka, M.; Ailabouni, R.; Tran, P. Synovasure 'quick test' is not as accurate as the laboratory-based alpha-defensin immunoassay: A systematic review and meta-analysis. Bone Jt. J. 2018, 100, 66–72. [Google Scholar] [CrossRef]
- Gehrke, T.; Lausmann, C.; Citak, M.; Bonanzinga, T.; Frommelt, L.; Zahar, A. The accuracy of the alpha defensin lateral flow device for diagnosis of periprosthetic joint infection: Comparison with a gold standard. J. Bone Jt. Surg. Am. 2018, 100, 42–48. [Google Scholar] [CrossRef]
- Eriksson, H.K.; Nordström, J.; Gabrysch, K.; Hailer, N.P.; Lazarinis, S. Does the alpha-defensin immunoassay or the lateral flow test have better diagnostic value for periprosthetic joint infection? A systematic review. Clin. Orthop. Relat. R 2018, 476, 1065. [Google Scholar] [CrossRef]
- Kuiper, J.W.P.; Verberne, S.J.; Vos, S.J.; van Egmond, P.W. Does the alpha defensin ELISA test perform better than the alpha defensin lateral flow test for PJI diagnosis? A systematic review and meta-analysis of prospective studies. Clin. Orthop. Relat. Res. 2020, 478, 1333–1344. [Google Scholar] [CrossRef]
- Kleeman-Forsthuber, L.T.; Johnson, R.M.; Brady, A.C.; Pollet, A.K.; Dennis, D.A.; Jennings, J.M. Alpha-defensin offers limited utility in routine workup of periprosthetic joint infection. J. Arthroplast. 2021, 36, 1746–1752. [Google Scholar] [CrossRef]
- Bingham, J.; Clarke, H.; Spangehl, M.; Schwartz, A.; Beauchamp, C.; Goldberg, B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 4006–4009. [Google Scholar] [CrossRef]
- Kasparek, M.F.; Kasparek, M.; Boettner, F.; Faschingbauer, M.; Hahne, J.; Dominkus, M. Intraoperative diagnosis of periprosthetic joint infection using a novel alpha-defensin lateral flow assay. J. Arthroplast. 2016, 31, 2871–2874. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Holinka, J.; Gamper, J.; Staats, K.; Bohler, C.; Kubista, B.; Windhager, R. Qualitative alpha-defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty. Bone Jt. J. 2017, 99, 66–72. [Google Scholar] [CrossRef]
- Okroj, K.T.; Calkins, T.E.; Kayupov, E.; Kheir, M.M.; Bingham, J.S.; Beauchamp, C.P.; Parvizi, J.; Della Valle, C.J. The alpha-defensin test for diagnosing periprosthetic joint infection in the setting of an adverse local tissue reaction secondary to a failed metal-on-metal bearing or corrosion at the head-neck junction. J. Arthroplast. 2018, 33, 1896–1898. [Google Scholar] [CrossRef]
- Berger, P.; Van Cauter, M.; Driesen, R.; Neyt, J.; Cornu, O.; Bellemans, J. Diagnosis of prosthetic joint infection with alpha-defensin using a lateral flow device: A multicentre study. Bone Jt. J. 2017, 99, 1176–1182. [Google Scholar] [CrossRef]
- Suda, A.J.; Tinelli, M.; Beisemann, N.D.; Weil, Y.; Khoury, A.; Bischel, O.E. Diagnosis of periprosthetic joint infection using alpha-defensin test or multiplex-PCR: Ideal diagnostic test still not found. Int. Orthop. 2017, 41, 1307–1313. [Google Scholar] [CrossRef]
- Balato, G.; Franceschini, V.; Ascione, T.; Lamberti, A.; D'Amato, M.; Ensini, A.; Baldini, A. High performance of alpha-defensin lateral flow assay (Synovasure) in the diagnosis of chronic knee prosthetic infections. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1717–1722. [Google Scholar] [CrossRef]
- De Saint Vincent, B.; Migaud, H.; Senneville, E.; Loiez, C.; Pasquier, G.; Girard, J.; Putman, S. Diagnostic accuracy of the alpha defensin lateral flow device (Synovasure) for periprosthetic infections in microbiologically complex situations: A study of 42 cases in a French referral centre. Orthop. Traumatol. Surg. Res. 2018, 104, 427–431. [Google Scholar] [CrossRef]
- Riccio, G.; Cavagnaro, L.; Akkouche, W.; Carrega, G.; Felli, L.; Burastero, G. Qualitative alpha-defensin versus the main available tests for the diagnosis of periprosthetic joint infection: Best predictor test? J. Bone Infect. 2018, 3, 156–164. [Google Scholar] [CrossRef]
- Stone, W.Z.; Gray, C.F.; Parvataneni, H.K.; Al-Rashid, M.; Vlasak, R.G.; Horodyski, M.; Prieto, H.A. Clinical evaluation of synovial alpha defensin and synovial C-reactive protein in the diagnosis of periprosthetic joint infection. J. Bone Jt. Surg. Am. 2018, 100, 1184–1190. [Google Scholar] [CrossRef]
- Plate, A.; Stadler, L.; Sutter, R.; Anagnostopoulos, A.; Frustaci, D.; Zbinden, R.; Fucentese, S.F.; Zinkernagel, A.S.; Zingg, P.O.; Achermann, Y. Inflammatory disorders mimicking periprosthetic joint infections may result in false-positive alpha-defensin. Clin. Microbiol. Infect. 2018, 24, e1212.e1–e1212.e6. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Holinka, J.; Lang, S.; Stenicka, S.; Staats, K.; Hobusch, G.; Kubista, B.; Windhager, R. A comparative study of intraoperative frozen section and alpha defensin lateral flow test in the diagnosis of periprosthetic joint infection. Acta Orthop. 2019, 90, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Abdo, R.C.T.; Gobbi, R.G.; Leite, C.B.G.; Pasoto, S.G.; Leon, E.P.; Lima, A.; Bonfa, E.; Pecora, J.R.; Demange, M.K. Performance of alpha-defensin lateral flow test after synovial fluid centrifugation for diagnosis of periprosthetic knee infection. World J. Orthop. 2021, 12, 565–574. [Google Scholar] [CrossRef] [PubMed]
- De Saint Vincent, B.; Martinot, P.; Pascal, A.; Senneville, E.; Loiez, C.; Pasquier, G.; Girard, J.; Putman, S.; Migaud, H. Does the alpha-defensin lateral flow test conserve its diagnostic properties in a larger population of chronic complex periprosthetic infections? Enlargement to 112 tests, from 42 tests in a preliminary study, in a reference center. Orthop. Traumatol. Surg. Res. 2021, 107, 102912. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.Z.; Li, R.; Fu, J.; Chai, W.; Hao, L.B.; Chen, J.Y. Leukocyte esterase test and alpha-defensin test have similar accuracy for the diagnosis of periprosthetic joint infection. Int. Orthop. 2021, 45, 1677–1682. [Google Scholar] [CrossRef]
- Zeng, Y.Q.; Deng, S.; Zhu, X.Y.; Sun, X.B.; Feng, W.J.; Zeng, J.C.; Zhang, H.T.; Zeng, Y.R. Diagnostic accuracy of the synovial fluid alpha-defensin lateral flow test in periprosthetic joint infection: A meta-analysis. Orthop. Surg. 2021, 13, 708–718. [Google Scholar] [CrossRef]
- Kuiper, J.W.P.; Verberne, S.J.; van Egmond, P.W.; Slot, K.; Temmerman, O.P.P.; Vos, C.J. Are accuracy studies for periprosthetic joint infection diagnosis inherently flawed? And what to do with Schrödinger’s hips? A prospective analysis of the alpha defensin lateral-flow test in chronic painful hip arthroplasties. Hip. Pelvis. 2022, 34, 236–244. [Google Scholar] [CrossRef]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Parvizi, J. Diagnosing periprosthetic joint infection: Has the era of the biomarker arrived? Clin. Orthop. Relat. R 2014, 472, 3254–3262. [Google Scholar] [CrossRef]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Parvizi, J. Combined measurement of synovial fluid alpha-defensin and C-reactive protein levels: Highly accurate for diagnosing periprosthetic joint infection. J. Bone Jt. Surg. Am. 2014, 96, 1439–1445. [Google Scholar] [CrossRef]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Booth, R.E., Jr.; Parvizi, J. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin. Orthop. Relat. Res. 2015, 473, 198–203. [Google Scholar] [CrossRef]
- Frangiamore, S.J.; Gajewski, N.D.; Saleh, A.; Farias-Kovac, M.; Barsoum, W.K.; Higuera, C.A. Alpha-defensin accuracy to diagnose periprosthetic joint infection-best available test? J. Arthroplast. 2016, 31, 456–460. [Google Scholar] [CrossRef]
- Bonanzinga, T.; Zahar, A.; Dutsch, M.; Lausmann, C.; Kendoff, D.; Gehrke, T. How reliable is the alpha-defensin immunoassay test for diagnosing periprosthetic joint infection? A prospective study. Clin. Orthop. Relat. Res. 2017, 475, 408–415. [Google Scholar] [CrossRef]
- Kleiss, S.; Jandl, N.M.; Novo de Oliveira, A.; Ruther, W.; Niemeier, A. Diagnostic accuracy of alpha-defensin enzyme-linked immunosorbent assay in the clinical evaluation of painful hip and knee arthroplasty with possible prosthetic joint infection: A prospective study of 202 cases. Bone Jt. J. 2019, 101, 970–977. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Ni, M.; Fu, J.; Xu, C.; Chai, W.; Chen, J.Y. What is the performance of novel synovial biomarkers for detecting periprosthetic joint infection in the presence of inflammatory joint disease? Bone Jt. J. 2021, 103, 32–38. [Google Scholar] [CrossRef]
- Iorio, R.; Viglietta, E.; Mazza, D.; Petrucca, A.; Borro, M.; Iolanda, S.; Simmaco, M.; Ferretti, A. Accuracy and cost-effectivenss of a novel method for alpha defensins measurement in the diagnosis of periprosthetic joint infections. J. Arthroplast. 2021, 36, 3275–3281. [Google Scholar] [CrossRef]
- Parvizi, J.; Ghanem, E.; Menashe, S.; Barrack, R.L.; Bauer, T.W. Periprosthetic infection: What are the diagnostic challenges? J. Bone Jt. Surg. Am. 2006, 88 (Suppl. 4), 138–147. [Google Scholar] [CrossRef]
- Saleh, A.; Ramanathan, D.; Siqueira, M.B.P.; Klika, A.K.; Barsoum, W.K.; Rueda, C.A.H. The diagnostic utility of synovial fluid markers in periprosthetic joint infection: A systematic review and meta-analysis. J. Am. Acad. Orthop. Surg. 2017, 25, 763–772. [Google Scholar] [CrossRef]
- Lenski, M.; Scherer, M.A. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J. Arthroplast. 2014, 29, 1105–1109. [Google Scholar] [CrossRef]
- Parvizi, J.; McKenzie, J.C.; Cashman, J.P. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J. Arthroplast. 2012, 27, 12–16. [Google Scholar] [CrossRef]
- Parvizi, J.; Jacovides, C.; Adeli, B.; Jung, K.A.; Hozack, W.J. Mark B. Coventry Award: Synovial C-reactive protein: A prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin. Orthop. Relat. Res. 2012, 470, 54–60. [Google Scholar] [CrossRef]
- De Vecchi, E.; Villa, F.; Bortolin, M.; Toscano, M.; Tacchini, L.; Romano, C.L.; Drago, L. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: A prospective study. Clin. Microbiol. Infect. 2016, 22, 555–560. [Google Scholar] [CrossRef]
- Gallo, J.; Svoboda, M.; Zapletalova, J.; Proskova, J.; Juranova, J. Serum IL-6 in combination with synovial IL-6/CRP shows excellent diagnostic power to detect hip and knee prosthetic joint infection. PLoS ONE 2018, 13, e0199226. [Google Scholar] [CrossRef] [PubMed]
- Plate, A.; Anagnostopoulos, A.; Glanzmann, J.; Stadler, L.; Weigelt, L.; Sutter, R.; Kastli, M.; Zinkernagel, A.S.; Zingg, P.O.; Achermann, Y. Synovial C-reactive protein features high negative predictive value but is not useful as a single diagnostic parameter in suspected periprosthetic joint infection (PJI). J. Infect. 2019, 78, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Grzelecki, D.; Walczak, P.; Szostek, M.; Grajek, A.; Rak, S.; Kowalczewski, J. Blood and synovial fluid calprotectin as biomarkers to diagnose chronic hip and knee periprosthetic joint infections. Bone Jt. J. 2021, 103, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qin, L.; Wang, J.; Hu, N.; Huang, W. Combined serum and synovial C-reactive protein tests: A valuable adjunct to the diagnosis of chronic prosthetic joint infection. BMC Musculoskelet. Disord. 2021, 22, 670. [Google Scholar] [CrossRef] [PubMed]
- Praz, C.; Gubbiotti, L.; Buia, G.; Chapus, V.; Dunet, J.; Grandhomme, F.; Michon, J.; Rochcongar, G.; Hulet, C. Value of the synovial C-reactive protein test in the diagnosis of total hip and knee periprosthetic joint infections: A case-control study. Orthop. Traumatol. Surg. Res. 2021, 107, 102903. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Ploegmakers, J.J.W.; Kampinga, G.A.; Wagenmakers-Huizenga, L.; Jutte, P.C.; Muller Kobold, A.C. Synovial calprotectin: A potential biomarker to exclude a prosthetic joint infection. Bone Jt. J. 2017, 99, 660–665. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Ploegmakers, J.J.W.; Ottink, K.; Kampinga, G.A.; Wagenmakers-Huizenga, L.; Jutte, P.C.; Kobold, A.C.M. Synovial calprotectin: An inexpensive biomarker to exclude a chronic prosthetic joint infection. J. Arthroplast. 2018, 33, 1149–1153. [Google Scholar] [CrossRef]
- Salari, P.; Grassi, M.; Cinti, B.; Onori, N.; Gigante, A. Synovial fluid calprotectin for the preoperative diagnosis of chronic periprosthetic joint infection. J. Arthroplast. 2020, 35, 534–537. [Google Scholar] [CrossRef]
- Trotter, A.J.; Dean, R.; Whitehouse, C.E.; Mikalsen, J.; Hill, C.; Brunton-Sim, R.; Kay, G.L.; Shakokani, M.; Durst, A.Z.E.; Wain, J.; et al. Preliminary evaluation of a rapid lateral flow calprotectin test for the diagnosis of prosthetic joint infection. Bone Jt. Res. 2020, 9, 202–210. [Google Scholar] [CrossRef]
- Warren, J.; Anis, H.K.; Bowers, K.; Pannu, T.; Villa, J.; Klika, A.K.; Colon-Franco, J.; Piuzzi, N.S.; Higuera, C.A. Diagnostic utility of a novel point-of-care test of calprotectin for periprosthetic joint infection after total knee arthroplasty: A prospective cohort study. J. Bone Jt. Surg. Am. 2021, 103, 1009–1015. [Google Scholar] [CrossRef]
- Warren, J.A.; Klika, A.K.; Bowers, K.; Colon-Franco, J.; Piuzzi, N.S.; Higuera, C.A. Calprotectin lateral flow test: Consistent across criteria for ruling out periprosthetic joint infection. J. Arthroplast. 2022, 37, 1153–1158. [Google Scholar] [CrossRef]
- Cheok, T.; Smith, T.; Siddiquee, S.; Jennings, M.P.; Jayasekera, N.; Jaarsma, R.L. Synovial fluid calprotectin performs better than synovial fluid polymerase chain reaction and interleukin-6 in the diagnosis of periprosthetic joint infection: A systematic review and meta-analysis. Bone Jt. J. 2022, 104, 311–320. [Google Scholar] [CrossRef]
- Grassi, M.; Salari, P.; Farinelli, L.; D'Anzeo, M.; Onori, N.; Gigante, A. Synovial biomarkers to detect chronic periprosthetic joint infection: A pilot study to compare calprotectin rapid test, calprotectin ELISA immunoassay and leukocyte esterase test. J. Arthroplast. 2022, 37, 781–786. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Li, X.; Deng, Z.; Zheng, M.; Zheng, Q. The immune cell landscape in different anatomical structures of knee in osteoarthritis: A gene expression-based study. Biomed. Res. Int. 2020, 2020, 9647072. [Google Scholar] [CrossRef]
- Xing, J.; Li, J.; Yan, Z.; Li, Y.; Liu, X.; He, L.; Xu, T.; Wang, C.; Zhao, L.; Jie, K. Diagnostic accuracy of calprotectin in periprosthetic joint infection: A diagnostic meta-analysis. J. Orthop. Surg. Res. 2022, 17, 11. [Google Scholar] [CrossRef]
- Xie, K.; Dai, K.; Qu, X.; Yan, M. Serum and synovial fluid interleukin-6 for the diagnosis of periprosthetic joint infection. Sci. Rep. 2017, 7, 1496. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Deng, B. Serum versus synovial fluid interleukin-6 for periprosthetic joint infection diagnosis: A systematic review and meta-analysis of 30 diagnostic test accuracy studies. J. Orthop. Surg. Res. 2022, 17, 564. [Google Scholar] [CrossRef]
- Qin, L.; Du, C.; Yang, J.; Wang, H.; Su, X.; Wei, L.; Zhao, C.; Chen, C.; Chen, H.; Hu, N.; et al. Synovial fluid interleukin levels cannot distinguish between prosthetic joint infection and active rheumatoid arthritis after hip or knee arthroplasty. Diagnostics 2022, 12, 1196. [Google Scholar] [CrossRef]
- Su, X.; Chen, Y.; Zhan, Q.; Zhu, B.; Chen, L.; Zhao, C.; Yang, J.; Wei, L.; Xu, Z.; Wei, K.; et al. The ratio of Il-6 to Il-4 in synovial fluid of knee or hip performances a noteworthy diagnostic value in prosthetic joint infection. J. Clin. Med. 2022, 11, 6520. [Google Scholar] [CrossRef]
- Shahi, A.; Tan, T.L.; Kheir, M.M.; Tan, D.D.; Parvizi, J. Diagnosing periprosthetic joint infection: And the winner is? J. Arthroplast. 2017, 32, S232–S235. [Google Scholar] [CrossRef]
- Wang, C.; Li, R.; Wang, Q.; Wang, C. Synovial fluid leukocyte esterase in the diagnosis of peri-prosthetic joint infection: A systematic review and meta-analysis. Surg. Infect. 2018, 19, 245–253. [Google Scholar] [CrossRef]
- Chisari, E.; Yacovelli, S.; Goswami, K.; Shohat, N.; Woloszyn, P.; Parvizi, J. Leukocyte esterase versus ICM 2018 criteria in the diagnosis of periprosthetic joint infection. J. Arthroplast. 2021, 36, 2942–2945.e2941. [Google Scholar] [CrossRef] [PubMed]
- Logoluso, N.; Pellegrini, A.; Suardi, V.; Morelli, I.; Battaglia, A.G.; D'Anchise, R.; De Vecchi, E.; Zagra, L. Can the leukocyte esterase strip test predict persistence of periprosthetic joint infection at second-stage reimplantation? J. Arthroplast. 2022, 37, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.; Fernandez-Pittol, M.J.; Munoz-Mahamud, E.; Morata, L.; Bosch, J.; Vila, J.; Soriano, A.; Casals-Pascual, C. Evaluation of lipocalin-2 as a biomarker of periprosthetic joint infection. J. Arthroplast. 2019, 34, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Gallo, J.; Zapletalova, J.; Proskova, J.; Juranova, J.; Loveckova, Y. Glucose, lactate, NGAL and coefficient of energy balance in synovial fluid in patients with hip and knee prosthetic joint infection. Acta Chir. Orthop. Traumatol. Cech. 2022, 89, 16–26. [Google Scholar]
- Vicenti, G.; Bizzoca, D.; Nappi, V.; Pesce, V.; Solarino, G.; Carrozzo, M.; Moretti, F.; Dicuonzo, F.; Moretti, B. Serum biomarkers in the diagnosis of periprosthetic joint infection: Consolidated evidence and recent developments. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 43–50. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Ding, P.; Jiao, Q. The accuracy of D-dimer in the diagnosis of periprosthetic infections: A systematic review and meta-analysis. J. Orthop Surg. Res. 2022, 17, 99. [Google Scholar] [CrossRef]
- Piper, K.E.; Fernandez-Sampedro, M.; Steckelberg, K.E.; Mandrekar, J.N.; Karau, M.J.; Steckelberg, J.M.; Berbari, E.F.; Osmon, D.R.; Hanssen, A.D.; Lewallen, D.G.; et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS ONE 2010, 5, e9358. [Google Scholar] [CrossRef]
- Xie, K.; Qu, X.; Yan, M. Procalcitonin and alpha-defensin for diagnosis of periprosthetic joint infections. J. Arthroplast. 2017, 32, 1387–1394. [Google Scholar] [CrossRef]
- Dapunt, U.; Giese, T.; Maurer, S.; Stegmaier, S.; Prior, B.; Hansch, G.M.; Gaida, M.M. Neutrophil-derived MRP-14 is up-regulated in infectious osteomyelitis and stimulates osteoclast generation. J. Leukoc. Biol. 2015, 98, 575–582. [Google Scholar] [CrossRef]
- Shohat, N.; Goswami, K.; Fillingham, Y.; Tan, T.L.; Calkins, T.; Della Valle, C.J.; George, J.; Higuera, C.; Parvizi, J. Diagnosing periprosthetic joint infection in inflammatory arthritis: Assumption is the enemy of true understanding. J. Arthroplast. 2018, 33, 3561–3566. [Google Scholar] [CrossRef]
- Ren, Y.; Biedermann, L.; Gwinner, C.; Perka, C.; Kienzle, A. Serum and synovial markers in patients with rheumatoid arthritis and periprosthetic joint infection. J. Pers. Med. 2022, 12, 810. [Google Scholar] [CrossRef]
- George, J.; Jawad, M.; Curtis, G.L.; Samuel, L.T.; Klika, A.K.; Barsoum, W.K.; Higuera, C.A. Utility of serological markers for detecting persistent infection in two-stage revision arthroplasty in patients with inflammatory arthritis. J. Arthroplast. 2018, 33, S205–S208. [Google Scholar] [CrossRef]
- Saleh, A.; George, J.; Faour, M.; Klika, A.K.; Higuera, C.A. Serum biomarkers in periprosthetic joint infections. Bone Jt. Res. 2018, 7, 85–93. [Google Scholar] [CrossRef]
- Abdelbary, H.; Cheng, W.; Ahmadzai, N.; Carli, A.V.; Shea, B.J.; Hutton, B.; Fergusson, D.A.; Beaule, P.E. Combination tests in the diagnosis of chronic periprosthetic joint infection: Systematic review and development of a stepwise clinical decision-making tool. J. Bone Jt. Surg. Am. 2020, 102, 114–124. [Google Scholar] [CrossRef]
- Tunney, M.M.; Patrick, S.; Gorman, S.P.; Nixon, J.R.; Anderson, N.; Davis, R.I.; Hanna, D.; Ramage, G. Improved detection of infection in hip replacements. A currently underestimated problem. J. Bone Jt. Surg. Br. 1998, 80, 568–572. [Google Scholar] [CrossRef]
- Tunney, M.M.; Patrick, S.; Curran, M.D.; Ramage, G.; Hanna, D.; Nixon, J.R.; Gorman, S.P.; Davis, R.I.; Anderson, N. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J. Clin. Microbiol. 1999, 37, 3281–3290. [Google Scholar] [CrossRef]
- Moojen, D.J.; van Hellemondt, G.; Vogely, H.C.; Burger, B.J.; Walenkamp, G.H.; Tulp, N.J.; Schreurs, B.W.; de Meulemeester, F.R.; Schot, C.S.; van de Pol, I.; et al. Incidence of low-grade infection in aseptic loosening of total hip arthroplasty. Acta Orthop. 2010, 81, 667–673. [Google Scholar] [CrossRef]
- Piper, K.E.; Jacobson, M.J.; Cofield, R.H.; Sperling, J.W.; Sanchez-Sotelo, J.; Osmon, D.R.; McDowell, A.; Patrick, S.; Steckelberg, J.M.; Mandrekar, J.N.; et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 2009, 47, 1878–1884. [Google Scholar] [CrossRef]
- Vergidis, P.; Greenwood-Quaintance, K.E.; Sanchez-Sotelo, J.; Morrey, B.F.; Steinmann, S.P.; Karau, M.J.; Osmon, D.R.; Mandrekar, J.N.; Steckelberg, J.M.; Patel, R. Implant sonication for the diagnosis of prosthetic elbow infection. J. Shoulder Elbow. Surg. 2011, 20, 1275–1281. [Google Scholar] [CrossRef]
- Cazanave, C.; Greenwood-Quaintance, K.E.; Hanssen, A.D.; Karau, M.J.; Schmidt, S.M.; Gomez Urena, E.O.; Mandrekar, J.N.; Osmon, D.R.; Lough, L.E.; Pritt, B.S.; et al. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J. Clin. Microbiol. 2013, 51, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.S.; Parvizi, J. Diagnosis and treatment of culture-negative periprosthetic joint infection. J. Arthroplast. 2022, 37, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Eggleston, K.; Rotimi, V.; Zeckhauser, R.J. Antibiotic resistance as a global threat: Evidence from China, Kuwait and the United States. Glob. Health 2006, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, A.R. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States; Department of Health and Human Services: Atlanta, GA, USA, 2019. [Google Scholar]
- CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/drugresistance/covid19.html (accessed on 30 December 2022).
- Dapunt, U.; Maurer, S.; Giese, T.; Gaida, M.M.; Hansch, G.M. The macrophage inflammatory proteins MIP1alpha (CCL3) and MIP2alpha (CXCL2) in implant-associated osteomyelitis: Linking inflammation to bone degradation. Mediat. Inflamm. 2014, 2014, 728619. [Google Scholar] [CrossRef]
- Dapunt, U.; Giese, T.; Stegmaier, S.; Moghaddam, A.; Hansch, G.M. The osteoblast as an inflammatory cell: Production of cytokines in response to bacteria and components of bacterial biofilms. BMC Musculoskelet. Disord. 2016, 17, 243. [Google Scholar] [CrossRef]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Hartman, C.W.; Garvin, K.L.; Kielian, T. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J. Immunol. 2015, 194, 3861–3872. [Google Scholar] [CrossRef]
- Marazzi, M.G.; Randelli, F.; Brioschi, M.; Drago, L.; Romano, C.L.; Banfi, G.; Massaccesi, L.; Crapanzano, C.; Morelli, F.; Corsi Romanelli, M.M.; et al. Presepsin: A potential biomarker of PJI? A comparative analysis with known and new infection biomarkers. Int. J. Immunopathol. Pharmacol. 2018, 31, 394632017749356. [Google Scholar] [CrossRef]
- Zou, Q.; Wen, W.; Zhang, X.-C. Presepsin as a novel sepsis biomarker. World J. Emerg. Med. 2014, 5, 16. [Google Scholar] [CrossRef]
- Castello, L.M.; Baldrighi, M.; Molinari, L.; Salmi, L.; Cantaluppi, V.; Vaschetto, R.; Zunino, G.; Quaglia, M.; Bellan, M.; Gavelli, F.; et al. The role of osteopontin as a diagnostic and prognostic biomarker in sepsis and septic shock. Cells 2019, 8, 174. [Google Scholar] [CrossRef]
- Masters, T.L.; Bhagwate, A.V.; Dehankar, M.K.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Mandrekar, J.N.; Patel, R. Human transcriptomic response to periprosthetic joint infection. Gene 2022, 825, 146400. [Google Scholar] [CrossRef]
- Fröschen, F.S.; Schell, S.; Wimmer, M.D.; Hischebeth, G.T.; Kohlhof, H.; Gravius, S.; Randau, T.M. Synovial complement factors in patients with periprosthetic joint infection after undergoing revision arthroplasty of the hip or knee joint. Diagnostics 2021, 11, 434. [Google Scholar] [CrossRef]
- Fisher, C.R.; Salmons, H.I.; Mandrekar, J.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Patel, R. A 92 protein inflammation panel performed on sonicate fluid differentiates periprosthetic joint infection from non-infectious causes of arthroplasty failure. Sci. Rep. 2022, 12, 16135. [Google Scholar] [CrossRef]
- Fisher, C.F.; Mangalaparthi, K.K.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Pandey, A. Mass spectrometry-based proteomic profiling of sonicate fluid differentiates Staphylococcus aureus periprosthetic joint infection from non-infectious failure: A pilot study. medRxiv 2023. [Google Scholar] [CrossRef]
- Fisher, C.R.; Krull, J.E.; Bhagwate, A.; Masters, T.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Patel, R. Sonicate fluid cellularity predicted by transcriptomic deconvolution differentiates infectious from non-infectious arthroplasty failure. J. Bone Jt. Surg. Am. 2022, 105, 63–73. [Google Scholar] [CrossRef]
- Johnzon, C.F.; Ronnberg, E.; Pejler, G. The role of mast cells in bacterial infection. Am. J. Pathol. 2016, 186, 4–14. [Google Scholar] [CrossRef]
- Piliponsky, A.M.; Acharya, M.; Shubin, N.J. Mast cells in viral, bacterial, and fungal infection immunity. Int. J. Mol. Sci. 2019, 20, 2851. [Google Scholar] [CrossRef]
- Nigrovic, P.A.; Binstadt, B.A.; Monach, P.A.; Johnsen, A.; Gurish, M.; Iwakura, Y.; Benoist, C.; Mathis, D.; Lee, D.M. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc. Natl. Acad. Sci. USA 2007, 104, 2325–2330. [Google Scholar] [CrossRef]
- Nigrovic, P.A.; Lee, D.M. Synovial mast cells: Role in acute and chronic arthritis. Immunol. Rev. 2007, 217, 19–37. [Google Scholar] [CrossRef]
- Limberg, A.K.; Salib, C.G.; Tibbo, M.E.; Vargas-Hernandez, J.S.; Bettencourt, J.W.; Bayram, B.; Berry, C.E.; Dudakovic, A.; Bolon, B.; van Wijnen, A.J.; et al. Immune cell populations differ in patients undergoing revision total knee arthroplasty for arthrofibrosis. Sci. Rep. 2022, 12, 22627. [Google Scholar] [CrossRef] [PubMed]
- Korn, M.F.; Stein, R.R.; Dolf, A.; Shakeri, F.; Buness, A.; Hilgers, C.; Masson, W.; Gravius, S.; Kohlhof, H.; Burger, C.; et al. High-dimensional analysis of immune cell composition predicts periprosthetic joint infections and dissects its pathophysiology. Biomedicines 2020, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Paziuk, T.; Rondon, A.J.; Goswami, K.; Tan, T.L.; Parvizi, J. A novel adjunct indicator of periprosthetic joint infection: Platelet count and mean platelet volume. J. Arthroplast. 2020, 35, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Festa, E.; Ascione, T.; Bernasconi, A.; Di Gennaro, D.; Basso, M.A.; Guarino, A.; Balato, G. Diagnostic performance of neutrophil to lymphocyte ratio, monocyte to lymphocyte ratio, platelet to lymphocyte ratio, and platelet to mean platelet volume ratio in periprosthetic hip and knee infections: A systematic review and meta-analysis. Diagnostics 2022, 12, 2033. [Google Scholar] [CrossRef]
- Sahin, E.; Karaismailoglu, B.; Ozsahin, M.K.; Guven, M.F.; Kaynak, G. Low value of platelet count to mean platelet volume ratio to diagnose chronic PJI: A case control study. Orthop. Traumatol. Surg. Res. 2021, 107, 102899. [Google Scholar] [CrossRef]
- Heim, C.E.; Vidlak, D.; Odvody, J.; Hartman, C.W.; Garvin, K.L.; Kielian, T. Human prosthetic joint infections are associated with myeloid-derived suppressor cells (MDSCs): Implications for infection persistence. J. Orthop. Res. 2018, 36, 1605–1613. [Google Scholar] [CrossRef]
- Heim, C.E.; Bosch, M.E.; Yamada, K.J.; Aldrich, A.L.; Chaudhari, S.S.; Klinkebiel, D.; Gries, C.M.; Alqarzaee, A.A.; Li, Y.; Thomas, V.C.; et al. Lactate production by Staphylococcus aureus biofilm inhibits HDAC11 to reprogramme the host immune response during persistent infection. Nat. Microbiol. 2020, 5, 1271–1284. [Google Scholar] [CrossRef]
- Bosch, M.E.; Bertrand, B.P.; Heim, C.E.; Alqarzaee, A.A.; Chaudhari, S.S.; Aldrich, A.L.; Fey, P.D.; Thomas, V.C.; Kielian, T. Staphylococcus aureus ATP synthase promotes biofilm persistence by influencing innate immunity. mBio 2020, 11, e01581-20. [Google Scholar] [CrossRef]
- Deo, R.C. Machine learning in medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Handelman, G.S.; Kok, H.K.; Chandra, R.V.; Razavi, A.H.; Lee, M.J.; Asadi, H. eDoctor: Machine learning and the future of medicine. J. Intern. Med. 2018, 284, 603–619. [Google Scholar] [CrossRef]
- Weiskittel, T.M.; Correia, C.; Yu, G.T.; Ung, C.Y.; Kaufmann, S.H.; Billadeau, D.D.; Li, H. The trifecta of single-cell, systems-biology, and machine-learning approaches. Genes 2021, 12, 1098. [Google Scholar] [CrossRef]
- Wiens, J.; Shenoy, E.S. Machine learning for healthcare: On the verge of a major shift in healthcare epidemiology. Clin. Infect. Dis. 2018, 66, 149–153. [Google Scholar] [CrossRef]
- Polisetty, T.S.; Jain, S.; Pang, M.; Karnuta, J.M.; Vigdorchik, J.M.; Nawabi, D.H.; Wyles, C.C.; Ramkumar, P.N. Concerns surrounding application of artificial intelligence in hip and knee arthroplasty: A review of literature and recommendations for meaningful adoption. Bone Jt. J. 2022, 104, 1292–1303. [Google Scholar] [CrossRef]
- Klemt, C.; Laurencin, S.; Uzosike, A.C.; Burns, J.C.; Costales, T.G.; Yeo, I.; Habibi, Y.; Kwon, Y.M. Machine learning models accurately predict recurrent infection following revision total knee arthroplasty for periprosthetic joint infection. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2582–2590. [Google Scholar] [CrossRef]
- Fu, S.; Wyles, C.C.; Osmon, D.R.; Carvour, M.L.; Sagheb, E.; Ramazanian, T.; Kremers, W.K.; Lewallen, D.G.; Berry, D.J.; Sohn, S.; et al. Automated detection of periprosthetic joint infections and data elements using natural language processing. J. Arthroplast. 2021, 36, 688–692. [Google Scholar] [CrossRef]
- Shohat, N.; Goswami, K.; Tan, T.L.; Yayac, M.; Soriano, A.; Sousa, R.; Wouthuyzen-Bakker, M.; Parvizi, J.; ESCMID Study Group of Implant Associated Infections; Northern Infection Network of Joint Arthroplasty. 2020 Frank Stinchfield Award: Identifying who will fail following irrigation and debridement for prosthetic joint infection. Bone Jt. J. 2020, 102, 11–19. [Google Scholar] [CrossRef]



| Biomarker | Knee/Hip/Other | Time Since Arthroplasty | Cut-Point | Sensitivity (%) a | Specificity (%) a | Citation |
|---|---|---|---|---|---|---|
| Total nucleated cell count—cutoff values in cells/µL | ||||||
| Mason et al., 2003 | 440/-/- | NR | 2500 50,000 | 69 19 | 98 100 | [84] |
| Trampuz et al., 2004 | 133/-/- | >6 months | 1700 | 94 (80–99) | 88 (80–93) | [85] |
| Zmistowski et al., 2012 | 153/-/- | NR | 3000 | 94 | 93 | [86] |
| Dinneen et al., 2013 | 48/27/- | NR | 1590 | 90 (78–100) | 91 (83–100) | [87] |
| Wyles et al., 2013 | -/39/- | NR | 3000 | 100 (40–100) | 57 (85–100) | [88] |
| Gallo et al., 2017 | 203/188/- | >7 months | 3450 | 95 | 95 | [89] |
| Higuera et al., 2017 | -/453/- | ≥3 months | 3966 | 90 | 91 | [90] |
| Kim et al., 2017 | 197/-/- | >7 days | 11,200 16,000 | 100 (73–100) 75 (43–95) | 99 (96–100) 100 (98–100) | [91] |
| Lee et al., 2017 | 33 studies | Pooled | Pooled | 89 (86–91) | 86 (80–90) | [92] |
| Shahi et al., 2017 | 836 total | NR | 10,000 | 86 | 83 | [93] |
| Sousa et al., 2017 | 40/15/- | >1 month | 1463 2064 | 100 91 | 7275 | [94] |
| Balato et al., 2018 | 250/-/- | >90 days | 3000 | 81 (74–86) | 91 (86–95) | [95] |
| De Vecchi et al., 2018 | 45/21/- | NR | 1600 3000 | 100 (87–100) 94 (78–99) | 82 (65–93) 91 (75–98) | [96] |
| Kuo et al., 2018 | 131/83/- | NR | 835 | 84 (65–96) | 78 (72–84) | [97] |
| Tahta et al., 2018 | 38/-/- | >3 months | 2347 | 86 (70–100) | 76 (63–98) | [98] |
| Carli et al., 2019 | 26 studies | Pooled | Pooled | 93 | 90 | [99] |
| Dijkman et al., 2020 | 80/-/- | NR | 2575 | 92 | 84 | [100] |
| Mihalič et al., 2020 | 25/24/- | NR | 1700 | 82 (55–100) | 97 (92–100) | [101] |
| Sharma et al., 2020 | 93/14/- | NR | 1100 | 89 | 98 | [102] |
| Ivy et al., 2021 | 74/25/- | NR | 1700 | 83 (59–96) | 81 (70–89) | [103] |
| Levent et al., 2021 | 143/116/- | NR | 3000 | 88 | 88 | [104] |
| van den Kieboom et al., 2021 | 43/101/- | NR | 3000 4552 | 87 (66–97) 86 | 78 (66–87) 85 | [105] |
| Baker et al., 2022 | 358/36/- | >90 days | 3000 | 92 | 99 | [106] |
| Huang et al., 2022 | 39/39/- | NR | 3005 | 90 (78–97) | 100 (88–100) | [107] |
| Lazic et al., 2022 | 4/10/- | NR | 4550 | 40 (12–74) | 100 (79–100) | [108] |
| Dilley et al., 2023 | 485/245/- | >6 weeks | 5600 | 72 | 86 | [109] |
| Polymorphonuclear (PMN) percentage—cutoff values in % of total white blood cell count | ||||||
| Mason et al., 2003 | 440/-/- | NR | 6080 | 7657 | 89100 | [84] |
| Trampuz et al., 2004 | 133/-/- | >6 months | 65 | 97 (85–100) | 98 (93–100) | [85] |
| Zmistowski et al., 2012 | 153/-/- | NR | 75 | 83 | 88 | [86] |
| Dinneen et al., 2013 | 48/27/- | NR | 65 | 90 (80–100) | 87 (76–97) | [87] |
| Wyles et al., 2013 | -/39/- | NR | 80 | 100 (40–100) | 97 (81–100) | [88] |
| Gallo et al., 2017 | 203/188/- | >7 months | 75 | 93 | 91 | [89] |
| Higuera et al., 2017 | -/453/- | ≥3 months | 80 | 92 | 86 | [90] |
| Lee et al., 2017 | 33 studies | Pooled | Pooled | 89 (82–93) | 86 (77–92) | [92] |
| Sousa et al., 2017 | 40/15/- | >1 month | 7881 | 8778 | 7275 | [94] |
| Balato et al., 2018 | 250/-/- | >90 days | 80 | 84 (77–89) | 95 (90–98) | [95] |
| Mihalič et al., 2020 | 25/24/- | NR | 65 | 82 (55–100) | 97 (92–100) | [101] |
| Qin et al., 2020 | 24/26/- | NR | 70 | 92 (74–99) | 80 (59–93) | [110] |
| Qin et al., 2020 | 45/48/- | >6 weeks | 70 | 89 (75–97) | 84 (72–92) | [111] |
| Sharma et al., 2020 | 93/14/- | NR | 72 | 92 | 91 | [102] |
| Ivy et al., 2021 | 74/25/- | NR | 65 | 90 (65–99) | 87 (78–94) | [103] |
| van den Kieboom et al., 2021 | 43/101/- | NR | 80 | 79 | 63 | [105] |
| Wang et al., 2021 | 45/48/- | >6 weeks | 70 | 95 (82–99) | 93 (83–98) | [112] |
| Qin et al., 2022 | 30/40/- | >2.5 years | 70 | 89 | 80 | [113] |
| Dilley et al., 2023 | 485/245/- | >6 weeks | 82 | 81 | 78 | [109] |
| Assay | Knee/Hip/Other | Cut-Point | Sensitivity (%) a | Specificity (%) a | Citation |
|---|---|---|---|---|---|
| Lateral flow | |||||
| Bingham et al., 2014 | 61/-/- | NA | 100 (79–100) | 95 (83–99) | [130] |
| Kasparek et al., 2016 | 29/11/- | NA | 67 (35–89) | 93 (75–99) | [131] |
| Sigmund et al., 2017 | 17/30/- | NA | 69 (46–92) | 94 (86–100) | [132] |
| Okroj et al., 2018 | -/26/- | NA | 100 | 68 | [133] |
| Berger et al., 2017 | 85/36/- | NA | 97 (85–100) | 97 (90–99) | [134] |
| Suda et al., 2017 | 19/11/- | NA | 77 | 82 | [135] |
| Balato et al., 2018 | 51/-/- | NA | 88 (75–95) | 97 (87–100) | [136] |
| de Saint Vincent et al., 2018 | 23/13/3 | NA | 89 | 91 | [137] |
| Gehrke et al., 2018 | 99/96/- | NA | 92 (84–97) | 100 (97–100) | [126] |
| Renz et al., 2018 | 151/61/- | NA | 84 (71–94) | 96 (92–99) | [120] |
| Riccio et al., 2018 | 49/22/2 | NA | 85 (70–94) | 97 (84–100) | [138] |
| Sigmund et al., 2018 | 54/17/- | NA | 77 (49–92) | 98 (90–100) | [121] |
| Stone et al., 2018 | 121/62/- | NA | 81 (65–92) | 96 (91–99) | [139] |
| Tahta et al., 2018 | 38/-/- | NA | 92 (80–100) | 98 (90–100) | [98] |
| Plate et al., 2018 | 60/49/- | NA | 90 (68–99) | 92 (85–97) | [140] |
| Carli et al., 2019 | 9 studies | NA | 96 | 82 | [99] |
| Sigmund et al., 2019 | 48/53/- | NA | 69 (51–83) | 94 (85–98) | [141] |
| Sharma et al., 2020 | 93/14/- | NA | 88 | 95 | [102] |
| Abdo et al., 2021 | 53/-/- | NA | 86 (65–97) | 100 (89–100) | [142] |
| de Saint Vincent et al., 2021 | 59/39/8 | NA | 96 | 91 | [143] |
| Deirmengian et al., 2021 | 203/102/- | NA | 94 (84–99) | 95 (91–97) | [115] |
| Ivy et al., 2021 | 74/25/- | NA | 83 (59–96) | 94 (86–98) | [103] |
| Yu et al., 2021 | 82/48/- | NA | 83 | 86 | [144] |
| Zeng et al., 2021 | 1443 total (pooled) | NA | 83 (77–88) | 95 (93–97) | [145] |
| Baker et al., 2022 | 358/36/- | NA | 99 | 87 | [106] |
| Kuiper et al., 2022 | -/57/- | NA | 83 (36–100) | 92 (81–98) | [146] |
| Enzyme-linked immunoassay (ELISA)—cutoff values in mg/L | |||||
| Deirmengian et al., 2014 | 84/11/- | 4.8 | 100 (88–100) | 100 (95–100) | [147] |
| Deirmengian et al., 2014 | 116/33/- | 5.2 | 97 (86–100) | 96 (90–99) | [148] |
| Deirmengian et al., 2015 | 43/3/- | 1.6 | 100 (85–100) | 100 (85–100) | [149] |
| Frangiamore et al., 2016 | 78 total(1st stage) 38 total (2nd stage) | 5.2 5.2 | 100 (86–100) 67 (12–95) | 98 (90–100) 97 (83–99) | [150] |
| Bonanzinga et al., 2017 | 65/91/- | 5.2 | 97 (92–99) | 97 (92–99) | [151] |
| De Vecchi et al., 2018 | 45/21/- | 5.2 | 84 (67–94) | 94 (79–99) | [96] |
| Sigmund et al., 2018 | 54/17/- | 5.2 | 85 (56–97) | 98 (90–100) | [121] |
| Carli et al., 2019 | 9 studies | Pooled | 97 | 87 | [99] |
| Kleiss et al., 2019 | 112/90/- | 5.2 | 78 (67–89) | 97 (93–99) | [152] |
| Abdo et al., 2021 | 53/-/- | 5.2 | 96 (77–100) | 100 (89–100) | [142] |
| Deirmengian et al., 2021 | 203/102/- | 5.2 | 89 (76–96) | 98 (94–99) | [115] |
| Ivy et al., 2021 | 74/25/- | 5.2 | 83 (59–96) | 96 (90–99) | [103] |
| Levent et al., 2021 | 143/116/- | 5.2 | 92 | 92 | [104] |
| Li et al., 2021 | 17/33 | 35.5 | 96 | 100 | [153] |
| Mass spectrometry | |||||
| Iorio et al., 2021 | 88/50/- | 5.2 mg/L | 93 (85–98) | 96 (89–99) | [154] |
| Balato et al., 2022 | 125/-/- | 1 µg/L | 100 (96–100) | 97 (90–98) | [124] |
| Biomarker | Knee/Hip/Other | Cut-Point | Sensitivity (%) a | Specificity (%) a | Citation |
|---|---|---|---|---|---|
| C-reactive protein (CRP)—cutoff values in mg/L | |||||
| Parvizi et al., 2012 | 43/12/- | 9.5 | 83 | 95 | [158] |
| Parvizi et al., 2012 | 66/-/- | 3.7 | 84 | 97 | [159] |
| Wyles et al., 2013 | -/39/- | 8 | 75 (19–99) | 68 (50–83) | [88] |
| Deirmengian et al., 2014 | 84/11/- | 12.2 | 90 (73–98) | 97 (90–100) | [147] |
| Deirmengian et al., 2014 | 116/33/- | 3 | 98 (86–100) | 79 (70–86) | [148] |
| De Vecchi et al., 2016 | 84/45/- | 10 | 82 (61–93) | 94 (87–98) | [160] |
| Kim et al., 2017 | 197/-/- | 34.9 74.5 | 100 (74–100) 58 (28–85) | 91 (83–95) 100 (97–100) | [91] |
| Lee et al., 2017 | 33 studies | Pooled | 85 (78–90) | 88 (78–94) | [92] |
| Sousa et al., 2017 | 40/15/- | 1.6 6.7 8.0 | 91 78 74 | 88 94 97 | [94] |
| De Vecchi et al., 2018 | 45/21/- | 1.0 | 88 (70–96) | 97 (83–100) | [96] |
| Gallo et al., 2018 | 116/124/- | 8.8 | 92 (73–99) | 100 (95–100) | [161] |
| Tahta et al., 2018 | 38/-/- | 11.7 | 76 (62–97) | 90 (80–100) | [98] |
| Carli et al., 2019 | 9 studies | Pooled | 93 | 89 | [99] |
| Plate et al., 2019 | 91/80/21 | 2.9 | 88 | 82 | [162] |
| Sharma et al., 2020 | 93/14/- | 5.6 | 80 | 92 | [102] |
| Baker et al., 2022 | 358/36/- | 6.9 | 74 | 98 | [106] |
| Grzelecki et al., 2021 | 50/35/- | 6.9 | 64 | 95 | [163] |
| Li et al., 2021 | 17/33/- | 9.0 | 76 | 96 | [153] |
| Wang et al., 2021 | 36/61/- | 7.3 | 85 (70–94) | 93 (83–98) | [164] |
| Praz et al., 2021 | 91/102/- | 2.74.4 | 85 (71–93) 83 (71–94.3) | 77 (68–84) 88 (82–94) | [165] |
| Qin et al., 2022 | 30/40/- | 11.6 | 89 | 49 | [113] |
| Calprotectin—cutoff values in mg/L | |||||
| Wouthuyzen-Bakker et al., 2017 | 10/45/6 | 50 (LF) | 89 (69–98) | 90 (78–96) | [166] |
| Wouthuyzen-Bakker et al., 2018 | 12/21/1 | 50 (LF) | 87 (60–98) | 92 (78–98) | [167] |
| Salari et al., 2020 | 76/-/- | 50 ELISA | 100 (100–100) | 95 (89–100) | [168] |
| Trotter et al., 2020 | 17/42/- | 14 (LF) | 75 (53–90) | 76 (60–87) | [169] |
| Grzelecki et al., 2021 | 50/35/- | 1.5 | 96 | 95 | [163] |
| Warren et al., 2021, 2022 | 123/-/- | 14 (LF) 14 (ELISA) 50 (LF) 50 (ELISA) | 98 98 98 98 | 87 83 96 96 | [170,171] |
| Cheok et al., 2022 | 5 studies | Pooled | 94 (82–98) | 93 (85–97) | [172] |
| Grassi et al., 2022 | 93/-/- | 50 (LF) 50 (ELISA) | 97 (87–100) 92 (79–98) | 94 (84–99) 100 (93–100) | [173] |
| Hantouly et al., 2022 | 8 studies | Pooled | 92 (84–98) | 93 (84–99) | [174] |
| Lazic et al., 2022 | 4/10/- | 50 (LF) | 67 (40–93) | 79 (57–100) | [108] |
| Xing et al., 2022 | 7 studies | Pooled | 94 (87–98) | 93 (87–96) | [175] |
| Interleukin-6 (Il-6)—cutoff values in ng/mL | |||||
| Deirmengian et al., 2014 | 84/11/- | 2.3 | 89 (71–98) | 97 (89–100) | [147] |
| Lee et al., 2017 | 33 studies | Pooled | 81 (70–89) | 94 (88–97) | [92] |
| Xie et al., 2017 | 8 studies | Pooled | 91 (82–96) | 90 (84–95) | [176] |
| Gallo et al., 2018 | 116/124/- | 21.0 | 68 (47–85) | 95 (87–99) | [161] |
| Carli et al., 2019 | 5 studies | Pooled | 97 | 84 | [99] |
| Mihalič et al., 2020 | 25/24/- | 2.3 | 73 (45–100) | 95 (87–100) | [101] |
| Qin et al., 2020 | 45/48/- | 1.86 | 95 (82–99) | 93 (83–98) | [111] |
| Sharma et al., 2020 | 93/14/- | 0.417 | 74 | 88 | [102] |
| Cheok et al., 2022 | 6 studies | Pooled | 86 (74–92) | 94 (90–96) | [172] |
| Li et al., 2022 | 30 studies | Pooled | 87 (75–93) | 90 (85–93) | [177] |
| Qin et al., 2022 | 63/39/- | 1.3 | 90 (74–97) | 89 (73–96) | [178] |
| Qin et al., 2022 | 30/40/- | 2.0 | 91 | 97 | [113] |
| Su et al., 2022 | 78/102/- | 1.2 | 91 (79–97) | 52 (38–66) | [179] |
| Leukocyte esterase (LE) | |||||
| Deirmengian et al., 2015 | 43/3/- | + | 69 (41–89) | 100 (84–100) | [149] |
| De Vecchi et al., 2016 | 84/45/- | + | 93 (74–99) | 97 (91–99) | [160] |
| Lee et al., 2017 | 33 studies | Pooled | 77 | 95 | [92] |
| Shahi et al., 2017 | 659 total | + | 75 | 91 | [180] |
| De Vecchi et al., 2018 | 45/21/- | + + + | 94 (79–99) 56 (38–56) | 97 (83–100) 100 (87–100) | [96] |
| Wang et al., 2018 | 11 studies | Pooled | 90 (76–96) | 97 (95–98) | [181] |
| Carli et al., 2019 | 9 studies 10 studies | + + + | 97 84 | 9396 | [99] |
| Dijkman et al., 2020 | 89/-/- | + + | 39 | 88 | [100] |
| Sharma et al., 2020 | 93/14/- | + + | 81 90 | 9584 | [102] |
| Chisari et al., 2021 | 226/33/- | + + + | 74 51 | 91100 | [182] |
| Grzelecki et al., 2021 | 50/35/- | + + | 82 | 98 | [163] |
| Levent et al., 2021 | 143/116/- | + + | 78 | 91 | [104] |
| Yu et al., 2021 | 82/48/- | + + | 80 | 95 | [144] |
| Grassi et al., 2022 | 93/-/- | + | 46 (30 -63) | 94 (84–99) | [173] |
| Logoluso et al., 2022 | 21/58/- | + | 82 | 99 | [183] |
| Lipocalin | |||||
| Vergara et al., 2018 | 54/18/- | 152 ng/mL | 86 | 77 | [184] |
| Dijkman et al., 2020 | 89/-/- | 740 ng/mL | 92 | 83 | [100] |
| Li et al., 2021 | 17/33/- | 763 ng/mL | 100 | 100 | [153] |
| Huang et al., 2022 | 39/39/- | 263 ng/mL | 93 (77–99) | 98 (89–100) | [107] |
| Svoboda et al., 2022 | 56/33/- | 998 µg/mL | 100 | 100 | [185] |
| Patient-Related | Sample-Related | Treatment-Related | Failure-Related |
|---|---|---|---|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, C.R.; Patel, R. Profiling the Immune Response to Periprosthetic Joint Infection and Non-Infectious Arthroplasty Failure. Antibiotics 2023, 12, 296. https://doi.org/10.3390/antibiotics12020296
Fisher CR, Patel R. Profiling the Immune Response to Periprosthetic Joint Infection and Non-Infectious Arthroplasty Failure. Antibiotics. 2023; 12(2):296. https://doi.org/10.3390/antibiotics12020296
Chicago/Turabian StyleFisher, Cody R., and Robin Patel. 2023. "Profiling the Immune Response to Periprosthetic Joint Infection and Non-Infectious Arthroplasty Failure" Antibiotics 12, no. 2: 296. https://doi.org/10.3390/antibiotics12020296
APA StyleFisher, C. R., & Patel, R. (2023). Profiling the Immune Response to Periprosthetic Joint Infection and Non-Infectious Arthroplasty Failure. Antibiotics, 12(2), 296. https://doi.org/10.3390/antibiotics12020296






