Development of a Clinical Score to Stratify the Risk for Carbapenem-Resistant Enterobacterales Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Setting, Patients and Study Design
4.2. Definitions
4.3. Microbiological Studies
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Center | 1 | 2 | 3 | 4 * | 5 | 6 | 7 | 8 | 9 * | 10 | 11 | 12 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients included | |||||||||||||
| n | 71 | 66 | 60 | 7 | 26 | 44 | 58 | 18 | 9 | 31 | 34 | 19 | 443 |
| % | 16.0 | 14.9 | 13.5 | 1.6 | 5.9 | 9.9 | 13.1 | 4.1 | 2.0 | 7.0 | 7.7 | 4.3 | 100 |
| CRE | |||||||||||||
| n | 10 | 2 | 13 | 0 | 1 | 13 | 15 | 0 | 0 | 1 | 2 | 2 | 59 |
| % | 14.1 | 3.0 | 21.7 | 0.0 | 3.8 | 29.5 | 25.9 | 0.0 | 0.0 | 3.2 | 5.9 | 10.5 | 13.3 |
| blaKPC | |||||||||||||
| n | 10 | 2 | 13 | 0 | 1 | 9 | 15 | 0 | 0 | 0 | 2 | 2 | 54 |
| % | 14.1 | 3.0 | 21.7 | 0.0 | 3.8 | 20.4 | 25.9 | 0.0 | 0.0 | 0.0 | 5.9 | 10.5 | 12.2 |
| blaOXA | |||||||||||||
| n | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 5 |
| % | 0 | 0 | 0 | 0 | 0 | 9.1 | 0 | 0 | 0 | 3.2 | 0 | 0 | 1.1 |
| Variables | HM n = 266 n (%) | HSCT n = 85 n (%) | ST n = 92 n (%) | p-Value * |
|---|---|---|---|---|
| Stage of underlying cancer | ||||
| Recently diagnosed | 130 (48.9) | 4 (4.7) | 25 (27.2) | <0.0001 |
| Complete remission | 25 (9.4) | 48 (56.5) | 3 (3.3) | <0.0001 |
| Partial remission | 23 (8.6) | 13 (15.3) | 26 (28.3) | <0.0001 |
| Refractory | 27 (10.2) | 7 (8.2) | 12 (13) | 0.56 |
| Relapse | 61 (22.9) | 13 (15.3) | 26 (28.3) | 0.11 |
| Recent chemotherapy (1 month prior to bacteremia) | 193 (72.6) | 59 (69.4) | 80 (87) | 0.01 |
| Recent radiotherapy (1 month prior to bacteremia) | 9 (3.4) | 8 (9.4) | 14 (15.2) | <0.0001 |
| Receiving steroids during bacteremia | 96 (36.1) | 32 (37.6) | 22 (23.9) | 0.07 |
| Receiving biological agents | 27 (10.2) | 19 (22.4) | 6 (6.5) | 0.002 |
References
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef] [PubMed]
- Capone, A.; Giannella, M.; Fortini, D.; Giordano, A.; Meledandri, M.; Ballardini, M.; Venditti, M.; Bordi, E.; Capozzi, D.; Balice, M.P.; et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin. Microbiol. Infect. 2013, 19, E23–E30. [Google Scholar] [CrossRef]
- Castanheira, M.; Deshpande, L.M.; Mendes, R.E.; Canton, R.; Sader, H.S.; Jones, R.N. Variations in the Occurrence of Resistance Phenotypes and Carbapenemase Genes Among Enterobacteriaceae Isolates in 20 Years of the SENTRY Antimicrobial Surveillance Program. Open Forum Infect. Dis. 2019, 6 (Suppl. S1), S23–S33. [Google Scholar] [CrossRef]
- García-Betancur, J.C.; Appel, T.M.; Esparza, G.; Gales, A.C.; Levy-Hara, G.; Cornistein, W.; Vega, S.; Nuñez, D.; Cuellar, L.; Bavestrello, L.; et al. Update on the epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev. Anti-Infect. Ther. 2021, 19, 197–213. [Google Scholar] [CrossRef]
- Thomas, G.R.; Corso, A.; Pasterán, F.; Shal, J.; Sosa, A.; Pillonetto, M.; de Souza Peral, R.T.; Hormazábal, J.C.; Araya, P.; Saavedra, S.Y.; et al. Increased Detection of Carbapenemase-Producing Enterobacterales Bacteria in Latin America and the Caribbean during the COVID-19 Pandemic. Emerg. Infect. Dis. 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Villegas, M.V.; Pallares, C.J.; Escandón-Vargas, K.; Hernández-Gómez, C.; Correa, A.; Álvarez, C.; Rosso, F.; Matta, L.; Luna, C.; Zurita, J.; et al. Characterization and Clinical Impact of Bloodstream Infection Caused by Carbapenemase-Producing Enterobacteriaceae in Seven Latin American Countries. PLoS ONE 2016, 11, e0154092. [Google Scholar] [CrossRef]
- Averbuch, D.; Tridello, G.; Hoek, J.; Mikulska, M.; Akan, H.; Yanez San Segundo, L.; Pabst, T.; Özçelik, T.; Klyasova, G.; Donnini, I.; et al. Antimicrobial Resistance in Gram-Negative Rods Causing Bacteremia in Hematopoietic Stem Cell Transplant Recipients: Intercontinental Prospective Study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin. Infect. Dis. 2017, 65, 1819–1828. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Pagano, L.; Candoni, A.; Pastore, D.; Cattaneo, C.; Fanci, R.; Nosari, A.; Caira, M.; Spadea, A.; Busca, A.; et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: An Italian multicentre prospective survey. Clin. Microbiol. Infect. 2015, 21, 337–343. [Google Scholar] [CrossRef]
- Herrera, F.; Laborde, A.; Jordán, R.; Berruezo, L.; Roccia Rossi, I.; Valledor, A.; Lambert, S.; Pereyra, M.; Nenna, A.; Dictar, M.; et al. Current Epidemiology of Bacteremia in Patients with Hematological Malignancies and Hematopoietic Stem Cell Transplantation and the Impact of Antibiotic Resistance on Survival. In Proceedings of the 31st of European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Vienna, Austria, 9–12 July 2021. [Google Scholar]
- Tofas, P.; Skiada, A.; Angelopoulou, M.; Sipsas, N.; Pavlopoulou, I.; Tsaousi, S.; Pagoni, M.; Kotsopoulou, M.; Perlorentzou, S.; Antoniadou, A.; et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: Analysis of 50 cases. Int. J. Antimicrob. Agents 2016, 47, 335–339. [Google Scholar] [CrossRef]
- Freire, M.P.; Pierrotti, L.C.; Filho, H.H.; Ibrahim, K.Y.; Magri, A.S.; Bonazzi, P.R.; Hajar, L.; Diz, M.P.; Pereira, J.; Hoff, P.M.; et al. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in cancer patients. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Rossolini, G.M.; Piciocchi, A.; Bertaina, A.; Pisapia, G.; Pastore, D.; Sica, S.; Severino, A.; Cudillo, L.; Ciceri, F.; et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: A nationwide retrospective survey from Italy. Bone Marrow Transplant. 2015, 50, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, E.M.; Pagano, L.; Martino, B.; Candoni, A.; Di Blasi, R.; Nadali, G.; Fianchi, L.; Delia, M.; Sica, S.; Perriello, V.; et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: Clinical impact of carbapenem resistance in a multicentre prospective survey. Am. J. Hematol. 2016, 91, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.; Laborde, A.; Torre, V.; Jordán, R.; Roccia Rossi, I.; Valledor, A.; Costantini, P.; Dictar, M.; Nenna, A.; Caeiro, J.P.; et al. Bacteremia due to Enterobacteriaceae in cancer patients: Etiology, clinical features and outcome depending on antimicrobial resistance. Data from ROCAS Study. In Proceedings of the 29th of European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Amsterdam, The Netherlands, 13–16 April 2019. [Google Scholar]
- Girmenia, C.; Bertaina, A.; Piciocchi, A.; Perruccio, K.; Algarotti, A.; Busca, A.; Cattaneo, C.; Raiola, A.M.; Guidi, S.; Iori, A.P.; et al. Incidence, Risk Factors and Outcome of Pre-engraftment Gram-Negative Bacteremia After Allogeneic and Autologous Hematopoietic Stem Cell Transplantation: An Italian Prospective Multicenter Survey. Clin. Infect. Dis. 2017, 65, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Micozzi, A.; Gentile, G.; Minotti, C.; Cartoni, C.; Capria, S.; Ballarò, D.; Santilli, S.; Pacetti, E.; Grammatico, S.; Bucaneve, G.; et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: Factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect. Dis. 2017, 17, 203. [Google Scholar] [CrossRef]
- Satlin, M.J.; Cohen, N.; Ma, K.C.; Gedrimaite, Z.; Soave, R.; Askin, G.; Chen, L.; Kreiswirth, B.N.; Walsh, T.J.; Seo, S.K. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J. Infect. 2016, 73, 336–345. [Google Scholar] [CrossRef]
- Giannella, M.; Pascale, R.; Gutiérrez-Gutiérrez, B.; Cano, A.; Viale, P. The use of predictive scores in the management of patients with carbapenem-resistant Klebsiella pneumoniae infection. Expert Rev. Anti-Infect. Ther. 2019, 17, 265–273. [Google Scholar] [CrossRef]
- Giannella, M.; Trecarichi, E.M.; De Rosa, F.G.; Del Bono, V.; Bassetti, M.; Lewis, R.E.; Losito, A.R.; Corcione, S.; Saffioti, C.; Bartoletti, M.; et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: A prospective observational multicentre study. Clin. Microbiol. Infect. 2014, 20, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Gracia-Ahufinger, I.; Pérez-Nadales, E.; Causse, M.; Castón, J.J.; Guzman-Puche, J.; Torre-Giménez, J.; Kindelán, L. Risks of Infection and Mortality Among Patients Colonized with Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae: Validation of Scores and Proposal for Management. Clin. Infect. Dis. 2018, 66, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Bartoletti, M.; Morelli, M.C.; Tedeschi, S.; Cristini, F.; Tumietto, F.; Pasqualini, E.; Danese, I.; Campoli, C.; Lauria, N.D.; et al. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: The importance of pre- and posttransplant colonization. Am. J. Transplant. 2015, 15, 1708–1715. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Hu, H.; Zhang, S.; Wei, J.; Yang, Q.; Qu, T. Clinical Characteristics and Risk Factors for Bloodstream Infection Due to Carbapenem-Resistant Klebsiella pneumoniae in Patients with Hematologic Malignancies. Infect. Drug Resist. 2020, 13, 3233–3242. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.; Chen, Z.; Hou, H.; Shen, N.; Wang, Z.; Wang, F.; Sun, Z. Construction of a Risk Prediction Model for Subsequent Bloodstream Infection in Intestinal Carriers of Carbapenem-Resistant Enterobacteriaceae: A Retrospective Study in Hematology Department and Intensive Care Unit. Infect. Drug Resist. 2021, 14, 815–824. [Google Scholar] [CrossRef]
- Wu, Q.; Qian, C.; Yin, H.; Liu, F.; Wu, Y.; Li, W.; Xia, L.; Ma, L.; Hong, M. A Novel Risk Predictive Scoring Model for Predicting Subsequent Infection After Carbapenem-Resistant Gram-Negative Bacteria Colonization in Hematological Malignancy Patients. Front. Oncol. 2022, 12, 897479. [Google Scholar] [CrossRef]
- Falcone, M.; Bassetti, M.; Tiseo, G.; Giordano, C.; Nencini, E.; Russo, A.; Graziano, E.; Tagliaferri, E.; Leonildi, A.; Barnini, S.; et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit. Care 2020, 24, 29. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar] [CrossRef]
- van Duin, D.; Lok, J.J.; Earley, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 2018, 66, 163–171. [Google Scholar] [CrossRef]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-Avibactam Use for Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae Infections: A Retrospective Observational Multicenter Study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef]
- Tsolaki, V.; Mantzarlis, K.; Mpakalis, A.; Malli, E.T.; Tsimpoukas, F.; Tsirogianni, A.; Papagiannitsis, C.; Zygoulis, P.; Papadonta, M.E.; Petinaki, E.; et al. Ceftazidime-Avibactam To Treat Life-Threatening Infections by Carbapenem-Resistant Pathogens in Critically Ill Mechanically Ventilated Patients. Antimicrob. Agents Chemother. 2020, 64, e02320-19. [Google Scholar] [CrossRef]
- Castón, J.J.; Lacort-Peralta, I.; Martín-Dávila, P.; Loeches, B.; Tabares, S.; Temkin, L.; Torre-Cisneros, J.; Paño-Pardo, J.R. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int. J. Infect. Dis. 2017, 59, 118–123. [Google Scholar] [CrossRef]
- Meng, H.; Han, L.; Niu, M.; Xu, L.; Xu, M.; An, Q.; Lu, J. Risk Factors for Mortality and Outcomes in Hematological Malignancy Patients with Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections. Infect. Drug Resist. 2022, 15, 4241–4251. [Google Scholar] [CrossRef]
- Mularoni, A.; Martucci, G.; Douradinha, B.; Campanella, O.; Hazen, B.; Medaglia, A.; Arena, G.; Gruttadauria, S.; Tuzzolino, F.; Arcadipane, A.; et al. Epidemiology and successful containment of a carbapenem-resistant Enterobacteriaceae outbreak in a Southern Italian Transplant Institute. Transpl. Infect. Dis. 2019, 21, e13119. [Google Scholar] [CrossRef]
- Pillinger, K.E.; Bouchard, J.; Withers, S.T.; Mediwala, K.; McGee, E.U.; Gibson, G.M.; Bland, C.M.; Bookstaver, P.B. Inpatient Antibiotic Stewardship Interventions in the Adult Oncology and Hematopoietic Stem Cell Transplant Population: A Review of the Literature. Ann. Pharmacother. 2020, 54, 594–610. [Google Scholar] [CrossRef]
- Battipaglia, G.; Malard, F.; Rubio, M.T.; Ruggeri, A.; Mamez, A.C.; Brissot, E.; Giannotti, F.; Dulery, R.; Joly, A.C.; Baylatry, M.T.; et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica 2019, 104, 1682–1688. [Google Scholar] [CrossRef]
- Pession, A.; Zama, D.; Muratore, E.; Leardini, D.; Gori, D.; Guaraldi, F.; Prete, A.; Turroni, S.; Brigidi, P.; Masetti, R. Fecal Microbiota Transplantation in Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Systematic Review. J. Pers. Med. 2021, 11, 100. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.; Wingard, J.R.; et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health care-associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients With E. coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994, Erratum in JAMA 2019, 321, 2370. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Perez, F.; Adachi, J.; Bonomo, R.A. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin. Infect. Dis. 2014, 59 (Suppl. S5), S335–S339. [Google Scholar] [CrossRef]
- Protocolo de PCR-Multiplex Para la Detección de Carbapenemasas. Available online: http://antimicrobianos.com.ar/2019/10/protocolo-de-pcr-multiplex-para-la-deteccion-de-carbapenemasas/ (accessed on 30 November 2022).
- Gutiérrez-Gutiérrez, B.; Salamanca, E.; de Cueto, M.; Hsueh, P.R.; Viale, P.; Paño-Pardo, J.R.; Venditti, M.; Tumbarello, M.; Daikos, G.; Pintado, V.; et al. A Predictive Model of Mortality in Patients With Bloodstream Infections due to Carbapenemase-Producing Enterobacteriaceae. Mayo Clin. Proc. 2016, 91, 1362–1371, Erratum in Mayo Clin. Proc. 2016, 91, 1843. [Google Scholar] [CrossRef]
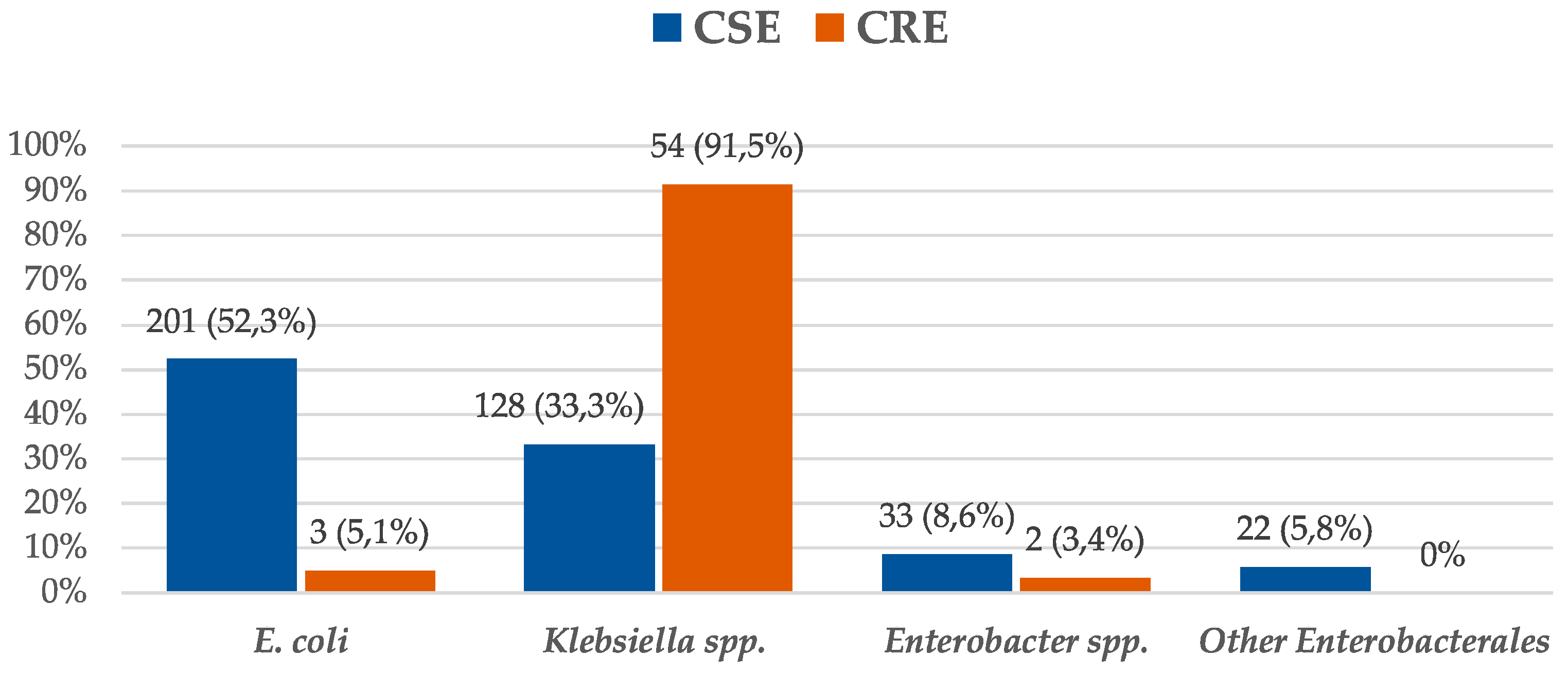
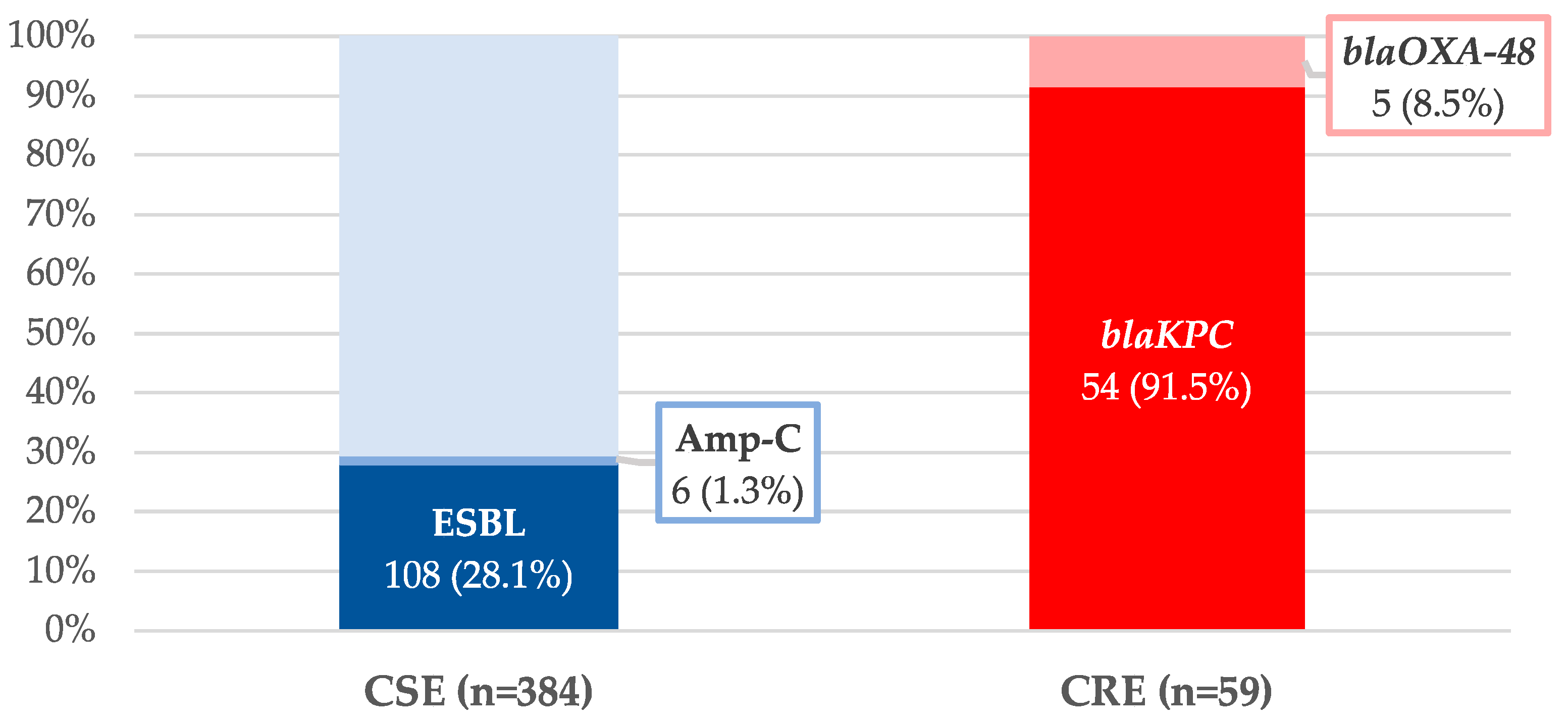
| Variables | CSE n = 384 n (%) | CRE n = 59 n (%) | p-Value * |
|---|---|---|---|
| Age (years) (median, IQR) | 55.5 (37–66) | 51 (39–64) | 0.31 |
| Male gender | 216 (56.3) | 35 (59.3) | 0.65 |
| Inclusion criteria | |||
| Hematologic malignancy | 226 (58.8) | 40 (67.8) | 0.19 |
| Solid malignancy | 89 (23.2) | 3 (5.1) | 0.001 |
| Hematopoietic Stem Cell Transplant | 69 (18) | 16 (27.1) | 0.09 |
| Stage of underlying cancer | |||
| Recently diagnosed | 126 (32.8) | 33(55.9) | 0.001 |
| Complete remission | 65 (17) | 11(18.6) | 0.74 |
| Partial remission | 57 (14.8) | 5 (8.5) | 0.23 |
| Refractory | 43 (11.2) | 3 (5.1) | 0.17 |
| Relapse | 93 (24.2) | 7 (11.9) | 0.03 |
| Recent chemotherapy (1 month prior to bacteremia) | 290 (75.5) | 42 (71.2) | 0.47 |
| Recent radiotherapy (1 month prior to bacteremia) | 28 (7.3) | 3 (5.1) | 0.78 |
| Receiving steroids during bacteremia | 125 (32.5) | 35 (59.3) | 0.13 |
| Charlson comorbidity index (median, IQR) | 2 (2–3) | 2 (2–3) | 0.21 |
| Neutropenia | 258 (67.2) | 52 (88.1) | 0.001 |
| High risk by MASCC score | 222 (86) | 49 (94.2) | 0.10 |
| Neutropenia duration (days) (IQR) | 11 (6–19) | 16 (10–24) | 0.004 |
| Recent hospitalization (1 month prior to bacteremia) | 204 (53.1) | 32 (54.2) | 0.87 |
| Recent antibiotic use (1 month prior to bacteremia) | 158 (41.1) | 45 (76.3) | <0.001 |
| Fluoroquinolone prophylaxis | 64 (16.7) | 13 (22) | 0.31 |
| Variables | CSE n (%) | CRE n (%) | p-Value * |
|---|---|---|---|
| Nosocomial infection | 241 (62.8) | 55 (93.2) | <0.001 |
| Bacteremia with clinical source | 286 (74.5) | 34 (57.6) | 0.007 |
| Abdominal | 115 (29.9) | 14 (23.7) | 0.32 |
| Central venous catheter | 40 (10.4) | 10 (16.9) | 0.14 |
| Urinary tract | 55 (14.3) | 2 (3.4) | 0.02 |
| Adequate EAT | 352 (91.7) | 28 (47.5) | <0.001 |
| Empirical antibiotic monotherapy | 283 (73.6) | 38 (64.4) | 0.13 |
| Combined definitive antibiotic treatment | 22 (5.7) | 57 (96.6) | <0.001 |
| APACHE II score the day of bacteremia (median, IQR) | 13 (9–17) | 12 (8–17) | 0.16 |
| PITT score the day of bacteremia (median, IQR) | 0 (0–2) | 0 (0–2) | 0.39 |
| Intensive care unit admission required | 86 (22.4) | 29 (49.1) | <0.001 |
| Shock development | 83 (21.6) | 30 (50.8) | <0.001 |
| Breakthrough bacteremia | 20 (5.2) | 14 (23.7) | <0.001 |
| 7-day mortality | 45 (11.7) | 21 (35.6) | <0.001 |
| Infection-related | 37 (82.2) | 21 (100) | 0.03 |
| 30-day mortality | 68 (17.7) | 32 (54.2) | <0.001 |
| Infection-related | 47 (69.1) | 27 (84.4) | 0.08 |
| More than 24-h delay in adequate antibiotic treatment | 27 (7) | 26 (44.1) | <0.001 |
| Variables | Univariate Analysis | Multivariate Analysis | Points | ||
|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | ||
| Recent carbapenems use | 4.1 (2.3–7.7) | 0.001 | |||
| >7 days of antibiotic use prior to bacteremia | 4.6 (2.6–4.1) | 0.001 | 4.65 (2.29–9.46) | <0.001 | 2 |
| Neutropenia | 3.6 (1.6–8.2) | 0.002 | |||
| ≥10-days of hospitalization prior to bacteremia | 4.4 (2.3–8.6) | 0.001 | 4.03 (1.88–8.66) | <0.001 | 2 |
| Recent intensive care unit admission | 3 (1.4–6.8) | 0.006 | |||
| Central venous catheter in place | 3.4 (1.7–6.7) | 0.002 | |||
| Previous colonization with KPC-CPE | 10.6 (4.4–25.5) | 0.001 | |||
| Recent colonization with KPC-CPE | 24.9 (1.3–55) | 0.001 | 33.08 (11.7–93.25) | <001 | 5 |
| Point of the Score | Sensitivity | Specificity | +LR | −LR | Positive Post-Test Probability | Negative Post-Test Probability |
|---|---|---|---|---|---|---|
| ≥0 | 100.00% | 0.00% | 1 | 13.30% | ||
| ≥2 | 94.92% | 40.21% | 1.5874 | 0.1265 | 19.58% | 1.90% |
| ≥4 | 76.27% | 83.81% | 4.7116 | 0.2831 | 41.95% | 4.16% |
| ≥5 | 42.37% | 97.13% | 14.7535 | 0.5933 | 69.36% | 8.34% |
| ≥7 | 35.59% | 98.43% | 22.7204 | 0.6543 | 77.71% | 9.12% |
| ≥9 | 20.34% | 99.22% | 25.9662 | 0.8029 | 79.93% | 10.97% |
| >9 | 0.00% | 100.00% | 1 | 0.00% | 13.30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, F.; Torres, D.; Laborde, A.; Berruezo, L.; Jordán, R.; Rossi, I.R.; Valledor, A.; Costantini, P.; Dictar, M.; Nenna, A.; et al. Development of a Clinical Score to Stratify the Risk for Carbapenem-Resistant Enterobacterales Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation. Antibiotics 2023, 12, 226. https://doi.org/10.3390/antibiotics12020226
Herrera F, Torres D, Laborde A, Berruezo L, Jordán R, Rossi IR, Valledor A, Costantini P, Dictar M, Nenna A, et al. Development of a Clinical Score to Stratify the Risk for Carbapenem-Resistant Enterobacterales Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation. Antibiotics. 2023; 12(2):226. https://doi.org/10.3390/antibiotics12020226
Chicago/Turabian StyleHerrera, Fabián, Diego Torres, Ana Laborde, Lorena Berruezo, Rosana Jordán, Inés Roccia Rossi, Alejandra Valledor, Patricia Costantini, Miguel Dictar, Andrea Nenna, and et al. 2023. "Development of a Clinical Score to Stratify the Risk for Carbapenem-Resistant Enterobacterales Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation" Antibiotics 12, no. 2: 226. https://doi.org/10.3390/antibiotics12020226
APA StyleHerrera, F., Torres, D., Laborde, A., Berruezo, L., Jordán, R., Rossi, I. R., Valledor, A., Costantini, P., Dictar, M., Nenna, A., Pereyra, M. L., Lambert, S., Benso, J., Poletta, F., Gonzalez Ibañez, M. L., Baldoni, N., Eusebio, M. J., Lovano, F., Barcán, L., ... Carena, A. Á., on behalf of the Argentine Group for the Study of Bacteremia in Cancer and Stem Cell Transplant (ROCAS) Study. (2023). Development of a Clinical Score to Stratify the Risk for Carbapenem-Resistant Enterobacterales Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation. Antibiotics, 12(2), 226. https://doi.org/10.3390/antibiotics12020226





