Antimicrobial Activities and Mode of Flavonoid Actions
Abstract
1. Introduction
2. Results and Discussion
2.1. Antimicrobial Activity of Flavonoids
2.1.1. Antibacterial Activity
2.1.2. Antifungal Activity
2.2. Lipophilicity: LogP, CrippenLogP (CLogP)
2.3. Mode of Flavonoids Actions
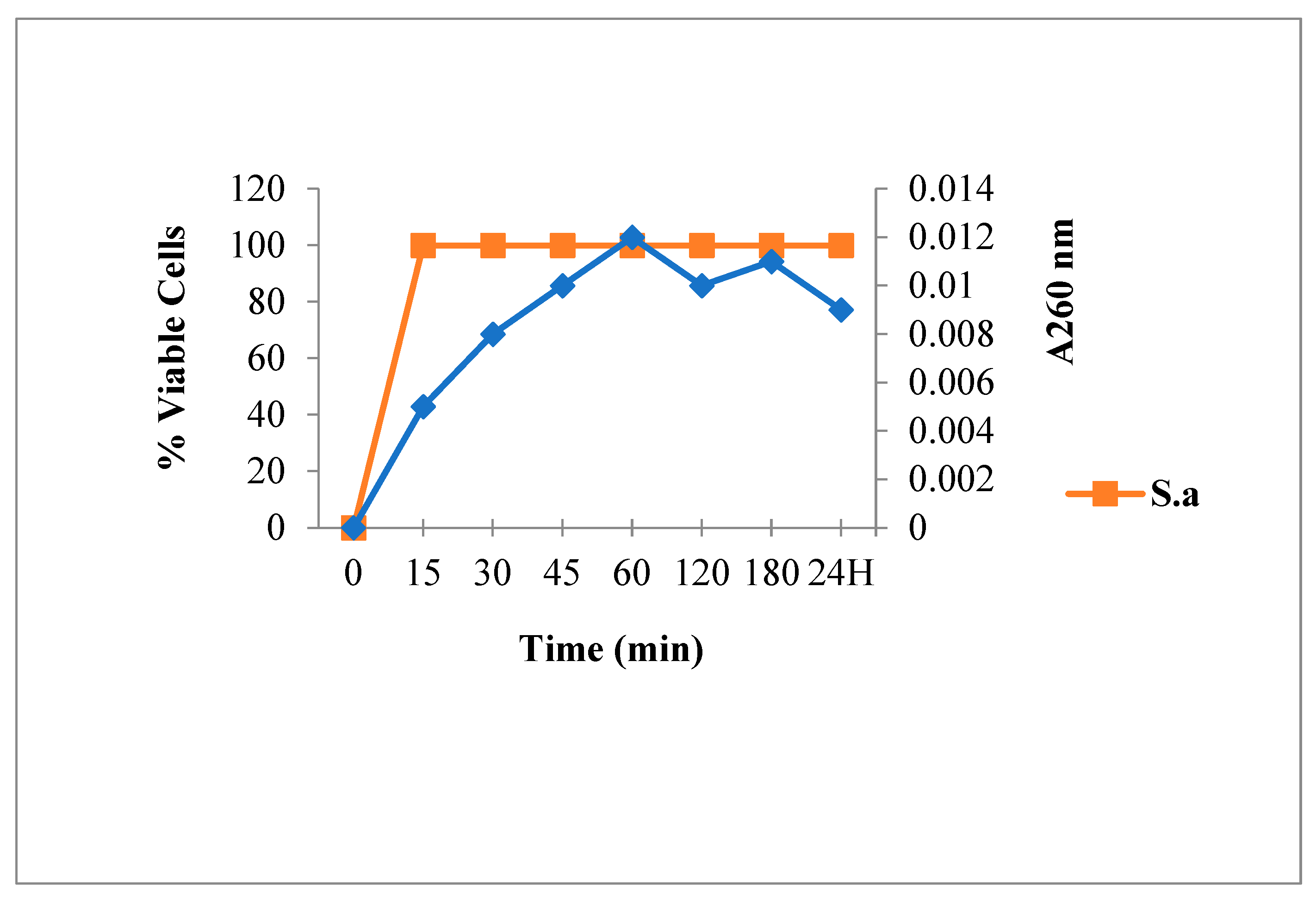
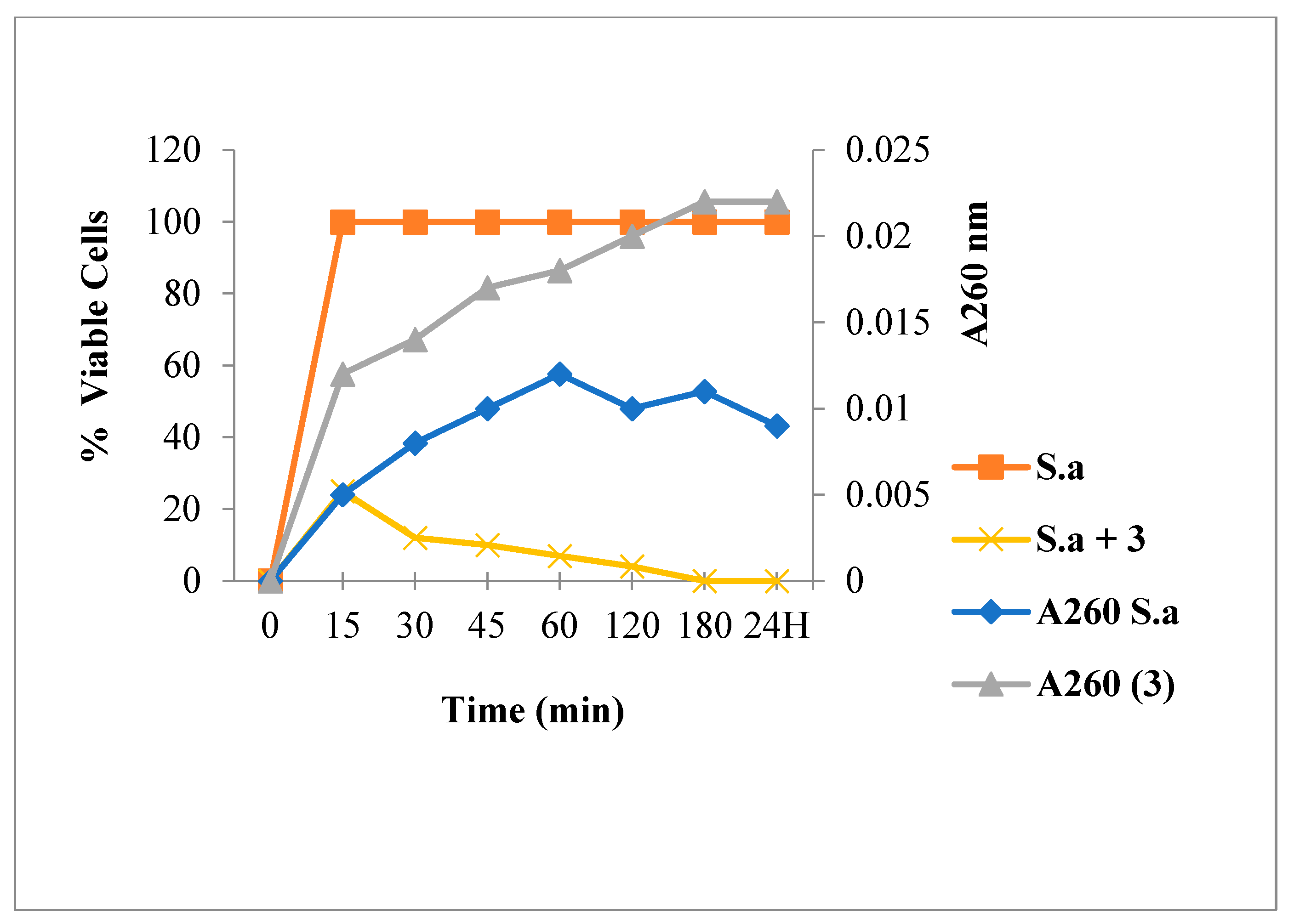
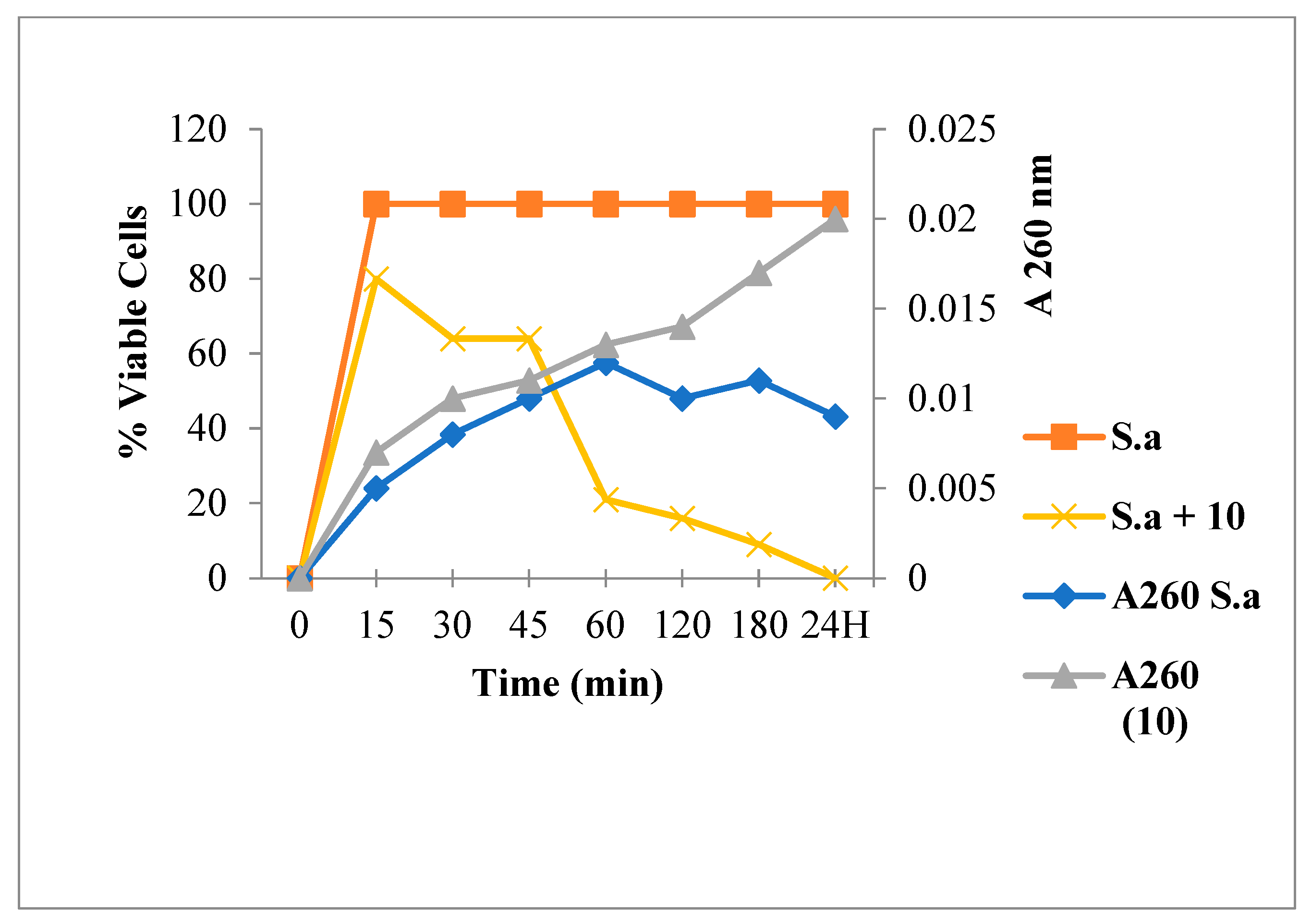
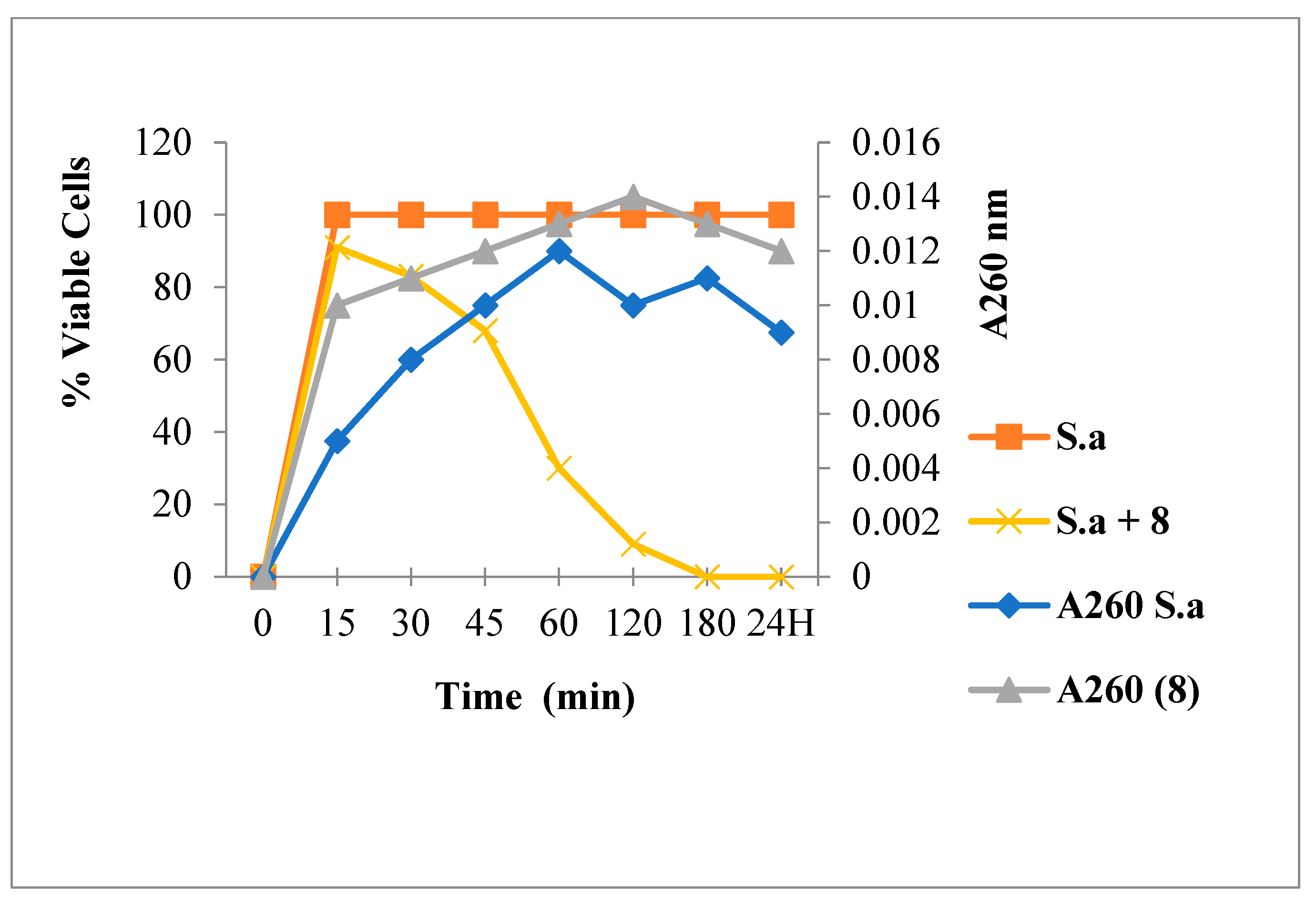
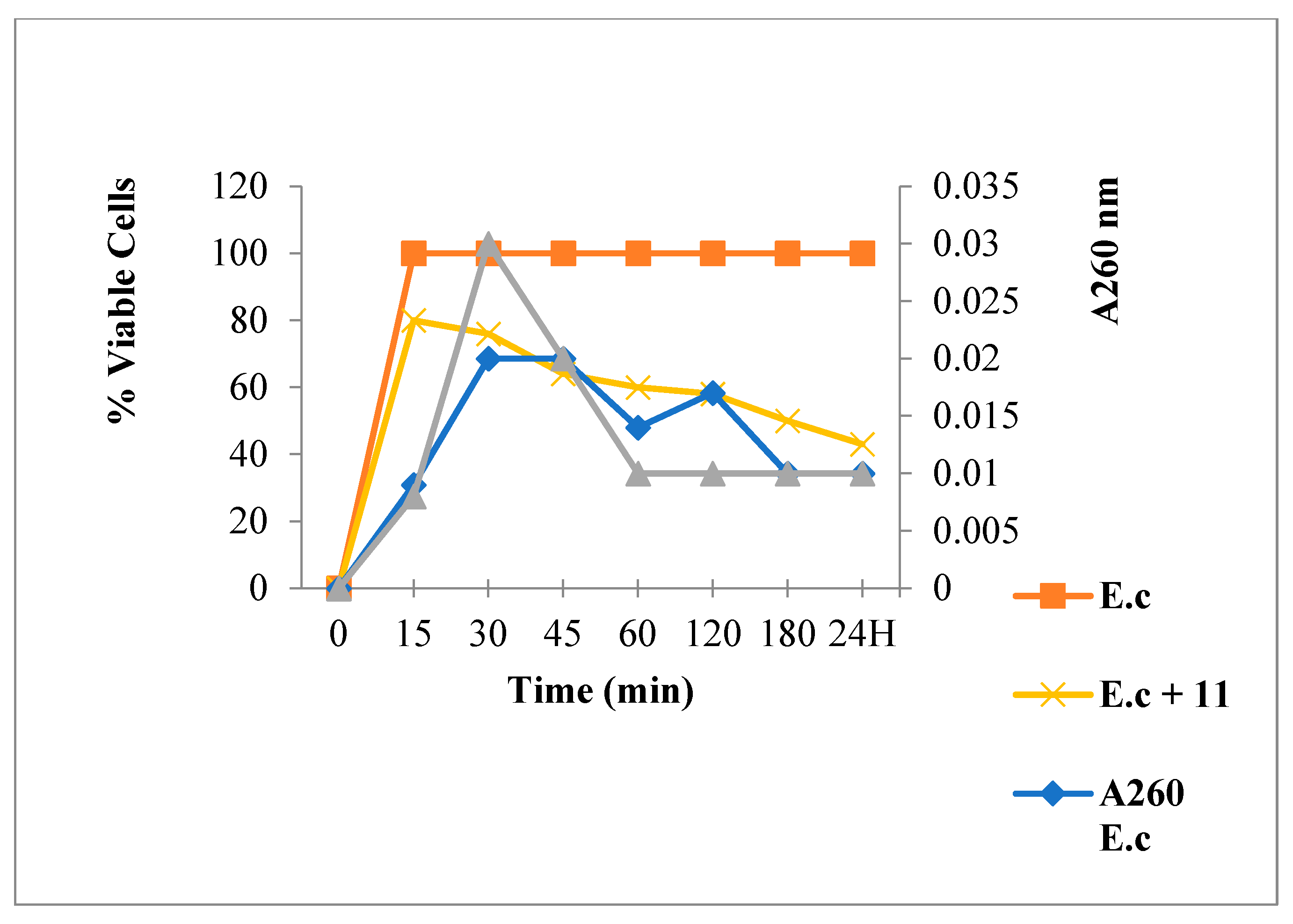
2.3.1. Leakage of 260 nm Absorbing Material for S. aureus
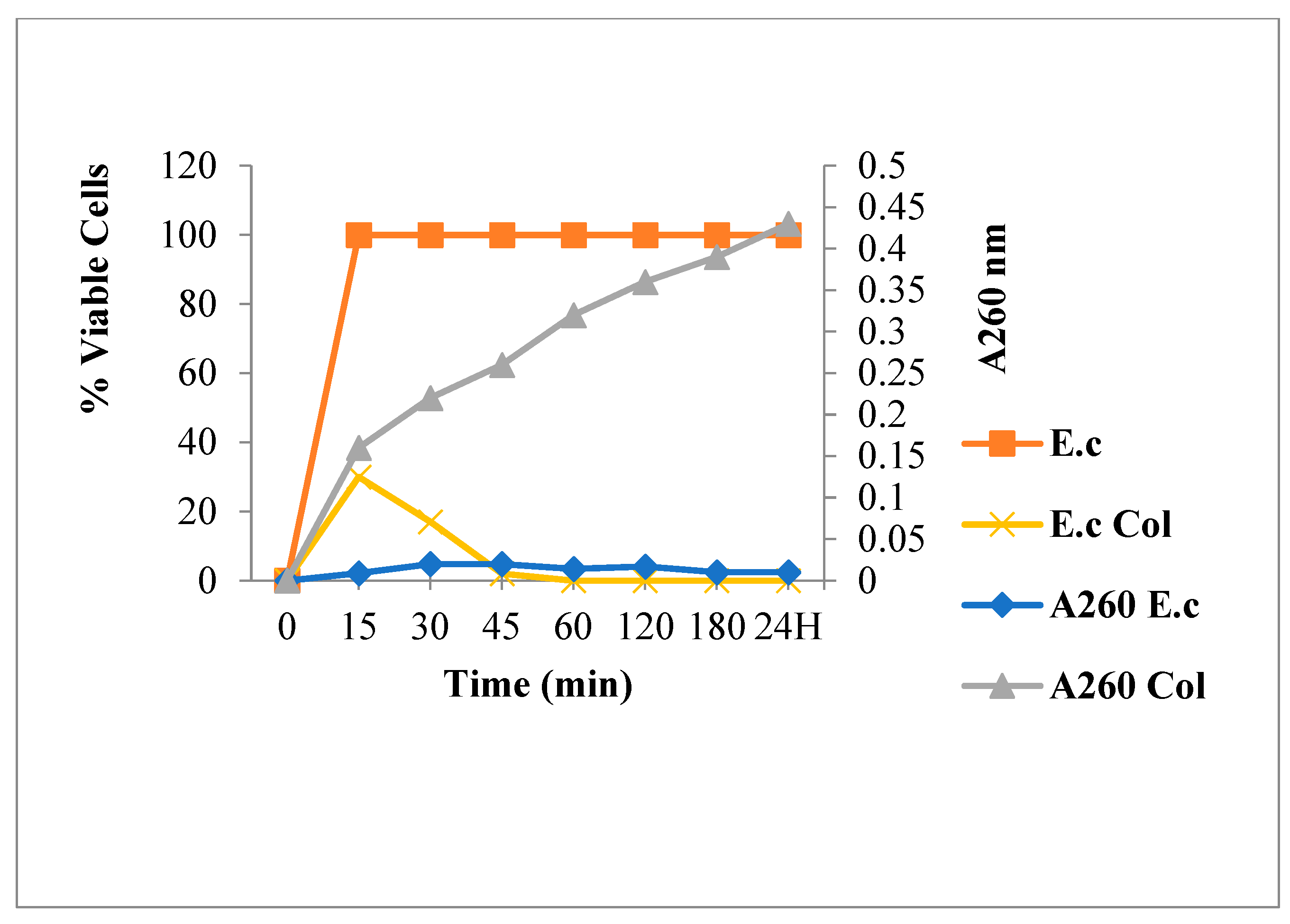
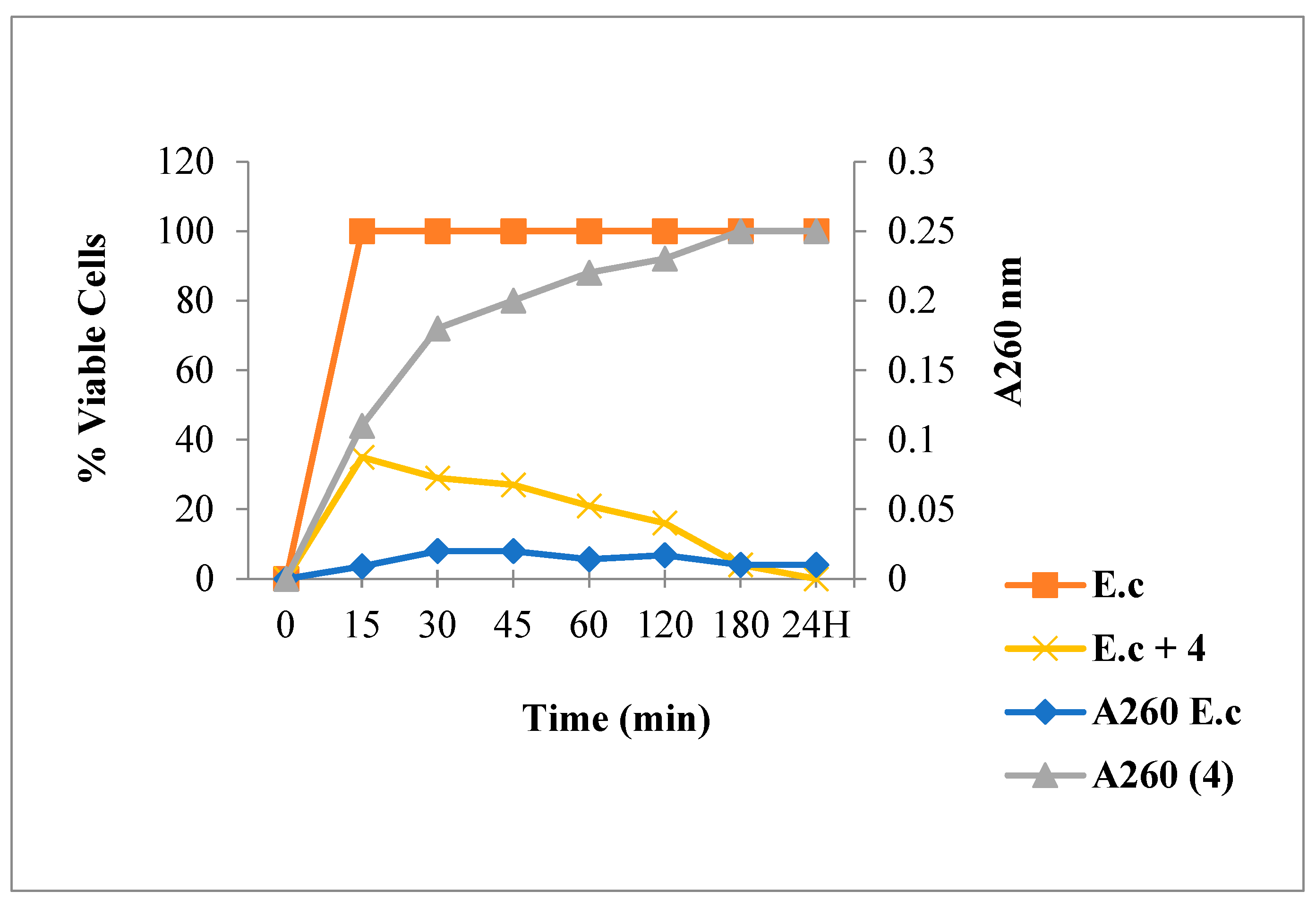
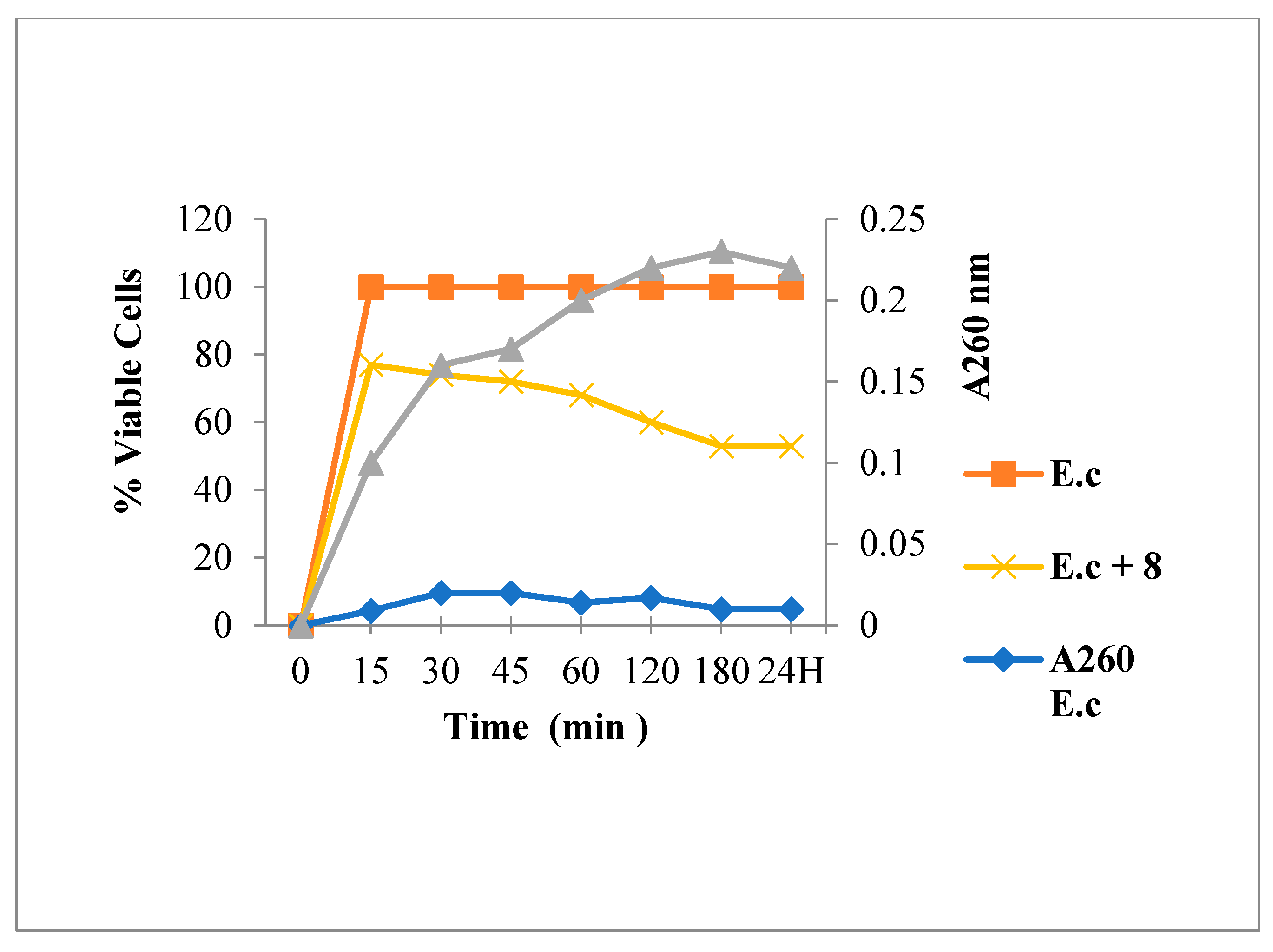
2.3.2. Nucleic Acid Release for E. coli
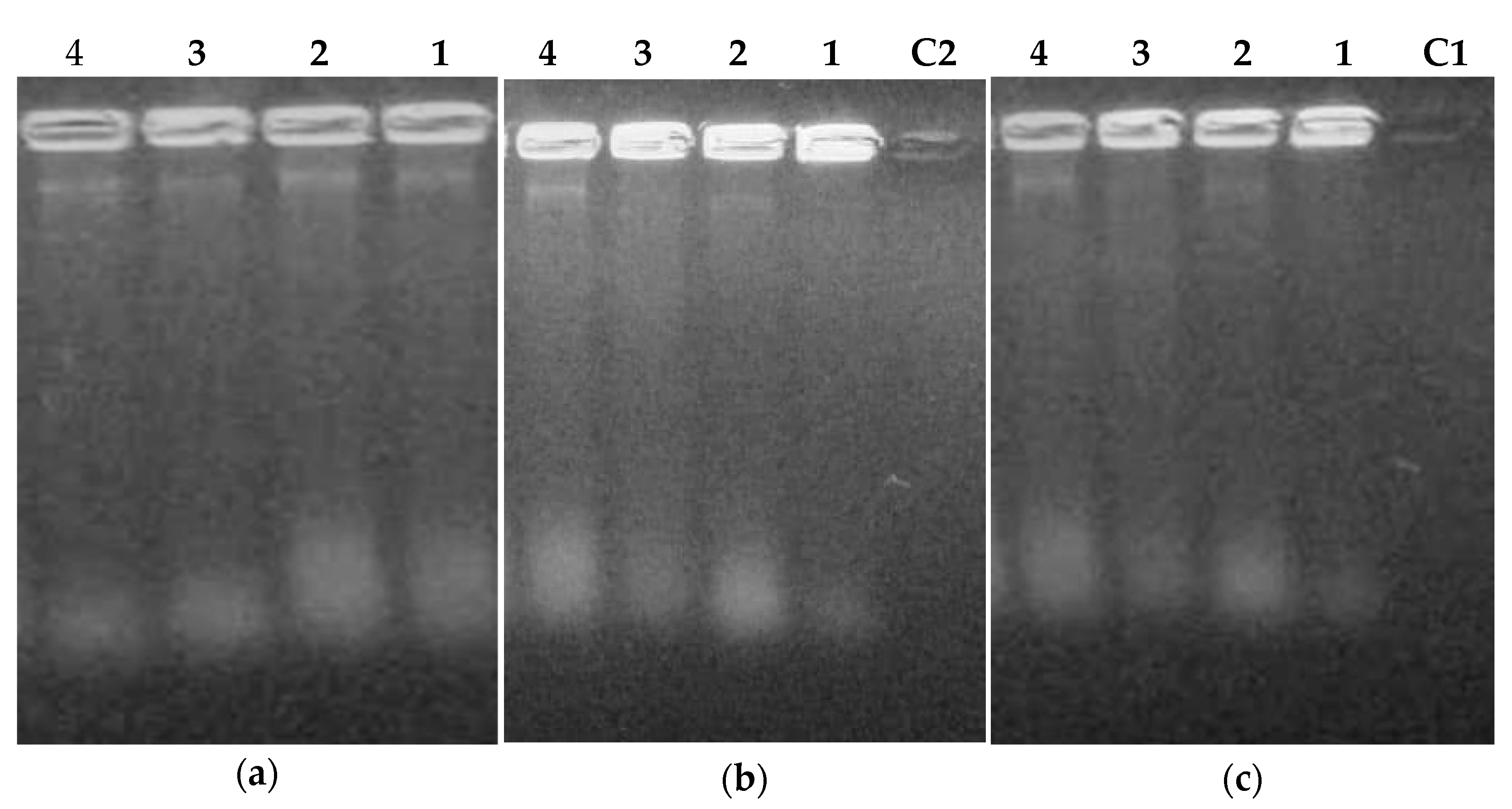
2.3.3. Extraction and Purification of Nucleic Acids from Flavonoids Treated Bacteria
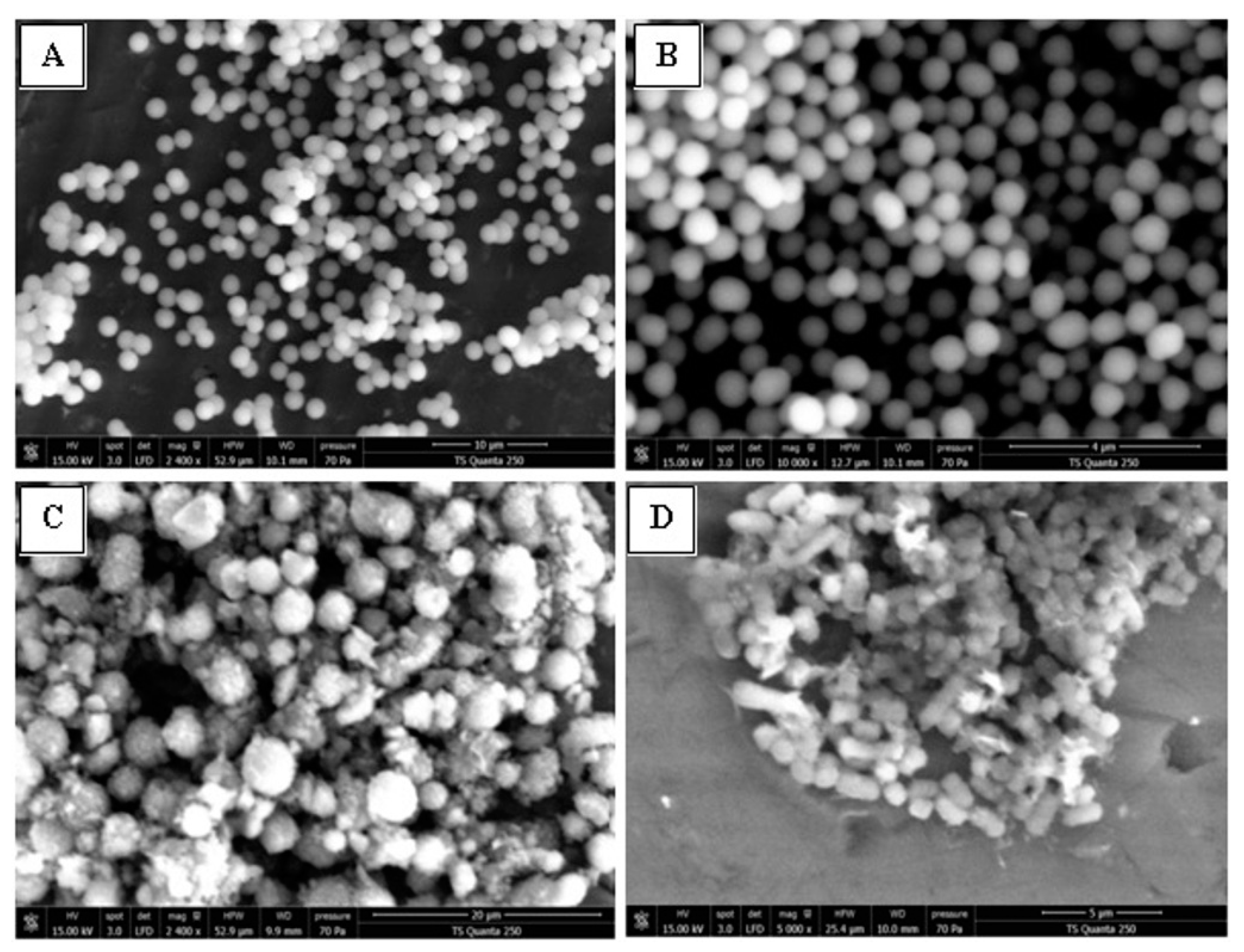
2.3.4. SEM Analysis, Cell Membrane Permeability and Morphological Changes
3. Material and Methods
3.1. Microbial Strains Origin
3.2. Antibacterial Activity
3.3. Antifungal Activity
3.4. Determination of Minimal Bactericidal Concentration (MBC) and Minimal Fungicidal Concentration (MFC)
3.5. Calcul of Lipophilicity
3.6. Mode of Action of Flavonoids
3.6.1. Leakage of 260-nm Absorbing Material
3.6.2. Bacterial Viability Determination
3.6.3. Bacteria Lysis by Flavonoids, DNA, and RNA Extraction
Extraction and Purification of Nucleic Acids
Agarose Gel Electrophoresis
3.6.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect Contr. 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L. Resistance in Gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 2006, 119, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Ozcelik, B.; Ozgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Zintars, G.B.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Isenman, H.; Fisher, D. Advances in prevention and treatment of vancomycin-resistant Enterococcus infection. Curr. Opin. Infect. Dis. 2016, 29, 577–582. [Google Scholar] [CrossRef]
- Alanis, A.L. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Publishes List of Bacteria for Which Now Antibiotics are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 16 December 2022).
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Brink, A.J. Epidemiology of carbapenem-resistant Gram-negative infections. Curr. Opin. 2019, 32, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Takamura, Y.; Yamamoto, M.; Inoue, Y.; Takagaki, R.; Takahashi, K.; Demizu, S.; Kajiyama, K.; Hiraga, Y.; Kinoshita, T. Identification of antimicrobial and antioxidant constituents from licorice of Russian and Xinjiang origin. Chem. Pharm. Bull. 1989, 37, 2528–2530. [Google Scholar] [CrossRef]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef]
- Boubakeur, B.; Tirtouil, A.; Meddah, B.; Khadem, H. The evaluation of the effect of synthetic flavonoids on growth of pathogenic and probiotic bacteria. J. Chem. Pharm. Res. 2015, 7, 228–236. [Google Scholar]
- Ohemeng, K.A.; Schwender, C.F.; Fu, K.P.; Barrett, J.F. DNA gyrase inhibitory and antibacterial activity of some flavones. Bioorg. Med. Chem. Lett. 1993, 3, 225–230. [Google Scholar] [CrossRef]
- Lal, K.; Yadav, P.; Kumar, A.; Kumar, A.; Paul, A.K. Design, synthesis, characterization, antimicrobial evaluation and molecular modeling studies of some dehydroacetic acid-chalcone-1,2,3-triazole hybrids. Bioorg. Chem. 2018, 77, 236–244. [Google Scholar] [CrossRef]
- Ayman, M.; El-Messery, S.M.; Habib, E.E.; Al-Rashood, S.T.; Almehizia, A.A.; Alkahtani, H.M.; Hassan, G.S. Targeting microbial resistance: Synthesis, antibacterial evaluation, DNA binding and modeling study of new chalcone-based dithiocarbamate derivatives. Bioorg. Chem. 2019, 85, 282–292. [Google Scholar] [CrossRef]
- Yadav, P.; Lal, K.; Kumar, L.; Kumar, A.; Kumar, A.; Paul, A.K.; Kumar, R. Synthesis, crystal structure and antimicrobial potential of some fluorinated chalcone-1,2,3-triazole conjugates. Eur. J. Med. Chem. 2018, 155, 263–274. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Iinuma, M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165. [Google Scholar] [CrossRef]
- Awolola, G.V.; Koorbanally, N.A.; Chenia, H.; Shode, F.O.; Baijnath, H. Antibacterial and anti-biofilm activity of flavonoids and triterpenes isolated from the extracts of Ficus Sansibarica warb. Subsp. Sansibarica (Moraceae) extracts. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gaur, R.; Sharma, A.; Akther, J.; Saini, M.; Bhakuni, R.S.; Pathania, R. A novel bifunctional chalcone inhibits multi-drug resistant Staphylococcus aureus and potentiates the activity of fluoroquinolones. Bioorg. Chem. 2019, 83, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihai, C.T.; Sârbu, L.G.; Birsa, L.M. A novel synthetic flavonoid with potent antibacterial properties: In vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Javed, K.; Ahmad, S.; Rathish, I.G.; Singh, S.; Chaitanya, M.; Arunasree, K.M.; Alama, M.S. Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur. J. Med. Chem. 2013, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Alcara´z, L.E.; Blanco, S.E.; Puig, O.N. Antibacterial activity of flavonoids agains methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring Pharmacological Significance of Chalcone Scaffold: A Review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef]
- Kumar, R.; Mohanakrishnan, D.; Sharma, A.; Kaushik, N.K.; Kalia, K.; Sinha, A.K.; Sahal, D. Reinvestigation of structure-activity relationship of methoxylated chalcones as antimalarials: Synthesis and evaluation of 2,4,5- trimethoxy substituted patterns as lead candidates derived from abundantly available natural -asarone. Eur. J. Med. Chem. 2010, 45, 5292–5301. [Google Scholar] [CrossRef]
- Yang, H.-M.; Shin, H.-R.; Cho, S.-H.; Bang, S.C.; Song, G.Y.; Ju, J.H.; Kim, M.K.; Lee, S.H.; Ryu, J.C.; Kim, Y.; et al. Structural requirement of chalcones for the inhibitory activity of interleukin-5. Bioorg. Med. Chem. 2007, 15, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Do, T.H.; Tran, N.C.; Ngo, T.D.; Huynh, T.N.P.; Tran, C.D.; Thai, K.M. Synthesis and anti Methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorg. Med. Chem. Lett. 2012, 22, 4555–4560. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Nguyen, T.T.N.; Do, T.H.; Huynh, T.N.P.; Tran, C.D.; Thai, K.M. Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics. Molecules 2012, 17, 6684–6696. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, A.; Mkhalid, I.A.I.; Mishra, R.; Rashid, M. Synthesis and antimicrobial activity of bischalcone derivatives. Med. Chem. Res. 2012, 22, 1578–1586. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Hopf, H.; Jones, P.G.; Sarbu, L.G.; Babii, C.; Mihai, A.C.; Stefan, M.; Birsa, L.M. Antibacterial structure–activity relationship studies of several tricyclic sulfur-containing flavonoids. Beilstein J. Org. Chem. 2016, 12, 1065–1071. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Sarbu, L.G.; Hopf, H.; Jones, P.G.; Babii, C.; Stefan, M.; Birsa, M.L. The influence of halogen substituents on the biological properties of sulfur-containing flavonoids. Bioorg. Med. Chem. 2016, 24, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Pasqualetti, M.; Provenzano, G.; Tempesta, S. Ecofriendly synthesis of halogenated flavonoids and evaluation of their antifungal activity. NJC 2015, 39, 2980–2987. [Google Scholar] [CrossRef]
- Mahalle, P.R.; Khaty, N.T. Synthesis of Some Bromo-Substituted 3-Aroyl Flavanones and Flavones. E-J. Chem. 2010, 7, 1359–1361. [Google Scholar] [CrossRef]
- Hasan, A.; Rasheed, L.; Malik, A. Synthesis and characterization of variably halogenated chalcones and flavonols and their antifungal activity. As. J. Chem. 2007, 19, 937–948. [Google Scholar]
- Vasanthanathan, P.; Lakshmi, M.; Babu, M.A.; Gupta, A.K.; Kaskhedikar, S.G. QSAR study of 3-Phenyl-5-acyloxymethyl -2H, 5H-furan-2ones as antifungal agents: The dominant role of electronic parameter. Chem. Pharm. Bull. 2006, 54, 583–587. [Google Scholar] [CrossRef]
- Dixon, R.A.; Chopra, I. Polymixin B and nonapeptide alter cytoplasmic membrane permeability in Escherichia.coli. J. Antimicrob. Chemother. 1986, 18, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Chung, H.Y.; Kang, S.S.; Jung, M.J.; Kim, J.W.; Jung, H.A. The structure–activity relationship of flavonoids as scavengers of peroxynitrite. Phytother. Res. 2002, 16, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Farkas, O.; Jakus, J.; Héberger, K. Quantitative structure–antioxidant activity relationships of flavonoid compounds. Molecules 2004, 9, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; McKeever, L.C.; Malik, N.S. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microb. 2017, 8, 113. [Google Scholar] [CrossRef]
- Babii, C.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihasan, M.; Birsa, L.M.; Stefan, M. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. App. Microb. 2016, 120, 630–637. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Zeng, X.; Xu, X.; Brennan, C. Membrane and genomic DNA dual-targeting of citrus flavonoid 2 naringenin against Staphylococcus aureus. Integr. Biol. 2017, 9, 820–829. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; To, I.V.; Rangel, A.O.S.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, A.; Kehri, K.H.; Sharma, B.; Pandey, A.K. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 9–16. [Google Scholar] [CrossRef]
- Kelly, E.H.; Anthony, R.T.; Dennis, J.B. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, A.; Bouhalleb, G.; Legros, J.; Rezgui, F. Tetrahydronaphthalene as a precursor of new series of chalcones, flavanones and flavones. Turk. J. Chem. 2018, 42, 237–246. [Google Scholar] [CrossRef]
- Biolog. Available online: https://www.biolog.com/products-portfolio-overview/phenotype-microarrays-for-microbial-cells/ (accessed on 16 December 2022).
- Bochner, B.R. New technologies to assess genotype-phenotype relationships. Nat. Rev. Genet. 2003, 4, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Mbah, J.; Ngemenya, M.N.; Abawah, A.; Babiaka, S.B.; Nubed, L.N.; Nyongbela, K.D.; Lemuh, N.; Efange, S.M. Bioassay-guided discovery of antibacterial agents: In vitro screening of Peperomia vulcanica, Peperomia fernandopoioana and Scleria striatinux. Ann. Microbiol. Antimicrob. 2012, 11, 102–113. [Google Scholar] [CrossRef]
- Wildman, S.A.; Crippen, G.M. Prediction of physicochemical parameters by atomic contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Nobmann, P.; Bourke, P.; Dunne, J.; Henehan, G. In vitro antimicrobial activity and mechanism of action of novel carbohydrate fatty acid derivatives against staphylococcus aureus MRSA. J. Appl. Microbiol. 2010, 108, 2152–2161. [Google Scholar] [CrossRef]
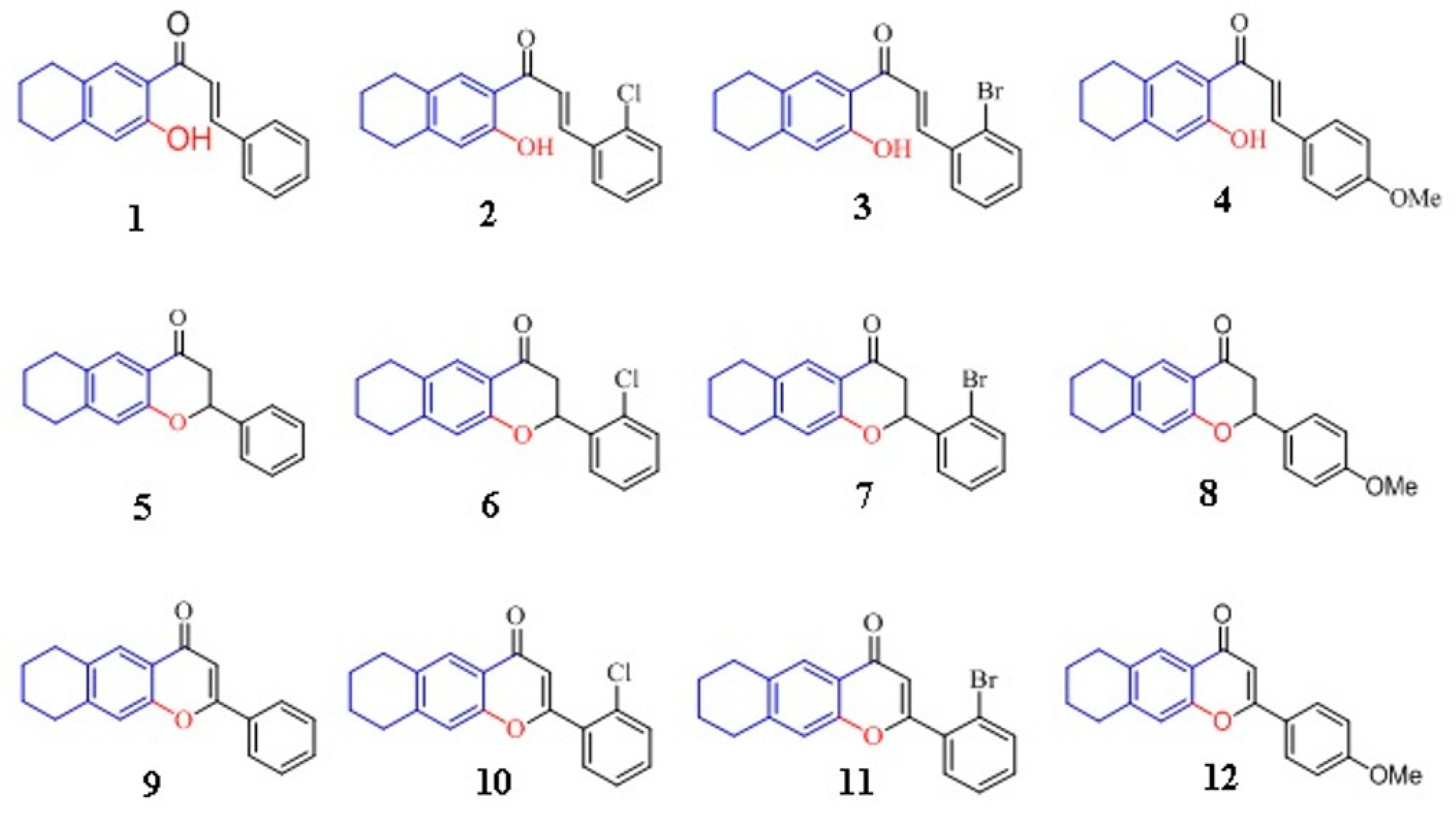



| E. coli | Salmonella spp | P. aeruginosa | ||||
|---|---|---|---|---|---|---|
| Compound | MIC | MBC | MIC | MBC | MIC | MBC |
| 1 | 250 | 250 | 125 | 500 | 125 | 500 |
| 2 | 250 | 250 | 125 | ≥500 | 125 | ≥500 |
| 3 | 250 | 250 | 250 | ≥500 | 125 | 500 |
| 4 | 125 | 125 | 250 | 500 | 125 | ≥500 |
| 5 | ≥500 | ≥500 | 500 | >500 | ≥500 | >500 |
| 6 | 250 | ≥500 | 125 | 500 | 250 | ≥500 |
| 7 | 500 | ≥500 | 500 | >500 | 250 | ≥500 |
| 8 | 250 | ≥500 | 250 | >500 | 125 | ≥500 |
| 9 | 250 | 500 | 125 | ≥500 | 250 | ≥500 |
| 10 | 250 | 500 | 250 | ≥500 | 250 | ≥500 |
| 11 | 125 | 500 | 250 | ≥500 | 125 | 500 |
| 12 | 250 | 500 | ≥500 | ≥500 | 250 | ≥500 |
| Amp | ≤3.9 | ≤3.9 | 62.5 | 62.5 | 500 | >500 |
| Van | 250 | 250 | 250 | 250 | 250 | 250 |
| S. aureusa | S. aureusb | S. aureusc | S. aureusd | E. faecium | B. cereus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cpd | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| 1 | 125 | 250 | 62.5 | 250 | 125 | >500 | 125 | >500 | 125 | >500 | 125 | >500 |
| 2 | 62.5 | 250 | 62.5 | 62.5 | 125 | 500 | 31.25 | 125 | 62.5 | 500 | 31.25 | >250 |
| 3 | 31.25 | 500 | 31.25 | 31.25 | 250 | 500 | 31.25 | 62.5 | 125 | >500 | 125 | 500 |
| 4 | 62.5 | ≥500 | 62.5 | 125 | 250 | >500 | 62.5 | >500 | 125 | >500 | 125 | 500 |
| 5 | 62.5 | ≥500 | 31.25 | 250 | 125 | >500 | 125 | 1000 | 500 | ≥1000 | 125 | 500 |
| 6 | 62.5 | 500 | 31.25 | 250 | 500 | >500 | 500 | ≥1000 | 500 | ≥1000 | 250 | >500 |
| 7 | 62.5 | 250 | 15.62 | ≥125 | 500 | >500 | 125 | ≥1000 | 500 | ≥1000 | ≥500 | >500 |
| 8 | 62.5 | 250 | 15.62 | ≥125 | 125 | >500 | 62.5 | ≥1000 | 62.5 | ≥500 | 62.5 | >500 |
| 9 | 250 | ≥1000 | 62.5 | ≥500 | 250 | >500 | 250 | ≥1000 | 250 | ≥1000 | 250 | >500 |
| 10 | 31.25 | ≥250 | 31.25 | 250 | ≥ 500 | >500 | 31.25 | 125 | 500 | ≥1000 | 500 | 500 |
| 11 | 125 | 1000 | 62.5 | 250 | 125 | 500 | 62.5 | 125 | 250 | 500 | 62.5 | 500 |
| 12 | 62.5 | 250 | 62.5 | 62.5 | 125 | >500 | 62.5 | ≥500 | 250 | 250 | 62.5 | 500 |
| Amp | ≥500 | >500 | ≤3.9 | ≤3.9 | 15.62 | 15.62 | ≤3.9 | ≤3.9 | ≥500 | >500 | 250 | 250 |
| Van | ≤3.9 | ≤3.9 | ≤3.9 | ≤3.9 | ≤3.9 | - | ≤3.9 | ≤3.9 | - | - | ≤3.9 | ≤3.9 |
| A. niger | A. flavus | P. expansum | ||||
|---|---|---|---|---|---|---|
| Cpd | MIC | MFC | MIC | MFC | MIC | MFC |
| 1 | 15.62 | 250 | 31.25 | ≥500 | 62.5 | 250 |
| 2 | 15.62 | 62.5 | 15.62 | ≥500 | 125 | 500 |
| 3 | 15.62 | 62.5 | 7.81 | 250 | 62.5 | 250 |
| 4 | 31.25 | 31.25 | 62.5 | 250 | 125 | 500 |
| 5 | 31.25 | 31.25 | 31.25 | 250 | 250 | ≥500 |
| 6 | 125 | 125 | 62.5 | 500 | 125 | 500 |
| 7 | 31.25 | 31.25 | 62.5 | 250 | 125 | ≥500 |
| 8 | 62.5 | 62.5 | 31.25 | 125 | 125 | 125 |
| 9 | 125 | 250 | 125 | 500 | 125 | 500 |
| 10 | 62.5 | 62.5 | 15.62 | ≥500 | 125 | 500 |
| 11 | 125 | 125 | 31.25 | 500 | 125 | 500 |
| 12 | 62.5 | 62.5 | 31.25 | 250 | 62.5 | 62.5 |
| Fluconazole | 62.5 | 62.5 | 62.5 | 62.5 | 7.81 | 7.81 |
| LogP | CLogP | ||
| Chalcones | 1 | 4.51 | 5.5296 |
| 2 | 4.38 | 5.4486 | |
| 3 | 4.94 | 5.9099 | |
| 4 | 4.94 | 5.515 | |
| Flavanones | 5 | 5.06 | 6.2426 |
| 6 | 4.11 | 5.0469 | |
| 7 | 3.98 | 4.9659 | |
| 8 | 5.21 | 5.615 | |
| Flavones | 9 | 5.33 | 6.3926 |
| 10 | 4.67 | 5.7599 | |
| 11 | 4.38 | 5.052 | |
| 12 | 4.26 | 5.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thebti, A.; Meddeb, A.; Ben Salem, I.; Bakary, C.; Ayari, S.; Rezgui, F.; Essafi-Benkhadir, K.; Boudabous, A.; Ouzari, H.-I. Antimicrobial Activities and Mode of Flavonoid Actions. Antibiotics 2023, 12, 225. https://doi.org/10.3390/antibiotics12020225
Thebti A, Meddeb A, Ben Salem I, Bakary C, Ayari S, Rezgui F, Essafi-Benkhadir K, Boudabous A, Ouzari H-I. Antimicrobial Activities and Mode of Flavonoid Actions. Antibiotics. 2023; 12(2):225. https://doi.org/10.3390/antibiotics12020225
Chicago/Turabian StyleThebti, Amal, Ahmed Meddeb, Issam Ben Salem, Coulibaly Bakary, Sami Ayari, Farhat Rezgui, Khadija Essafi-Benkhadir, Abdellatif Boudabous, and Hadda-Imene Ouzari. 2023. "Antimicrobial Activities and Mode of Flavonoid Actions" Antibiotics 12, no. 2: 225. https://doi.org/10.3390/antibiotics12020225
APA StyleThebti, A., Meddeb, A., Ben Salem, I., Bakary, C., Ayari, S., Rezgui, F., Essafi-Benkhadir, K., Boudabous, A., & Ouzari, H.-I. (2023). Antimicrobial Activities and Mode of Flavonoid Actions. Antibiotics, 12(2), 225. https://doi.org/10.3390/antibiotics12020225






