Green Synthesis of Characterized Silver Nanoparticle Using Cullen tomentosum and Assessment of Its Antibacterial Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Cullen tomentosum Silver Nanoparticle
2.1.1. UV-Visible Spectrophotometry
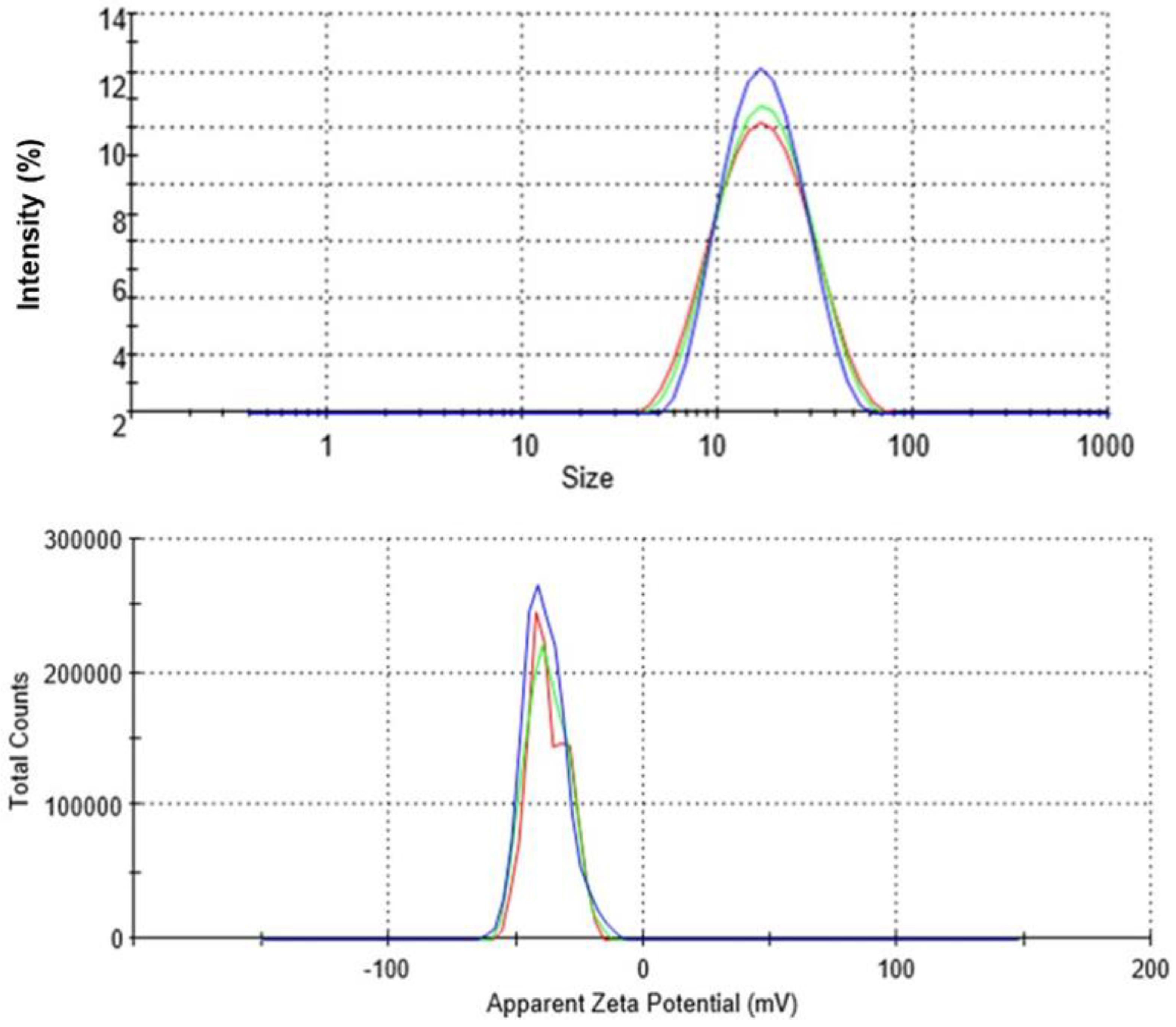
2.1.2. Dynamic Light Scattering (DLS)
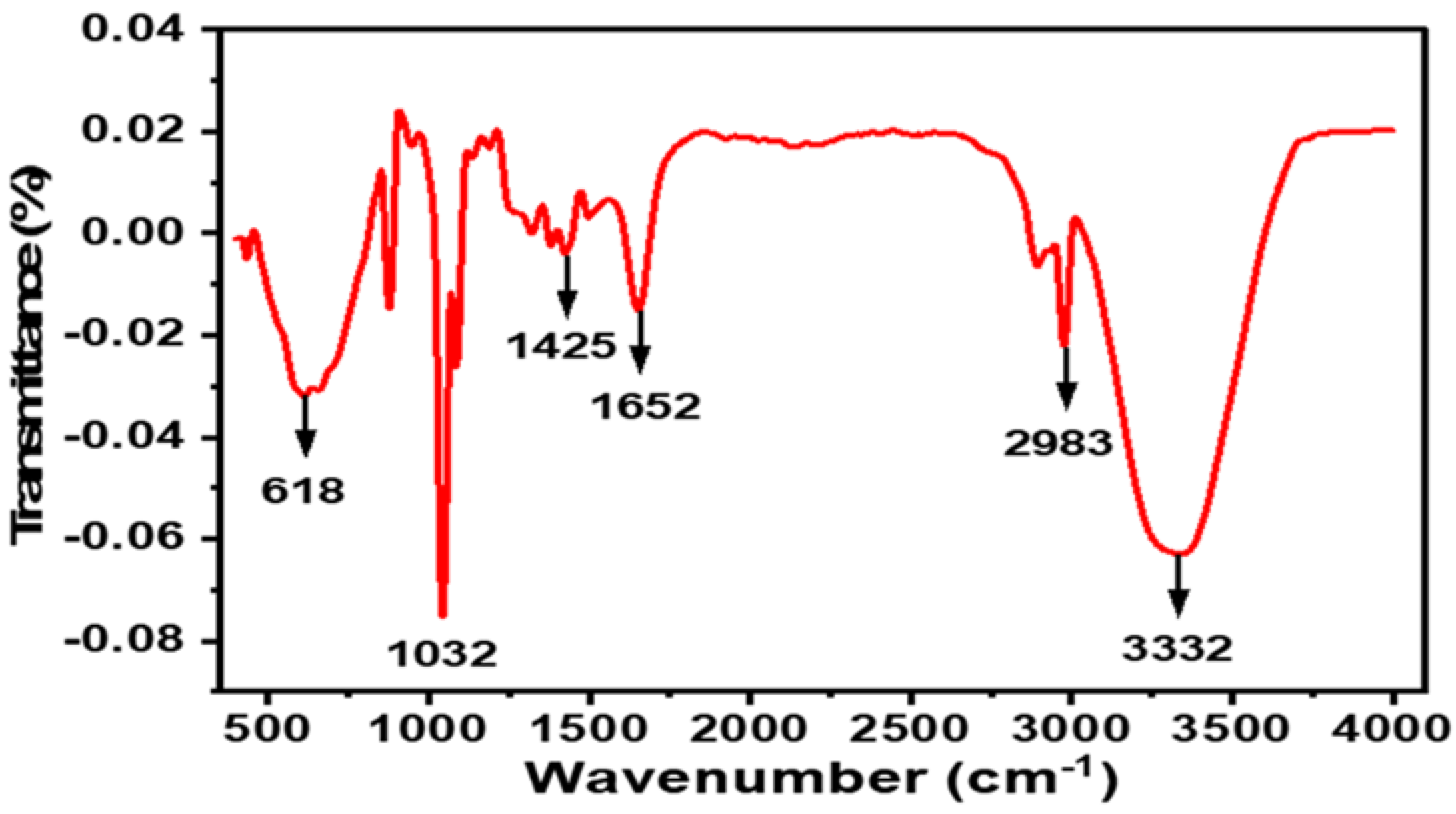
2.1.3. FTIR Spectroscopy
2.1.4. SEM-EDX and TEM Analyses
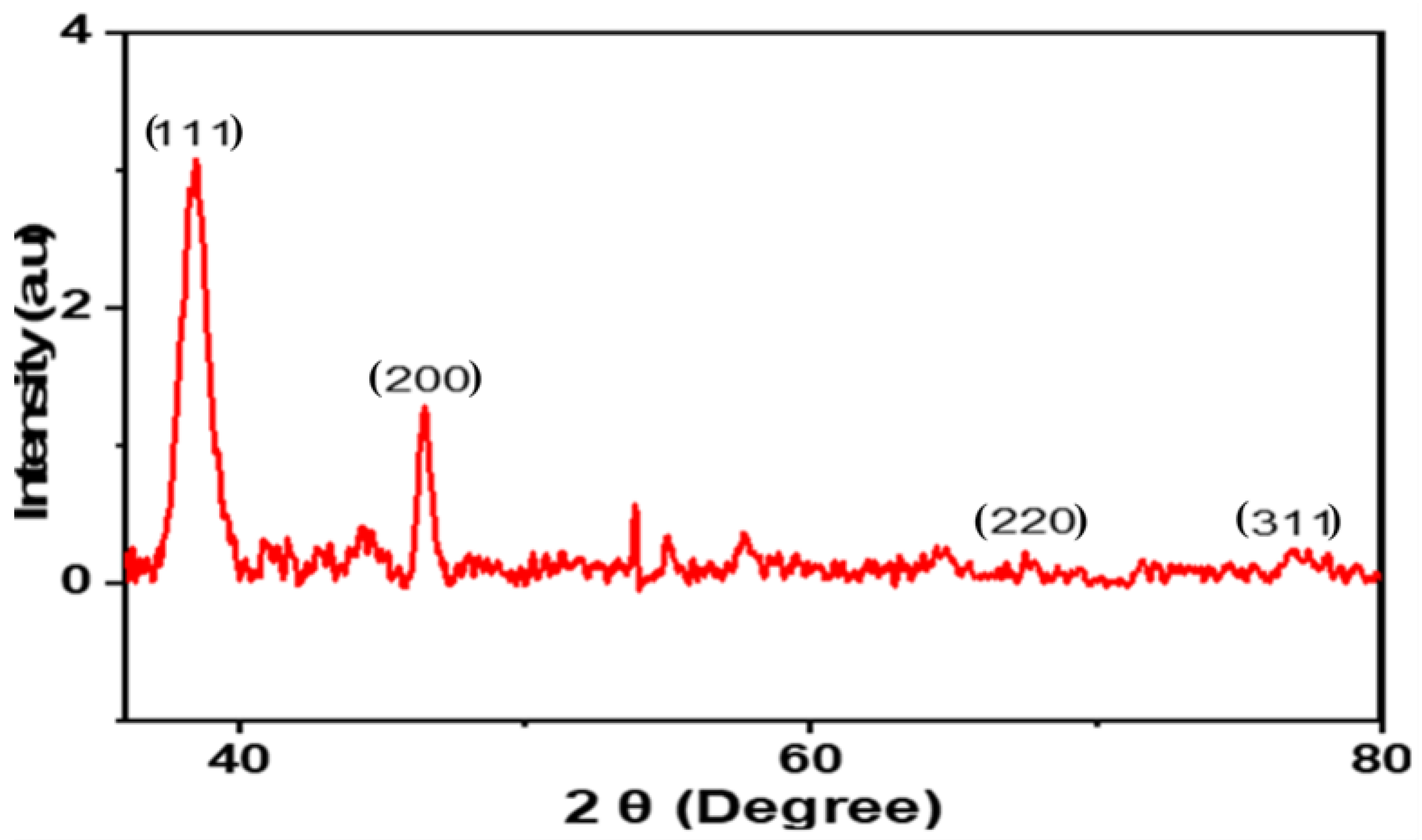
2.1.5. X-ray Diffraction (XRD)
2.2. Antibacterial Potency of Extracts and Cullen tomentosum Silver Nanoparticle (CTAgNP)
2.3. Phytochemical Profiling
3. Materials and Methods
3.1. Collection of Plant Material and Extraction
3.2. Synthesis of Cullen tomentosum Silver Nanoparticle (CTAgNP)
3.3. Characterization of the Cullen tomentosum Silver Nanoparticle (CTAgNP)
3.4. Antibacterial Assay
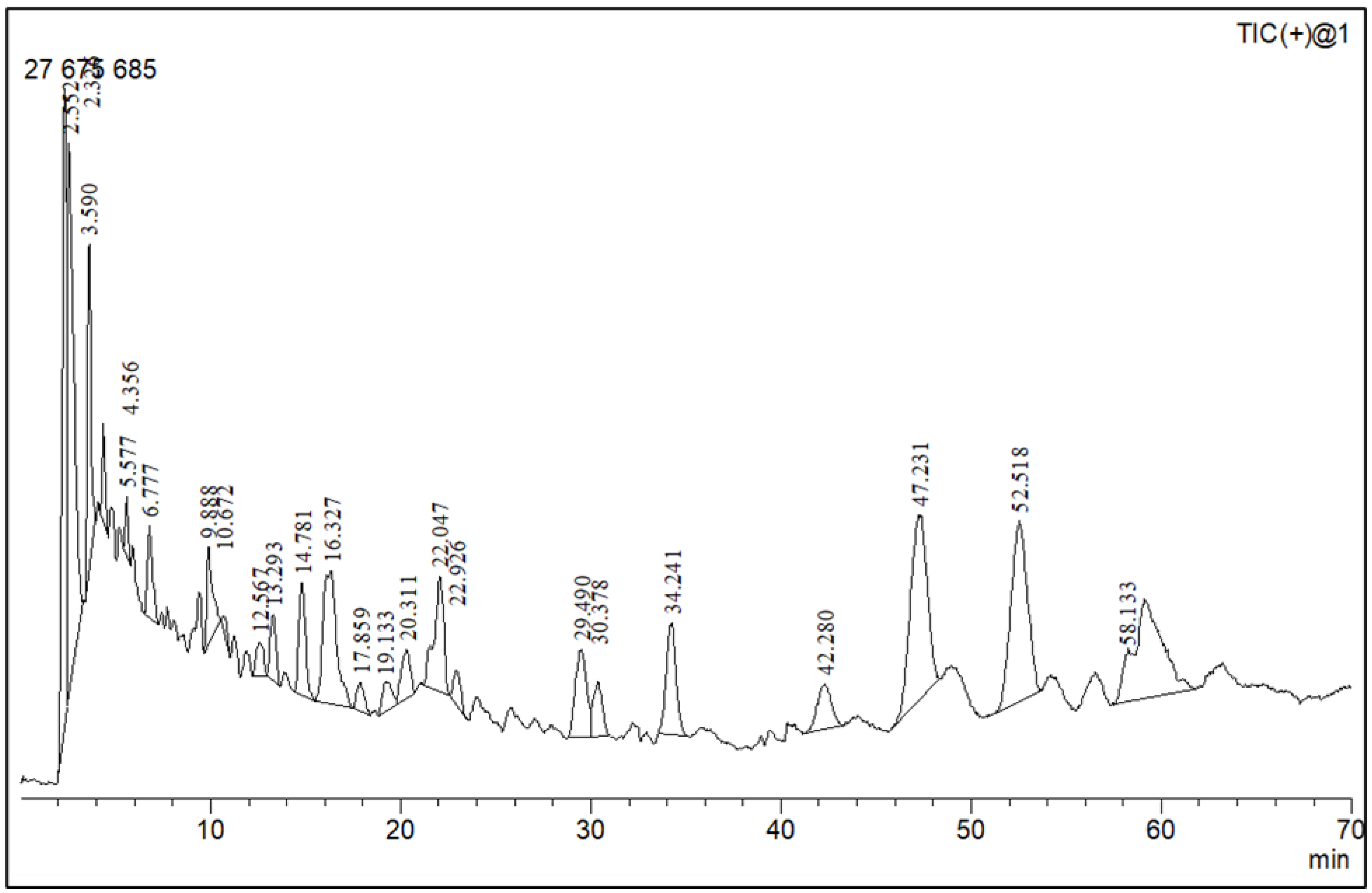
3.5. Phytochemical Profile Using Liquid Chromatography–Mass Spectroscopy (LC–MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Yadav, S.; Rawal, G.; Baxi, M. An overview of the latest lnfectious diseases around the world. J. Community Health Manag. 2016, 3, 41–43. [Google Scholar]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Ajose, D.J.; Abolarinwa, T.O.; Oluwarinde, B.O.; Montso, P.K.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Application of plant-derived nanoparticles (PDNP) in food-producing animals as a bio-control agent against antimicrobial-resistant pathogens. Biomedicines 2022, 10, 2426. [Google Scholar] [CrossRef] [PubMed]
- Keabadile, O.P.; Aremu, A.O.; Elugoke, S.E.; Fayemi, O.E. Green and traditional synthesis of Copper oxide nanoparticles-comparative Study. Nanomaterials 2020, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Jang, H.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Din, M.I.; Rehan, R. Synthesis, characterization, and applications of copper nanoparticles. Anal. Lett. 2017, 50, 50–62. [Google Scholar] [CrossRef]
- Ghosh, S.; Shah, S.; Webster, T. Recent trends in fungal mediated biosynthesis of nanoparticles. Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Jee, S.-C.; Kim, M.; Shinde, S.K.; Ghodake, G.S.; Sung, J.-S.; Kadam, A.A. Assembling ZnO and Fe3O4 nanostructures on halloysite nanotubes for anti-bacterial assessments. Appl. Surf. Sci. 2020, 509, 145358. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233. [Google Scholar] [CrossRef]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic applications of biostable silver nanoparticles synthesized using peel extract of Benincasa hispida: Antibacterial and anticancer activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Xia, Y. Transformation of silver nanospheres into nanobelts and triangular nanoplates through a thermal process. Nano Lett. 2003, 3, 675–679. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Mohan Mantravadi, K.; Nageswara Rao, G.V.S. Strategies to synthesise copper oxide nanoparticles and their bio applications—A review. Mater. Sci. Technol. 2018, 34, 2214–2222. [Google Scholar] [CrossRef]
- Mohan, S.; Singh, Y.; Verma, D.K.; Hasan, S.H. Synthesis of CuO nanoparticles through green route using Citrus limon juice and its application as nanosorbent for Cr(VI) remediation: Process optimization with RSM and ANN-GA based model. Process Saf. Environ. Prot. 2015, 96, 156–166. [Google Scholar] [CrossRef]
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Kitture, R.; Kale, S.; Bellare, J.; Chopade, B.A. Novel platinum–palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activities. Int. J. Nanomed. 2015, 10, 7477. [Google Scholar]
- Sumitha, S.; Vidhya, R.; Lakshmi, M.S.; Prasad, K.S. Leaf extract mediated green synthesis of copper oxide nanoparticles using Ocimum tenuiflorum and its characterization. Int. J. Chem. Sci. 2016, 14, 2016. [Google Scholar]
- Kumar, B.V.; Naik, H.S.B.; Girija, D.; Kumar, B.V. ZnO nanoparticle as catalyst for efficient green one-pot synthesis of coumarins through Knoevenagel condensation. J. Chem. Sci. 2011, 123, 615–621. [Google Scholar] [CrossRef]
- Zhang, D.E.; Ni, X.M.; Zheng, H.G.; Li, Y.; Zhang, X.J.; Yang, Z.P. Synthesis of needle-like nickel nanoparticles in water-in-oil microemulsion. Mater. Lett. 2005, 59, 2011–2014. [Google Scholar] [CrossRef]
- Padalia, H.; Moteriya, P.; Chanda, S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef]
- Shankar, S.; Leejae, S.; Jaiswal, L.; Voravuthikunchai, S.P. Metallic nanoparticles augmented the antibacterial potency of Rhodomyrtus tomentosa acetone extract against Escherichia coli. Microb. Pathog. 2017, 107, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.S.; Ren, C.Y. Synthesis of nanosized silver particles by chemical reduction method. Mater. Chem. Phys. 2000, 64, 241–246. [Google Scholar] [CrossRef]
- Maragoni, V.; Maragoni, V.; Ayodhya, D.-D.; Madhusudhan, A.; Amrutham, S.; Guttena, V.; Mangatayaru, K. A Novel Green Synthesis of Silver Nanoparticles Using Gum Karaya: Characterization, Antimicrobial and Catalytic Activity Studies. J. Clust. Sci. 2013, 25, 409–422. [Google Scholar]
- Jabir, M.S.; Hussien, A.A.; Sulaiman, G.M.; Yaseen, N.Y.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A.; Rizwana, H. Green synthesis of silver nanoparticles from Eriobotrya japonica extract: A promising approach against cancer cells proliferation, inflammation, allergic disorders and phagocytosis induction. Artif. Cells Nanomed. Biotechnol. 2021, 49, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Bowler, P.G.; Walker, M.; Parsons, D. Controlling wound bioburden with a novel silver-containing Hydrofiber® dressing. Wound Repair Regen. 2004, 12, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Silver, S.; Phung, L.T. Bacterial heavy metal resistance: New surprises. Annu. Rev. Microbiol. 1996, 50, 753–789. [Google Scholar] [CrossRef] [PubMed]
- von Staden, L. Cullen tomentosum (Thunb.) J.W.Grimes. National Assessment: Red List of South African Plants, Version 2020.1. Available online: http://redlist.sanbi.org/species.php?species=374-9 (accessed on 14 October 2022).
- Kaholongo, L.T. Screening of Indigenous Forage Legumes as Potential Fodder Crops and Protein Source for Livestock in Central Namibia. Masters’s Thesis, University of Namibia, Windhoek, Namibia, 2016. [Google Scholar]
- Van Wyk, B.-E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2000. [Google Scholar]
- Asong, J.A.; Ndhlovu, P.T.; Khosana, N.S.; Aremu, A.O.; Otang-Mbeng, W. Medicinal plants used for skin-related diseases among the Batswanas in Ngaka Modiri Molema District Municipality, South Africa. S. Afr. J. Bot. 2019, 126, 11–20. [Google Scholar] [CrossRef]
- Banerjee, P.P.; Bandyopadhyay, A.; Harsha, S.N.; Policegoudra, R.S.; Bhattacharya, S.; Karak, N.; Chattopadhyay, A. Mentha arvensis (Linn.)-mediated green silver nanoparticles trigger caspase 9-dependent cell death in MCF7 and MDA-MB-231 cells. Breast Cancer: Targets Ther. 2017, 9, 265. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Rao, N.H.; N, L.; Pammi, S.V.N.; Kollu, P.; S, G.; P, L. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C 2016, 62, 553–557. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.C.; Rusch, P.; Scheper, T.; Walter, J.-G. Hypericum perforatum L.-mediated green synthesis of silver nanoparticles exhibiting antioxidant and anticancer activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef]
- Algotiml, R.; Gab-Alla, A.; Seoudi, R.; Abulreesh, H.H.; El-Readi, M.Z.; Elbanna, K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci. Rep. 2022, 12, 1–18. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Bharali, P.; Das, S.; Bhandari, N.; Das, A.K.; Kalta, M.C. Sunlight induced biosynthesis of silver nanoparticle from the bark extract of Amentotaxus assamica DK Ferguson and its antibacterial activity against Escherichia coli and Staphylococcus aureus. IET Nanobiotechnol. 2019, 13, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Mihoubi, W.; Sahli, E.; Gargouri, A.; Amiel, C. FTIR spectroscopy of whole cells for the monitoring of yeast apoptosis mediated by p53 over-expression and its suppression by Nigella sativa extracts. PLoS ONE 2017, 12, e0180680. [Google Scholar] [CrossRef] [PubMed]
- Dumas, P.; Miller, L. The use of synchrotron infrared microspectroscopy in biological and biomedical investigations. Vib. Spectrosc. 2003, 32, 3–21. [Google Scholar] [CrossRef]
- Kuhire, S.S.; Nagane, S.S.; Wadgaonkar, P.P. Poly (ether urethane) s from aromatic diisocyanates based on lignin-derived phenolic acids. Polym. Int. 2017, 66, 892–899. [Google Scholar] [CrossRef]
- Basavarajappa, D.S.; Kumar, R.S.; Almansour, A.I.; Chakraborty, B.; Bhat, M.P.; Nagaraja, S.K.; Hiremath, H.; Perumal, K.; Nayaka, S. Biofunctionalized silver nanoparticles synthesized from Passiflora vitifolia leaf extract and evaluation of its antimicrobial, antioxidant and anticancer activities. Biochem. Eng. J. 2022, 187, 108517. [Google Scholar] [CrossRef]
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia pectinata (Willd.) C. Presl.): Characterization and antimicrobial studies. Heliyon 2019, 5, e01543. [Google Scholar] [CrossRef]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Camacho-López, M.A.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.; Odebunmi, E. A novel zerovalent manganese for removal of copper ions: Synthesis, characterization and adsorption studies. Appl. Water Sci. 2017, 7, 1409–1427. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Rizvi, S.M.D.; Hussain, T.; Mehmood, K.; Rafi, Z.; Moin, A.; Abu Lila, A.S.; Alshammari, F.; Khafagy, E.-S.; Rahamathulla, M.; et al. Cefotaxime mediated synthesis of gold nanoparticles: Characterization and antibacterial activity. Polymers 2022, 14, 771. [Google Scholar] [CrossRef]
- Mollick, M.M.R.; Rana, D.; Dash, S.K.; Chattopadhyay, S.; Bhowmick, B.; Maity, D.; Mondal, D.; Pattanayak, S.; Roy, S.; Chakraborty, M. Studies on green synthesized silver nanoparticles using Abelmoschus esculentus (L.) pulp extract having anticancer (in vitro) and antimicrobial applications. Arab. J. Chem. 2019, 12, 2572–2584. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.-S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.K.; Khan, S.; Lila, A.S.A.; Arab, H.H.; Hegazy, W.A. Synthesis of gold nanoparticles by using green machinery: Characterization and in vitro toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, W.-H. Mango peel extract mediated novel route for synthesis of silver nanoparticles and antibacterial application of silver nanoparticles loaded onto non-woven fabrics. Ind. Crops Prod. 2013, 48, 81–88. [Google Scholar] [CrossRef]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Suib, S.L. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir 2011, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Katerere, D.R.; Eloff, J.N. Anti-bacterial and anti-oxidant activity of Hypoxis hemerocallidea (Hypoxidaceae): Can leaves be substituted for corms as a conservation strategy? S. Afr. J. Bot. 2008, 74, 613–616. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Afolayan, A.J. In vitro antibacterial and time-kill evaluation of the Erythrina caffra Thunb. extract against bacteria associated with diarrhoea. Sci. World J. 2012, 2012, 738314. [Google Scholar] [CrossRef] [PubMed]
- Obistioiu, D.; Cocan, I.; Tîrziu, E.; Herman, V.; Negrea, M.; Cucerzan, A.; Neacsu, A.G.; Cozma, A.L.; Nichita, I.; Hulea, A.; et al. Phytochemical profile and microbiological activity of some plants belonging to the Fabaceae family. Antibiotics 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and Its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49(D1), D1388–D1395. [Google Scholar] [CrossRef]
- Singh, D.; Kumari, K.; Ahmed, S. Natural herbal products for cancer therapy. In Understanding Cancer: From Basics to Therapeutics; Jain, B., Pandey, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 257–268. [Google Scholar]
- Stringlis, I.A.; de Jonge, R.; Pieterse, C.M.J. The age of coumarins in plant-microbe interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef]
- Kavanaugh, K.M.; Aisen, A.M.; Fechner, K.P.; Wroblewski, L.; Chenevert, T.L.; Buda, A.J. Effects of diltiazem on phosphate metabolism in ischemic and reperfused myocardium using phosphorus31 nuclear magnetic resonance spectroscopy in vivo. Am. Heart J. 1989, 118, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Pethakamsetty, L.; Kothapenta, K.; Nammi, H.R.; Ruddaraju, L.K.; Kollu, P.; Yoon, S.G.; Pammi, S.V.N. Green synthesis, characterization and antimicrobial activity of silver nanoparticles using methanolic root extracts of Diospyros sylvatica. J. Environ. Sci. 2017, 55, 157–163. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Fuchs, S.; Pané-Farré, J.; Kohler, C.; Hecker, M.; Engelmann, S. Anaerobic gene expression in Staphylococcus aureus. J. Bacteriol. 2007, 189, 4275–4289. [Google Scholar] [CrossRef]
- Drewnowska, J.M.; Stefanska, N.; Czerniecka, M.; Zambrowski, G.; Swiecicka, I. Potential enterotoxicity of phylogenetically diverse Bacillus cereus sensu lato soil isolates from different geographical locations. Appl. Environ. Microbiol. 2020, 86, e03032-19. [Google Scholar] [CrossRef] [PubMed]
- Esmkhani, M.; Shams, S. Cutaneous infection due to Bacillus cereus: A case report. BioMed Cent. Infect. Dis. 2022, 22, 393. [Google Scholar] [CrossRef]
- Michelotti, F.; Bodansky, H.J. Bacillus cereus causing widespread necrotising skin infection in a diabetic person. Pract. Diabetes 2015, 32, 169–170a. [Google Scholar] [CrossRef]
- Swiecicka, I. Natural occurrence of Bacillus thuringiensis and Bacillus cereus in eukaryotic organisms: A case for symbiosis. Biocontrol Sci. Technol. 2008, 18, 221–239. [Google Scholar] [CrossRef]
- McGaw, L.J.; Van der Merwe, D.; Eloff, J.N. In vitro anthelmintic, antibacterial and cytotoxic effects of extracts from plants used in South African ethnoveterinary medicine. Vet. J. 2007, 173, 366–372. [Google Scholar] [CrossRef]
- Seepe, H.A.; Ramakadi, T.G.; Lebepe, C.M.; Amoo, S.O.; Nxumalo, W. Antifungal activity of isolated compounds from the leaves of Combretum erythrophyllum (Burch.) Sond. and Withania somnifera (L.) Dunal against Fusarium pathogens. Molecules 2021, 26, 4732. [Google Scholar] [CrossRef]


| Sample | Minimum Inhibitory Concentration (mg/mL) | |
|---|---|---|
| Bacillus cereus | Staphylococcus aureus | |
| Acetone extract | 2.6 | 3.1 |
| Silver nanoparticle (CTAgNP) | 1.5 | 2.6 |
| Peak Number | RT * | PA # (%) | Structure of Compound | Name of Compound (m/z Cloud Library) | Similarity Index (%) |
|---|---|---|---|---|---|
| 1 | 2.326 | 11.63 | 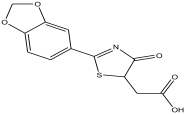 | 2-(2-(benzo[d][1,3] dioxol-6-yl)-4,5-dihydro-4-oxothiazol-5-yl)acetic acid | 81.3 |
| 2 | 2.552 | 11.988 | 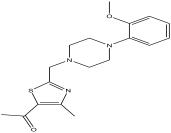 | 1-(2-((4-(2-methoxyphenyl) piperazin-1-yl)methyl)-4-methylthiazol-5-yl)ethanone | 82.2 |
| 3 | 3.59 | 4.545 | 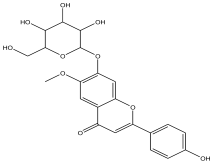 | 2-(4-hydroxyphenyl)-6-methoxy-7-(tetrahydro-3,4,5-trihydroxy-6-(hydroxymethyl)-2H-pyran-2-yloxy)-4H-chromen-4-one | 56.8 |
| 4 | 4.356 | 1.053 |  | 5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 90.3 |
| 5 | 5.577 | 0.588 | 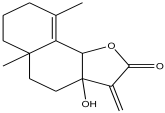 | 3,3a,4,5,5a,6,7,8-octahydro-3a-hydroxy-5a,9-dimethyl-3-methylenenaphtho[1,2-b]furan-2(9bH)-one | 84.8 |
| 6 | 6.777 | 1.683 | 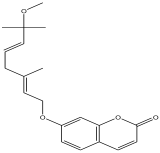 | 7-((2E,5E)-7-methoxy-3,7-dimethylocta-2,5-dienyloxy)-2H-chromen-2-one | 84.3 |
| 7 | 9.888 | 2.19 | 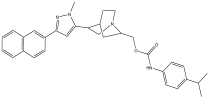 | (3-(1-methyl-3-(naphthalen-6-yl)-1H-pyrazol-5-yl)quinuclidin-7-yl)methyl 4-isopropylphenylcarbamate | 81.5 |
| 8 | 10.672 | 0.252 | 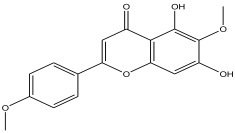 | 5,7-dihydroxy-6-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one | 81.2 |
| 9 | 12.567 | 0.945 | 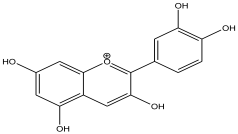 | Cyanidin | 89.2 |
| 10 | 13.293 | 1.414 | 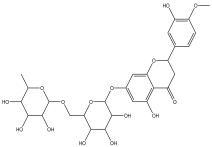 | No name identity from NIST Library | 95.7 |
| 11 | 14.781 | 2.77 | 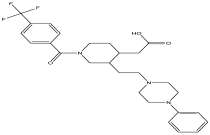 | No name identity from NIST library | 90.6 |
| 12 | 16.327 | 6.629 | 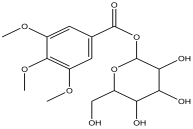 | tetrahydro-3,4,5-trihydroxy-6-(hydroxymethyl)-2H-pyran-2-yl 3,4,5-trimethoxybenzoate | 89.1 |
| 13 | 17.859 | 0.823 | 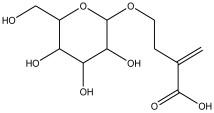 | 2-methylene-4-(tetrahydro-3,4,5-trihydroxy-6-(hydroxymethyl)-2H-pyran-2-yloxy) butanoic acid | 80.1 |
| 14 | 19.133 | 1.078 | 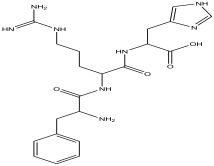 | 2-(guanidine)-3-(1H-imidazol-4-yl) propanoic acid | 99.4 |
| 15 | 20.311 | 1.654 | 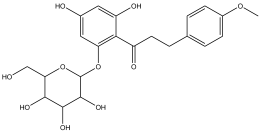 | No name identity equivalent in NIST library | 93.8 |
| 16 | 22.047 | 4.224 | 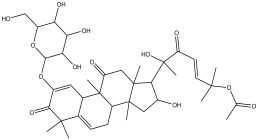 | No name identity equivalent in NIST library | 80.1 |
| 17 | 22.926 | 0.907 | 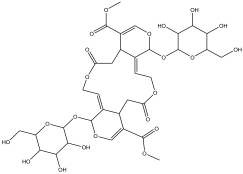 | No name identity equivalent in NIST library | 90.6 |
| 18 | 29.49 | 3.729 | 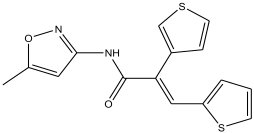 | (E)-N-(5-methylisoxazol-3-yl)-3-(thiophen-2-yl)-2-(thiophen-3-yl)acrylamide | 95.8 |
| 19 | 30.378 | 1.804 | 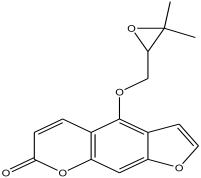 | 4-((3,3-dimethyloxiran-2-yl)methoxy)-7H-furo[3,2-g]chromen-7-one | 88.9 |
| 20 | 34.241 | 4.11 |  | (Z)-6-(bromomethylene)-tetrahydro-3-(naphthalen-5-yl)pyran-2-one | 91.2 |
| 21 | 42.28 | 2.368 |  | N-(3-(tetrahydro-2H-pyran-4-yl)-1-hydroxy-1-phenylpropan-2-yl)decanamide | 84.6 |
| 22 | 47.231 | 11.648 | 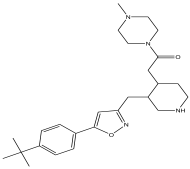 | 2-(3-((5-(4-tert-butylphenyl)isoxazol-3-yl) methyl) piperidin-4-yl)-1-(4-methylpiperazin-1-yl)ethanone | 85.3 |
| 23 | 52.518 | 11.169 | 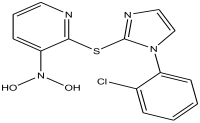 | 2-(1-(2-chlorophenyl)-1H-imidazol-2-ylthio)-N,N-dihydroxypyridin-3-amine | 87.9 |
| 24 | 58.133 | 10.802 | 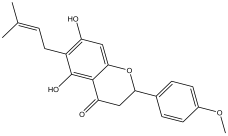 | 2,3-dihydro-5,7-dihydroxy-2-(4-methoxyphenyl)-6-(3-methylbut-2-enyl)chromen-4-one | 85.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asong, J.A.; Frimpong, E.K.; Seepe, H.A.; Katata-Seru, L.; Amoo, S.O.; Aremu, A.O. Green Synthesis of Characterized Silver Nanoparticle Using Cullen tomentosum and Assessment of Its Antibacterial Activity. Antibiotics 2023, 12, 203. https://doi.org/10.3390/antibiotics12020203
Asong JA, Frimpong EK, Seepe HA, Katata-Seru L, Amoo SO, Aremu AO. Green Synthesis of Characterized Silver Nanoparticle Using Cullen tomentosum and Assessment of Its Antibacterial Activity. Antibiotics. 2023; 12(2):203. https://doi.org/10.3390/antibiotics12020203
Chicago/Turabian StyleAsong, John Awungnjia, Ebenezer Kwabena Frimpong, Hlabana Alfred Seepe, Lebogang Katata-Seru, Stephen Oluwaseun Amoo, and Adeyemi Oladapo Aremu. 2023. "Green Synthesis of Characterized Silver Nanoparticle Using Cullen tomentosum and Assessment of Its Antibacterial Activity" Antibiotics 12, no. 2: 203. https://doi.org/10.3390/antibiotics12020203
APA StyleAsong, J. A., Frimpong, E. K., Seepe, H. A., Katata-Seru, L., Amoo, S. O., & Aremu, A. O. (2023). Green Synthesis of Characterized Silver Nanoparticle Using Cullen tomentosum and Assessment of Its Antibacterial Activity. Antibiotics, 12(2), 203. https://doi.org/10.3390/antibiotics12020203









