Colistin Effects on Emphysematous Lung in an LPS-Sepsis Model
Abstract
:1. Introduction
2. Results
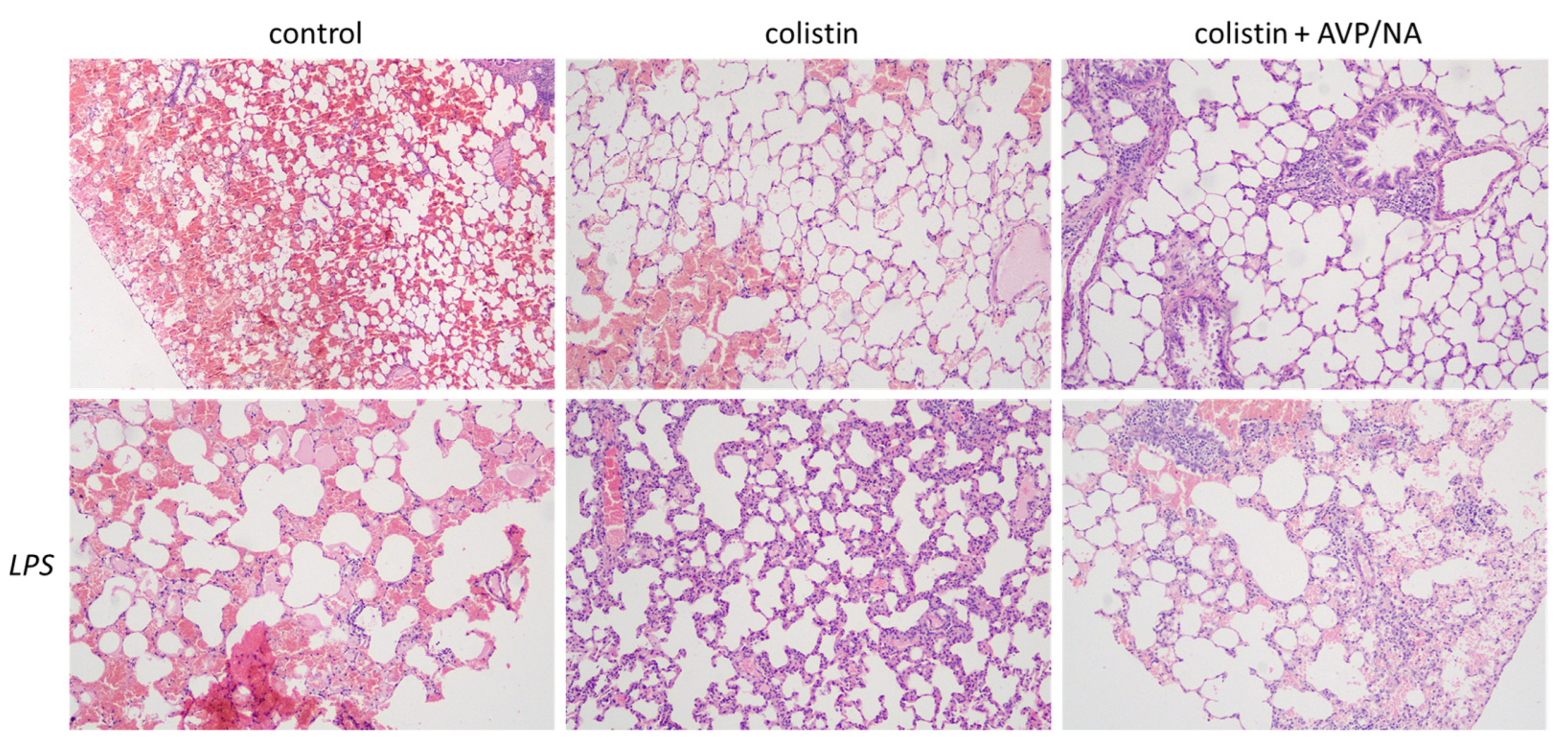
2.1. Histopathological Evaluations
2.2. Colistin Tissue Levels
2.3. Cytokine Production
2.4. Signaling Pathways Activated
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animal Treatment
4.3. Tissues Processing
4.4. Histopathological Evaluation
4.5. Colistin Levels Evaluation
4.6. Cytokine Concentration Evaluation
4.7. Protein Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamoru, T.; Gen, Y.; Hiroyuki, K.; Hiroki, T. Classification of centrilobular emphysema based on CT-pathologic correlations. Open Respir. Med. J. 2012, 6, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Alfageme, I.; Reyes, N.; Merino, M.; Reina, A.; Gallego, J.; Lima, J.; Palacios, Z. The effect of airflow limitation on the cause of death in patients with COPD. Chron. Respir. Dis. 2010, 7, 135–145. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Salvatore, D.; Buzzetti, R.; Baldo, E.; Forneris, M.P.; Lucidi, V.; Manunza, D.; Marinelli, I.; Messore, B.; Neri, A.S.; Raia, V.; et al. An overview of international literature from cystic fibrosis registries. Part 3. Disease incidence, genotype/phenotype correlation, microbiology, pregnancy, clinical complications, lung transplantation, and miscellanea. J. Cyst. Fibros. 2011, 10, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, D.P.; Steinfort, C. Effect of long-term nebulized colistin on lung function and quality of life in patients with chronic bronchial sepsis. Intern. Med. J. 2007, 37, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. BMC Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, A.; Hagart, K.L.; Klöckner, A.; Becce, M.; Evans, L.E.; Furniss, R.C.D.; Mavridou, D.A.; Murphy, R.; Stevens, M.M.; Davies, J.C.; et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. eLife 2021, 10, e65836. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, H.; Liu, Y.H.; Feng, Y. Towards Understanding MCR-like Colistin Resistance. Trends Microbiol. 2018, 26, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Souli, M.; Galani, I.; Giamarellou, H. Colistin: Still a lifesaver for the 21st century? Expert Opin. Drug Metab. Toxicol. 2017, 13, 59–71. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lai, C.-C.; Wang, Y.-H.; Wang, C.-Y.; Wang, H.-C.; Yu, C.-J.; Chen, L.; Taiwan Clinical Trial Consortium for Respiratory Diseases (TCORE). The Impact of Sepsis on the Outcomes of COPD Patients: A Population-Based Cohort Study. J. Clin. Med. 2018, 7, 393. [Google Scholar] [CrossRef]
- Zhu, Y.; Monsel, A.; Roberts, J.A.; Pontikis, K.; Mimoz, O.; Rello, J.; Qu, J.; Rouby, J.-J.; on behalf of the European Investigator Network for Nebulized Antibiotics in Ventilator-Associated Pneumonia (ENAVAP). Nebulized Colistin in Ventilator-Associated Pneumonia and Tracheobronchitis: Historical Background, Pharmacokinetics and Perspectives. Microorganisms 2021, 9, 1154. [Google Scholar] [CrossRef]
- Voynow, J.A.; Shinbashi, M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules 2021, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Elphick, H.; Pettitt, E.; Everard, M.L.; Evans, G.S. Colistin stimulates the activity of neutrophil elastase and Pseudomonas aeruginosa elastase. Eur. Respir. J. 2002, 19, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.-B.; He, Z.-H. Animal models of emphysema. Chin. Med. J. 2019, 132, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Stamatiou, R.; Vasilaki, A.; Tzini, D.; Tsolaki, V.; Zacharouli, K.; Ioannou, M.; Fotakopoulos, G.; Sgantzos, M.; Makris, D. Critical-Illness: Combined Effects of Colistin and Vasoactive Drugs: A Pilot Study. Antibiotics 2023, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Ma, Y.; Manrique-Caballero, C.L.; Li, H.; Emlet, D.R.; Li, S.; Baty, C.J.; Wen, X.; Kim-Campbell, N.; Frank, A.; et al. Activation of AMP-activated protein kinase during sepsis/inflammation improves survival by preserving cellular metabolic fitness. FASEB J. 2020, 34, 7036–7057. [Google Scholar] [CrossRef]
- Vachharajani, V.T.; Liu, T.; Brown, C.M.; Wang, X.; Buechler, N.L.; Wells, J.D.; Yoza, B.K.; McCall, C.E. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J. Leukoc. Biol. 2014, 96, 785–796. [Google Scholar] [CrossRef]
- Yang, Q.H.; Liu, D.W.; Wang, X.T.; Yang, R.L.; Shi, Y.; Long, Y.; Liu, H.Z.; He, H.W.; Zhou, X.; Tang, B. G1 cell cycle arrest signaling in hepatic injury after intraperitoneal sepsis in rats. Inflamm. Res. 2011, 60, 783–789. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Ding, W.; Jiang, G.; Lu, Z.; Li, L.; Wang, J.; Li, J.; Li, J. Autophagy regulates colistin-induced apoptosis in PC-12 cells. Antimicrob. Agents Chemother. 2015, 59, 2189–2197. [Google Scholar] [CrossRef]
- Mets, O.M.; Roothaan, S.M.; Bronsveld, I.; Luijk, B.; van de Graaf, E.A.; Vink, A.; de Jong, P.A. Emphysema Is Common in Lungs of Cystic Fibrosis Lung Transplantation Patients: A Histopathological and Computed Tomography Study. PLoS ONE 2015, 10, e0128062. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation during Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules. 2019, 24, 653. [Google Scholar] [CrossRef] [PubMed]
- Ott, L.W.; Resing, K.A.; Sizemore, A.W.; Heyen, J.W.; Cocklin, R.R.; Pedrick, N.M.; Woods, H.C.; Chen, J.Y.; Goebl, M.G.; Witzmann, F.A.; et al. Tumor Necrosis Factor-alpha- and interleukin-1-induced cellular responses: Coupling proteomic and genomic information. J. Proteome Res. 2007, 6, 2176–2185. [Google Scholar] [CrossRef]
- Bhutta, M.S.; Gallo, E.S.; Borenstein, R. Multifaceted Role of AMPK in Viral Infections. Cells 2021, 10, 1118. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Filippidis, A.; Zarogiannis, S.; Ioannou, M.; Gourgoulianis, K.; Molyvdas, P.A.; Hatzoglou, C. Transmembrane resistance and histology of isolated sheep leptomeninges. Neurol. Res. 2010, 32, 205–208. [Google Scholar] [CrossRef]
- Surti, P.V.; Kim, M.W.; Phan, L.M.T.; Kailasa, S.K.K.; Mungray, A.K.; Park, J.P.; Park, T.J. Progress on dot-blot assay as a promising analytical tool: Detection from molecules to cells. TrAC Trends Anal. Chem. 2022, 157, 116736. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. AMP-activated protein kinase: An energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp. Cell Res. 2023, 29, 113614. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef] [PubMed]
- McComb, S.; Chan, P.K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.-P.; Bornhauser, B.C. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci. Adv. 2019, 5, eaau9433. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wang, C.; Li, Z.; Sakamaki, T.; Pestell, R.G. Minireview: Cyclin D1, normal and abnormal functions. Endocrinology 2004, 145, 5439–5447. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamatiou, R.; Vasilaki, A.; Tzini, D.; Deskata, K.; Zacharouli, K.; Ioannou, M.; Sgantzos, M.; Zakynthinos, E.; Makris, D. Colistin Effects on Emphysematous Lung in an LPS-Sepsis Model. Antibiotics 2023, 12, 1731. https://doi.org/10.3390/antibiotics12121731
Stamatiou R, Vasilaki A, Tzini D, Deskata K, Zacharouli K, Ioannou M, Sgantzos M, Zakynthinos E, Makris D. Colistin Effects on Emphysematous Lung in an LPS-Sepsis Model. Antibiotics. 2023; 12(12):1731. https://doi.org/10.3390/antibiotics12121731
Chicago/Turabian StyleStamatiou, Rodopi, Anna Vasilaki, Dimitra Tzini, Konstantina Deskata, Konstantina Zacharouli, Maria Ioannou, Markos Sgantzos, Epaminondas Zakynthinos, and Demosthenes Makris. 2023. "Colistin Effects on Emphysematous Lung in an LPS-Sepsis Model" Antibiotics 12, no. 12: 1731. https://doi.org/10.3390/antibiotics12121731
APA StyleStamatiou, R., Vasilaki, A., Tzini, D., Deskata, K., Zacharouli, K., Ioannou, M., Sgantzos, M., Zakynthinos, E., & Makris, D. (2023). Colistin Effects on Emphysematous Lung in an LPS-Sepsis Model. Antibiotics, 12(12), 1731. https://doi.org/10.3390/antibiotics12121731






