Innovative Approaches for Maintaining and Enhancing Skin Health and Managing Skin Diseases through Microbiome-Targeted Strategies
Abstract
:1. Introduction
2. Prebiotics, Probiotics and Postbiotics
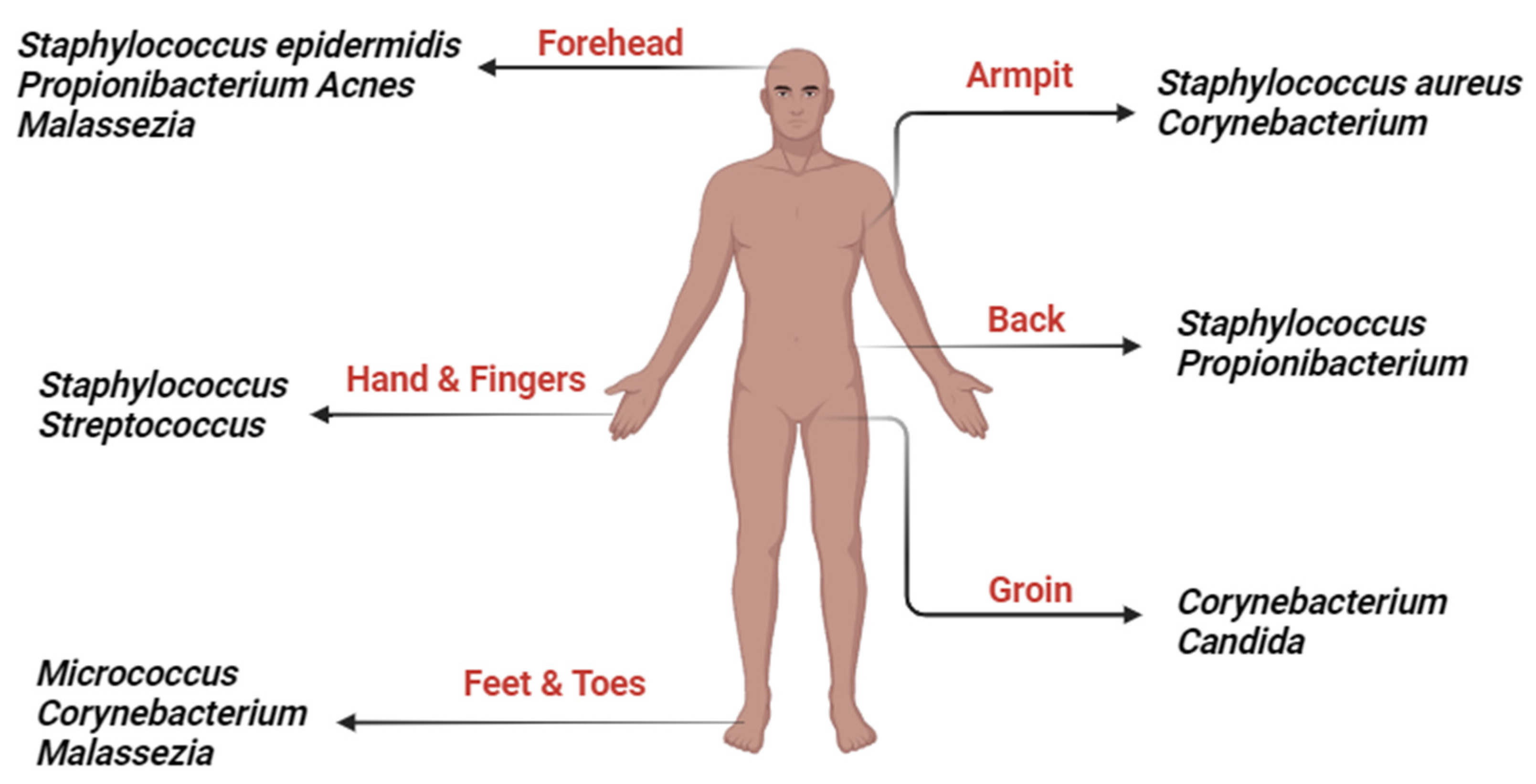
3. Skin Microbiome
4. Mechanisms of Action for Topical Prebiotics, Postbiotics and Probiotics
5. Clinical Verification and Effectiveness
6. Formulations and Delivery Methods
7. Challenges in Maintaining Probiotic and Prebiotic Viability and Stability
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dréno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Sanchez Viera, M.; Xiang, L.F.; Martin, R.; Bieber, T. Microbiome in healthy skin, update for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, P.; Li, Q.; Han, Z. Oxidative Stress and Gut Microbiome in Inflammatory Skin Diseases. Front. Cell Dev. Biol. 2022, 10, 849985. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Yang, S.-C.; Hsu, C.-Y.; Sung, J.-T.; Fang, J.-Y. The antibiofilm nanosystems for improved infection inhibition of microbes in skin. Molecules 2021, 26, 6392. [Google Scholar] [CrossRef]
- Lúcio, M.; Giannino, N.; Barreira, S.; Catita, J.; Gonçalves, H.; Ribeiro, A.; Fernandes, E.; Carvalho, I.; Pinho, H.; Cerqueira, F. Nanostructured lipid carriers enriched hydrogels for skin topical administration of quercetin and omega-3 fatty acid. Pharmaceutics 2023, 15, 2078. [Google Scholar] [CrossRef]
- Basu, B.; Garala, K.; Bhalodia, R.; Joshi, B.; Mehta, K. Solid lipid nanoparticles: A promising tool for drug delivery system. J. Pharm. Res. 2010, 3, 84–92. [Google Scholar]
- Zupančič, S.; Rijavec, T.; Lapanje, A.; Petelin, M.; Kristl, J.; Kocbek, P. Nanofibers with incorporated autochthonous bacteria as potential probiotics for local treatment of periodontal disease. Biomacromolecules 2018, 19, 4299–4306. [Google Scholar] [CrossRef]
- Chen, H.-J.; Lin, D.-a.; Liu, F.; Zhou, L.; Liu, D.; Lin, Z.; Yang, C.; Jin, Q.; Hang, T.; He, G. Transdermal delivery of living and biofunctional probiotics through dissolvable microneedle patches. ACS Appl. Bio Mater. 2018, 1, 374–381. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, J.; Rawding, P.; Bu, J.; Hong, S.; Hu, Q. Chemically and biologically engineered bacteria-based delivery systems for emerging diagnosis and advanced therapy. Adv. Mater. 2021, 33, 2102580. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine (s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Marcato, P.D.; Durán, N. New aspects of nanopharmaceutical delivery systems. J. Nanosci. Nanotechnol. 2008, 8, 2216–2229. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Mishra, D.; Hubenak, J.R.; Mathur, A.B. Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2013, 101, 3646–3660. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.-H.; Choi, S.-M.; Park, T.G. Thermally reversible pluronic/heparin nanocapsules exhibiting 1000-fold volume transition. Langmuir 2006, 22, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Battaglia, G. Exploiting endocytosis for nanomedicines. Cold Spring Harb. Perspect. Biol. 2013, 5, a016980. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; De Sarkar, S.; Pradhan, A.; Pati, A.K.; Pradhan, R.; Mondal, D.; Sen, S.; Ghosh, A.; Chatterjee, S.; Chatterjee, M. Levels of oxidative damage and proinflammatory cytokines are enhanced in patients with active vitiligo. Free Radic. Res. 2017, 51, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Guo, W.; Shi, Q.; Yang, Y.; Zhang, W.; Chen, X.; Kang, P.; Chen, J.; Cui, T.; Ma, J. SIRT3-dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced melanocyte degeneration in vitiligo. Theranostics 2019, 9, 1614–1633. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Męcińska-Jundziłł, K. Current aspects of vitiligo genetics. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2014, 31, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Krüger, C.; Schallreuter, K.U. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int. J. Dermatol. 2012, 51, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Lim, H.W.; Suzuki, T.; Katayama, I.; Hamzavi, I.; Lan, C.C.E.; Goh, B.K.; Anbar, T.; Silva de Castro, C.; Lee, A.Y. Revised classification/nomenclature of vitiligo and related issues: The Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012, 25, E1–E13. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef]
- Al-smadi, K.; Imran, M.; Leite-Silva, V.R.; Mohammed, Y. Vitiligo: A Review of Aetiology, Pathogenesis, Treatment, and Psychosocial Impact. Cosmetics 2023, 10, 84. [Google Scholar] [CrossRef]
- Alkhateeb, A.; Fain, P.R.; Thody, A.; Bennett, D.C.; Spritz, R.A. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003, 16, 208–214. [Google Scholar] [CrossRef]
- Laberge, G.; Mailloux, C.M.; Gowan, K.; Holland, P.; Bennett, D.C.; Fain, P.R.; Spritz, R.A. Early disease onset and increased risk of other autoimmune diseases in familial generalized vitiligo. Pigment Cell Res. 2005, 18, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Taieb, A.; Alomar, A.; Böhm, M.; Dell’Anna, M.L.; De Pase, A.; Eleftheriadou, V.; Ezzedine, K.; Gauthier, Y.; Gawkrodger, D.J.; Jouary, T. Guidelines for the management of vitiligo: The European Dermatology Forum consensus. Br. J. Dermatol. 2013, 168, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kwon, H.S.; Jung, H.M.; Lee, H.; Kim, G.M.; Yim, H.W.; Bae, J.M. Treatment outcomes of topical calcineurin inhibitor therapy for patients with vitiligo: A systematic review and meta-analysis. JAMA Dermatol. 2019, 155, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.J.; Domm, S.; Katz, H.I.; Lebwohl, M.; Mrowietz, U.; Kragballe, K.; International Psoriasis, Council. Topical corticosteroids in psoriasis: Strategies for improving safety. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Forschner, T.; Buchholtz, S.; Stockfleth, E. Current state of vitiligo therapy–evidence-based analysis of the literature. JDDG J. Dtsch. Dermatol. Ges. 2007, 5, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Al-Smadi, K.; Ali, M.; Alavi, S.E.; Jin, X.; Imran, M.; Leite-Silva, V.R.; Mohammed, Y. Using a Topical Formulation of Vitamin D for the Treatment of Vitiligo: A Systematic Review. Cells 2023, 12, 2387. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D. Heavy metals and heavy-metal antagonists. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed.; McGraw-Hill: New York, NY, USA, 2006; pp. 1753–1775. [Google Scholar]
- Wang, Y.; Li, S.; Li, C. Perspectives of new advances in the pathogenesis of vitiligo: From oxidative stress to autoimmunity. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Thio, H.B. The microbiome in psoriasis and psoriatic arthritis: The skin perspective. J. Rheumatol. Suppl. 2018, 94, 30–31. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis (Primer). Nat. Rev. Dis. Primers 2016, 2, 1. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 263–269. [Google Scholar] [CrossRef]
- Pothmann, A.; Illing, T.; Wiegand, C.; Hartmann, A.A.; Elsner, P. The microbiome and atopic dermatitis: A review. Am. J. Clin. Dermatol. 2019, 20, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.Y.; Gao, S.; Li, Y.R.; Wu, Y.F. Research progress of microbiome and pathogenesis of vitiligo. Life Res. 2021, 4, 13. [Google Scholar] [CrossRef]
- Pellicciotta, M.; Rigoni, R.; Falcone, E.L.; Holland, S.M.; Villa, A.; Cassani, B. The microbiome and immunodeficiencies: Lessons from rare diseases. J. Autoimmun. 2019, 98, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Mihranyan, A.; Ferraz, N.; Strømme, M. Current status and future prospects of nanotechnology in cosmetics. Prog. Mater. Sci. 2012, 57, 875–910. [Google Scholar] [CrossRef]
- Park, S.B.; Im, M.; Lee, Y.; Lee, J.H.; Lim, J.; Park, Y.-H.; Seo, Y.J. Effect of emollients containing vegetable-derived lactobacillus in the treatment of atopic dermatitis symptoms: Split-body clinical trial. Ann. Dermatol. 2014, 26, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ambrożej, D.; Kunkiel, K.; Dumycz, K.; Feleszko, W. The use of probiotics and bacteria-derived preparations in topical treatment of atopic dermatitis—A systematic review. J. Allergy Clin. Immunol. Pract. 2021, 9, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Bekiaridou, A.; Karlafti, E.; Oikonomou, I.M.; Ioannidis, A.; Papavramidis, T.S. Probiotics and their effect on surgical wound healing: A systematic review and new insights into the role of nanotechnology. Nutrients 2021, 13, 4265. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. The current trends and future perspectives of prebiotics research: A review. 3 Biotech 2012, 2, 115–125. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Sanders, M.E.; Gibson, G.R.; Gill, H.S.; Guarner, F. Probiotics: Their potential to impact human health. Counc. Agric. Sci. Technol. Issue Pap. 2007, 36, 1–20. [Google Scholar]
- França, K. Topical probiotics in dermatological therapy and skincare: A concise review. Dermatol. Ther. 2021, 11, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Swaby, A.M.; Agellon, L.B. The Potential Role of Commensal Microbes in Optimizing Nutrition Care Delivery and Nutrient Metabolism. Recent Prog. Nutr. 2022, 2, 1–16. [Google Scholar] [CrossRef]
- Novik, G.; Savich, V. Beneficial microbiota. Probiotics and pharmaceutical products in functional nutrition and medicine. Microbes Infect. 2020, 22, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Rad, A.H.; Ghasempour, Z.; Sabahi, S.; Kafil, H.S.; Hasannezhad, P.; Rahbar Saadat, Y.; Shahbazi, N. The biological activities of postbiotics in gastrointestinal disorders. Crit. Rev. Food Sci. Nutr. 2022, 62, 5983–6004. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2009, 28, 623–667. [Google Scholar] [CrossRef]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar] [CrossRef]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef]
- Bay, L.; Barnes, C.J.; Fritz, B.G.; Thorsen, J.; Restrup, M.E.M.; Rasmussen, L.; Sørensen, J.K.; Hesselvig, A.B.; Odgaard, A.; Hansen, A.J. Universal dermal microbiome in human skin. MBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Ogai, K.; Nana, B.C.; Lloyd, Y.M.; Arios, J.P.; Jiyarom, B.; Awanakam, H.; Esemu, L.F.; Hori, A.; Matsuoka, A.; Nainu, F. Skin microbiome profile of healthy Cameroonians and Japanese. Sci. Rep. 2022, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, M. Skin barrier function and the microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M. The Human Microbiota in Health and Disease: An Ecological and Community-Based Approach; Garland Science: New York, NY, USA, 2018. [Google Scholar]
- Harrison, O.J.; Linehan, J.L.; Shih, H.-Y.; Bouladoux, N.; Han, S.-J.; Smelkinson, M.; Sen, S.K.; Byrd, A.L.; Enamorado, M.; Yao, C. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019, 363, eaat6280. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, V.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Sánchez-Pellicer, P.; Agüera-Santos, J.; Navarro-Moratalla, L. Probiotics in the therapeutic arsenal of dermatologists. Microorganisms 2021, 9, 1513. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Fischbach, M.A. What lives on our skin: Ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov. Today Dis. Mech. 2013, 10, e83–e89. [Google Scholar] [CrossRef]
- Günther, J.; Seyfert, H.-M. The first line of defence: Insights into mechanisms and relevance of phagocytosis in epithelial cells. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 555–565. [Google Scholar]
- Nazir, Y.; Hussain, S.A.; Abdul Hamid, A.; Song, Y. Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. BioMed Res. Int. 2018, 2018, 3428437. [Google Scholar] [CrossRef]
- Cogen, A.L.; Yamasaki, K.; Sanchez, K.M.; Dorschner, R.A.; Lai, Y.; MacLeod, D.T.; Torpey, J.W.; Otto, M.; Nizet, V.; Kim, J.E. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Investig. Dermatol. 2010, 130, 192–200. [Google Scholar] [CrossRef]
- Gibbs, N.K.; Norval, M. Urocanic acid in the skin: A mixed blessing? J. Investig. Dermatol. 2011, 131, 14–17. [Google Scholar] [CrossRef]
- Sfriso, R.; Egert, M.; Gempeler, M.; Voegeli, R.; Campiche, R. Revealing the secret life of skin-with the microbiome you never walk alone. Int. J. Cosmet. Sci. 2020, 42, 116–126. [Google Scholar] [CrossRef]
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef]
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the gut-skin axis—Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019, 28, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Mohammed, Y.; Prow, T.W. Advances and controversies in studying sunscreen delivery and toxicity. Adv. Drug Deliv. Rev. 2020, 153, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Mohammed, Y.; Namjoshi, S.; Grice, J.; Sakran, W.; Roberts, M. Mechanistic evaluation of hydration effects on the human epidermal permeation of salicylate esters. AAPS J. 2017, 19, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Tapfumaneyi, P.; Imran, M.; Mohammed, Y.; Roberts, M.S. Recent advances and future prospective of topical and transdermal delivery systems. Front. Drug Deliv. 2022, 2, 957732. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Alekseyenko, A.V.; Perez-Perez, G.I.; De Souza, A.; Strober, B.; Gao, Z.; Bihan, M.; Li, K.; Methé, B.A.; Blaser, M.J. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013, 1, 31. [Google Scholar] [CrossRef]
- Gallo, R.L.; Nakatsuji, T. Microbial symbiosis with the innate immune defense system of the skin. J. Investig. Dermatol. 2011, 131, 1974–1980. [Google Scholar] [CrossRef]
- Chilicka, K.; Dzieńdziora-Urbińska, I.; Szyguła, R.; Asanova, B.; Nowicka, D. Microbiome and probiotics in acne vulgaris—A narrative review. Life 2022, 12, 422. [Google Scholar] [CrossRef]
- Ni, Q.; Ye, Z.; Wang, Y.; Chen, J.; Zhang, W.; Ma, C.; Li, K.; Liu, Y.; Liu, L.; Han, Z. Gut microbial dysbiosis and plasma metabolic profile in individuals with vitiligo. Front. Microbiol. 2020, 11, 592248. [Google Scholar] [CrossRef]
- Ganju, P.; Nagpal, S.; Mohammed, M.H.; Nishal Kumar, P.; Pandey, R.; Natarajan, V.T.; Mande, S.S.; Gokhale, R.S. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016, 6, 18761. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome–host metabolic signal disruption in health and disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Younes, J.A.; Van der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Microbiol. 2011, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Fischbach, M.A. Community health care: Therapeutic opportunities in the human microbiome. Sci. Transl. Med. 2011, 3, ps12–ps78. [Google Scholar] [CrossRef] [PubMed]
- Caselli, M.; Vaira, G.; Calo, G.; Papini, F.; Holton, J.; Vaira, D. Structural bacterial molecules as potential candidates for an evolution of the classical concept of probiotics. Adv. Nutr. 2011, 2, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, B.A. Modern condition and prospective host microecology investigations. Microb. Ecol. Health Dis. 2007, 19, 145–149. [Google Scholar]
- Shenderov, B.A. Metabiotics: Novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 2013, 24, 20399. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, D.N. Microbial ecology of human skin in health and disease. J. Investig. Dermatol. Symp. Proc. 2001, 6, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Foulongne, V.; Sauvage, V.; Hebert, C.; Dereure, O.; Cheval, J.; Gouilh, M.A.; Pariente, K.; Segondy, M.; Burguière, A.; Manuguerra, J.-C. Human skin microbiota: High diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS ONE 2012, 7, e38499. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J.M.; Brüggemann, H. Bacterial skin commensals and their role as host guardians. Benef. Microbes. 2014, 5, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Frizon, L.; Demeda, V.F. Ocular surface microbiome in health and disease. Asia-Pac. J. Ophthalmol. 2020, 9, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R.; Maarouf, M.; Hendricks, A.J.; Lee, D.E.; Shi, V.Y. Topical probiotics: The unknowns behind their rising popularity. Dermatol. Online J. 2019, 25, 13030. [Google Scholar] [CrossRef]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and human aging: Probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef]
- Bzioueche, H.; Sjödin, K.S.; West, C.E.; Khemis, A.; Rocchi, S.; Passeron, T.; Tulic, M.K. Analysis of matched skin and gut microbiome of patients with vitiligo reveals deep skin dysbiosis: Link with mitochondrial and immune changes. J. Investig. Dermatol. 2021, 141, 2280–2290. [Google Scholar] [CrossRef]
- Tulic, M.K.; Cavazza, E.; Cheli, Y.; Jacquel, A.; Luci, C.; Cardot-Leccia, N.; Hadhiri-Bzioueche, H.; Abbe, P.; Gesson, M.; Sormani, L. Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat. Commun. 2019, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Dellacecca, E.R.; Cosgrove, C.; Mukhatayev, Z.; Akhtar, S.; Engelhard, V.H.; Rademaker, A.W.; Knight, K.L.; Le Poole, I.C. Antibiotics drive microbial imbalance and vitiligo development in mice. J. Investig. Dermatol. 2020, 140, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.H.; Tester, R.F. Impact of prebiotics and probiotics on skin health. Benef. Microbes. 2014, 5, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wallen-Russell, C.; Wallen-Russell, S. Topical Probiotics Do Not Satisfy New Criteria for Effective Use Due to Insufficient Skin Microbiome Knowledge. Cosmetics 2021, 8, 90. [Google Scholar] [CrossRef]
- Renuka, S.R.; Kumar, N.A.; Manoharan, D.; Naidu, D.K. Probiotics: A Review on Microbiome That Helps for Better Health–A Dermatologist’s Perspective. J. Pharmacol. Pharmacother. 2023, 0976500X231175225. [Google Scholar] [CrossRef]
- Fuchs-Tarlovsky, V.; Marquez-Barba, M.F.; Sriram, K. Probiotics in dermatologic practice. Nutrition 2016, 32, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Reisch, M.S. Cosmetics: The next microbiome frontier. Mitsui Chem. Catal. Sci. Award 2017, 95, 30–34. [Google Scholar]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The concept of postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hanamizu, T.; Iizuka, R.; Chiba, K. Bifidobacterium-fermented soy milk extract stimulates hyaluronic acid production in human skin cells and hairless mouse skin. Ski. Pharmacol. Physiol. 2003, 16, 108–116. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hanamizu, T.; Sone, T.; Chiba, K.; Kinoshita, T.; Yoshikawa, S. Topical application of Bifidobacterium-fermented soy milk extract containing genistein and daidzein improves rheological and physiological properties of skin. J. Cosmet. Sci. 2004, 55, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Frei, R.; Akdis, M.; O’Mahony, L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015, 31, 153–158. [Google Scholar] [CrossRef]
- Myles, I.A.; Castillo, C.R.; Barbian, K.D.; Kanakabandi, K.; Virtaneva, K.; Fitzmeyer, E.; Paneru, M.; Otaizo-Carrasquero, F.; Myers, T.G.; Markowitz, T.E. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci. Transl. Med. 2020, 12, eaaz8631. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.; Rabiee, B.; Festok, M.; Gaspari, M.; Chaudhry, A. Combination of Topical Tacrolimus, Antioxidants, and Probiotics in the Treatment of Periorbital Vitiligo; Trinity Health Mid-Atlantic, Nazareth Hospital, Department of Ophthalmology: Darby, PA, USA, 2022; Available online: https://scholarcommons.towerhealth.org/cgi/viewcontent.cgi?article=1008&context=schc_researchday (accessed on 30 October 2023).
- Podrini, C.; Schramm, L.; Marianantoni, G.; Apolinarska, J.; McGuckin, C.; Forraz, N.; Milet, C.; Desroches, A.-L.; Payen, P.; D’Aguanno, M. Topical Administration of Lactiplantibacillus plantarum (SkinDuoTM) Serum Improves Anti-Acne Properties. Microorganisms 2023, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Simmering, R.; Breves, R. Prebiotic cosmetics. In Nutrition for Healthy Skin: Strategies for Clinical and Cosmetic Practice; Springer: Berlin/Heidelberg, Germany, 2010; pp. 137–147. [Google Scholar]
- Baral, K.C.; Bajracharya, R.; Lee, S.H.; Han, H.-K. Advancements in the pharmaceutical applications of probiotics: Dosage forms and formulation technology. Int. J. Nanomed. 2021, 16, 7535–7556. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C. Live biotherapeutic products and probiotics for the skin. Adv. NanoBiomed Res. 2021, 1, 2100118. [Google Scholar] [CrossRef]
- Jung, N.; Namjoshi, S.; Mohammed, Y.; Grice, J.E.; Benson, H.A.; Raney, S.G.; Roberts, M.S.; Windbergs, M. Application of confocal Raman microscopy for the characterization of topical semisolid formulations and their penetration into human skin ex vivo. Pharm. Res. 2022, 39, 935–948. [Google Scholar] [CrossRef]
- Ayichew, T.; Belete, A.; Alebachew, T.; Tsehaye, H.; Berhanu, H.; Minwuyelet, A. Bacterial probiotics their importances and limitations: A review. J. Nutr. Health Sci. 2017, 4, 202. [Google Scholar]
- Akbarzadeh, A.; Alirezaei, P.; Doosti-Irani, A.; Mehrpooya, M.; Nouri, F. The Efficacy of Lactocare® Synbiotic on the Clinical Symptoms in Patients with Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Dermatol. Res. Pract. 2022, 2022, 4549134. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kalailingam, P.; Delcour, J.A.; Fogliano, V.; Thanabalu, T. Olive-Derived Antioxidant Dietary Fiber Modulates Gut Microbiota Composition and Attenuates Atopic Dermatitis Like Inflammation in Mice. Mol. Nutr. Food Res. 2023, 67, 2200127. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Altermann, E.; Hutkins, R.; Cano, R.; Klaenhammer, T.R. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2003, 100, 8957–8962. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Klaenhammer, T.R. Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Annu. Rev. Food Sci. Technol. 2015, 6, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Florowska, A.; Krygier, K.; Florowski, T.; Dłużewska, E. Prebiotics as functional food ingredients preventing diet-related diseases. Food Funct. 2016, 7, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Vahedi, K.; Achour, L.; Attar, A.; Salfati, J.; Pochart, P.; Marteau, P.; Flourie, B.; Bornet, F.; Rambaud, J.-C. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal Bifidobacteria in healthy humans. J. Nutr. 1999, 129, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Shan, C.; Liu, S.; Zheng, H.; Liu, C.; Liu, M.; Jin, F.; Wang, L. Skin resistance to UVB-induced oxidative stress and hyperpigmentation by the topical use of Lactobacillus helveticus NS8-fermented milk supernatant. J. Appl. Microbiol. 2017, 123, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Golkar, N.; Ashoori, Y.; Heidari, R.; Omidifar, N.; Abootalebi, S.N.; Mohkam, M.; Gholami, A. A novel effective formulation of bioactive compounds for wound healing: Preparation, in vivo characterization, and comparison of various postbiotics cold creams in a rat model. Evid.-Based Complement. Altern. Med. 2021, 2021, 8577116. [Google Scholar] [CrossRef]
- Catic, T.; Pehlivanovic, B.; Pljakic, N.; Balicevac, A. The moisturizing efficacy of a proprietary dermo-cosmetic product (cls02021) versus placebo in a 4-week application period. Med. Arch. 2022, 76, 108. [Google Scholar] [CrossRef]
- Dimarzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.G.; Giuliani, M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.; Kim, W.J.; Chung, D.-K. Effect of paraprobiotic prepared from Kimchi-derived Lactobacillus plantarum K8 on skin moisturizing activity in human keratinocyte. J. Funct. Foods 2020, 75, 104244. [Google Scholar] [CrossRef]
- Cui, H.; Guo, C.; Wang, Q.; Feng, C.; Duan, Z. A pilot study on the efficacy of topical lotion containing anti-acne postbiotic in subjects with mild-to-moderate acne. Front. Med. 2022, 9, 1064460. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Liboutet, M.; Cheilian, S.; Fagot, D.; Juchaux, F.; Breton, L. Vitreoscilla filiformis extract for topical skin care: A review. Front. Cell. Infect. Microbiol. 2021, 11, 1253. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.R.; Shah, K.; Beede, K. Novel topical application of a postbiotic, LactoSporin®, in mild to moderate acne: A randomized, comparative clinical study to evaluate its efficacy, tolerability and safety. Cosmetics 2020, 7, 70. [Google Scholar] [CrossRef]
- Cui, H.; Feng, C.; Guo, C.; Duan, Z. Development of novel topical anti-acne cream containing postbiotics for mild-to-moderate acne: An observational study to evaluate its efficacy. Indian J. Dermatol. 2022, 67, 667–673. [Google Scholar] [PubMed]
- de Jesus, G.F.A.; Rossetto, M.P.; Voytena, A.; Feder, B.; Borges, H.; da Costa Borges, G.; Feuser, Z.P.; Dal-Bó, S.; Michels, M. Clinical evaluation of paraprobiotic-associated Bifidobacterium lactis CCT 7858 anti-dandruff shampoo efficacy: A randomized placebo-controlled clinical trial. Int. J. Cosmet. Sci. 2023, 45, 572–580. [Google Scholar] [CrossRef]
- Rinaldi, F.; Trink, A.; Pinto, D. Efficacy of postbiotics in a PRP-like cosmetic product for the treatment of alopecia area Celsi: A randomized double-blinded parallel-group study. Dermatol. Ther. 2020, 10, 483–493. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Recent advances in understanding the structure and function of the human microbiome. Front. Microbiol. 2022, 13, 825338. [Google Scholar] [CrossRef]
- Gueniche, A.; Perin, O.; Bouslimani, A.; Landemaine, L.; Misra, N.; Cupferman, S.; Aguilar, L.; Clavaud, C.; Chopra, T.; Khodr, A. Advances in microbiome-derived solutions and methodologies are founding a new era in skin health and care. Pathogens 2022, 11, 121. [Google Scholar] [CrossRef]
- Holland, K.T.; Bojar, R.A. Cosmetics: What is their influence on the skin microflora? Am. J. Clin. Dermatol. 2002, 3, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Kohneshahri, S.R.A.; Nabizadeh, F. The effect of unit operation and adjunct probiotic culture on physicochemical, biochemical, and textural properties of Dutch Edam cheese. Lebensm.-Wiss. Technol. 2022, 155, 112859. [Google Scholar] [CrossRef]



| Probiotics | Oral | Topical | Benefits Claimed |
|---|---|---|---|
| Lactobacillus | |||
| Lactobacillus Ferment Essence | A skincare brand containing Lactobacillus ferment for skin nourishment | ||
| Probiotic Complex with Lactobacillus Acidophilus | a health brand promoting overall well-being, including potential benefits for the skin | ||
| Bifidobacterium | |||
| BifidoBalance Cream | A skincare company formulated with Bifidobacterium to support skin microbiome balance | ||
| Gut Health Probiotic Blend with Bifidobacterium | A nutritional supplement brand aimed at promoting gut health with potential skin benefits | ||
| Streptococcus thermophilus | |||
| Thermal Probiotic Cream | Skincare line featuring Streptococcus thermophilus for enhancing the diversity of the skin microbiome | ||
| Saccharomyces boulardii | |||
| Saccharomyces boulardii Probiotic Capsules | A wellness brand specifically designed to support gut health and potentially improve skin conditions | ||
| Probiotic Blends | |||
| Probiotic Power Serum | A skincare brand incorporating a blend of Lactobacillus, Bifidobacterium, and Streptococcus thermophilus for a comprehensive skin health approach | ||
| Daily Probiotic Blend Capsules | A health and wellness company offering a mix of various probiotic strains for overall health, including potential benefits for the skin |
| Treatment Modality | Delivery Method | Key Components | Results and Applications |
|---|---|---|---|
| Probiotics | Topical application | Live bacteria (Probiotics) | Reduction of skin lesions in AD; potential for repigmentation |
| Prebiotics | Oral administration | Substances promoting beneficial bacteria growth (e.g., fructooligosaccharides) | Improved psoriasis scores when combined with topical hydrocortisone; positive effects on gut microbiota linked to AD |
| Symbiotics | Oral administration | Combination of probiotics and prebiotics (e.g., Lactocare®) | Improved psoriasis scores when used alongside topical hydrocortisone |
| Postbiotics | Topical application | Cell-free supernatants, lysates, bioactive peptides | Acceleration of epithelization, reduction of skin inflammation, antioxidant capabilities against UVB-induced damage; multiple positive effects on skin health |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Smadi, K.; Leite-Silva, V.R.; Filho, N.A.; Lopes, P.S.; Mohammed, Y. Innovative Approaches for Maintaining and Enhancing Skin Health and Managing Skin Diseases through Microbiome-Targeted Strategies. Antibiotics 2023, 12, 1698. https://doi.org/10.3390/antibiotics12121698
AL-Smadi K, Leite-Silva VR, Filho NA, Lopes PS, Mohammed Y. Innovative Approaches for Maintaining and Enhancing Skin Health and Managing Skin Diseases through Microbiome-Targeted Strategies. Antibiotics. 2023; 12(12):1698. https://doi.org/10.3390/antibiotics12121698
Chicago/Turabian StyleAL-Smadi, Khadeejeh, Vania Rodrigues Leite-Silva, Newton Andreo Filho, Patricia Santos Lopes, and Yousuf Mohammed. 2023. "Innovative Approaches for Maintaining and Enhancing Skin Health and Managing Skin Diseases through Microbiome-Targeted Strategies" Antibiotics 12, no. 12: 1698. https://doi.org/10.3390/antibiotics12121698
APA StyleAL-Smadi, K., Leite-Silva, V. R., Filho, N. A., Lopes, P. S., & Mohammed, Y. (2023). Innovative Approaches for Maintaining and Enhancing Skin Health and Managing Skin Diseases through Microbiome-Targeted Strategies. Antibiotics, 12(12), 1698. https://doi.org/10.3390/antibiotics12121698






