Multidrug-Resistant Escherichia coli Strains to Last Resort Human Antibiotics Isolated from Healthy Companion Animals in Valencia Region
Abstract
1. Introduction
2. Results
2.1. Epidemiological Results
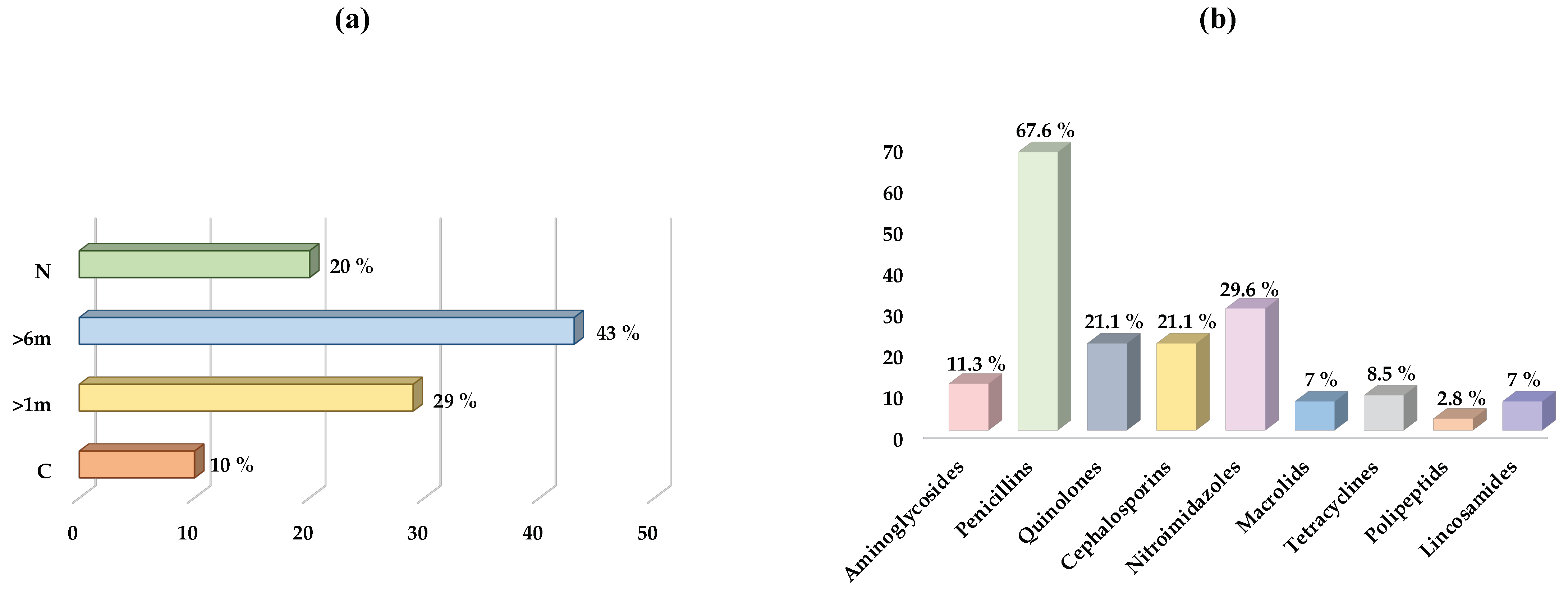
2.1.1. Dogs
2.1.2. Cats
2.2. Antimicrobial Susceptibility from E. coli Strains
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Epidemiological Data Collection
4.3. Sample Collection
4.4. E. coli Isolation
4.5. Antimicrobial Susceptibility Testing
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS). Report 2022; World Health Organization: Geneva, Switzerland, 2022; Volume 2.
- FEDIAF. FEDIAF—European Pet and Food Industry. Annual Report. 2023. Available online: https://europeanpetfood.org/about/annual-report/ (accessed on 7 July 2023).
- Overgaauw, P.A.M.; Vinke, C.M.; van Hagen, M.A.E.; Lipman, L.J.A. A One Health Perspective on the Human-Companion Animal Relationship with Emphasis on Zoonotic Aspects. Int. J. Environ. Res. Public. Health 2020, 17, 3789. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Hermes, J.; Cuny, C.; Wieler, L.H.; Vincze, S.; Abou Elnaga, Y.; Stamm, I.; Kopp, P.A.; Kohn, B.; Witte, W.; et al. Sharing More than Friendship—Nasal Colonization with Coagulase-Positive Staphylococci (CPS) and Co-Habitation Aspects of Dogs and Their Owners. PLoS ONE 2012, 7, e35197. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S.; et al. Complexities in Understanding Antimicrobial Resistance across Domesticated Animal, Human, and Environmental Systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef]
- Lorenzo-Rebenaque, L.; López-Fernández, S.; Marco-Jiménez, F.; Montoro-Dasi, L.; Marin, C.; Vega, S.; Martínez-Manzanares, E.; Fariñas, F. Zoonotic Parasites in Playgrounds in Southern Spain: A One Health Approach. Microorganisms 2023, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hao, J.; Yang, J.; Tong, C.; Xie, L.; Xiao, D.; Zeng, Z.; Xiong, W. The Co-Occurrence of Antibiotic Resistance Genes between Dogs and Their Owners in Families. iMeta 2022, 1, e21. [Google Scholar] [CrossRef]
- Pinello, K.C.; Palmieri, C.; Ruiz, J.; Zaidan Dagli, M.L.; Niza-Ribeiro, J. Risks and Benefits of the Interaction with Companion Animals. In One Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 113–153. [Google Scholar]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M.; et al. Public Health Risk of Antimicrobial Resistance Transfer from Companion Animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef]
- Marco-Fuertes, A.; Marin, C.; Lorenzo-Rebenaque, L.; Vega, S.; Montoro-Dasi, L. Antimicrobial Resistance in Companion Animals: A New Challenge for the One Health Approach in the European Union. Vet. Sci. 2022, 9, 208. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Markovic, R.; Peric, D.; Laudanovic, M.; Baltic, B.; Radulovic, S.; Baltic, M.Z.; Sefer, D. Antimicrobial Growth Promoters in Feed—Possibilities and Necessity. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012029. [Google Scholar] [CrossRef]
- Mendelson, M.; Sharland, M.; Mpundu, M. Antibiotic Resistance: Calling Time on the ‘Silent Pandemic’. JAC-Antimicrobial Resist. 2022, 4, dlac016. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. EU Protocol for Harmonised Monitoring of Antimicrobial Resistance in Human Salmonella and Campylobacter Isolates EU Protocol for Harmonised Monitoring of Antimicrobial Resistance in Human Salm Onella and Cam Pylobacter Isolates; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2016.
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net). Annual Epidemiological Report for 2021; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2022.
- European Centre for Disease Prevention and Control. European Antimicrobial Resistance Surveillance Network (EARS-Net). Available online: https://www.ecdc.europa.eu/en/about-us/networks/disease-networks-and-laboratory-networks/ears-net-data (accessed on 19 July 2023).
- EFSA. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar] [CrossRef]
- World Health Organization. WHO Medically Important List. A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use, 7th ed.; World Health Organization: Geneva, Switzerland, 2023.
- European Medicines Agency. Categorisation of Antibiotics Used in Animals Promotes Responsible Use to Protect Public and Animal Health. Available online: https://www.ema.europa.eu/en/news/categorisation-antibiotics-used-animals-promotes-responsible-use-protect-public-animal-health (accessed on 6 December 2022).
- Mader, R.; Demay, C.; Jouvin-Marche, E.; Ploy, M.C.; Barraud, O.; Bernard, S.; Lacotte, Y.; Pulcini, C.; Weinbach, J.; Berling, C.; et al. Defining the Scope of the European Antimicrobial Resistance Surveillance Network in Veterinary Medicine (EARS-Vet): A Bottom-up and One Health Approach. J. Antimicrob. Chemother. 2022, 77, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.; Muñoz Madero, C.; Aasmäe, B.; Bourély, C.; Broens, E.M.; Busani, L.; Callens, B.; Collineau, L.; Crespo-Robledo, P.; Damborg, P.; et al. Review and Analysis of National Monitoring Systems for Antimicrobial Resistance in Animal Bacterial Pathogens in Europe: A Basis for the Development of the European Antimicrobial Resistance Surveillance Network in Veterinary Medicine (EARS-Vet). Front. Microbiol. 2022, 13, 838490. [Google Scholar] [CrossRef] [PubMed]
- PRAN Proyecto Piloto PRAN—Pequeños Animales|Plan Nacional de Resistencias a Los Antibióticos—PRAN. Available online: https://www.resistenciaantibioticos.es/es/lineas-de-accion/control/programas-de-reduccion-en-sanidad-animal/proyecto-piloto-pran-pequenos-animales (accessed on 7 July 2023).
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2021; European Medicines Agency: Amsterdam, The Netherlands, 2022.
- World Organization for Animal Health. Annual Report of Antimicrobial Resistances; World Organization for Animal Health: Paris, France, 2023. [Google Scholar]
- BelVet—SAC. Belgian Veterinary Surveillance of Antibacterial Consumption National Consumption Report; BelVet—SAC: Ghent, Belgium, 2018. [Google Scholar]
- DANMAP. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Available online: https://www.danmap.org/reports/2022 (accessed on 30 October 2023).
- Simonsen, G.S. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway; Norm-Vet: Tromsø/Oslo, Norway, 2021; pp. 1890–9965. [Google Scholar]
- Aspevall, O.; Obeid, R.; Tao, W.; Nilsson, O.; Pringle, M. A Report on Swedish Antibiotic Sales and Resistance in Human Medicine (Swedres) and Swedish Veterinary Antibiotic Resistance Monitoring (Svarm); SWEDRES|SVARM: Solna, Sweden, 2021. [Google Scholar]
- UK Veterinary Antibiotic Resistance and Sales Surveillance Report. 2020. Available online: www.gov.uk/government/organisations/veterinary-medicines-directorate (accessed on 30 October 2023).
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial Usage and Resistance in Companion Animals: A Cross-Sectional Study in Three European Countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Tompson, A.C.; Mateus, A.L.P.; Brodbelt, D.C.; Chandler, C.I.R. Understanding Antibiotic Use in Companion Animals: A Literature Review Identifying Avenues for Future Efforts. Front. Vet. Sci. 2021, 8, 719547. [Google Scholar] [CrossRef] [PubMed]
- De Briyne, N.; Atkinson, J.; Borriello, S.P.; Pokludová, L. Antibiotics Used Most Commonly to Treat Animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef]
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial Resistance: One Health Approach. Vet. World 2022, 15, 743. [Google Scholar] [CrossRef]
- Despotovic, M.; de Nies, L.; Busi, S.B.; Wilmes, P. Reservoirs of Antimicrobial Resistance in the Context of One Health. Curr. Opin. Microbiol. 2023, 73, 102291. [Google Scholar] [CrossRef]
- Cantón, R.; Akova, M.; Langfeld, K.; Torumkuney, D. Relevance of the Consensus Principles for Appropriate Antibiotic Prescribing in 2022. J. Antimicrob. Chemother. 2022, 77, i2–i9. [Google Scholar] [CrossRef]
- European Medicines Agency. Reflection Paper on the Risk of Antimicrobial Resistance Transfer from Companion Animals; European Medicines Agency: Amsterdam, The Netherlands, 2015.
- Mader, R.; Damborg, P.; Amat, J.P.; Bengtsson, B.; Bourély, C.; Broens, E.M.; Busani, L.; Crespo-Robledo, P.; Filippitzi, M.E.; Fitzgerald, W.; et al. Building the European Antimicrobial Resistance Surveillance Network in Veterinary Medicine (EARS-Vet). Eurosurveillance 2021, 26, 2001359. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, X.; Li, R.; Han, Y.; Hao, G.; Wang, G.; Sun, S. Companion Animals as Potential Reservoirs of Antibiotic Resistant Diarrheagenic Escherichia coli in Shandong, China. Antibiotics 2022, 11, 828. [Google Scholar] [CrossRef]
- Algammal, A.M.; El-Tarabili, R.M.; Alfifi, K.J.; Al-Otaibi, A.S.; Hashem, M.E.A.; El-Maghraby, M.M.; Mahmoud, A.E. Virulence Determinant and Antimicrobial Resistance Traits of Emerging MDR Shiga Toxigenic E. coli in Diarrheic Dogs. AMB Express 2022, 12, 34. [Google Scholar] [CrossRef]
- Haulisah, N.A.; Hassan, L.; Jajere, S.M.; Ahmad, N.I.; Bejo, S.K. High Prevalence of Antimicrobial Resistance and Multidrug Resistance among Bacterial Isolates from Diseased Pets: Retrospective Laboratory Data (2015–2017). PLoS ONE 2022, 17, e0277664. [Google Scholar] [CrossRef]
- Jackson, C.R.; Davis, J.A.; Frye, J.G.; Barrett, J.B.; Hiott, L.M. Diversity of Plasmids and Antimicrobial Resistance Genes in Multidrug-Resistant Escherichia coli Isolated from Healthy Companion Animals. Zoonoses Public Health 2015, 62, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Feng, M.; Liao, S.; Zheng, Z.; Jia, C.; Zhou, X.; Nambiar, R.B.; Ma, Z.; Yue, M. A Cross-Sectional Study of Companion Animal-Derived Multidrug-Resistant Escherichia coli in Hangzhou, China. Microbiol. Spectr. 2023, 11, e0211322. [Google Scholar] [CrossRef]
- Ma, S.; Chen, S.; Lyu, Y.; Huang, W.; Liu, Y.; Dang, X.; An, Q.; Song, Y.; Jiao, Y.; Gong, X.; et al. China Antimicrobial Resistance Surveillance Network for Pets (CARPet), 2018 to 2021. One Health Adv. 2023, 1, 7. [Google Scholar] [CrossRef]
- Ekakoro, J.E.; Kenitra Hendrix, G.; Guptill, L.F.; Ruple, A. Antimicrobial Susceptibility and Risk Factors for Resistance among Escherichia coli Isolated from Canine Specimens Submitted to a Diagnostic Laboratory in Indiana, 2010–2019. PLoS ONE 2022, 17, e0263949. [Google Scholar] [CrossRef] [PubMed]
- Galarce, N.; Arriagada, G.; Sánchez, F.; Escobar, B.; Miranda, M.; Matus, S.; Vilches, R.; Varela, C.; Zelaya, C.; Peralta, J.; et al. Phenotypic and Genotypic Antimicrobial Resistance in Escherichia coli Strains Isolated from Household Dogs in Chile. Front. Vet. Sci. 2023, 10, 1233127. [Google Scholar] [CrossRef]
- Belas, A.; Menezes, J.; Gama, L.T.; Pomba, C. Sharing of Clinically Important Antimicrobial Resistance Genes by Companion Animals and Their Human Household Members. Microb. Drug Resist. 2020, 26, 1174–1185. [Google Scholar] [CrossRef]
- Wedley, A.L.; Dawson, S.; Maddox, T.W.; Coyne, K.P.; Pinchbeck, G.L.; Clegg, P.; Nuttall, T.; Kirchner, M.; Williams, N.J. Carriage of Antimicrobial Resistant Escherichia coli in Dogs: Prevalence, Associated Risk Factors and Molecular Characteristics. Vet. Microbiol. 2017, 199, 23–30. [Google Scholar] [CrossRef]
- Fayez, M.; Elmoslemany, A.; Al Romaihi, A.A.; Azzawi, A.Y.; Almubarak, A.; Elsohaby, I. Prevalence and Risk Factors Associated with Multidrug Resistance and Extended-Spectrum β-Lactamase Producing E. coli Isolated from Healthy and Diseased Cats. Antibiotics 2023, 12, 229. [Google Scholar] [CrossRef]
- Furuya, Y.; Matsuda, M.; Harada, S.; Kumakawa, M.; Shirakawa, T.; Uchiyama, M.; Akama, R.; Ozawa, M.; Kawanishi, M.; Shimazaki, Y.; et al. Nationwide Monitoring of Antimicrobial-Resistant Escherichia coli and Enterococcus spp. Isolated From Diseased and Healthy Dogs and Cats in Japan. Front. Vet. Sci. 2022, 9, 916461. [Google Scholar] [CrossRef]
- Fernandes, V.; Cunha, E.; Nunes, T.; Silva, E.; Tavares, L.; Mateus, L.; Oliveira, M. Antimicrobial Resistance of Clinical and Commensal Escherichia coli Canine Isolates: Profile Characterization and Comparison of Antimicrobial Susceptibility Results According to Different Guidelines. Vet. Sci. 2022, 9, 284. [Google Scholar] [CrossRef]
- Walas, N.; Müller, N.F.; Parker, E.; Henderson, A.; Capone, D.; Brown, J.; Barker, T.; Graham, J.P. Phylodynamics Uncovers the Transmission of Antibiotic-Resistant Escherichia coli between Canines and Humans in an Urban Environment. bioRxiv 2023. bioRxiv:2023.06.01.543064. [Google Scholar] [CrossRef]
- Habib, I.; Mohteshamuddin, K.; Mohamed, M.Y.I.; Lakshmi, G.B.; Abdalla, A.; Bakhit Ali Alkaabi, A. Domestic Pets in the United Arab Emirates as Reservoirs for Antibiotic-Resistant Bacteria: A Comprehensive Analysis of Extended-Spectrum Beta-Lactamase Producing Escherichia coli Prevalence and Risk Factors. Animals 2023, 13, 1587. [Google Scholar] [CrossRef]
- Li, Y.; Fernández, R.; Durán, I.; Molina-López, R.A.; Darwich, L. Antimicrobial Resistance in Bacteria Isolated From Cats and Dogs From the Iberian Peninsula. Front. Microbiol. 2021, 11, 621597. [Google Scholar] [CrossRef]
- Thungrat, K.; Price, S.B.; Carpenter, D.M.; Boothe, D.M. Antimicrobial Susceptibility Patterns of Clinical Escherichia coli Isolates from Dogs and Cats in the United States: January 2008 through January 2013. Vet. Microbiol. 2015, 179, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Fridman, D.; Johnstone, J.; Langford, B.J.; Lee, S.M.; Macfadden, D.M.; Mponponsuo, K.; Patel, S.N.; Schwartz, K.L.; Brown, K.A. Antimicrobial Resistance and Mortality Following E. coli Bacteremia. eClinicalMedicine 2022, 56, 101781. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Albarracin, B.; Altier, C.; Gröhn, Y.T.; Cazer, C. Antimicrobial Resistance Trends among Canine Escherichia coli Isolated at a New York Veterinary Diagnostic Laboratory between 2007 and 2020. Prev. Vet. Med. 2022, 208, 105767. [Google Scholar] [CrossRef]
- Yousfi, M.; Mairi, A.; Touati, A.; Hassissene, L.; Brasme, L.; Guillard, T.; De Champs, C. Extended Spectrum β-Lactamase and Plasmid Mediated Quinolone Resistance in Escherichia coli Fecal Isolates from Healthy Companion Animals in Algeria. J. Infect. Chemother. 2016, 22, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Courtice, R.; Sniatynski, M.; Rubin, J.E. Characterization of Antimicrobial-Resistant Escherichia coli Causing Urinary Tract Infections in Dogs: Passive Surveillance in Saskatchewan, Canada 2014 to 2018. J. Vet. Intern. Med. 2021, 35, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Penicillins—Infectious Diseases—MSD Manual Professional Edition. Available online: https://www.msdmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/penicillins (accessed on 26 July 2023).
- Tseng, C.H.; Liu, C.W.; Liu, P.Y. Extended-Spectrum β-Lactamases (ESBL) Producing Bacteria in Animals. Antibiotics 2023, 12, 661. [Google Scholar] [CrossRef]
- Seo, K.W. Development of a Method for the Fast Detection of Extended-Spectrum β-Lactamase- and Plasmid-Mediated AmpC β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Dogs and Cats in the USA. Animals 2023, 13, 649. [Google Scholar] [CrossRef]
- Mavrides, D.E.; Morgan, A.L.; Na, J.G.; Graham, P.A.; McHugh, T.D. Antimicrobial Resistance Profiles of Bacteria Associated with Lower Respiratory Tract Infections in Cats and Dogs in England. Vet. Rec. 2022, 190, e779. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.D.; Mavrides, D.E.; Graham, P.A.; McHugh, T.D. Results of Urinary Bacterial Cultures and Antibiotic Susceptibility Testing of Dogs and Cats in the UK. J. Small Anim. Pract. 2021, 62, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Hase, A.; Matsuo, M.; Horimoto, T.; Ogasawara, J. Prevalence and Genetic Characterization of Cephalosporinresistant Enterobacteriaceae among Dogs and Cats in an Animal Shelter. J. Med. Microbiol. 2019, 68, 339–345. [Google Scholar] [CrossRef]
- Harada, K.; Nakai, Y.; Kataoka, Y. Mechanisms of Resistance to Cephalosporin and Emergence of O25b-ST131 Clone Harboring CTX-M-27 β-Lactamase in Extraintestinal Pathogenic Escherichia coli from Dogs and Cats in Japan. Microbiol. Immunol. 2012, 56, 480–485. [Google Scholar] [CrossRef]
- Rzewuska, M.; Czopowicz, M.; Kizerwetter-Świda, M.; Chrobak, D.; Błaszczak, B.; Binek, M. Multidrug Resistance in Escherichia coli Strains Isolated from Infections in Dogs and Cats in Poland (2007–2013). Sci. World J. 2015, 2015, 408205. [Google Scholar] [CrossRef] [PubMed]
- Woerde, D.J.; Reagan, K.L.; Byrne, B.A.; Weimer, B.C.; Epstein, S.E.; Schlesener, C.; Huang, B.C.; Sykes, J.E. Characteristics of Extended-Spectrum β-Lactamase Producing Enterobacterales Isolated from Dogs and Cats, 2011–2021. Vet. Sci. 2023, 10, 178. [Google Scholar] [CrossRef]
- Saputra, S.; Jordan, D.; Mitchell, T.; Wong, H.S.; Abraham, R.J.; Kidsley, A.; Turnidge, J.; Trott, D.J.; Abraham, S. Antimicrobial Resistance in Clinical Escherichia coli Isolated from Companion Animals in Australia. Vet. Microbiol. 2017, 211, 43–50. [Google Scholar] [CrossRef]
- Stege, P.B.; Hordijk, J.; Sandholt, A.K.S.; Zomer, A.L.; Viveen, M.C.; Rogers, M.R.C.; Salomons, M.; Wagenaar, J.A.; Mughini-Gras, L.; Willems, R.J.L.; et al. Gut Colonization by ESBL-Producing Escherichia coli in Dogs Is Associated with a Distinct Microbiome and Resistome Composition. Microbiol. Spectr. 2023, 11, e0006323. [Google Scholar] [CrossRef]
- Schnepf, A.; Kramer, S.; Wagels, R.; Volk, H.A.; Kreienbrock, L. Evaluation of Antimicrobial Usage in Dogs and Cats at a Veterinary Teaching Hospital in Germany in 2017 and 2018. Front. Vet. Sci. 2021, 8, 689018. [Google Scholar] [CrossRef]
- Lhermie, G.; La Ragione, R.M.; Weese, J.S.; Olsen, J.E.; Christensen, J.P.; Guardabassi, L.; on behalf of the ESCMID Study Group for Veterinary Microbiology (ESGVM). Indications for the Use of Highest Priority Critically Important Antimicrobials in the Veterinary Sector. J. Antimicrob. Chemother. 2020, 75, 1671–1680. [Google Scholar] [CrossRef]
- Mahase, E. Superbug Spreads in European Hospitals as Resistance to Last Resort Antibiotics Grows. BMJ 2019, 366, l4942. [Google Scholar] [CrossRef] [PubMed]
- Vanstokstraeten, R.; Piérard, D.; Crombé, F.; De Geyter, D.; Wybo, I.; Muyldermans, A.; Seyler, L.; Caljon, B.; Janssen, T.; Demuyser, T. Genotypic Resistance Determined by Whole Genome Sequencing versus Phenotypic Resistance in 234 Escherichia coli Isolates. Sci. Rep. 2023, 13, 449. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Chen, S.; Schwarz, S.; Cao, Y.; Dang, X.; Zhai, W.; Zou, Z.; Shen, J.; Lyu, Y.; et al. Low Prevalence of Colistin-Resistant Escherichia coli from Companion Animals, China, 2018–2021. One Health Adv. 2023, 1, 14. [Google Scholar] [CrossRef]
- Tocalli, L.; Boselli, A.; Rimoldi, S.G.; Gismondo, M.R. EPIDEMIOLOGICAL OBSERVATORY: Spreading of ESBL and Carbapenemase Positive Strains in the Period between January 2007–June 2012, at the Hospital—University Campus—Hospital “Luigi Sacco” in Milan. Microbiol. Medica 2012, 27, 3. [Google Scholar] [CrossRef][Green Version]
- Debergh, H.; Maex, M.; Garcia-Graells, C.; Boland, C.; Saulmont, M.; Van Hoorde, K.; Saegerman, C. First Belgian Report of Ertapenem Resistance in an ST11 Klebsiella Pneumoniae Strain Isolated from a Dog Carrying BlaSCO-1 and BlaDHA-1 Combined with Permeability Defects. Antibiotics 2022, 11, 1253. [Google Scholar] [CrossRef]
- Murugan, M.S.; Sinha, D.K.; Vinodh Kumar, O.R.; Yadav, A.K.; Pruthvishree, B.S.; Vadhana, P.; Nirupama, K.R.; Bhardwaj, M.; Singh, B.R. Epidemiology of Carbapenem-Resistant Escherichia coli and First Report of BlaVIM Carbapenemases Gene in Calves from India. Epidemiol. Infect. 2019, 147, e159. [Google Scholar] [CrossRef]
- Abdus Sobur, M.; Al Momen Sabuj, A.; Sarker, R.; Taufiqur Rahman, A.M.M.; Lutful Kabir, S.M.; Tanvir Rahman, M. Antibiotic-Resistant Escherichia coli and Salmonella spp. Associated with Dairy Cattle and Farm Environment Having Public Health Significance. Vet. World 2019, 12, 984. [Google Scholar] [CrossRef]
- Lartigue, M.F.; Poirel, L.; Poyart, C.; Réglier-Poupet, H.; Nordmann, P. Ertapenem Resistance of Escherichia coli. Emerg. Infect. Dis. 2007, 13, 315. [Google Scholar] [CrossRef]
- Nittayasut, N.; Yindee, J.; Boonkham, P.; Yata, T.; Suanpairintr, N.; Chanchaithong, P. Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and Blandm-5-Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand. Antibiotics 2021, 10, 1374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ji, X.; Liang, B.; Jiang, B.; Li, Y.; Yuan, T.; Zhu, L.; Liu, J.; Guo, X.; Sun, Y. Antimicrobial Resistance and Prevalence of Extended Spectrum β-Lactamase-Producing Escherichia coli from Dogs and Cats in Northeastern China from 2012 to 2021. Antibiotics 2022, 11, 1506. [Google Scholar] [CrossRef] [PubMed]
- Darwich, L.; Seminati, C.; Burballa, A.; Nieto, A.; Durán, I.; Tarradas, N.; Molina-López, R.A. Antimicrobial Susceptibility of Bacterial Isolates from Urinary Tract Infections in Companion Animals in Spain. Vet. Rec. 2021, 188, e60. [Google Scholar] [CrossRef] [PubMed]
- Tigecycline—Infectious Diseases—MSD Manual Professional Edition. Available online: https://www.msdmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/tigecycline (accessed on 27 July 2023).
- Sato, T.; Harada, K.; Usui, M.; Tsuyuki, Y.; Shiraishi, T.; Tamura, Y.; Yokota, S.I. Tigecycline Susceptibility of Klebsiella Pneumoniae Complex and Escherichia coli Isolates from Companion Animals: The Prevalence of Tigecycline-Nonsusceptible K. Pneumoniae Complex, Including Internationally Expanding Human Pathogenic Lineages. Microb. Drug Resist. 2018, 24, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.D. How Old Is My Dog? Identification of Rational Age Groupings in Pet Dogs Based Upon Normative Age-Linked Processes. Front. Vet. Sci. 2021, 8, 321. [Google Scholar] [CrossRef]
- AniCura Edad y Fases de La Vida de Un Gato: De Cachorro a Anciano|AniCura España. Available online: https://www.anicura.es/consejos-de-salud/gato/consejos-de-salud/fases-de-la-vida-de-un-gato/ (accessed on 21 July 2023).
- Hetsa, B.A.; Ateba, T.P.; Moroane, T.; Nyirenda, M.; Gopane, R.E.; Ateba, C.N. Detection of Antibiotic Resistant Enterobacteriaceae from Dogs in the North West University Animal Health Hospital. Life Sci. J. 2022, 19, 19–26. [Google Scholar] [CrossRef]
- Shnaiderman-Torban, A.; Navon-Venezia, S.; Baron, H.; Abu-Ahmad, W.; Arielly, H.; Zizelski Valenci, G.; Nissan, I.; Paitan, Y.; Steinman, A. Prevalence and Molecular Characterization of Extended-Spectrum β-Lactamase Producing Enterobacterales in Healthy Community Dogs in Israel. Antibiotics 2022, 11, 1069. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Graves, S.; Piepho, H.; Selzer, L.; Dorai-Raj, S. MultcompView: Visualizations of Paired Comparisons. R Package Version 0.1-9. Available online: https://cran.r-project.org/web/packages/multcompView/index.html (accessed on 27 July 2023).

| Dog | Cat | Dog | Cat | |||
|---|---|---|---|---|---|---|
| Antibiotic Group | % AMR/Group | % AMR/Group | Antibiotic | EMA | % AMR/Antibiotic | % AMR/Antibiotic |
| Aminoglycosides | 15.7 a ± 1.4 | 23.2 a ± 2.5 | Amikacin | C | 17.6 a,b,c ± 3.8 | 23.2 a,b,c,d ± 4.3 |
| Gentamicin | C | 8.8 c,g ± 2.8 | 21.1 a,b,c ± 4.2 | |||
| Tobramycin | C | 20.6 a,b,f ± 4 | 25.3 b,c,d ± 4.5 | |||
| Carbapenemases | 5.9 b ± 1.2 | 9.5 b,c ± 2.1 | Ertapenem | A | 5.9 g ± 2.3 | 12.6 a,f ± 3.4 |
| Meropenem | A | 5.9 g ± 2.3 | 6.3 f,g ± 2.5 | |||
| Cephalosporins | 22.4 c ± 4.1 | 21.7 a ± 1.6 | Cefepime | B | 15.7 a,c ± 3.6 | 17.9 a,b ± 3.9 |
| Cefixime | B | 23.5 a,b,f,h ± 4.2 | 30.5 c,d,e ± 4.7 | |||
| Cefotaxime | B | 28.4 b,f,h ± 4.5 | 16.8 a,b ± 3.8 | |||
| Cefoxitin | C | 23.5 a,b,f,h ± 4.2 | 21.1 a,b,c ± 4.2 | |||
| Cefuroxime | C | 21.6 a,b,f ± 4.1 | 18.9 a,b,c ± 4 | |||
| Cefalexin | C | 23.5 a,b,f,h ± 4.2 | 24.2 b,c,d ± 4.4 | |||
| Ceftazidime | B | 20.6 a,b,f ± 4 | 21.1 a,b,c ± 4.2 | |||
| Nitrofurans | 14.7 a ± 3.5 | 4.2 c,d ± 2.1 | Nitrofurantoin | D | 14.7 a ± 3.5 | 4.2 c,d ± 2.1 |
| Penicillins | 45.8 d ± 1.9 | 25.5 a ± 2.2 | Ampicillin | D | 62.7 d ± 4.8 | 38.9 e ± 5 |
| Amoxicillin/ Clavulanic acid | C | 43.1 e ± 4.9 | 18.9 a,b,c ± 4 | |||
| Piperacillin/ Tazobactam | A | 16.7 a,c ± 3.7 | 4.2 g ± 2.1 | |||
| Ticarcillin | D | 60.8 d ± 4.8 | 40 e ± 5 | |||
| Quinolones | 23.9 c ± 2.1 | 25.6 a ± 3.8 | Ciprofloxacin (FQ) | B | 22.5 a,b,f ± 4.1 | 21.1 a,b,c ± 4.2 |
| Levofloxacin (FQ) | B | 19.6 a,b,f ± 3.9 | 21.1 a,b,c ± 4.2 | |||
| Nalidixic acid (Q) | B | 29.4 f,h ± 4.5 | 34.7 d,e ± 4.9 | |||
| Folate inhibitor pathway | 35.3 e ± 4.7 | 16.8 a,b ± 3.8 | Sulfamethoxazole/Trimethoprim | D | 35.3 e,h ± 4.7 | 16.8 a,b ± 3.8 |
| Glycylcycline | 2.9 b ± 1 | 1.1 d ± 1 | Tigecycline | A | 2.9 b ± 1 | 1.1 d ± 1 |
| N of AB Groups | n of Dog Isolates (%) | n of Cat Isolates (%) | N of Isolates (%) | AMR Patterns |
|---|---|---|---|---|
| 0 | - | - | 12 (6.1%) | - |
| 1 | 11 (10.8%) | 9 (9.5%) | 20 (10.2%) | PEN |
| 4 (3.9%) | 5 (5.3%) | 9 (4.6%) | CEPHA | |
| 2 (2.1%) | 6 (6.3%) | 8 (4.1%) | QUIN | |
| - | 3 (3.2%) | 3 (1.5%) | AMINO | |
| 2 | 2 (2.1%) | - | 2 (1.1%) | PEN-AMINO |
| 7 (6.9%) | 3 (3.2%) | 10 (5.1%) | PEN-CEPHA | |
| 3 (2.9%) | 3 (3.2%) | 6 (3.0%) | PEN-QUIN | |
| 9 (8.8%) | 4 (4.2%) | 13 (6.6%) | PEN-FOL | |
| 1 (1.1%) | - | 1 (0.5%) | CEPHA-NITRO | |
| 1 (1.1%) | - | 1 (0.5%) | CEPHA-QUIN | |
| 2 (2.1%) | - | 2 (1.1%) | QUIN-FOL | |
| - | 1 (1.1%) | 1 (0.5%) | ANIMO-FOL | |
| - | 2 (2.1%) | 2 (1.1%) | AMINO-QUIN | |
| 3 | 3 (2.9%) | 2 (2.1%) | 5 (2.5%) | PEN-AMINO-CEPHA |
| 1 (1.1%) | - | 1 (0.5%) | PEN-AMINO-NITRO | |
| 2 (2.1%) | 2 (2.1%) | 4 (2.0%) | PEN-AMINO-QUIN | |
| 4 (3.9%) | 4 (4.2%) | 8 (4.1%) | PEN-CEPHA-QUIN | |
| 5 (5.0%) | 1 (1.1%) | 6 (3.0%) | PEN-CEPHA-FOL | |
| 2 (2.1%) | - | 2 (1.1%) | PEN-NITRO-FOL | |
| 4 (3.9%) | 1 (1.1%) | 5 (2.5%) | PEN-QUIN-FOL | |
| 1 (1.1%) | 1 (1.1%) | 2 (1.1%) | PEN-QUIN-NITRO | |
| 1 (1.1%) | - | 1 (0.5%) | PEN-CARB-GLYC | |
| 1 (1.1%) | 1 (1.1%) | 2 (1.1%) | AMINO-CEPHA-QUIN | |
| 4 | 3 (2.9%) | 3 (3.2%) | 6 (3.0%) | PEN-AMINO-CEPHA-QUIN |
| 2 (2.1%) | - | 2 (1.1%) | PEN-AMINO-CEPHA-FOL | |
| 1 (1.1%) | 1 (1.1%) | 2 (1.1%) | PEN-AMINO-QUIN-FOL | |
| 2 (2.1%) | - | 2 (1.1%) | PEN-AMINO-NITRO-FOL | |
| 2 (2.1%) | - | 2 (1.1%) | PEN-CEPHA-QUIN-FOL | |
| - | 1 (1.1%) | 1 (0.5%) | AMINO-CEPHA-QUIN-FOL | |
| - | 1 (1.1%) | 1 (0.5%) | AMINO-CEPHA-QUIN-CARB | |
| 5 | 5 (5.0%) | 3 (3.2%) | 8 (4.1%) | PEN-AMINO-CEPHA-QUIN-CARB |
| 1 (1.1%) | 2 (2.1%) | 3 (1.5%) | PEN-AMINO-CEPHA-QUIN-FOL | |
| - | 1 (1.1%) | 1 (0.5%) | PEN-AMINO-QUIN-FOL-CARB | |
| - | 1 (1.1%) | 1 (0.5%) | PEN-AMINO-CEPHA-NITRO-FOL | |
| 1 (1.1%) | - | 1 (0.5%) | PEN-CEPHA-QUIN-NITRO-CARB | |
| 1 (1.1%) | - | 1 (0.5%) | PEN-CEPHA-QUIN-NITRO-FOL | |
| 6 | 1 (1.1%) | 1 (1.1%) | 1 (0.5%) | PEN-AMINO-CEPHA-QUIN-NITRO-CARB |
| 2 (2.1%) | - | 2 (1.1%) | PEN-AMINO-CEPHA-QUIN-NITRO-FOL | |
| - | 5 (5.3%) | 5 (2.5%) | PEN-AMINO-CEPHA-QUIN-FOL-CARB | |
| - | 1 (1.1%) | 1 (0.5%) | PEN-AMINO-CEPHA-QUIN-FOL-GLYC | |
| 7 | 1 (1.1%) | 1 (1.1%) | 2 (1.1%) | PEN-AMINO-CEPHA-QUIN-NITRO-FOL-CARB |
| 8 | 2 (2.1%) | - | 2 (1.1%) | PEN-AMINO-CEPHA-QUIN-NITRO-FOL-CARB-GLYC |
| Antibiotic Group | Antibiotic | Abbreviation | Concentration | EUCAST Breakpoints |
|---|---|---|---|---|
| Aminoglycosides | Amikacin | AMI | 2–32 μg/mL | >8 μg/mL |
| Gentamicin | GEN | 0.5–8 μg/mL | >2 μg/mL | |
| Tobramycin | TOB | 0.5–8 μg/mL | >2 μg/mL | |
| Carbapenemases | Ertapenem | ERT | 0.12–2 μg/mL | >0.5 μg/mL |
| Meropenem | MER | 0.12–16 μg/mL | >8 μg/mL | |
| Cephalosporins | Cefepime | CEP | 0.5–8 μg/mL | >4 μg/mL |
| Cefixime | CIX | 0.5–2 μg/mL | >1 μg/mL | |
| Cefotaxime | CTA | 0.5–4 μg/mL | >2 μg/mL | |
| Cefoxitin | CXI | 2–16 μg/mL | >8 μg/mL | |
| Cefuroxime | CUR | 2–16 μg/mL | >8 μg/mL | |
| Cefalexin | CLE | 8–32 μg/mL | >16 μg/mL | |
| Ceftazidime | CTZ | 0.5–8 μg/mL | >4 μg/mL | |
| Nitrofurans | Nitrofurantoin | NIT | 32–64 μg/mL | >64 μg/mL |
| Penicillins | Ampicillin | AMP | 2–16 μg/mL | >8 μg/mL |
| Amoxicillin/ Clavulanic acid | AMC | 2/2–32/2 μg/mL | >8 μg/mL | |
| Piperacillin/ Tazobactam | PIT | 2/4–32/4 μg/mL | >8 μg/mL | |
| Ticarcillin | TIC | 4–32 μg/mL | >16 μg/mL | |
| Quinolones | Ciprofloxacin (FQ) | CIP | 0.12–1 μg/mL | >0.5 μg/mL |
| Levofloxacin (FQ) | LEV | 0.25–2 μg/mL | >1 μg/mL | |
| Nalidixic acid (Q) | NAL | 16 μg/mL | >8 μg/mL | |
| Folate inhibitor pathway | Sulfamethoxazole/Trimethoprim | TRS | 1/19–8/152 μg/mL | >4 μg/mL |
| Glycylcycline | Tigecycline | TIG | 0.5–4 μg/mL | >0.5 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marco-Fuertes, A.; Jordá, J.; Marin, C.; Lorenzo-Rebenaque, L.; Montoro-Dasi, L.; Vega, S. Multidrug-Resistant Escherichia coli Strains to Last Resort Human Antibiotics Isolated from Healthy Companion Animals in Valencia Region. Antibiotics 2023, 12, 1638. https://doi.org/10.3390/antibiotics12111638
Marco-Fuertes A, Jordá J, Marin C, Lorenzo-Rebenaque L, Montoro-Dasi L, Vega S. Multidrug-Resistant Escherichia coli Strains to Last Resort Human Antibiotics Isolated from Healthy Companion Animals in Valencia Region. Antibiotics. 2023; 12(11):1638. https://doi.org/10.3390/antibiotics12111638
Chicago/Turabian StyleMarco-Fuertes, Ana, Jaume Jordá, Clara Marin, Laura Lorenzo-Rebenaque, Laura Montoro-Dasi, and Santiago Vega. 2023. "Multidrug-Resistant Escherichia coli Strains to Last Resort Human Antibiotics Isolated from Healthy Companion Animals in Valencia Region" Antibiotics 12, no. 11: 1638. https://doi.org/10.3390/antibiotics12111638
APA StyleMarco-Fuertes, A., Jordá, J., Marin, C., Lorenzo-Rebenaque, L., Montoro-Dasi, L., & Vega, S. (2023). Multidrug-Resistant Escherichia coli Strains to Last Resort Human Antibiotics Isolated from Healthy Companion Animals in Valencia Region. Antibiotics, 12(11), 1638. https://doi.org/10.3390/antibiotics12111638







