Antimicrobial Activities of Aztreonam-Avibactam and Comparator Agents against Enterobacterales Analyzed by ICU and Non-ICU Wards, Infection Sources, and Geographic Regions: ATLAS Program 2016–2020
Abstract
:1. Introduction
2. Results
2.1. Distribution of Isolates
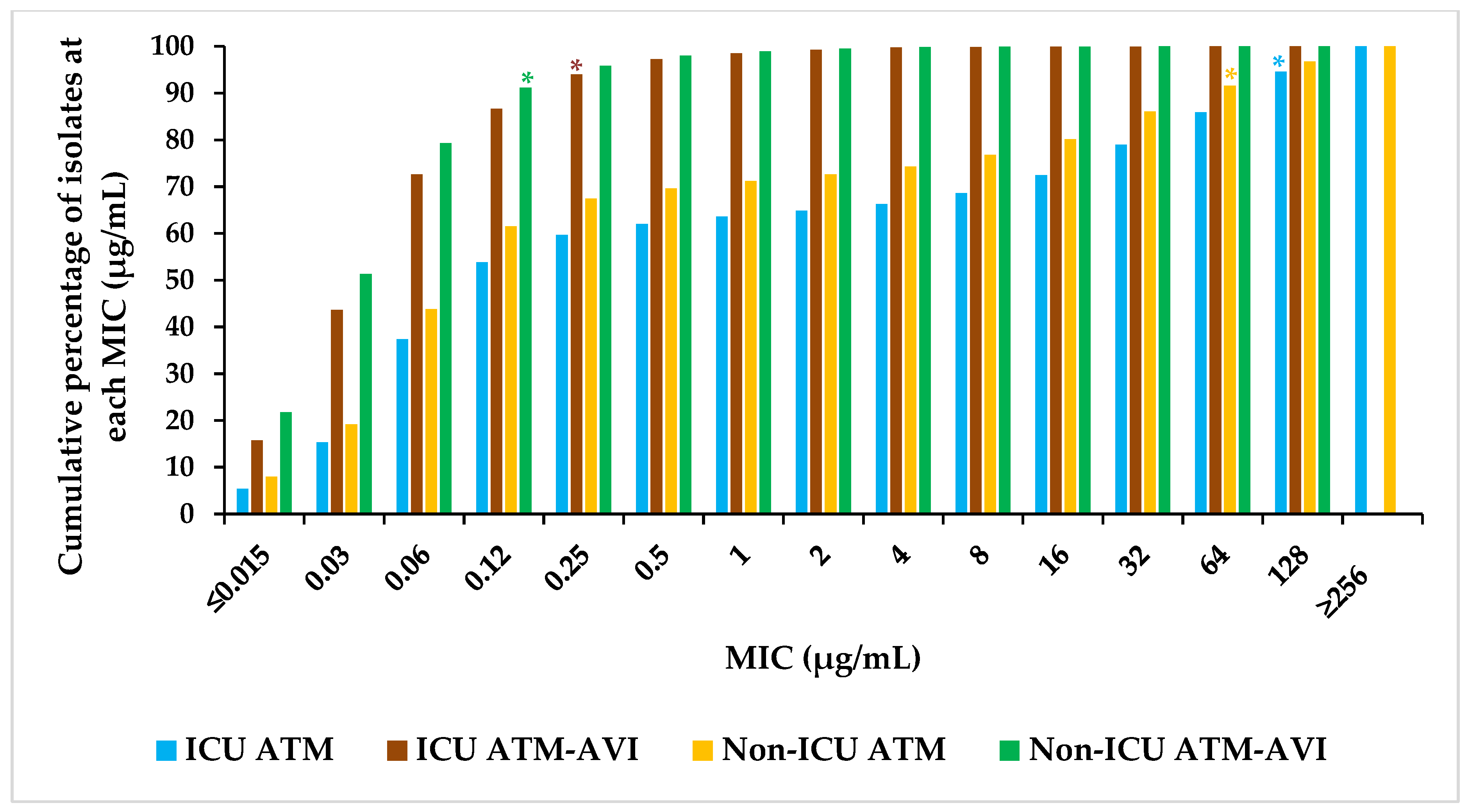
2.2. Activity of ATM-AVI and Other Antimicrobials against Enterobacterales across Wards
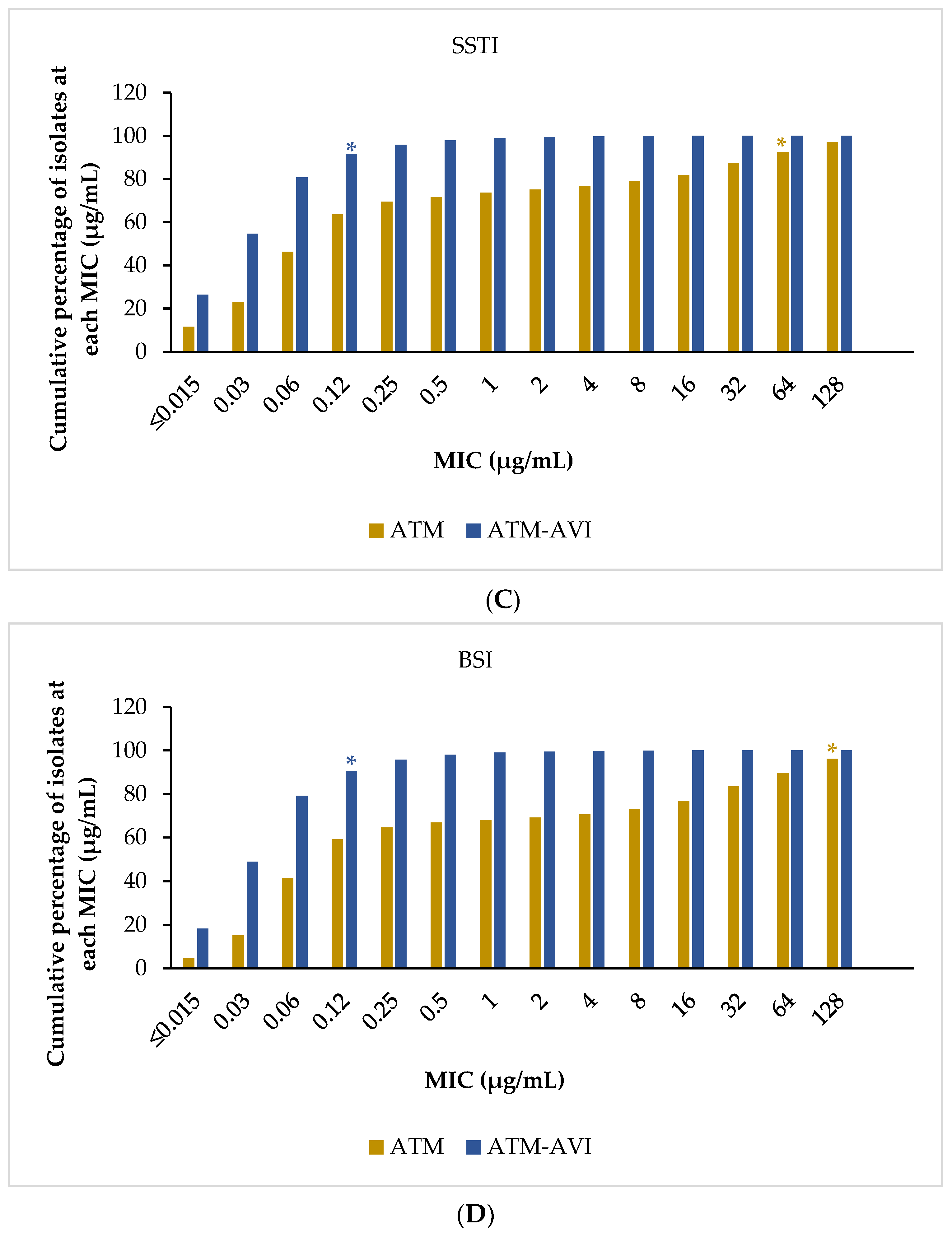
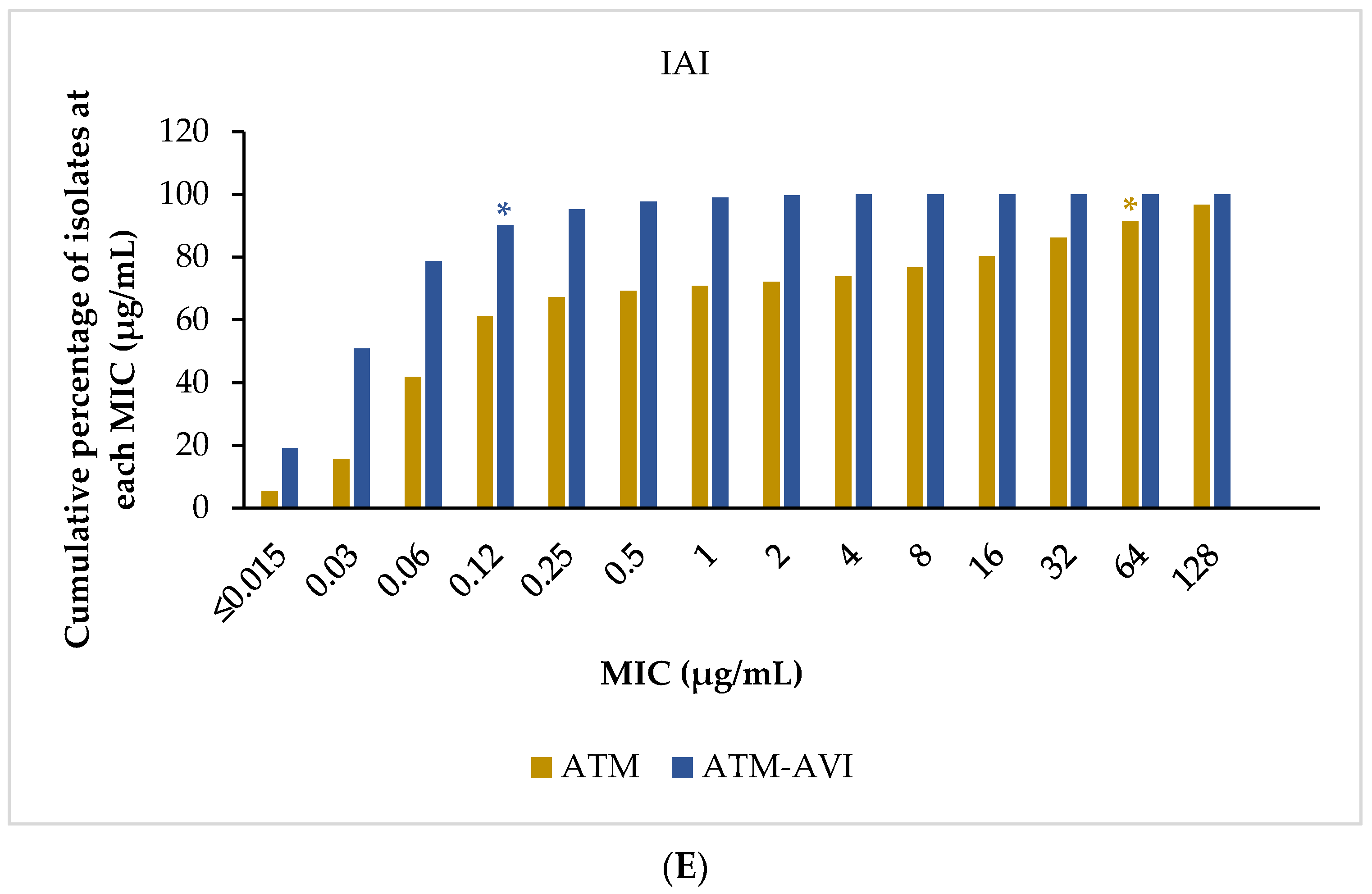
2.3. Activity of ATM-AVI and Other Antimicrobials against Enterobacterales across Infection Sources
2.4. Activity of ATM-AVI and Other Antimicrobials against Specific Resistance Phenotypes: MDR, ESBL-Positive, CRE, and MBL-Positive
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Antimicrobial Susceptibility Testing
4.3. Resistance Phenotypes Definitions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynch, J.P.; Clark, N.M.; Zhanel, G.G. Escalating antimicrobial resistance among Enterobacteriaceae: Focus on carbapenemases. Expert Opin. Pharmacother. 2021, 22, 1455–1474. [Google Scholar] [CrossRef]
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front. Med. 2019, 6, 74. [Google Scholar] [CrossRef]
- Sader, H.S.; Mendes, R.E.; Arends, S.J.R.; Carvalhaes, C.G.; Castanheira, M. Antimicrobial activities of aztreonam-avibactam and comparator agents tested against Enterobacterales from European hospitals analysed by geographic region and infection type (2019–2020). Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 477–487. [Google Scholar] [CrossRef]
- Sheu, C.C.; Chang, Y.T.; Lin, S.Y.; Chen, Y.H.; Hsueh, P.R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Noster, J.; Thelen, P.; Hamprecht, A. Detection of Multidrug-Resistant Enterobacterales—From ESBLs to Carbapenemases. Antibiotics 2021, 10, 1140. [Google Scholar] [CrossRef]
- Stewardson, A.J.; Allignol, A.; Beyersmann, J.; Graves, N.; Schumacher, M.; Meyer, R.; Tacconelli, E.; De Angelis, G.; Farina, C.; Pezzoli, F.; et al. The health and economic burden of bloodstream infections caused by antimicrobial-susceptible and non-susceptible Enterobacteriaceae and Staphylococcus aureus in European hospitals, 2010 and 2011: A multicentre retrospective cohort study. Eurosurveillance 2016, 21, 30319. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 15 June 2023).
- Dong, L.T.; Espinoza, H.V.; Espinoza, J.L. Emerging superbugs: The threat of Carbapenem Resistant Enterobacteriaceae. AIMS Microbiol. 2020, 6, 176–182. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Carmeli, Y. Carbapenem-Resistant Enterobacteriaceae: A Potential Threat. JAMA 2008, 300, 2911–2913. [Google Scholar] [CrossRef]
- Tesfa, T.; Mitiku, H.; Edae, M.; Assefa, N. Prevalence and incidence of carbapenem-resistant K. pneumoniae colonization: Systematic review and meta-analysis. Syst. Rev. 2022, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Tansarli, G.S.; Karageorgopoulos, D.E.; Vardakas, K.Z. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg. Infect. Dis. 2014, 20, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chen, J.; Sun, S.; Deng, S. Mortality-Related Risk Factors and Novel Antimicrobial Regimens for Carbapenem-Resistant Enterobacteriaceae Infections: A Systematic Review. Infect. Drug Resist. 2022, 15, 6907–6926. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Lourida, P.; Poulikakos, P.; Rafailidis, P.I.; Tansarli, G.S. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: Systematic evaluation of the available evidence. Antimicrob. Agents Chemother. 2014, 58, 654–663. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; De Rosa, F.G.; Giannella, M.; Giacobbe, D.R.; Bassetti, M.; Losito, A.R.; Bartoletti, M.; Del Bono, V.; Corcione, S.; et al. Infections caused by KPC-producing Klebsiella pneumoniae: Differences in therapy and mortality in a multicentre study. J. Antimicrob. Chemother. 2015, 70, 2133–2143. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Ma, X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 18. [Google Scholar] [CrossRef]
- Alizadeh, N.; Ahangarzadeh Rezaee, M.; Samadi Kafil, H.; Hasani, A.; Soroush Barhaghi, M.H.; Milani, M.; Yeganeh Sefidan, F.; Memar, M.Y.; Lalehzadeh, A.; Ghotaslou, R. Evaluation of Resistance Mechanisms in Carbapenem-Resistant Enterobacteriaceae. Infect. Drug Resist. 2020, 13, 1377–1385. [Google Scholar] [CrossRef]
- Bush, K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef]
- Goodman, K.E.; Simner, P.J.; Tamma, P.D.; Milstone, A.M. Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant Enterobacteriaceae (CRE). Expert Rev. Anti-Infect. Ther. 2016, 14, 95–108. [Google Scholar] [CrossRef]
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef]
- Boyd, S.E.; Livermore, D.M.; Hooper, D.C.; Hope, W.W. Metallo-β-Lactamases: Structure, Function, Epidemiology, Treatment Options, and the Development Pipeline. Antimicrob. Agents Chemother. 2020, 64, e00397-20. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Heffernan, A.J.; Naicker, S.; Henderson, A.; Chowdhury, M.A.H.; Roberts, J.A.; Sime, F.B. Epidemiology of extended-spectrum β-lactamase and metallo-β-lactamase-producing Escherichia coli in South Asia. Future Microbiol. 2021, 16, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Daikos, G.L.; Petrikkos, P.; Psichogiou, M.; Kosmidis, C.; Vryonis, E.; Skoutelis, A.; Georgousi, K.; Tzouvelekis, L.S.; Tassios, P.T.; Bamia, C.; et al. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob. Agents Chemother. 2009, 53, 1868–1873. [Google Scholar] [CrossRef]
- de Jager, P.; Chirwa, T.; Naidoo, S.; Perovic, O.; Thomas, J. Nosocomial Outbreak of New Delhi Metallo-β-Lactamase-1-Producing Gram-Negative Bacteria in South Africa: A Case-Control Study. PLoS ONE 2015, 10, e0123337. [Google Scholar] [CrossRef]
- Snyder, B.M.; Montague, B.T.; Anandan, S.; Madabhushi, A.G.; Pragasam, A.K.; Verghese, V.P.; Balaji, V.; Simões, E.A.F. Risk factors and epidemiologic predictors of blood stream infections with New Delhi Metallo-b-lactamase (NDM-1) producing Enterobacteriaceae. Epidemiol. Infect. 2019, 147, e137. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Biedenbach, D.J.; Kazmierczak, K.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob. Agents Chemother. 2015, 59, 4239–4248. [Google Scholar] [CrossRef]
- Shields, R.K.; Doi, Y. Aztreonam Combination Therapy: An Answer to Metallo-β-Lactamase-Producing Gram-Negative Bacteria? Clin. Infect. Dis. 2020, 71, 1099–1101. [Google Scholar] [CrossRef]
- Tamma, P.D.; Doi, Y.; Bonomo, R.A.; Johnson, J.K.; Simner, P.J. A Primer on AmpC β-Lactamases: Necessary Knowledge for an Increasingly Multidrug-resistant World. Clin. Infect. Dis. 2019, 69, 1446–1455. [Google Scholar] [CrossRef]
- Crandon, J.L.; Nicolau, D.P. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-β-lactamase producers. Antimicrob. Agents Chemother. 2013, 57, 3299–3306. [Google Scholar] [CrossRef]
- Oberoi, L.; Singh, N.; Sharma, P.; Aggarwal, A. ESBL, MBL and Ampc β Lactamases Producing Superbugs—Havoc in the Intensive Care Units of Punjab India. J. Clin. Diagn. Res. 2013, 7, 70–73. [Google Scholar] [CrossRef]
- Veeraraghavan, B.; Pragasam, A.K.; Bakthavatchalam, Y.D.; Anandan, S.; Swaminathan, S.; Sundaram, B. Colistin-sparing approaches with newer antimicrobials to treat carbapenem-resistant organisms: Current evidence and future prospects. Indian J. Med. Microbiol. 2019, 37, 72–90. [Google Scholar] [CrossRef]
- Cornely, O.A.; Cisneros, J.M.; Torre-Cisneros, J.; Rodríguez-Hernández, M.J.; Tallón-Aguilar, L.; Calbo, E.; Horcajada, J.P.; Queckenberg, C.; Zettelmeyer, U.; Arenz, D.; et al. Pharmacokinetics and safety of aztreonam/avibactam for the treatment of complicated intra-abdominal infections in hospitalized adults: Results from the REJUVENATE study. J. Antimicrob. Chemother. 2020, 75, 618–627. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03580044 (accessed on 8 August 2023).
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT03329092 (accessed on 8 August 2023).
- Karlowsky, J.A.; Kazmierczak, K.M.; de Jonge, B.L.M.; Hackel, M.A.; Sahm, D.F.; Bradford, P.A. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob. Agents Chemother. 2017, 61, e00472-17. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Bradford, P.A.; Stone, G.G.; de Jonge, B.L.M.; Sahm, D.F. In Vitro Activity of Ceftazidime-Avibactam and Aztreonam-Avibactam against OXA-48-Carrying Enterobacteriaceae Isolated as Part of the International Network for Optimal Resistance Monitoring (INFORM) Global Surveillance Program from 2012 to 2015. Antimicrob. Agents Chemother. 2018, 62, e00592-18. [Google Scholar] [CrossRef]
- Sader, H.S.; Carvalhaes, C.G.; Arends, S.J.R.; Castanheira, M.; Mendes, R.E. Aztreonam/avibactam activity against clinical isolates of Enterobacterales collected in Europe, Asia and Latin America in 2019. J. Antimicrob. Chemother. 2021, 76, 659–666. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Stone, G.; Kantecki, M.; Arhin, F.F. In vitro activity of aztreonam/avibactam against isolates of Enterobacterales collected globally from ATLAS in 2019. J. Glob. Antimicrob. Resist. 2022, 30, 214–221. [Google Scholar] [CrossRef]
- Esposito, S.; Stone, G.G.; Papaparaskevas, J. In vitro activity of aztreonam/avibactam against a global collection of Klebsiella pneumoniae collected from defined culture sources in 2016 and 2017. J. Glob. Antimicrob. Resist. 2021, 24, 14–22. [Google Scholar] [CrossRef]
- ATLAS Surveillance. Antimicrobial Testing Leadership and Surveillance. Available online: https://atlas-surveillance.com/ (accessed on 21 April 2023).
- Singh, R.; Kim, A.; Tanudra, M.A.; Harris, J.J.; McLaughlin, R.E.; Patey, S.; O’Donnell, J.P.; Bradford, P.A.; Eakin, A.E. Pharmacokinetics/pharmacodynamics of a β-lactam and β-lactamase inhibitor combination: A novel approach for aztreonam/avibactam. J. Antimicrob. Chemother. 2015, 70, 2618–2626. [Google Scholar] [CrossRef]
- Sadek, M.; Juhas, M.; Poirel, L.; Nordmann, P. Genetic Features Leading to Reduced Susceptibility to Aztreonam-Avibactam among Metallo-β-Lactamase-Producing Escherichia coli Isolates. Antimicrob. Agents Chemother. 2020, 64, e01659-20. [Google Scholar] [CrossRef]
- Estabrook, M.; Kazmierczak, K.M.; Wise, M.; Arhin, F.F.; Stone, G.G.; Sahm, D.F. Molecular characterization of clinical isolates of Enterobacterales with elevated MIC values for aztreonam-avibactam from the INFORM global surveillance study, 2012–2017. J. Glob. Antimicrob. Resist. 2021, 24, 316–320. [Google Scholar] [CrossRef]
- Sader, H.S.; Mendes, R.E.; Carvalhaes, C.G.; Kimbrough, J.H.; Castanheira, M. Changing Epidemiology of Carbapenemases Among Carbapenem-Resistant Enterobacterales From United States Hospitals and the Activity of Aztreonam-Avibactam Against Contemporary Enterobacterales (2019–2021). Open Forum Infect. Dis. 2023, 10, ofad046. [Google Scholar] [CrossRef]
- Li, H.; Estabrook, M.; Jacoby, G.A.; Nichols, W.W.; Testa, R.T.; Bush, K. In vitro susceptibility of characterized β-lactamase-producing strains tested with avibactam combinations. Antimicrob. Agents Chemother. 2015, 59, 1789–1793. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Biedenbach, D.J.; Hackel, M.; Rabine, S.; de Jonge, B.L.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. Global Dissemination of blaKPC into Bacterial Species beyond Klebsiella pneumoniae and In Vitro Susceptibility to Ceftazidime-Avibactam and Aztreonam-Avibactam. Antimicrob. Agents Chemother. 2016, 60, 4490–4500. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Rabine, S.; Hackel, M.; McLaughlin, R.E.; Biedenbach, D.J.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. Multiyear, Multinational Survey of the Incidence and Global Distribution of Metallo-β-Lactamase-Producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 1067–1078. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Bouchillon, S.K.; Benaouda, A.; Soraa, N.; Zerouali, K.; Mohamed, N.; Alami, T.; Sahm, D.F. Antimicrobial susceptibility testing of clinical isolates of Gram-negative bacilli collected in Morocco by the ATLAS Global Surveillance Program from 2018 to 2020. J. Glob. Antimicrob. Resist. 2022, 30, 23–30. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2023. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.0; The European Committee on Antimicrobial Susceptibility Testing: Basel, Switzerland, 2023; Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 8 August 2023).
- Al-Sweih, N.; Jamal, W.; Mokaddas, E.; Habashy, N.; Kurdi, A.; Mohamed, N. Evaluation of the in vitro activity of ceftaroline, ceftazidime/avibactam and comparator antimicrobial agents against clinical isolates from paediatric patients in Kuwait: ATLAS data 2012–19. JAC Antimicrob. Resist. 2021, 3, dlab159. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Zekiy, A.O.; Krutova, M.; Gholami, M.; Kouhsari, E.; Sholeh, M.; Ghafouri, Z.; Maleki, F. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: Narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1003–1022. [Google Scholar] [CrossRef]

| Wards n (%) | Infection Sources n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | n a,b | ICU | Non-ICU | n a,c | RTI | UTI | SSTI | BSI | IAI | |
| Global | ||||||||||
| Enterobacterales d | 116,602 | 109,197 | 25,093 | 70,054 | 116,247 | 25,575 | 27,254 | 22,040 | 23,450 | 16,873 |
| (23.0) | (64.2) | (22.0) | (23.4) | (19.0) | (20.2) | (14.5) | ||||
| MDR CLSI | 36,305 | 34,330 | 9202 | 21,691 | 36,258 | 7772 | 8955 | 6381 | 7836 | 5212 |
| (26.8) | (63.2) | (21.4) | (24.7) | (17.6) | (21.6) | (14.4) | ||||
| MDR EUCAST | 39,700 | 37,488 | 9857 | 23,771 | 39,643 | 8393 | 9815 | 6927 | 8651 | 5728 |
| (26.3) | (63.4) | (21.2) | (24.8) | (17.5) | (21.8) | (14.4) | ||||
| ESBL | 20,303 | 19,149 | 5312 | 11,837 | 20,266 | 4463 | 5093 | 3508 | 4227 | 2855 |
| (27.7) | (61.8) | (22) | (25.1) | (17.3) | (20.9) | (14.1) | ||||
| CRE CLSI | 5576 | 5206 | 2058 | 2771 | 5564 | 1475 | 1142 | 870 | 1328 | 706 |
| (39.5) | (53.2) | (26.5) | (20.5) | (15.6) | (23.9) | (12.7) | ||||
| CRE EUCAST | 4388 | 4101 | 1681 | 2120 | 4379 | 1190 | 892 | 620 | 1096 | 551 |
| (41.0) | (51.7) | (27.2) | (20.4) | (14.2) | (25.0) | (12.6) | ||||
| MBL-positive | 1877 | 1765 | 728 | 909 | 1874 | 436 | 487 | 327 | 430 | 193 |
| (41.3) | (51.5) | (23.3) | (26.0) | (17.4) | (22.9) | (10.3) | ||||
| Africa–Middle East | ||||||||||
| Enterobacterales | 8990 | 8531 | 1839 | 5812 | 8964 | 1555 | 2268 | 2390 | 1565 | 1151 |
| (21.6) | (68.1) | (17.3) | (25.3) | (26.7) | (17.5) | (12.8) | ||||
| MDR CLSI | 3627 | 3414 | 805 | 2298 | 3616 | 576 | 1010 | 852 | 762 | 413 |
| (23.9) | (67.3) | (15.9) | (27.9) | (23. 6) | (21.1) | (11.4) | ||||
| MDR EUCAST | 3935 | 3709 | 869 | 2501 | 3923 | 626 | 1072 | 946 | 820 | 454 |
| (23.4) | (67.4) | (16.0) | (27.3) | (24.1) | (20.9) | (11.6) | ||||
| ESBL | 2176 | 2055 | 503 | 1369 | 2175 | 390 | 565 | 510 | 467 | 237 |
| (24.5) | (66.6) | (17.9) | (26.0) | (23.4) | (21.5) | (10.9) | ||||
| CRE CLSI | 337 | 312 | 115 | 176 | 337 | 68 | 76 | 63 | 88 | 41 |
| (36.9) | (56.4) | (20.2) | (22.6) | (18.7) | (26.1) | (12.2) | ||||
| CRE EUCAST | 252 | 229 | 89 | 121 | 252 | 54 | 51 | 44 | 73 | 30 |
| (38.9) | (52.8) | (21.4) | (20.2) | (17.5) | (29.0) | (11.9) | ||||
| MBL-positive | 190 | 177 | 58 | 104 | 190 | 24 | 59 | 38 | 54 | 15 |
| (32.8) | (58.8) | (12.6) | (31.1) | (20.0) | (28.4) | (7.9) | ||||
| Asia–Pacific | ||||||||||
| Enterobacterales | 21,653 | 20,577 | 4052 | 14,268 | 21,618 | 5525 | 5339 | 3239 | 4247 | 3182 |
| (19.7) | (69.3) | (25.6) | (24.7) | (15.0) | (19.6) | (14.7) | ||||
| MDR CLSI | 8781 | 8404 | 2029 | 5605 | 8775 | 2127 | 2354 | 1239 | 1766 | 1277 |
| (24.1) | (66.7) | (24.2) | (26.8) | (14.1) | (20.1) | (14.6) | ||||
| MDR EUCAST | 9441 | 9018 | 2135 | 6044 | 9435 | 2264 | 2540 | 1336 | 1908 | 1375 |
| (23.7) | (67.0) | (24.0) | (26.9) | (14.2) | (20.2) | (14.6) | ||||
| ESBL | 4106 | 3881 | 1025 | 2472 | 4098 | 1023 | 1105 | 619 | 819 | 526 |
| (26.4) | (63.7) | (25.0) | (27.0) | (15.1) | (20.0) | (12.8) | ||||
| CRE CLSI | 1691 | 1588 | 653 | 831 | 1686 | 536 | 398 | 206 | 365 | 172 |
| (41.1) | (52.3) | (31.8) | (23.6) | (12.2) | (21.6) | (10.2) | ||||
| CRE EUCAST | 1531 | 1438 | 602 | 744 | 1526 | 479 | 356 | 184 | 346 | 153 |
| (41.9) | (51.7) | (31.4) | (23.3) | (12.1) | (22.7) | (10.0) | ||||
| MBL-positive | 735 | 684 | 317 | 325 | 733 | 185 | 207 | 109 | 176 | 56 |
| (46.3) | (47.5) | (25.2) | (28.2) | (14.9) | (24.0) | (7.6) | ||||
| Europe | ||||||||||
| Enterobacterales | 55,919 | 52,177 | 12,919 | 32,797 | 55,688 | 12,770 | 12,086 | 11,008 | 10,938 | 8116 |
| (24.8) | (62.9) | (22.9) | (21.7) | (19.8) | (19.6) | (14.6) | ||||
| MDR CLSI | 14,785 | 13,944 | 4126 | 8606 | 14,764 | 3473 | 3297 | 2731 | 3140 | 2056 |
| (29.6) | (61.7) | (23.5) | (22.3) | (18.5) | (21.3) | (13.9) | ||||
| MDR EUCAST | 16,435 | 15,474 | 4473 | 9583 | 16,408 | 3809 | 3692 | 2971 | 3540 | 2308 |
| (28.9) | (61.9) | (23.2) | (22.5) | (18.1) | (21.6) | (14.1) | ||||
| ESBL | 8807 | 8246 | 2453 | 5014 | 8785 | 2148 | 2154 | 1491 | 1735 | 1174 |
| (29.7) | (60.8) | (24.5) | (24.5) | (17.0) | (19.7) | (13.4) | ||||
| CRE CLSI | 2241 | 2078 | 824 | 1120 | 2238 | 607 | 394 | 381 | 533 | 302 |
| (39.7) | (53.9) | (27.1) | (17.6) | (17.0) | (23.8) | (13.5) | ||||
| CRE EUCAST | 1649 | 1534 | 637 | 801 | 1648 | 456 | 286 | 244 | 417 | 231 |
| (41.5) | (52.2) | (27.7) | (17.4) | (14.8) | (25.3) | (14.0) | ||||
| MBL-positive | 604 | 571 | 235 | 311 | 604 | 179 | 139 | 97 | 111 | 78 |
| (41.2) | (54.5) | (29.6) | (23.0) | (16.1) | (18.4) | (12.9) | ||||
| Latin America | ||||||||||
| Enterobacterales | 16,501 | 15,608 | 3752 | 9133 | 16,465 | 2714 | 4371 | 2926 | 3556 | 2814 |
| (24.0) | (58.5) | (16.5) | (26.6) | (17.8) | (21.6) | (17.1) | ||||
| MDR CLSI | 6640 | 6333 | 1736 | 3675 | 6633 | 1005 | 1706 | 1188 | 1548 | 1170 |
| (27.4) | (58.0) | (15.2) | (25.7) | (17.9) | (23.3) | (17.6) | ||||
| MDR EUCAST | 7083 | 6747 | 1825 | 3923 | 7074 | 1047 | 1844 | 1258 | 1656 | 1251 |
| (27.0) | (58.1) | (14.8) | (26.1) | (17.8) | (23.4) | (17.7) | ||||
| ESBL | 4115 | 3950 | 1077 | 2346 | 4110 | 637 | 999 | 751 | 906 | 798 |
| (27.3) | (59.4) | (15.5) | (24.3) | (18.3) | (22.0) | (19.4) | ||||
| CRE CLSI | 1133 | 1076 | 425 | 540 | 1130 | 207 | 241 | 187 | 321 | 164 |
| (39.5) | (50.2) | (18.3) | (21.3) | (16.5) | (28.4) | (14.5) | ||||
| CRE EUCAST | 864 | 819 | 333 | 398 | 862 | 168 | 181 | 136 | 248 | 122 |
| (40.7) | (48.6) | (19.5) | (21.0) | (15.8) | (28.8) | (14.2) | ||||
| MBL-positive | 326 | 313 | 113 | 155 | 326 | 41 | 76 | 78 | 89 | 41 |
| (36.1) | (49.5) | (12.6) | (23.3) | (23.9) | (27.3) | (12.6) | ||||
| North America | ||||||||||
| Enterobacterales | 13,539 | 12,304 | 2531 | 8044 | 13,512 | 3011 | 3190 | 2477 | 3144 | 1610 |
| (20.6) | (65.4) | (22.3) | (23.6) | (18.3) | (23.3) | (11.9) | ||||
| MDR CLSI | 2472 | 2235 | 506 | 1507 | 2470 | 591 | 588 | 371 | 620 | 296 |
| (22.6) | (67.4) | (23.9) | (23.8) | (15.0) | (25.1) | (12.0) | ||||
| MDR EUCAST | 2806 | 2540 | 555 | 1720 | 2803 | 647 | 667 | 416 | 727 | 340 |
| (21.9) | (67.7) | (23.1) | (23.8) | (14.8) | (25.9) | (12.1) | ||||
| ESBL | 1099 | 1017 | 254 | 636 | 1098 | 265 | 270 | 137 | 300 | 120 |
| (25.0) | (62.5) | (24.1) | (24.6) | (12.5) | (27.3) | (10.9) | ||||
| CRE CLSI | 174 | 152 | 41 | 104 | 173 | 57 | 33 | 33 | 21 | 27 |
| (27.0) | (68.4) | (32.9) | (19.1) | (19.1) | (12.1) | (15.6) | ||||
| CRE EUCAST | 92 | 81 | 20 | 56 | 91 | 33 | 18 | 12 | 12 | 15 |
| (24.7) | (69.1) | (36.3) | (19.8) | (13.2) | (13.2) | (16.5) | ||||
| MBL-positive | 22 | 20 | 5 | 14 | 21 | 7 | 6 | 5 | NA | 3 |
| (25.0) | (70.0) | (33.3) | (28.6) | (23.8) | (14.3) | |||||
| MIC90 (µg/mL) (% S, CLSI/%S, EUCAST a) | ||||
|---|---|---|---|---|
| ICU | Non-ICU | |||
| Global (N = 116,602) b | n c | n c | ||
| Aztreonam-avibactam d | 20,200 | 0.25 (99.9) | 56,533 | 0.12 (99.9) |
| Aztreonam | 20,799 | 128 (66.3/66.3) | 58,531 | 64 (74.3/74.3) |
| Amikacin | 25,093 | 8 (94.3/91.9) | 70,054 | 8 (97.3/95.5) |
| Cefepime | 25,093 | 64 (70.5/73.1) | 70,054 | 32 (77.2/79.5) |
| Ceftazidime | 25,093 | 128 (67.7/67.7) | 70,054 | 64 (75.4/75.4) |
| Ceftazidime–avibactam | 20,799 | 1 (96.1/96.1) | 58,532 | 0.5 (98.2/98.2) |
| Ceftriaxone | 10,736 | 32 (66.3/67.7) | 31,851 | 32 (71.0/72.1) |
| Ciprofloxacin | 14,357 | 8 (61.6/65.7) | 38,203 | 8 (64.5/68.8) |
| Colistin e,f | 17,848 | 1 (NA/95.9) | 48,942 | 1 (NA/97.4) |
| Gentamicin | 14,357 | 32 (77.6/76.7) | 38,203 | 32 (83.0/81.9) |
| Imipenem g | 20,799 | 4 (81.6/NA) | 58,532 | 2 (84.3/NA) |
| Levofloxacin | 25,093 | 16 (68.3/73.5) | 70,054 | 16 (69.1/74.0) |
| Meropenem | 25,093 | 0.5 (91.0/93.3) | 70,054 | 0.12 (95.5/97.0) |
| Piperacillin–tazobactam | 25,093 | 128 (73.0/73.0) | 70,054 | 64 (81.0/81.0) |
| Tigecycline h,i,j | 23,374 | 1 (97.8/98.2) | 62,681 | 1 (98.3/98.0) |
| Africa–Middle East (N = 8990) | ||||
| Aztreonam-avibactam d | 1686 | 0.25 (100) | 5361 | 0.12 (99.9) |
| Aztreonam | 1686 | 128 (63.0/63.0) | 5361 | 64 (68.8/68.8) |
| Amikacin | 1839 | 8 (96.1/93.1) | 5812 | 8 (97.6/95.5) |
| Cefepime | 1839 | 64 (64.1/66.9) | 5812 | 32 (68.9/71.7) |
| Ceftazidime | 1839 | 64 (62.6/62.6) | 5812 | 64 (69.2/69.2) |
| Ceftazidime–avibactam | 1686 | 0.5 (96.5/96.5) | 5361 | 0.5 (97.8/97.8) |
| Ceftriaxone | 584 | 32 (60.6/61.8) | 2365 | 32 (69.6/70.6) |
| Ciprofloxacin | 1255 | 8 (56.7/64.1) | 3447 | 8 (52.8/58.9) |
| Colistin e,f | 1443 | 1 (NA/97.1) | 4475 | 1 (NA/98.1) |
| Gentamicin | 1255 | 32 (74.4/73.7) | 3447 | 32 (74.4/73.0) |
| Imipenem g | 1686 | 2 (83.4/NA) | 5361 | 2 (84.7/NA) |
| Levofloxacin | 1839 | 16 (66.8/75.1) | 5812 | 16 (62.7/69.8) |
| Meropenem | 1839 | 0.5 (92.7/95.2) | 5812 | 0.12 (96.4/97.9) |
| Piperacillin–tazobactam | 1839 | 128 (74.2/74.2) | 5812 | 64 (79.8/79.8) |
| Tigecycline h,i,j | 1714 | 1 (97.9/97.7) | 5107 | 1 (98.4/97.9) |
| Asia–Pacific (N = 21,653) | ||||
| Aztreonam-avibactam d | 3184 | 0.5 (99.2) | 10,913 | 0.25 (99.8) |
| Aztreonam | 3783 | 128 (56.0/56.0) | 12,911 | 128 (68.7/68.7) |
| Amikacin | 4052 | 128 (86.3/84.2) | 14,268 | 8 (95.6/93.8) |
| Cefepime | 4052 | 64 (58.5/61.5) | 14,268 | 64 (71.6/74.4) |
| Ceftazidime | 4052 | 256 (55.6/55.6) | 14,268 | 128 (70.0/70.0) |
| Ceftazidime–avibactam | 3783 | 16 (90.0/90.0) | 12,911 | 0.5 (96.5/96.5) |
| Ceftriaxone | 1190 | 32 (56.8/58.4) | 5682 | 32 (63.0/64.3) |
| Ciprofloxacin | 2862 | 8 (48.1/53.4) | 8586 | 8 (56.4/62.2) |
| Colistin e,f | 3291 | 1 (NA/94.5) | 10,944 | 1 (NA/96.3) |
| Gentamicin | 2862 | 32 (69.0/67.9) | 8586 | 32 (78.9/77.6) |
| Imipenem g | 3783 | 16 (74.3/NA) | 12,911 | 2 (82.9/NA) |
| Levofloxacin | 4052 | 16 (55.1/61.0) | 14,268 | 16 (61.2/67.2) |
| Meropenem | 4052 | 32 (83.4/85.1) | 14,268 | 0.25 (93.9/94.8) |
| Piperacillin–tazobactam | 4052 | 128 (66.9/66.9) | 14,268 | 128 (80.0/80.0) |
| Tigecycline h,i,j | 3738 | 2 (96.7/95.8) | 12,776 | 1 (98.0/95.9) |
| Europe (N = 55,919) | ||||
| Aztreonam-avibactam d | 10,124 | 0.25 (100) | 26,062 | 0.12 (100) |
| Aztreonam | 10,124 | 128 (70.4/70.4) | 26,062 | 64 (77.8/77.8) |
| Amikacin | 12,919 | 8 (95.9/93.6) | 32,797 | 4 (97.6/96.0) |
| Cefepime | 12,919 | 32 (74.9/77.2) | 32,797 | 32 (80.8/82.8) |
| Ceftazidime | 12,919 | 128 (71.7/71.7) | 32,797 | 32 (78.2/78.2) |
| Ceftazidime–avibactam | 10,124 | 1 (97.6/97.6) | 26,062 | 0.5 (98.8/98.8) |
| Ceftriaxone | 6264 | 32 (68.5/69.9) | 16,521 | 32 (74.1/75.2) |
| Ciprofloxacin | 6655 | 8 (67.9/71.0) | 16,276 | 8 (70.3/73.5) |
| Colistin e,f | 8617 | 1 (NA/96.1) | 21,651 | 0.5 (NA/98.0) |
| Gentamicin | 6655 | 32 (81.4/80.6) | 16,276 | 32 (86.4/85.3) |
| Imipenem g | 10,124 | 4 (83.1/NA) | 26,062 | 2 (84.5/NA) |
| Levofloxacin | 12,919 | 16 (72.3/76.9) | 32,797 | 16 (73.8/77.8) |
| Meropenem | 12,919 | 0.25 (92.7/95.1) | 32,797 | 0.12 (96.0/97.6) |
| Piperacillin–tazobactam | 12,919 | 128 (73.6/73.6) | 32,797 | 64 (80.9/80.9) |
| Tigecycline h,i,j | 12,013 | 1 (98.1/98.8) | 29,332 | 1 (98.4/98.8) |
| Latin America (N = 16,501) | ||||
| Aztreonam-avibactam d | 3302 | 0.25 (100) | 7810 | 0.12 (100) |
| Aztreonam | 3302 | 128 (58.5/58.5) | 7810 | 128 (66.1/66.1) |
| Amikacin | 3752 | 16 (93.2/89.6) | 9133 | 8 (96.3/93.6) |
| Cefepime | 3752 | 64 (60.5/63.9) | 9133 | 64 (67/69.6) |
| Ceftazidime | 3752 | 128 (59.6/59.6) | 9133 | 64 (67.6/67.6) |
| Ceftazidime–avibactam | 3302 | 1 (96.5/96.5) | 7810 | 0.5 (98.0/98.0) |
| Ceftriaxone | 1630 | 64 (57.1/58.0) | 4392 | 32 (61.5/62.4) |
| Ciprofloxacin | 2122 | 8 (51.9/56.6) | 4741 | 8 (53.8/58.6) |
| Colistin e,f | 2828 | 1 (NA/94.9) | 6571 | 1 (NA/96.4) |
| Gentamicin | 2122 | 32 (69.9/68.6) | 4741 | 32 (75.5/74.2) |
| Imipenem g | 3302 | 8 (79.9/NA) | 7810 | 2 (82.8/NA) |
| Levofloxacin | 3752 | 16 (60.4/66.9) | 9133 | 16 (59.1/64.9) |
| Meropenem | 3752 | 8 (87.6/91.1) | 9133 | 0.25 (93.2/95.6) |
| Piperacillin–tazobactam | 3752 | 128 (69.1/69.1) | 9133 | 128 (77.1/77.1) |
| Tigecycline h,i,j | 3496 | 1 (97.8/98.8) | 8204 | 1 (98.5/98.2) |
| North America (N = 13,539) | ||||
| Aztreonam-avibactam d | 1904 | 0.12 (100) | 6387 | 0.12 (99.9) |
| Aztreonam | 1904 | 32 (81.2/81.2) | 6387 | 16 (85.9/85.9) |
| Amikacin | 2531 | 4 (99.1/97.6) | 8044 | 4 (99.5/98.6) |
| Cefepime | 2531 | 8 (86.8/89.3) | 8044 | 4 (90.0/91.7) |
| Ceftazidime | 2531 | 32 (82.9/82.9) | 8044 | 16 (86.4/86.4) |
| Ceftazidime–avibactam | 1904 | 0.5 (99.7/99.7) | 6388 | 0.5 (99.7/99.7) |
| Ceftriaxone | 1068 | 32 (81.6/83.3) | 2891 | 32 (84.7/86.1) |
| Ciprofloxacin | 1463 | 8 (77.1/80.5) | 5153 | 8 (77.6/80.7) |
| Colistin e,f | 1669 | 0.5 (NA/98.1) | 5301 | 0.5 (NA/97.9) |
| Gentamicin | 1463 | 2 (91.5/91.0) | 5153 | 2 (92.0/91.0) |
| Imipenem g | 1904 | 2 (89.8/NA) | 6388 | 2 (87.4/NA) |
| Levofloxacin | 2531 | 8 (81.6/84.7) | 8044 | 8 (80.4/83.5) |
| Meropenem | 2531 | 0.12 (98.1/99.2) | 8044 | 0.12 (98.6/99.3) |
| Piperacillin–tazobactam | 2531 | 32 (84.6/84.6) | 8044 | 16 (88.3/88.3) |
| Tigecycline h,i,j | 2413 | 1 (98.1/99.1) | 7262 | 1 (98.4/98.9) |
| MIC90 (µg/mL) (% S, CLSI/%S, EUCAST a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RTI | UTI | SSTI | BSI | IAI | ||||||
| Global (N = 116,602) b | n c | n c | n c | n c | n c | |||||
| Aztreonam-avibactam d | 20,197 | 0.25 (99.9) | 22,199 | 0.12 (99.9) | 18,117 | 0.12 (99.9) | 18,159 | 0.12 (99.9) | 13,341 | 0.12 (100) |
| Aztreonam | 20,979 | 128 (71.7/71.7) | 22,875 | 64 (73.0/73.0) | 18,504 | 64 (76.7/76.7) | 18,754 | 128 (70.6/70.6) | 13,842 | 64 (73.8/73.8) |
| Amikacin | 25,575 | 8 (95.9/94.1) | 27,254 | 8 (96.7/94.7) | 22,040 | 8 (97.4/95.6) | 23,450 | 8 (96.3/94.2) | 16,873 | 8 (97.5/95.7) |
| Cefepime | 25,574 | 32 (76.0/78.3) | 27,254 | 32 (75.1/77.6) | 22,040 | 32 (79.3/81.6) | 23,450 | 64 (73.6/75.8) | 16,873 | 32 (77.8/80.1) |
| Ceftazidime | 25,575 | 64 (73.1/73.1) | 27,254 | 64 (74.0/74.0) | 22,040 | 64 (77.2/77.2) | 23,450 | 64 (72.8/72.8) | 16,873 | 64 (75.4/75.4) |
| Ceftazidime–avibactam | 20,979 | 0.5 (97.6/97.6) | 22,876 | 0.5 (97.6/97.6) | 18,504 | 0.5 (98.1/98.1) | 18,754 | 0.5 (97.4/97.4) | 13,842 | 0.5 (98.3/98.3) |
| Ceftriaxone | 11,953 | 32 (69.5/70.9) | 12,972 | 32 (70.7/71.8) | 10,880 | 32 (73.3/74.7) | 7935 | 64 (69.6/70.7) | 8257 | 32 (71.1/72.1) |
| Ciprofloxacin | 13,621 | 8 (67.1/71.2) | 14,281 | 8 (58.9/62.9) | 11,160 | 8 (66.6/71.0) | 15,515 | 8 (62.9/67.3) | 8616 | 8 (67.3/71.1) |
| Colistin e,f | 17,835 | 1 (NA/96.4) | 18,726 | 1 (NA/97.7) | 14,116 | 1 (NA/97.0) | 16,592 | 1 (NA/96.9) | 12,544 | 1 (NA/97.6) |
| Gentamicin | 13,622 | 32 (82.7/81.8) | 14,282 | 32 (79.3/77.9) | 11,160 | 32 (83.0/81.8) | 15,515 | 32 (80.9/79.9) | 8616 | 32 (85.1/84) |
| Imipenem g | 20,979 | 2 (84.4/NA) | 22,876 | 2 (82.6/NA) | 18,504 | 4 (79.6/NA) | 18,754 | 2 (86.2/NA) | 13,842 | 2 (87.4/NA) |
| Levofloxacin | 25,574 | 16 (71.9/77.1) | 27,254 | 16 (65.3/70.0) | 22,040 | 16 (70.7/75.7) | 23,450 | 16 (68.0/73.0) | 16,873 | 16 (71.0/75.1) |
| Meropenem | 25,574 | 0.25 (93.6/95.4) | 27,254 | 0.12 (95.3/96.7) | 22,040 | 0.12 (95.5/97.2) | 23,450 | 0.12 (93.8/95.3) | 16,873 | 0.12 (95.2/96.7) |
| Piperacillin–tazobactam | 25,575 | 128 (76.5/76.5) | 27,254 | 64 (80.8/80.8) | 22,040 | 64 (82.3/82.3) | 23,450 | 128 (79.4/79.4) | 16,873 | 128 (80.4/80.4) |
| Tigecycline h,i,j | 24,093 | 1 (97.8/98.0) | 23,534 | 1 (98.6/98.1) | 18,467 | 1 (98.1/98.1) | 22,139 | 1 (98.5/98.3) | 15,808 | 1 (98.3/98.1) |
| Africa–Middle East (N = 8990) | ||||||||||
| Aztreonam-avibactam d | 1433 | 0.12 (100) | 2118 | 0.12 (99.9) | 2202 | 0.12 (100) | 1458 | 0.12 (99.9) | 1010 | 0.12 (99.9) |
| Aztreonam | 1433 | 64 (68.5/68.5) | 2118 | 128 (66.2/66.2) | 2202 | 64 (71.9/71.9) | 1458 | 64 (58.9/58.9) | 1010 | 64 (71.9/71.9) |
| Amikacin | 1555 | 8 (96.9/95.1) | 2268 | 8 (98.0/95.5) | 2390 | 8 (97.5/95.1) | 1565 | 8 (96.4/93.7) | 1151 | 8 (98.0/96.1) |
| Cefepime | 1555 | 32 (68.2/71.0) | 2268 | 64 (65.8/68.8) | 2390 | 32 (72.2/74.7) | 1565 | 64 (60.3/63.1) | 1151 | 32 (73.5/76.2) |
| Ceftazidime | 1555 | 64 (69.8/69.8) | 2268 | 64 (66.2/66.2) | 2390 | 64 (72.0/72.0) | 1565 | 64 (58.7/58.7) | 1151 | 64 (72.6/72.6) |
| Ceftazidime–avibactam | 1433 | 0.5 (98.3/98.3) | 2118 | 0.5 (96.9/96.9) | 2202 | 0.5 (98.1/98.1) | 1458 | 0.5 (95.9/95.9) | 1010 | 0.5 (98.4/98.4) |
| Ceftriaxone | 645 | 32 (65.6/66.2) | 917 | 32 (69.9/71.1) | 1059 | 32 (71.4/72.1) | 346 | 64 (58.1/59.5) | 475 | 32 (72.0/73.3) |
| Ciprofloxacin | 910 | 8 (60.1/66.5) | 1351 | 8 (46.4/51.9) | 1331 | 8 (55.2/62.2) | 1219 | 8 (52.5/59.2) | 676 | 8 (58.7/64.9) |
| Colistin e,f | 1209 | 1 (NA/97.8) | 1820 | 0.5 (NA/98.1) | 1705 | 1 (NA/97.5) | 1284 | 1 (NA/97.8) | 904 | 1 (NA/98.3) |
| Gentamicin | 910 | 32 (78.5/76.8) | 1351 | 32 (69.3/68) | 1331 | 32 (75.5/74.2) | 1219 | 32 (72.4/71.3) | 676 | 32 (79/77.7) |
| Imipenem g | 1433 | 2 (85.8/NA) | 2118 | 2 (85.3/NA) | 2202 | 2 (81.5/NA) | 1458 | 2 (85.5/NA) | 1010 | 2 (87.2/NA) |
| Levofloxacin | 1555 | 16 (69.6/77.9) | 2268 | 16 (59.1/64.8) | 2390 | 16 (63.5/71.3) | 1565 | 16 (62.6/70.9) | 1151 | 16 (66.5/72.9) |
| Meropenem | 1555 | 0.12 (94.9/96.5) | 2268 | 0.12 (96.1/97.8) | 2390 | 0.12 (96.9/98.2) | 1565 | 0.25 (93.6/95.3) | 1151 | 0.12 (95.9/97.4) |
| Piperacillin–tazobactam | 1555 | 128 (77.8/77.8) | 2268 | 64 (78.0/78.0) | 2390 | 32 (82.4/82.4) | 1565 | 128 (74.4/74.4) | 1151 | 64 (81.2/81.2) |
| Tigecycline h,i,j | 1447 | 1 (97.5/97.1) | 2005 | 1 (98.9/98.3) | 1967 | 1 (98.2/97.2) | 1472 | 1 (98.5/98.0) | 1064 | 1 (98.4/98.4) |
| Asia–Pacific (N = 21,653) | ||||||||||
| Aztreonam-avibactam d | 4243 | 0.25 (99.7) | 4297 | 0.25 (99.8) | 2666 | 0.25 (99.7) | 2995 | 0.25 (99.8) | 2336 | 0.25 (99.8) |
| Aztreonam | 5025 | 128 (66.0/66.0) | 4973 | 128 (66.9/66.9) | 3053 | 128 (71.8/71.8) | 3590 | 128 (62.8/62.8) | 2837 | 128 (67.3/67.3) |
| Amikacin | 5525 | 8 (92.4/90.7) | 5339 | 8 (93.3/91.1) | 3239 | 8 (95.1/93.2) | 4247 | 8 (93.0/91.2) | 3182 | 8 (96.0/94.3) |
| Cefepime | 5524 | 64 (69.1/71.9) | 5339 | 64 (68.2/71.3) | 3239 | 32 (73.0/75.6) | 4247 | 64 (65.6/68.3) | 3182 | 32 (72.0/74.8) |
| Ceftazidime | 5525 | 128 (65.8/65.8) | 5339 | 128 (67.9/67.9) | 3239 | 128 (70.4/70.4) | 4247 | 128 (66.7/66.7) | 3182 | 128 (69.3/69.3) |
| Ceftazidime–avibactam | 5025 | 1 (95.2/95.2) | 4973 | 1 (94.7/94.7) | 3053 | 1 (95.8/95.8) | 3590 | 1 (93.9/93.9) | 2837 | 0.5 (96.8/96.8) |
| Ceftriaxone | 2200 | 32 (61.8/63.2) | 2064 | 32 (63.6/65.1) | 1426 | 32 (63.2/64.8) | 1315 | 64 (65.2/66.1) | 1370 | 32 (61.4/62.9) |
| Ciprofloxacin | 3324 | 8 (57.9/63.8) | 3274 | 8 (48.4/53.4) | 1813 | 8 (60.0/65.8) | 2932 | 8 (50.9/56.8) | 1812 | 8 (58.6/64) |
| Colistin e,f | 4375 | 1 (NA/95.3) | 4105 | 1 (NA/97.3) | 2324 | 1 (NA/95.7) | 3207 | 1 (NA/95.6) | 2563 | 1 (NA/95.7) |
| Gentamicin | 3325 | 32 (78.7/77.7) | 3275 | 32 (72.6/71) | 1813 | 32 (78.5/77.1) | 2932 | 32 (74.3/73.1) | 1812 | 32 (80.7/79.5) |
| Imipenem g | 5025 | 4 (81.7/NA) | 4973 | 4 (79.7/NA) | 3053 | 4 (76.3/NA) | 3590 | 4 (83.2/NA) | 2837 | 2 (86.0/NA) |
| Levofloxacin | 5524 | 16 (62.6/69.2) | 5339 | 16 (55.4/60.5) | 3239 | 16 (63.0/69.1) | 4247 | 16 (58.3/64.0) | 3182 | 16 (63.0/69.0) |
| Meropenem | 5524 | 2 (89.9/91.3) | 5339 | 0.25 (92.2/93.3) | 3239 | 0.25 (93/94.3) | 4247 | 0.5 (91.2/91.9) | 3182 | 0.25 (94.3/95.2) |
| Piperacillin–tazobactam | 5525 | 128 (72.7/72.7) | 5339 | 128 (78.9/78.9) | 3239 | 128 (81/81) | 4247 | 128 (79.1/79.1) | 3182 | 128 (80.2/80.2) |
| Tigecycline h,i,j | 5231 | 2 (96.8/95.3) | 4556 | 1 (98.2/95.8) | 2632 | 1 (97.5/96.5) | 4001 | 1 (98.3/96.3) | 2957 | 1 (98.2/96.3) |
| Europe (N = 55,919) | ||||||||||
| Aztreonam-avibactam d | 9864 | 0.25 (99.9) | 9238 | 0.12 (100) | 8802 | 0.12 (100) | 8206 | 0.12 (100) | 6411 | 0.12 (100) |
| Aztreonam | 9864 | 128 (74.1/74.1) | 9238 | 64 (75.4/75.4) | 8802 | 64 (79.7/79.7) | 8206 | 64 (75.5/75.5) | 6411 | 64 (78.2/78.2) |
| Amikacin | 12,770 | 4 (96.7/94.9) | 12,086 | 4 (97.3/95.6) | 11,008 | 4 (97.8/96.3) | 10,938 | 8 (97.3/95.2) | 8116 | 4 (97.9/96.2) |
| Cefepime | 12,770 | 32 (78.7/80.8) | 12,086 | 32 (77.9/80.2) | 11,008 | 32 (82.9/85.0) | 10,938 | 32 (77.8/79.8) | 8116 | 32 (82.1/84.2) |
| Ceftazidime | 12,770 | 64 (75.6/75.6) | 12,086 | 32 (75.9/75.9) | 11,008 | 32 (80.3/80.3) | 10,938 | 32 (76.4/76.4) | 8116 | 32 (78.9/78.9) |
| Ceftazidime–avibactam | 9864 | 0.5 (98.1/98.1) | 9238 | 0.5 (98.5/98.5) | 8802 | 0.5 (99.0/99.0) | 8206 | 0.5 (98.6/98.6) | 6411 | 0.5 (98.8/98.8) |
| Ceftriaxone | 6733 | 32 (71.0/72.5) | 6790 | 32 (71.6/72.6) | 5886 | 32 (76.4/77.7) | 4099 | 64 (72/73.2) | 4242 | 32 (75.7/76.6) |
| Ciprofloxacin | 6037 | 8 (71.9/75.0) | 5296 | 8 (65.3/68.5) | 5122 | 8 (71.7/74.8) | 6839 | 8 (69.0/72.3) | 3874 | 8 (73.4/76.3) |
| Colistin e,f | 8335 | 1 (NA/96.7) | 7398 | 0.5 (NA/97.9) | 6738 | 0.5 (NA/97.6) | 7273 | 0.5 (NA/97.6) | 5797 | 0.5 (NA/98.2) |
| Gentamicin | 6037 | 32 (85.0/84.3) | 5296 | 32 (83.6/82.1) | 5122 | 32 (86.5/85.4) | 6839 | 32 (83.9/83.0) | 3874 | 16 (88.7/87.8) |
| Imipenem g | 9864 | 2 (84.9/NA) | 9238 | 2 (82.7/NA) | 8802 | 2 (80.6/NA) | 8206 | 2 (87.1/NA) | 6411 | 2 (87.8/NA) |
| Levofloxacin | 12,770 | 16 (75.1/79.6) | 12,086 | 16 (70.1/74.5) | 11,008 | 16 (74.5/78.7) | 10,938 | 16 (71.4/75.6) | 8116 | 16 (77.0/80.0) |
| Meropenem | 12,770 | 0.25 (94.5/96.4) | 12,086 | 0.12 (96.2/97.6) | 11,008 | 0.12 (95.8/97.8) | 10,938 | 0.12 (94.5/96.2) | 8116 | 0.12 (95.6/97.2) |
| Piperacillin–tazobactam | 12,770 | 128 (76.4/76.4) | 12,086 | 64 (80.7/80.7) | 11,008 | 64 (82.2/82.2) | 10,938 | 128 (79.0/79.0) | 8116 | 128 (80.6/80.6) |
| Tigecycline h,i,j | 11,994 | 1 (98.2/98.6) | 10,422 | 1 (98.5/99.0) | 9333 | 1 (98.3/98.8) | 10,331 | 1 (98.3/98.9) | 7602 | 1 (98.3/98.7) |
| Latin America (N = 16,501) | ||||||||||
| Aztreonam-avibactam d | 2369 | 0.25 (100) | 3973 | 0.12 (100) | 2538 | 0.12 (100) | 3021 | 0.25 (100) | 2358 | 0.12 (100) |
| Aztreonam | 2369 | 128 (66.1/66.1) | 3973 | 128 (70.4/70.4) | 2538 | 128 (66.7/66.7) | 3021 | 128 (60.3/60.3) | 2358 | 128 (64.3/64.3) |
| Amikacin | 2714 | 8 (95.8/93.3) | 4371 | 8 (96.2/93.6) | 2926 | 8 (95.9/92.9) | 3556 | 8 (94.3/91.1) | 2814 | 8 (96.8/93.8) |
| Cefepime | 2714 | 64 (68.1/70.5) | 4371 | 32 (69.9/72.6) | 2926 | 32 (67.6/70.4) | 3556 | 64 (62.9/65.8) | 2814 | 64 (66/68.9) |
| Ceftazidime | 2714 | 64 (67.1/67.1) | 4371 | 64 (71.4/71.4) | 2926 | 64 (67.1/67.1) | 3556 | 128 (63.4/63.4) | 2814 | 64 (66.9/66.9) |
| Ceftazidime–avibactam | 2369 | 1 (98.2/98.2) | 3973 | 0.5 (98.1/98.1) | 2538 | 0.5 (96.7/96.7) | 3021 | 1 (97.0/97.0) | 2358 | 0.5 (98.2/98.2) |
| Ceftriaxone | 1190 | 32 (64.6/65.3) | 1996 | 32 (66.8/67.8) | 1500 | 32 (62.4/64.3) | 1327 | 64 (60.2/60.7) | 1472 | 64 (60.0/60.3) |
| Ciprofloxacin | 1524 | 8 (58.3/62.3) | 2375 | 8 (51.5/55.4) | 1426 | 8 (52.7/58.4) | 2229 | 8 (53.1/58.3) | 1342 | 8 (56.3/60.4) |
| Colistin e,f | 2003 | 1 (NA/95.6) | 3316 | 0.5 (NA/97.4) | 1950 | 1 (NA/95.5) | 2614 | 1 (NA/95.0) | 2148 | 0.5 (NA/97.4) |
| Gentamicin | 1524 | 32 (75.5/74.6) | 2375 | 32 (74.7/73.2) | 1426 | 32 (71.9/70.6) | 2229 | 32 (74.3/73.1) | 1342 | 32 (77.8/76.5) |
| Imipenem g | 2369 | 4 (84.0/NA) | 3973 | 2 (82.7/NA) | 2538 | 4 (77.0/NA) | 3021 | 4 (82.8/NA) | 2358 | 2 (86.3/NA) |
| Levofloxacin | 2714 | 16 (65.7/71.8) | 4371 | 16 (57.2/62.2) | 2926 | 16 (58.7/65.0) | 3556 | 16 (61.8/68.4) | 2814 | 16 (58.9/63.9) |
| Meropenem | 2714 | 0.25 (91.6/93.8) | 4371 | 0.12 (93.8/95.9) | 2926 | 0.25 (92.8/95.4) | 3556 | 2 (89.8/93.0) | 2814 | 0.25 (93.3/95.7) |
| Piperacillin–tazobactam | 2714 | 128 (75.8/75.8) | 4371 | 128 (78.6/78.6) | 2926 | 128 (76.7/76.7) | 3556 | 128 (73.7/73.7) | 2814 | 128 (76.1/76.1) |
| Tigecycline h,i,j | 2550 | 1 (98.0/98.5) | 3797 | 1 (99.0/98.8) | 2447 | 1 (98.3/97.8) | 3373 | 1 (98.5/98.4) | 2644 | 1 (98.5/98.2) |
| North America (N = 13,539) | ||||||||||
| Aztreonam-avibactam d | 2288 | 0.25 (99.9) | 2573 | 0.12 (99.9) | 1909 | 0.12 (99.8) | 2479 | 0.12 (100) | 1226 | 0.12 (100) |
| Aztreonam | 2288 | 32 (81.9/81.9) | 2573 | 16 (86.2/86.2) | 1909 | 8 (88.9/88.9) | 2479 | 32 (85.2/85.2) | 1226 | 16 (85.9/85.9) |
| Amikacin | 3011 | 4 (98.9/97.3) | 3190 | 4 (99.4/98.6) | 2477 | 4 (99.8/98.9) | 3144 | 4 (99.4/98.4) | 1610 | 4 (99.4/98.9) |
| Cefepime | 3011 | 4 (88.2/90.3) | 3190 | 4 (89.7/91.3) | 2477 | 1 (91.8/93.9) | 3144 | 8 (88.4/89.8) | 1610 | 2 (90.8/92.7) |
| Ceftazidime | 3011 | 32 (83.3/83.3) | 3190 | 16 (86.6/86.6) | 2477 | 8 (89.5/89.5) | 3144 | 16 (86.0/86.0) | 1610 | 16 (86.8/86.8) |
| Ceftazidime–avibactam | 2288 | 0.5 (99.7/99.7) | 2574 | 0.5 (99.7/99.7) | 1909 | 0.5 (99.5/99.5) | 2479 | 0.25 (99.9/99.9) | 1226 | 0.5 (99.8/99.8) |
| Ceftriaxone | 1185 | 32 (81.9/83.6) | 1205 | 32 (84.6/86.1) | 1009 | 8 (87.0/88.8) | 848 | 32 (84.4/85.9) | 698 | 32 (85.1/86.5) |
| Ciprofloxacin | 1826 | 8 (78.5/81.5) | 1985 | 8 (76.6/80.0) | 1468 | 4 (81.3/84.7) | 2296 | 8 (75.2/79.1) | 912 | 8 (81.4/83.7) |
| Colistin e,f | 1913 | 0.5 (NA/98.0) | 2087 | 0.5 (NA/98.2) | 1399 | 0.5 (NA/97.8) | 2214 | 0.5 (NA/98.3) | 1132 | 0.5 (NA/98.5) |
| Gentamicin | 1826 | 4 (90.5/89.5) | 1985 | 2 (91.2/90.2) | 1468 | 2 (93.6/92.7) | 2296 | 2 (91.4/90.6) | 912 | 2 (93.8/93.1) |
| Imipenem g | 2288 | 2 (87.6/NA) | 2574 | 2 (85.6/NA) | 1909 | 2 (81.5/NA) | 2479 | 1 (91.9/NA) | 1226 | 1 (91.0/NA) |
| Levofloxacin | 3011 | 8 (82.0/85.4) | 3190 | 16 (79.4/82.9) | 2477 | 4 (84.7/87.7) | 3144 | 16 (78.8/82.3) | 1610 | 16 (81.2/83.5) |
| Meropenem | 3011 | 0.12 (97.9/98.9) | 3190 | 0.12 (98.8/99.4) | 2477 | 0.12 (98.6/99.5) | 3144 | 0.12 (99.2/99.6) | 1610 | 0.12 (97.8/99.1) |
| Piperacillin–tazobactam | 3011 | 32 (84.4/84.4) | 3190 | 16 (89.8/89.8) | 2477 | 8 (90.8/90.8) | 3144 | 16 (89.9/89.9) | 1610 | 16 (86.8/86.8) |
| Tigecycline h,i,j | 2871 | 1 (97.9/99.1) | 2754 | 1 (98.6/98.1) | 2088 | 1 (98.0/98.9) | 2962 | 1 (99.0/99.4) | 1541 | 1 (98.7/98.6) |
| ICU | Non-ICU | |||||||
|---|---|---|---|---|---|---|---|---|
| CLSI | EUCAST | CLSI | EUCAST | |||||
| All Enterobacterales (N = 116,602) a | n b | MIC90 (µg/mL) (% S) | n b | MIC90 (mg/L) (% S c) | n b | MIC90 (µg/mL) (% S) | n b | MIC90 (mg/L) (% S c) |
| MDR (CLSI/EUCAST, N = 36,305/39,700) | ||||||||
| Aztreonam-avibactam d | 8289 | 0.5 (99.7) | 8828 | 0.5 (99.7) | 19,564 | 0.5 (99.8) | 21,258 | 0.5 (99.8) |
| Aztreonam | 8673 | 256 (20.3) | 9232 | 256 (24.2) | 20,599 | 128 (28.2) | 22,380 | 128 (32.9) |
| Amikacin | 9202 | 128 (84.6) | 9857 | 64 (80) | 21,691 | 16 (91.6) | 23,771 | 16 (87.7) |
| Cefepime | 9202 | 64 (27.4) | 9857 | 64 (36.1) | 21,691 | 64 (34.7) | 23,771 | 64 (44.2) |
| Ceftazidime | 9202 | 256 (21.2) | 9857 | 256 (24.6) | 21,691 | 256 (29.6) | 23,771 | 128 (33.9) |
| Ceftazidime–avibactam | 8673 | 4 (90.8) | 9232 | 4 (91.3) | 20,600 | 2 (94.9) | 22,381 | 1 (95.3) |
| Ceftriaxone | 2730 | 64 (7.8) | 3005 | 64 (13.2) | 7054 | 64 (8.7) | 7950 | 64 (14.8) |
| Ciprofloxacin | 6472 | 8 (24) | 6852 | 8 (32.4) | 14,637 | 8 (24.2) | 15,821 | 8 (32.7) |
| Colistin e,f | 7947 | 1 (NA) | 8462 | 1 (93) | 18,505 | 1 (NA) | 20,142 | 1 (95.4) |
| Gentamicin | 6472 | 32 (51.3) | 6852 | 32 (52.3) | 14,637 | 32 (57.5) | 15,821 | 32 (58.2) |
| Imipenem g | 8673 | 16 (70.0) | 9232 | 16 (NA) | 20,600 | 8 (77.5) | 22,381 | 8 (NA) |
| Levofloxacin | 9202 | 16 (31.6) | 9857 | 16 (43.1) | 21,691 | 16 (30.5) | 23,771 | 16 (41.1) |
| Meropenem | 9202 | 32 (75.6) | 9857 | 32 (83) | 21,691 | 8 (85.8) | 23,771 | 8 (91.1) |
| Piperacillin–tazobactam | 9202 | 256 (35.5) | 9857 | 256 (37.6) | 21,691 | 128 (48.3) | 23,771 | 128 (50.1) |
| Tigecycline h,i,j | 8647 | 2 (96.1) | 9270 | 2 (96.5) | 19,821 | 1 (96.8) | 21,774 | 1 (95.8) |
| ESBL (N = 20,303) | ||||||||
| Aztreonam-avibactam d | 4616 | 0.25 (99.7) | 4616 | 0.25 (99.7) | 10,130 | 0.25 (99.9) | 10,130 | 0.25 (99.9) |
| Aztreonam | 4769 | 256 (6.7) | 4769 | 256 (6.7) | 10,416 | 256 (9.6) | 10,416 | 256 (9.6) |
| Amikacin | 5312 | 128 (82.6) | 5312 | 128 (76.8) | 11,837 | 16 (91.1) | 11,837 | 16 (86.0) |
| Cefepime | 5312 | 64 (9.3) | 5312 | 64 (13.7) | 11,837 | 64 (11.7) | 11,837 | 64 (17.7) |
| Ceftazidime | 5312 | 256 (11.7) | 5312 | 256 (11.7) | 11,837 | 256 (16.9) | 11,837 | 256 (16.9) |
| Ceftazidime–avibactam | 4769 | 128 (87.7) | 4769 | 128 (87.7) | 10,416 | 2 (93.2) | 10,416 | 2 (93.2) |
| Ceftriaxone | 2322 | 64 (3.6) | 2322 | 64 (4.7) | 6308 | 64 (3.8) | 6308 | 64 (5.3) |
| Ciprofloxacin | 2990 | 8 (9.7) | 2990 | 8 (15.3) | 5529 | 8 (10.7) | 5529 | 8 (16.1) |
| Colistin e,f | 4622 | 1 (NA) | 4622 | 1 (93.3) | 10,054 | 1 (NA) | 10,054 | 1 (96.2) |
| Gentamicin | 2990 | 32 (39.8) | 2990 | 32 (38.9) | 5529 | 32 (47.6) | 5529 | 32 (46.8) |
| Imipenem g | 4769 | 16 (68.0) | 4769 | 16 (NA) | 10,416 | 8 (80.4) | 10,416 | 8 (NA) |
| Levofloxacin | 5312 | 16 (22.2) | 5312 | 16 (32.6) | 11,837 | 16 (22.8) | 11,837 | 16 (31.3) |
| Meropenem | 5312 | 32 (72.2) | 5312 | 32 (79.2) | 11,837 | 16 (84.4) | 11,837 | 16 (89.6) |
| Piperacillin–tazobactam | 5312 | 256 (38.2) | 5312 | 256 (38.2) | 11,837 | 256 (51.1) | 11,837 | 256 (51.1) |
| Tigecycline h,i,j | 5187 | 2 (96.5) | 5187 | 2 (97.9) | 11,507 | 1 (97.1) | 11,507 | 1 (97.3) |
| CRE (CLSI/EUCAST, N = 5576/4388) | ||||||||
| Aztreonam-avibactam d | 1715 | 1 (99.0) | 1401 | 1 (99.0) | 2288 | 1 (99.4) | 1720 | 1 (99.4) |
| Aztreonam | 1842 | 256 (8.6) | 1517 | 256 (6.9) | 2479 | 256 (10.0) | 1899 | 256 (8.5) |
| Amikacin | 2058 | 128 (50.5) | 1681 | 128 (33.4) | 2771 | 128 (60.7) | 2120 | 128 (43.2) |
| Cefepime | 2058 | 64 (2.2) | 1681 | 64 (2.1) | 2771 | 64 (2.6) | 2120 | 64 (1.9) |
| Ceftazidime | 2058 | 256 (3.5) | 1681 | 256 (2.6) | 2771 | 256 (4.2) | 2120 | 256 (2.7) |
| Ceftazidime–avibactam | 1842 | 256 (59.3) | 1517 | 256 (58.1) | 2479 | 256 (61.9) | 1899 | 256 (59.2) |
| Ceftriaxone | 580 | 64 (0.7) | 446 | 64 (0.2) | 904 | 64 (0.8) | 651 | 64 (0.8) |
| Ciprofloxacin | 1478 | 8 (5.8) | 1235 | 8 (5.4) | 1867 | 8 (5.9) | 1469 | 8 (5.5) |
| Colistin e,f | 1701 | 16 (NA) | 1414 | 16 (81.5) | 2317 | 16 (NA) | 1796 | 16 (83.9) |
| Gentamicin | 1478 | 32 (27.9) | 1235 | 32 (24) | 1867 | 32 (35.7) | 1469 | 32 (31.4) |
| Imipenem g | 1842 | 16 (1.3) | 1517 | 16 (NA) | 2479 | 16 (3.1) | 1899 | 16 (5.3) |
| Levofloxacin | 2058 | 16 (8.7) | 1681 | 16 (8.4) | 2771 | 16 (9) | 2120 | 16 (9.3) |
| Meropenem | 2058 | 32 (0) | 1681 | 32 (0) | 2771 | 32 (0) | 2120 | 32 (0) |
| Piperacillin–tazobactam | 2058 | 256 (0.8) | 1681 | 256 (0.5) | 2771 | 256 (1.1) | 2120 | 256 (0.5) |
| Tigecycline h,i,j | 1973 | 2 (93.7) | 1621 | 2 (89.9) | 2668 | 2 (93.7) | 2062 | 2 (81.2) |
| MBL-positive (N = 1877) | ||||||||
| Aztreonam-avibactam d | 693 | 1 (98.7) | 693 | 1 (98.7) | 878 | 1 (99.7) | 878 | 1 (99.7) |
| Aztreonam | 725 | 256 (15.3) | 725 | 256 (15.3) | 900 | 256 (19.3) | 900 | 256 (19.3) |
| Amikacin | 728 | 128 (38.6) | 728 | 128 (28.9) | 909 | 128 (55.1) | 909 | 128 (44.0) |
| Cefepime | 728 | 64 (0.4) | 728 | 64 (0.7) | 909 | 64 (1.4) | 909 | 64 (3.3) |
| Ceftazidime | 728 | 256 (0.1) | 728 | 256 (0.1) | 909 | 256 (0) | 909 | 256 (0) |
| Ceftazidime–avibactam | 725 | 256 (2.1) | 725 | 256 (2.1) | 900 | 256 (2.7) | 900 | 256 (2.7) |
| Ceftriaxone | 89 | 32 (0) | 89 | 32 (0) | 195 | 32 (0) | 195 | 32 (0) |
| Ciprofloxacin | 639 | 8 (7.7) | 639 | 8 (9.9) | 714 | 8 (6) | 714 | 8 (10.5) |
| Colistin e,f | 631 | 2 (NA) | 631 | 2 (90.3) | 802 | 1 (NA) | 802 | 1 (91.8) |
| Gentamicin | 639 | 32 (23.5) | 639 | 32 (21.8) | 714 | 32 (33.1) | 714 | 32 (31.5) |
| Imipenem g | 725 | 16 (1.4) | 725 | 16 (NA) | 900 | 16 (1.2) | 900 | 16 (NA) |
| Levofloxacin | 728 | 16 (12.9) | 728 | 16 (18.8) | 909 | 16 (11.2) | 909 | 16 (18.5) |
| Meropenem | 728 | 32 (3.2) | 728 | 32 (20.5) | 909 | 32 (4.5) | 909 | 32 (26.2) |
| Piperacillin–tazobactam | 728 | 256 (1.2) | 728 | 256 (1.2) | 909 | 256 (1.1) | 909 | 256 (1.1) |
| Tigecycline h,i,j | 657 | 2 (94.8) | 657 | 2 (92.0) | 824 | 2 (93.0) | 824 | 2 (92.4) |
| RTI | UTI | SSTI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLSI | EUCAST | CLSI | EUCAST | CLSI | EUCAST | |||||||
| All Enterobacterales (N = 116,602) a | n b | MIC90 (µg/mL) (% S) | n b | MIC90 (mg/L) (% S c) | n b | MIC90 (µg/mL) (% S) | n b | MIC90 (mg/L) (% Sc) | n b | MIC90 (µg/mL) (% S) | n b | MIC90 (mg/L) (% S c) |
| MDR (CLSI/EUCAST, N = 36,305/39,700) | ||||||||||||
| Aztreonam-avibactam d | 6968 | 0.5 (99.7) | 7469 | 0.5 (99.7) | 8127 | 0.5 (99.8) | 8810 | 0.25 (99.8) | 5888 | 0.5 (99.7) | 6364 | 0.5 (99.8) |
| Aztreonam | 7359 | 256 (20.9) | 7884 | 256 (25.0) | 8547 | 128 (29.2) | 9262 | 128 (33.5) | 6070 | 128 (30.0) | 6562 | 128 (34.3) |
| Amikacin | 7772 | 64 (86.9) | 8393 | 32 (82.8) | 8955 | 16 (90.3) | 9815 | 16 (86.4) | 6381 | 16 (91.4) | 6927 | 16 (87.1) |
| Cefepime | 7772 | 64 (29.9) | 8393 | 64 (38.8) | 8955 | 64 (32.8) | 9815 | 64 (42.5) | 6381 | 64 (35.5) | 6927 | 64 (45.2) |
| Ceftazidime | 7772 | 256 (22.1) | 8393 | 256 (25.8) | 8955 | 256 (30.3) | 9815 | 256 (34.4) | 6381 | 256 (30.1) | 6927 | 256 (34) |
| Ceftazidime–avibactam | 7359 | 2 (93.3) | 7884 | 2 (93.7) | 8548 | 2 (93.6) | 9263 | 2 (94.1) | 6070 | 2 (94.2) | 6562 | 2 (94.6) |
| Ceftriaxone | 2709 | 64 (7.2) | 3010 | 64 (13.0) | 2853 | 64 (7.2) | 3257 | 64 (13.8) | 2363 | 32 (9.8) | 2626 | 32 (17.6) |
| Ciprofloxacin | 5063 | 8 (24.6) | 5383 | 8 (32.7) | 6102 | 8 (20.6) | 6558 | 8 (28.2) | 4018 | 8 (25.8) | 4301 | 8 (34.4) |
| Colistin e,f | 6734 | 1 (NA) | 7212 | 1 (93.2) | 7530 | 1 (NA) | 8173 | 1 (95.8) | 5195 | 1 (NA) | 5634 | 1 (94.9) |
| Gentamicin | 5063 | 32 (54.7) | 5383 | 32 (55.2) | 6102 | 32 (53.6) | 6558 | 32 (54.3) | 4018 | 32 (54.8) | 4301 | 32 (55.2) |
| Imipenem g | 7359 | 16 (72.8) | 7884 | 16 (NA) | 8548 | 8 (77.1) | 9263 | 8 (NA) | 6070 | 8 (73.2) | 6562 | 8 (NA) |
| Levofloxacin | 7772 | 16 (31.2) | 8393 | 16 (43.3) | 8955 | 16 (27) | 9815 | 16 (36.8) | 6381 | 16 (31.7) | 6927 | 16 (43.2) |
| Meropenem | 7772 | 32 (79.3) | 8393 | 16 (85.9) | 8955 | 8 (85.9) | 9815 | 8 (91.0) | 6381 | 8 (84.5) | 6927 | 8 (91.1) |
| Piperacillin–tazobactam | 7772 | 256 (35.9) | 8393 | 256 (37.3) | 8955 | 128 (50.5) | 9815 | 128 (52.4) | 6381 | 128 (48.1) | 6927 | 128 (49.7) |
| Tigecycline h,i,j | 7337 | 2 (95.6) | 7927 | 2 (95.7) | 7995 | 1 (97.2) | 8790 | 1 (96.0) | 5566 | 1 (96.4) | 6060 | 1 (96.0) |
| ESBL (N = 20,303) | ||||||||||||
| Aztreonam-avibactam d | 3875 | 0.25 (99.8) | 3875 | 0.25 (99.8) | 4309 | 0.25 (99.8) | 4309 | 0.25 (99.8) | 3118 | 0.25 (99.8) | 3118 | 0.25 (99.8) |
| Aztreonam | 3967 | 256 (7.8) | 3967 | 256 (7.8) | 4486 | 256 (9.9) | 4486 | 256 (9.9) | 3186 | 256 (11.2) | 3186 | 256 (11.2) |
| Amikacin | 4463 | 64 (86.2) | 4463 | 64 (80.6) | 5093 | 32 (89.8) | 5093 | 32 (84.7) | 3508 | 16 (90.5) | 3508 | 16 (85.2) |
| Cefepime | 4463 | 64 (11.2) | 4463 | 64 (16.3) | 5093 | 64 (11.0) | 5093 | 64 (17.6) | 3508 | 64 (12.9) | 3508 | 64 (19.0) |
| Ceftazidime | 4463 | 256 (12.8) | 4463 | 256 (12.8) | 5093 | 256 (17.6) | 5093 | 256 (17.6) | 3508 | 256 (17.2) | 3508 | 256 (17.2) |
| Ceftazidime–avibactam | 3967 | 4 (90.9) | 3967 | 4 (90.9) | 4486 | 2 (92.3) | 4486 | 2 (92.3) | 3186 | 2 (91.9) | 3186 | 2 (91.9) |
| Ceftriaxone | 2390 | 64 (4.1) | 2390 | 64 (5.4) | 2621 | 64 (3.4) | 2621 | 64 (5.0) | 2028 | 64 (4.5) | 2028 | 64 (6.9) |
| Ciprofloxacin | 2073 | 8 (8.6) | 2073 | 8 (14) | 2472 | 8 (10.3) | 2472 | 8 (14.9) | 1480 | 8 (10.7) | 1480 | 8 (17.2) |
| Colistin e,f | 3852 | 1 (NA) | 3852 | 1 (93.8) | 4320 | 1 (NA) | 4320 | 1 (96.1) | 3024 | 1 (NA) | 3024 | 1 (95.9) |
| Gentamicin | 2073 | 32 (42.6) | 2073 | 32 (41.6) | 2472 | 32 (46.1) | 2472 | 32 (45.2) | 1480 | 32 (42.0) | 1480 | 32 (41.3) |
| Imipenem g | 3967 | 16 (72.7) | 3967 | 16 (NA) | 4486 | 16 (80.0) | 4486 | 16 (NA) | 3186 | 8 (78) | 3186 | 8 (NA) |
| Levofloxacin | 4463 | 16 (22.7) | 4463 | 16 (33.6) | 5093 | 16 (20.9) | 5093 | 16 (27.9) | 3508 | 16 (23.1) | 3508 | 16 (33.3) |
| Meropenem | 4463 | 16 (77.5) | 4463 | 16 (83.6) | 5093 | 16 (84.4) | 5093 | 16 (88.9) | 3508 | 16 (82.3) | 3508 | 16 (89.1) |
| Piperacillin–tazobactam | 4463 | 256 (39.3) | 4463 | 256 (39.3) | 5093 | 128 (52.6) | 5093 | 128 (52.6) | 3508 | 256 (50.2) | 3508 | 256 (50.2) |
| Tigecycline h,i,j | 4368 | 2 (96.2) | 4368 | 2 (98.0) | 4937 | 1 (97.6) | 4937 | 1 (97.2) | 3356 | 1 (96.6) | 3356 | 1 (97.5) |
| CRE (CLSI/EUCAST, N = 5576/4388) | ||||||||||||
| Aztreonam-avibactam d | 1200 | 1 (99.2) | 955 | 1 (99.1) | 967 | 1 (99.2) | 749 | 2 (98.9) | 747 | 1 (99.1) | 528 | 0.5 (99.2) |
| Aztreonam | 1328 | 256 (7.6) | 1071 | 256 (5.4) | 1044 | 256 (14.6) | 820 | 256 (12.7) | 779 | 256 (11.9) | 560 | 256 (10.2) |
| Amikacin | 1475 | 128 (53.9) | 1190 | 128 (39.5) | 1142 | 128 (53.7) | 892 | 128 (37.3) | 870 | 128 (61.4) | 620 | 128 (41.5) |
| Cefepime | 1475 | 64 (3.0) | 1190 | 64 (2.2) | 1142 | 64 (2.5) | 892 | 64 (1.7) | 870 | 64 (2.9) | 620 | 64 (2.4) |
| Ceftazidime | 1475 | 256 (4.2) | 1190 | 256 (2.9) | 1142 | 256 (3.5) | 892 | 256 (2.4) | 870 | 256 (5.8) | 620 | 256 (3.4) |
| Ceftazidime–avibactam | 1328 | 256 (66.0) | 1071 | 256 (64.3) | 1044 | 256 (51.8) | 820 | 256 (48.8) | 779 | 256 (59.1) | 560 | 256 (54.6) |
| Ceftriaxone | 465 | 64 (0.9) | 357 | 64 (0.6) | 331 | 64 (1.2) | 234 | 64 (0.9) | 303 | 64 (1.0) | 198 | 64 (1.0) |
| Ciprofloxacin | 1010 | 8 (4.2) | 833 | 8 (3.8) | 811 | 8 (4.3) | 658 | 8 (3.3) | 567 | 8 (7.2) | 422 | 8 (7.8) |
| Colistin e,f | 1248 | 16 (NA) | 1015 | 16 (82.0) | 931 | 16 (NA) | 743 | 16 (83.2) | 722 | 8 (NA) | 519 | 16 (83.2) |
| Gentamicin | 1010 | 32 (31.5) | 833 | 32 (27.1) | 811 | 32 (26.4) | 658 | 32 (21.7) | 567 | 32 (36.0) | 422 | 32 (30.3) |
| Imipenem g | 1328 | 16 (1.9) | 1071 | 16 (4.8) | 1044 | 16 (3.3) | 820 | 16 (6.5) | 779 | 16 (3.2) | 560 | 16 (NA) |
| Levofloxacin | 1475 | 16 (6.9) | 1190 | 16 (7.0) | 1142 | 16 (7.5) | 892 | 16 (8.7) | 870 | 16 (11.3) | 620 | 16 (12.6) |
| Meropenem | 1475 | 32 (0) | 1190 | 32 (0) | 1142 | 32 (0) | 892 | 32 (0) | 870 | 32 (0) | 620 | 32 (0) |
| Piperacillin–tazobactam | 1475 | 256 (1.3) | 1190 | 256 (0.7) | 1142 | 256 (1.3) | 892 | 256 (0.7) | 870 | 256 (1.2) | 620 | 256 (0.3) |
| Tigecycline h,i,j | 1448 | 2 (93.1) | 1177 | 2 (76.4) | 1049 | 2 (94.7) | 827 | 2 (87.3) | 825 | 2 (92.6) | 589 | 2 (83.7) |
| MBL-positive (N = 1877) | ||||||||||||
| Aztreonam-avibactam d | 423 | 0.5 (99.5) | 423 | 0.5 (99.5) | 468 | 2 (98.7) | 468 | 2 (98.7) | 316 | 0.5 (99.7) | 316 | 0.5 (99.7) |
| Aztreonam | 436 | 256 (15.1) | 436 | 256 (15.1) | 483 | 256 (24.4) | 483 | 256 (24.4) | 325 | 256 (19.7) | 325 | 256 (19.7) |
| Amikacin | 436 | 128 (45.9) | 436 | 128 (35.3) | 487 | 128 (45.2) | 487 | 128 (37.0) | 327 | 128 (53.8) | 327 | 128 (39.5) |
| Cefepime | 436 | 64 (0) | 436 | 64 (0.7) | 487 | 64 (1.2) | 487 | 64 (3.5) | 327 | 64 (0.9) | 327 | 64 (2.1) |
| Ceftazidime | 436 | 256 (0) | 436 | 256 (0) | 487 | 256 (0) | 487 | 256 (0) | 327 | 256 (0) | 327 | 256 (0) |
| Ceftazidime–avibactam | 436 | 256 (2.1) | 436 | 256 (2.1) | 483 | 256 (1.5) | 483 | 256 (1.5) | 325 | 256 (4.9) | 325 | 256 (4.9) |
| Ceftriaxone | 78 | 32 (0) | 78 | 32 (0) | 74 | 32 (0) | 74 | 32 (0) | 78 | 32 (0) | 78 | 32 (0) |
| Ciprofloxacin | 358 | 8 (5.6) | 358 | 8 (8.7) | 413 | 8 (5.1) | 413 | 8 (8.0) | 249 | 8 (6.4) | 249 | 8 (10.0) |
| Colistin e,f | 398 | 4 (NA) | 398 | 4 (89.7) | 403 | 4 (NA) | 403 | 4 (89.8) | 284 | 1 (NA) | 284 | 1 (94.7) |
| Gentamicin | 358 | 32 (27.7) | 358 | 32 (26.5) | 413 | 32 (22.5) | 413 | 32 (21.1) | 249 | 32 (32.5) | 249 | 32 (31.3) |
| Imipenem g | 436 | 16 (1.6) | 436 | 16 (10.1) | 483 | 16 (0.8) | 483 | 16 (NA) | 325 | 16 (1.5) | 325 | 16 (NA) |
| Levofloxacin | 436 | 16 (11.0) | 436 | 16 (16.7) | 487 | 16 (9.5) | 487 | 16 (17.0) | 327 | 16 (12.5) | 327 | 16 (21.4) |
| Meropenem | 436 | 32 (4.6) | 436 | 32 (22.3) | 487 | 32 (3.9) | 487 | 32 (22.0) | 327 | 32 (3.7) | 327 | 32 (28.4) |
| Piperacillin–tazobactam | 436 | 256 (1.8) | 436 | 256 (1.8) | 487 | 256 (1.4) | 487 | 256 (1.4) | 327 | 256 (0.9) | 327 | 256 (0.9) |
| Tigecycline h,i,j | 410 | 2 (94.2) | 410 | 2 (91.7) | 416 | 2 (94.7) | 416 | 2 (91.8) | 289 | 2 (93.1) | 289 | 2 (95.5) |
| a Includes Enterobacter cloacae, Enterobacter hormaechi, Enterobacter kobei, Enterobacter ludwigii, Enterobacter asburiae, Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella aerogenes, Citrobacter koseri, Citrobacter freundii, Morganella morganii, Serratia marcescens, Proteus mirabilis, Citrobacter amalonaticus, Citrobacter braakii, Citrobacter farmer, Citrobacter spp., Enterobacter bugandensis, Enterobacter xiangfangensis, Klebsiella variicola, Proteus hauseri, Proteus vulgaris, Providencia alcalifaciens, Providencia rettgeri, Providencia spp., Providencia stuartii, Raoultella ornithinolytica. b Not all drugs in the panel were tested every year. c Data include percentage isolates susceptible at increased exposure. d No breakpoints available from CLSI and EUCAST. Values expressed are indicative of the cumulative percentage of isolates inhibited at ≤8 mg/L for comparison purposes. e Susceptible category for colistin not available for CLSI breakpoints (only intermediate and resistant isolates are available). f Data for colistin do not include isolates of Morganella morganii, Proteus hauseri, Proteus mirabilis, Proteus vulgaris, Providencia alcalifaciens, Providencia rettgeri, Providencia spp., Providencia stuartii, and Serratia marcescens because of their intrinsic resistance. g Data for imipenem not available per EUCAST. h Data for tigecycline do not include isolates of Morganella morganii, Proteus hauseri, Proteus mirabilis, Proteus vulgaris, Providencia alcalifaciens, Providencia rettgeri, Providencia spp., and Providencia stuartii due to their intrinsic resistance. i Data for tigecycline were calculated based on FDA-approved breakpoints for CLSI. j EUCAST data for susceptibility to tigecycline are limited to E. coli and C. Koseri; denominator (n): MDR: RTI = 1940, UTI = 4113, SSTI = 2498; ESBL: RTI = 1023, UTI = 2179, SSTI = 1432; CRE: RTI = 55, UTI = 102, SSTI = 49; MBL-positive: RTI = 36, UTI = 85, SSTI = 44. MIC, minimum inhibitory concentration; N, total number of isolates; n, number of isolates from infection sources; NA, not available; RTI, respiratory tract infection; SSTI, skin and soft tissue infection; UTI, urinary tract infection. | ||||||||||||
| BSI | IAI | |||||||||||
| CLSI | EUCAST | CLSI | EUCAST | |||||||||
| All Enterobacterales (N = 116,602) a | n b | MIC90 (µg/mL) (% S) | n b | MIC90 (mg/L) (% S c) | n b | MIC90 (µg/mL) (% S) | n b | MIC90 (mg/L) (% S c) | ||||
| MDR (CLSI/EUCAST, N = 36,305/39,700) | ||||||||||||
| Aztreonam-avibactam d | 7004 | 0.5 (99.8) | 7600 | 0.25 (99.8) | 4574 | 0.5 (99.9) | 4994 | 0.5 (99.9) | ||||
| Aztreonam | 7364 | 128 (25.9) | 7990 | 128 (31.1) | 4837 | 128 (26.6) | 5279 | 128 (31.5) | ||||
| Amikacin | 7836 | 32 (89.2) | 8651 | 16 (85.0) | 5212 | 16 (92.3) | 5728 | 16 (88.1) | ||||
| Cefepime | 7836 | 64 (30.4) | 8651 | 64 (39.6) | 5212 | 64 (36.4) | 5728 | 64 (46.3) | ||||
| Ceftazidime | 7836 | 256 (27.5) | 8651 | 128 (32.1) | 5212 | 256 (29.9) | 5728 | 256 (34.5) | ||||
| Ceftazidime–avibactam | 7364 | 2 (93.4) | 7990 | 2 (93.9) | 4837 | 2 (95.2) | 5279 | 2 (95.6) | ||||
| Ceftriaxone | 1529 | 64 (8.6) | 1812 | 64 (13.5) | 1907 | 64 (10.2) | 2131 | 64 (16.2) | ||||
| Ciprofloxacin | 6307 | 8 (22.7) | 6839 | 8 (31.9) | 3305 | 8 (29.3) | 3597 | 8 (38.2) | ||||
| Colistin e,f | 6821 | 1 (NA) | 7403 | 1 (94.8) | 4536 | 1 (NA) | 4976 | 1 (95.4) | ||||
| Gentamicin | 6307 | 32 (54.5) | 6839 | 32 (56.0) | 3305 | 32 (62.5) | 3597 | 32 (63.3) | ||||
| Imipenem g | 7364 | 16 (76.2) | 7990 | 16 (NA) | 4837 | 8 (79.6) | 5279 | 8 (NA) | ||||
| Levofloxacin | 7836 | 16 (29.8) | 8651 | 16 (40.9) | 5212 | 16 (34.3) | 5728 | 16 (44) | ||||
| Meropenem | 7836 | 32 (81.4) | 8651 | 16 (87.3) | 5212 | 16 (84.6) | 5728 | 8 (90.4) | ||||
| Piperacillin–tazobactam | 7836 | 128 (46.4) | 8651 | 128 (48.8) | 5212 | 256 (46) | 5728 | 256 (48.2) | ||||
| Tigecycline h,i,j | 7414 | 2 (96.9) | 8196 | 1 (96.5) | 4940 | 1 (96.7) | 5453 | 1 (95.8) | ||||
| ESBL (N = 20,303) | ||||||||||||
| Aztreonam-avibactam b | 3433 | 0.25 (99.9) | 3433 | 0.25 (99.9) | 2397 | 0.25 (99.9) | 2397 | 0.25 (99.9) | ||||
| Aztreonam | 3560 | 256 (6.0) | 3560 | 256 (6.0) | 2458 | 256 (10.0) | 2458 | 256 (10.0) | ||||
| Amikacin | 4227 | 32 (88.5) | 4227 | 32 (83.1) | 2855 | 16 (91.2) | 2855 | 16 (85.9) | ||||
| Cefepime | 4227 | 64 (7.1) | 4227 | 64 (12.2) | 2855 | 64 (13.7) | 2855 | 64 (19.4) | ||||
| Ceftazidime | 4227 | 256 (14.5) | 4227 | 256 (14.5) | 2855 | 256 (18.1) | 2855 | 256 (18.1) | ||||
| Ceftazidime–avibactam | 3560 | 4 (90.4) | 3560 | 4 (90.4) | 2458 | 2 (93.7) | 2458 | 2 (93.7) | ||||
| Ceftriaxone | 1530 | 64 (2.4) | 1530 | 64 (3.5) | 1581 | 64 (3.9) | 1581 | 64 (4.9) | ||||
| Ciprofloxacin | 2697 | 8 (10.8) | 2697 | 8 (15.9) | 1274 | 8 (12.6) | 1274 | 8 (18.3) | ||||
| Colistin e,f | 3469 | 1 (NA) | 3469 | 1 (95.2) | 2411 | 1 (NA) | 2411 | 1 (95.8) | ||||
| Gentamicin | 2697 | 32 (44.4) | 2697 | 32 (43.5) | 1274 | 32 (51.3) | 1274 | 32 (50.2) | ||||
| Imipenem g | 3560 | 16 (75.8) | 3560 | 16 (NA) | 2458 | 8 (80.2) | 2458 | 8 (NA) | ||||
| Levofloxacin | 4227 | 16 (21.2) | 4227 | 16 (30.6) | 2855 | 16 (24.6) | 2855 | 16 (32) | ||||
| Meropenem | 4227 | 32 (79.4) | 4227 | 32 (84.8) | 2855 | 16 (83.2) | 2855 | 16 (88.8) | ||||
| Piperacillin–tazobactam | 4227 | 128 (48.7) | 4227 | 128 (48.7) | 2855 | 256 (51.5) | 2855 | 256 (51.5) | ||||
| Tigecycline h,i,j | 4150 | 2 (97.1) | 4150 | 2 (98.1) | 2812 | 1 (97.0) | 2812 | 1 (97.1) | ||||
| CRE (CLSI/EUCAST, N = 5576/4388) | ||||||||||||
| Aztreonam-avibactam b | 1080 | 0.5 (99.3) | 882 | 0.5 (99.4) | 549 | 1 (99.8) | 425 | 1 (99.8) | ||||
| Aztreonam | 1161 | 256 (8.4) | 959 | 256 (7.3) | 591 | 256 (6.9) | 463 | 256 (6.1) | ||||
| Amikacin | 1328 | 128 (56.3) | 1096 | 128 (37.9) | 706 | 128 (63.2) | 551 | 128 (41.7) | ||||
| Cefepime | 1328 | 64 (1.8) | 1096 | 64 (2.0) | 706 | 64 (2.0) | 551 | 64 (1.6) | ||||
| Ceftazidime | 1328 | 256 (3.8) | 1096 | 256 (3.2) | 706 | 256 (2.3) | 551 | 256 (1.6) | ||||
| Ceftazidime–avibactam | 1161 | 256 (60.6) | 959 | 256 (59.7) | 591 | 256 (66.3) | 463 | 256 (65) | ||||
| Ceftriaxone | 320 | 64 (0) | 254 | 64 (0.4) | 286 | 64 (0) | 227 | 64 (0.4) | ||||
| Ciprofloxacin | 1008 | 8 (7.2) | 842 | 8 (7.4) | 420 | 8 (7.4) | 324 | 8 (5.9) | ||||
| Colistin e,f | 1076 | 16 (NA) | 899 | 16 (83.0) | 566 | 16 (NA) | 451 | 16 (82.0) | ||||
| Gentamicin | 1008 | 32 (34.0) | 842 | 32 (30.3) | 420 | 32 (36.7) | 324 | 32 (32.7) | ||||
| Imipenem g | 1161 | 16 (1.7) | 959 | 16 (5.2) | 591 | 16 (2.9) | 463 | 16 (4.3) | ||||
| Levofloxacin | 1328 | 16 (10.1) | 1096 | 16 (11.3) | 706 | 16 (8.6) | 551 | 16 (8.7) | ||||
| Meropenem | 1328 | 32 (0) | 1096 | 32 (0) | 706 | 32 (0) | 551 | 32 (0) | ||||
| Piperacillin–tazobactam | 1328 | 256 (0.5) | 1096 | 256 (0.4) | 706 | 256 (0.9) | 551 | 256 (0.7) | ||||
| Tigecycline h,i,j | 1284 | 2 (94.1) | 1069 | 2 (89.4) | 689 | 2 (93.2) | 545 | 2 (83.7) | ||||
| MBL-positive (N = 1877) | ||||||||||||
| Aztreonam-avibactam b | 398 | 0.5 (99.5) | 398 | 0.5 (99.5) | 188 | 1 (99.5) | 188 | 1 (99.5) | ||||
| Aztreonam | 426 | 256 (15.7) | 426 | 256 (15.7) | 190 | 256 (15.8) | 190 | 256 (15.8) | ||||
| Amikacin | 430 | 128 (45.1) | 430 | 128 (35.4) | 193 | 128 (59.1) | 193 | 128 (50.3) | ||||
| Cefepime | 430 | 64 (0) | 430 | 64 (0.5) | 193 | 64 (3.6) | 193 | 64 (5.2) | ||||
| Ceftazidime | 430 | 256 (0) | 430 | 256 (0) | 193 | 256 (0.5) | 193 | 256 (0.5) | ||||
| Ceftazidime–avibactam | 426 | 256 (1.2) | 426 | 256 (1.2) | 190 | 256 (2.1) | 190 | 256 (2.1) | ||||
| Ceftriaxone | 44 | 32 (0) | 44 | 32 (0) | 52 | 32 (0) | 52 | 32 (0) | ||||
| Ciprofloxacin | 386 | 8 (9.3) | 386 | 8 (13.2) | 141 | 8 (7.8) | 141 | 8 (10.6) | ||||
| Colistin e,f | 372 | 2 (NA) | 372 | 2 (92.2) | 179 | 8 (NA) | 179 | 8 (89.4) | ||||
| Gentamicin | 386 | 32 (28.5) | 386 | 32 (25.4) | 141 | 32 (36.2) | 141 | 32 (33.3) | ||||
| Imipenem g | 426 | 16 (0.5) | 426 | 16 (NA) | 190 | 16 (2.1) | 190 | 16 (NA) | ||||
| Levofloxacin | 430 | 16 (15.1) | 430 | 16 (24.2) | 193 | 16 (11.4) | 193 | 16 (18.1) | ||||
| Meropenem | 430 | 32 (2.3) | 430 | 32 (19.3) | 193 | 32 (5.7) | 193 | 32 (30.1) | ||||
| Piperacillin–tazobactam | 430 | 128 (0.7) | 430 | 128 (0.7) | 193 | 256 (0.5) | 193 | 256 (0.5) | ||||
| Tigecycline h,i,j | 389 | 2 (95.1) | 389 | 2 (95.7) | 183 | 2 (91.8) | 183 | 2 (87.0) | ||||
| a Includes Enterobacter cloacae, Enterobacter hormaechi, Enterobacter kobei, Enterobacter ludwigii, Enterobacter asburiae, Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella aerogenes, Citrobacter koseri, Citrobacter freundii, Morganella morganii, Serratia marcescens, Proteus mirabilis, Citrobacter amalonaticus, Citrobacter braakii, Citrobacter farmer, Citrobacter spp., Enterobacter bugandensis, Enterobacter xiangfangensis, Klebsiella variicola, Proteus hauseri, Proteus vulgaris, Providencia alcalifaciens, Providencia rettgeri, Providencia spp., Providencia stuartii, Raoultella ornithinolytica. b Not all drugs in the panel were tested every year. c Data include percentage isolates susceptible at increased exposure. d No breakpoints available from CLSI and EUCAST. Values expressed are indicative of the cumulative percentage of isolates inhibited at ≤8 mg/L for comparison purposes. e Susceptible category for colistin not available for CLSI breakpoints (only intermediate and resistant isolates are available). f Data for colistin do not include isolates of Morganella morganii, Proteus hauseri, Proteus mirabilis, Proteus vulgaris, Providencia alcalifaciens, Providencia rettgeri, Providencia spp., Providencia stuartii, and Serratia marcescens because of their intrinsic resistance. g Data for imipenem not available per EUCAST. h Data for tigecycline do not include isolates of Morganella morganii, Proteus hauseri, Proteus mirabilis, Proteus vulgaris, Providencia alcalifaciens, Providencia rettgeri, Providencia spp., and Providencia stuartii due to their intrinsic resistance. i Data for tigecycline were calculated based on FDA approved breakpoints for CLSI. j EUCAST data for susceptibility to tigecycline are limited to E. coli and C. koseri; denominator (n): MDR: BSI = 3665, IAI = 2731; ESBL: BSI = 1711, IAI = 1428; CRE: BSI = 66, IAI = 43; MBL-positive: BSI = 47, IAI = 23. BSI, bloodstream infections; IAI, intra-abdominal infection; MIC, minimum inhibitory concentration; N, total number of isolates; n, number of isolates from infection sources; NA, not available. | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by Pfizer Inc. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piérard, D.; Hermsen, E.D.; Kantecki, M.; Arhin, F.F. Antimicrobial Activities of Aztreonam-Avibactam and Comparator Agents against Enterobacterales Analyzed by ICU and Non-ICU Wards, Infection Sources, and Geographic Regions: ATLAS Program 2016–2020. Antibiotics 2023, 12, 1591. https://doi.org/10.3390/antibiotics12111591
Piérard D, Hermsen ED, Kantecki M, Arhin FF. Antimicrobial Activities of Aztreonam-Avibactam and Comparator Agents against Enterobacterales Analyzed by ICU and Non-ICU Wards, Infection Sources, and Geographic Regions: ATLAS Program 2016–2020. Antibiotics. 2023; 12(11):1591. https://doi.org/10.3390/antibiotics12111591
Chicago/Turabian StylePiérard, Denis, Elizabeth D. Hermsen, Michal Kantecki, and Francis F. Arhin. 2023. "Antimicrobial Activities of Aztreonam-Avibactam and Comparator Agents against Enterobacterales Analyzed by ICU and Non-ICU Wards, Infection Sources, and Geographic Regions: ATLAS Program 2016–2020" Antibiotics 12, no. 11: 1591. https://doi.org/10.3390/antibiotics12111591
APA StylePiérard, D., Hermsen, E. D., Kantecki, M., & Arhin, F. F. (2023). Antimicrobial Activities of Aztreonam-Avibactam and Comparator Agents against Enterobacterales Analyzed by ICU and Non-ICU Wards, Infection Sources, and Geographic Regions: ATLAS Program 2016–2020. Antibiotics, 12(11), 1591. https://doi.org/10.3390/antibiotics12111591






