Enhancing Medical Students’ Confidence and Knowledge in Antibiotic Prescription and Administration through Virtual Education: A Quasi-Experimental Study
Abstract
:1. Introduction
2. Results
2.1. Participants’ Demographic Characteristics
2.2. Medical Students’ Confidence in Antibiotic Prescription and Administration
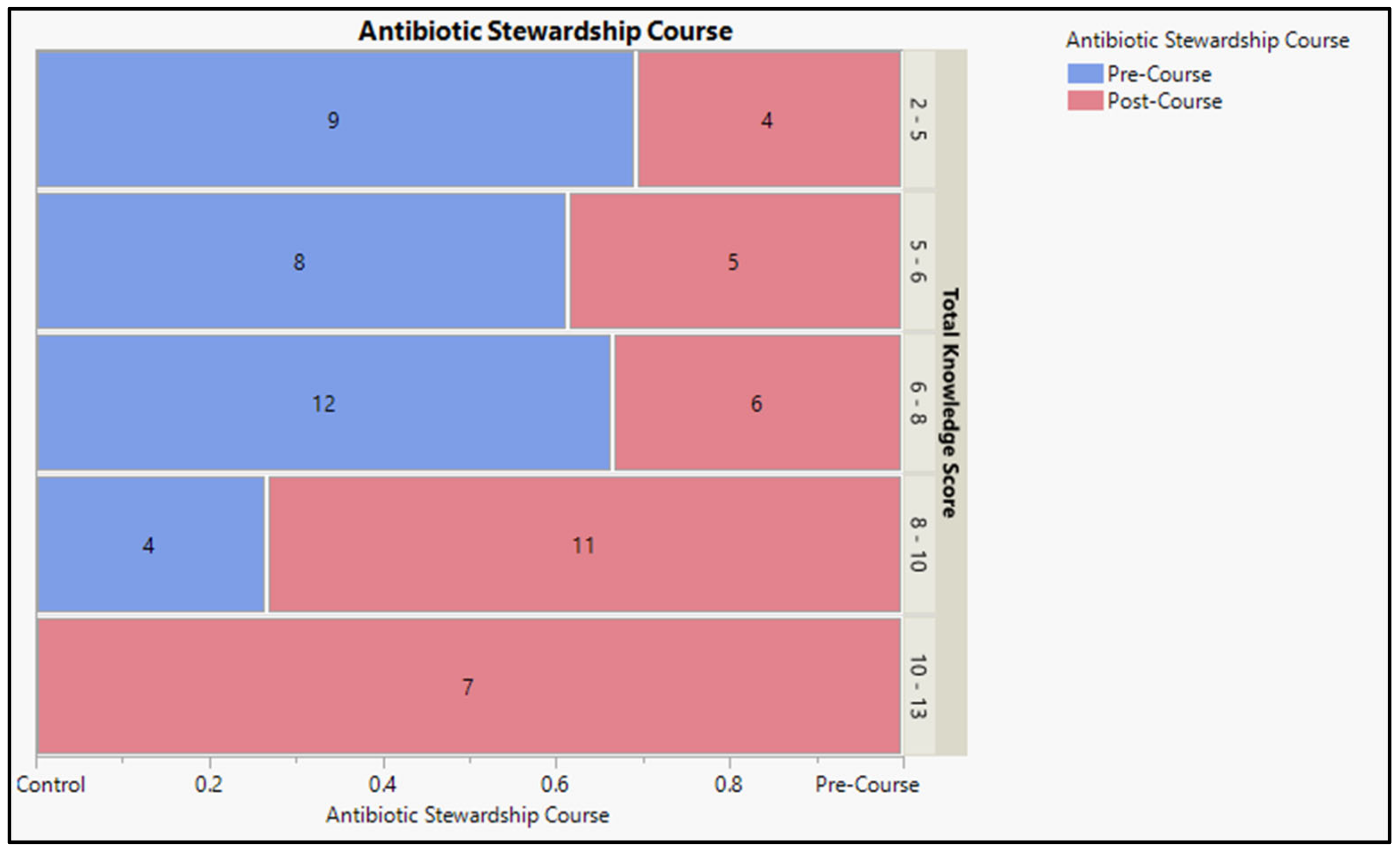
2.3. Medical Students’ Knowledge Associated with the Prescribing and Administering of Antibacterial Agents
2.3.1. Pre-Course Assessment Report
2.3.2. Post-Course Assessment Report
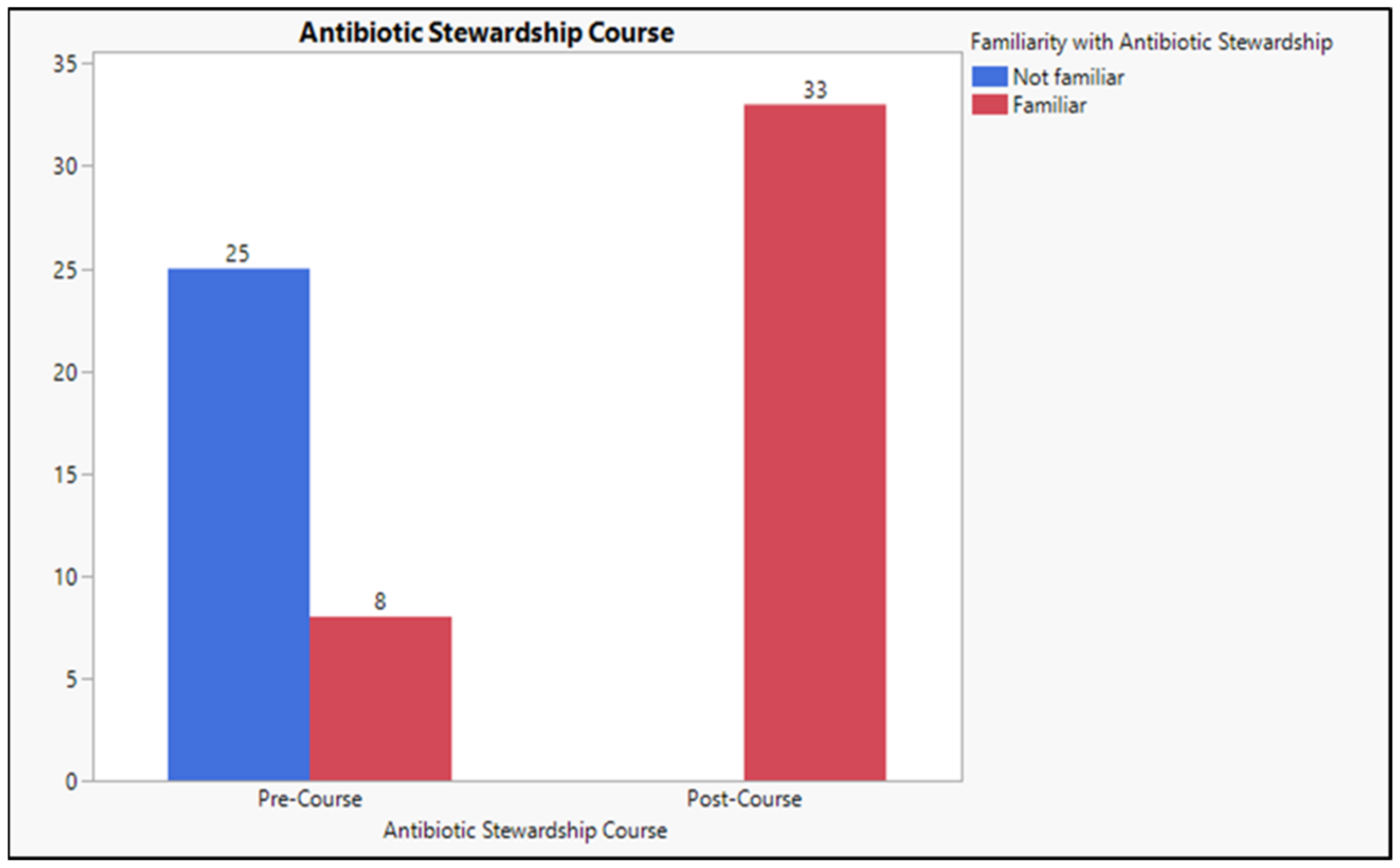
2.4. The Level of Familiarity with the “Antibiotic Stewardship” Term
2.5. Association of Confidence, Knowledge before and after the Antibiotic Stewardship Course
3. Materials and Methods
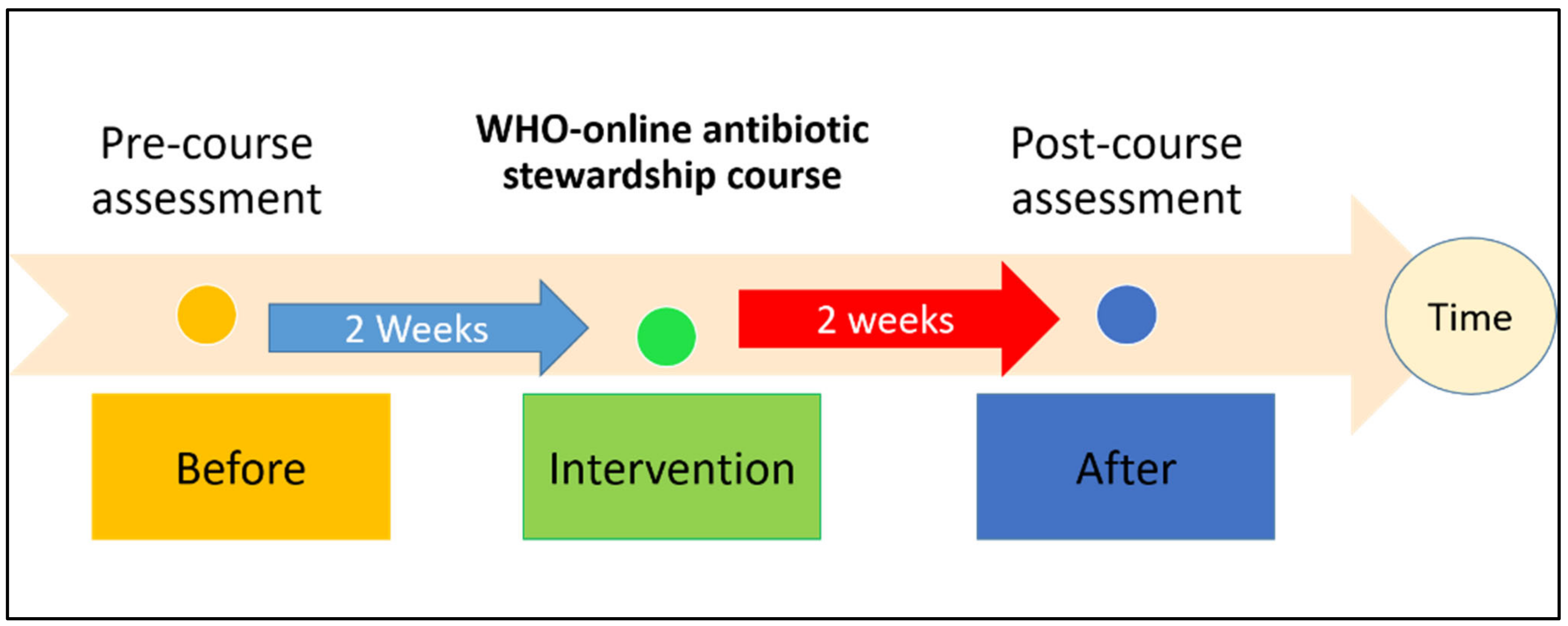
3.1. Study Design and Setting
3.2. Sample Size and Participant Sampling
3.3. WHO Antimicrobial Stewardship Course
3.4. Data Collection Tools
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sukumaran, V.; Senanayake, S. Bacterial skin and soft tissue infections. Aust. Prescr. 2016, 39, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Kissoon, N.; Limmathurotsakul, D.; Bory, S.; Mutahunga, B.; Seymour, C.W.; Angus, D.C.; West, T.E. The global burden of sepsis: Barriers and potential solutions. Crit. Care 2018, 22, 123305059. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, J.; Tabor, D.E.; Silver, J.S.; Nair, V.; Tovchigrechko, A.; Griffin, M.P.; Esser, M.T.; Sellman, B.; Jin, H. Microbial burden and viral exacerbations in a longitudinal multicenter COPD cohort. Respir. Res. 2020, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, N.P.; Acharya, S.P.; Mishra, S.K.; Parajuli, K.; Rijal, B.P.; Pokhrel, B.M. High burden of antimicrobial resistance among gram-negative bacteria causing healthcare-associated infections in a critical care unit of Nepal. Antimicrob. Resist. Infect. Control 2017, 6, 67. [Google Scholar] [CrossRef]
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.R.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.Z.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Ausubel, J.H.; Meyer, P.S.; Wernick, I.K. Death and the human environment: The United States in the 20th century. Technol. Soc. 2001, 23, 131–146. [Google Scholar] [CrossRef]
- Gandra, S.; Tseng, K.K.; Arora, A.; Bhowmik, B.; Robinson, M.L.; Panigrahi, B.; Laxminarayan, R.; Klein, E.Y. The Mortality Burden of Multidrug-resistant Pathogens in India: A Retrospective, Observational Study. Clin. Infect. Dis. 2019, 69, 563–570. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Dittmar, F. Re: Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Eur. Urol. 2022, 82, 658. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Klein, E.Y.; Levin, S.A.; Laxminarayan, R. Reply to Abat et al.: Improved policies necessary to ensure an effective future for antibiotics. Proc. Natl. Acad. Sci. USA 2018, 115, E8111–E8112. [Google Scholar] [PubMed]
- Fishman, N. Policy Statement on Antimicrobial Stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect. Control Hosp. Epidemiol. 2012, 33, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Auta, A. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef]
- Laxminarayan, R. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Alattas, H.A.; Alyami, S.H. Prescription of antibiotics for pulpal and periapical pathology among dentists in southern Saudi Arabia. J. Glob. Antimicrob. Resist. 2017, 9, 82–84. [Google Scholar] [CrossRef]
- Skodvin, B.; Aase, K.; Charani, E.; Holmes, A.; Smith, I. An antimicrobial stewardship program initiative: A qualitative study on prescribing practices among hospital doctors. Antimicrob. Resist. Infect. Control 2015, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Tebano, G.; Dyar, O.J.; Beovic, B.; Béraud, G.; Thilly, N.; Pulcini, C. Defensive medicine among antibiotic stewards: The international ESCMID AntibioLegalMap survey. J. Antimicrob. Chemother. 2018, 73, 1989–1996. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Silverberg, S.L.; Zannella, V.E.; Countryman, D.; Ayala, A.P.; Lenton, E.; Friesen, F.; Law, M. A review of antimicrobial stewardship training in medical education. Int. J. Med. Educ. 2017, 8, 353–374. [Google Scholar] [CrossRef]
- Liu, J.; Li, N.; Hao, J.; Li, Y.; Liu, A.; Wu, Y.; Cai, M. Impact of the Antibiotic Stewardship Program on Prevention and Control of Surgical Site Infection during Peri-Operative Clean Surgery. Surg. Infect. 2018, 19, 326–333. [Google Scholar] [CrossRef]
- El-Sokkary, R.H.; Badran, S.G.; El Seifi, O.S.; El-Fakharany, Y.M.; Tash, R.M.E. “Antibiotic prescribing etiquette” an elective course for medical students: Could we recruit potential physicians to fight resistance? BMC Med. Educ. 2023, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.A.R.; van de Pol, A.C.; Heltveit-Olsen, S.R.; Lindbæk, M.; Høye, S.; Lithén, S.S.; Sundvall, P.-D.; Sundvall, S.; Arnljots, E.S.; Gunnarsson, R.; et al. Effect of a Multifaceted Antibiotic Stewardship Intervention to Improve Antibiotic Prescribing for Suspected Urinary Tract Infections in Frail Older Adults (ImpresU): Pragmatic Cluster Randomised Controlled Trial in Four European Countries. BMJ 2023, 380, e072319. [Google Scholar] [CrossRef] [PubMed]
- Calò, F.; Onorato, L.; Macera, M.; Di Caprio, G.; Monari, C.; Russo, A.; Galdieri, A.; Giordano, A.; Cuccaro, P.; Coppola, N. Impact of an Education-Based Antimicrobial Stewardship Program on the Appropriateness of Antibiotic Prescribing: Results of a Multicenter Observational Study. Antibiotics 2021, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Morency-Potvin, P.; Schwartz, D.N.; Weinstein, R.A. Antimicrobial stewardship: How the microbiology laboratory can right the ship. Clin. Microbiol. Rev. 2017, 30, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Peebles, K.; Velloza, J.; Balkus, J.E.; McClelland, R.S.; Barnabas, R.V. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2019, 46, 304–311. [Google Scholar] [CrossRef]
- Prevel, R.; Berdaï, D.; Boyer, A. Antibiotics for Ceftriaxone-Resistant Gram-Negative Bacterial Bloodstream Infections. JAMA 2019, 321, 613. [Google Scholar] [CrossRef]
- Antimicrobial Stewardship: A Competency-Based Approach|OpenWHO n.d. Available online: https://openwho.org/courses/AMR-competency (accessed on 16 September 2020).
- Hsu, J.L. Building an Antibiotic Stewardship Program: An Interactive Teaching Module for Medical Students. MedEdPORTAL 2018, 14, 10726. [Google Scholar] [CrossRef]
- Abbo, L.M.; Cosgrove, S.E.; Pottinger, P.S.; Pereyra, M.; Sinkowitz-Cochran, R.; Srinivasan, A.; Webb, D.J.; Hooton, T.M. Medical students’ perceptions and knowledge about antimicrobial stewardship: How are we educating our future prescribers? Clin. Infect. Dis. 2013, 57, 631–638. [Google Scholar] [CrossRef]
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.A.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Wound 2021, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; McGregor, J.C.; Perencevich, E.N.; Furuno, J.P.; Zhu, J.; Peterson, D.E.; Finkelstein, J. The Use and Interpretation of Quasi-Experimental Studies in Medical Informatics. J. Am. Med. Inform. Assoc. 2006, 13, 16–23. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Ha, D.R.; Haste, N.M.; Gluckstein, D.P. The Role of Antibiotic Stewardship in Promoting Appropriate Antibiotic Use. Am. J. Lifestyle Med. 2017, 13, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Weier, N.; Thursky, K.; Zaidi, S.T.R. Antimicrobial Knowledge and Confidence amongst Final Year Medical Students in Australia. PLoS ONE 2017, 12, e0182460. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Smith, S.N.; Pugatch, M. Experimental and Quasi-Experimental Designs in Implementation Research. Psychiatry Res. 2020, 283, 112452. [Google Scholar] [CrossRef] [PubMed]
- Knapp, T.R. Why Is the One-Group Pre-test–Post-test Design Still Used? Clin. Nurs. Res. 2016, 25, 467–472. [Google Scholar] [CrossRef] [PubMed]

| Demographic Variables | N | (%) |
|---|---|---|
| Age (mean + SD) years | 22.6 ± 2.17 | |
| Gender | ||
| Male | 15 | 45.5% |
| Female | 18 | 54.6% |
| How Confident Do You Feel in the Following Scenarios When Prescribing an Antibiotic by Yourself? | Confidence Level | Antibiotic Stewardship | |||
|---|---|---|---|---|---|
| Pre-Course | Post-Course | ||||
| N | % | N | % | ||
| Unconfident | 12 | 36.4 | 3 | 9.1 |
| Unsure | 11 | 33.3 | 8 | 24.2 | |
| Confident | 10 | 30.3% | 22 | 66.7 | |
| Unconfident | 9 | 27.3 | 2 | 6.1 |
| Unsure | 11 | 33.3 | 7 | 21.2 | |
| Confident | 13 | 39.4 | 24 | 72.7 | |
| Unconfident | 14 | 42.4 | 7 | 21.2 |
| Unsure | 9 | 27.3 | 9 | 27.3 | |
| Confident | 10 | 30.3 | 17 | 51.5 | |
| Unconfident | 17 | 51.5 | 6 | 18.2 |
| Unsure | 14 | 42.4 | 13 | 39.4 | |
| Confident | 2 | 6.1 | 14 | 42.4 | |
| Unconfident | 16 | 48.5 | 3 | 9.1 |
| Unsure | 9 | 27.3 | 11 | 33.3 | |
| Confident | 8 | 24.2 | 19 | 57.6 | |
| Unconfident | 24 | 72.7 | 10 | 30.3 |
| Unsure | 7 | 21.2 | 15 | 45.5 | |
| Confident | 2 | 6.1 | 8 | 24.2 | |
| Unconfident | 21 | 63.6 | 7 | 21.2 |
| Unsure | 9 | 27.3 | 11 | 33.3 | |
| Confident | 3 | 9.1 | 15 | 45.5 | |
| Unconfident | 24 | 72.7 | 8 | 24.2 |
| Unsure | 6 | 18.2 | 13 | 39.4 | |
| Confident | 3 | 9.1 | 12 | 364 | |
| Unconfident | 24 | 72.7 | 8 | 24.2 |
| Unsure | 7 | 21.2 | 11 | 33.3 | |
| Confident | 2 | 6.1 | 14 | 42.4 | |
| Unconfident | 27 | 81.8 | 5 | 15.2 |
| Unsure | 4 | 12.1 | 12 | 36.4 | |
| Confident | 2 | 6.1 | 16 | 48.5 | |
| Unconfident | 12 | 36.4 | 2 | 6.1 |
| Unsure | 10 | 30.3 | 5 | 15.2 | |
| Confident | 11 | 33.3 | 26 | 78.8 | |
| Total Confidence Level | Unconfident | 5 | 15.2 | 1 | 3.0 |
| Unsure | 23 | 69.7 | 8 | 24.2 | |
| Confident | 5 | 15.2 | 24 | 72.7 | |
| Please Answer the Following Case Scenarios Regarding Antimicrobial Use and Resistance | Score | Antibiotic Stewardship | ||||
|---|---|---|---|---|---|---|
| Pre-Course | Post-Course | |||||
| N | % | N | % | |||
| Case 1: Areej, a 39-year-old female with mild COPD, was managed with PRN salbutamol MDI. She has no allergies and is not pregnant or breastfeeding. She presented to her doctor with pain and stinging when urinating and symptoms of urgency. She is diagnosed with an acute, uncomplicated urinary tract infection, and her doctor decides to prescribe her trimethoprim 300 mg daily. Over the next two days, Areej developed a fever and became increasingly unwell. She was diagnosed as having sepsis and was admitted to hospital. | How long should this treatment continue?
| Wrong Answer | 22 | 66.7 | 19 | 57.6 |
| Correct Answer | 11 | 33.3 | 14 | 42.4 | ||
What would be the most appropriate treatment?
| Wrong Answer | 11 | 33.3 | 6 | 18.2 | |
| Correct Answer | 22 | 66.7 | 27 | 81.8 | ||
| Case 2: Sara is a 2-year-old girl with a weight of 12 kg who has presented with ear pain in her left ear, which started the night before. She is healthy despite a history of asthma, can still play and eat, and has no medication allergies. Upon examination, you diagnose her with acute otitis media. Two days later, Sara returns for review. Her ear pain is no better, and she feels tired and irritated as she hasn’t been able to sleep for the past few days. | What is the most appropriate initial course of treatment?
| Wrong Answer | 17 | 51.5 | 11 | 33.3 |
| Correct Answer | 16 | 48.5 | 22 | 66.7 | ||
How long should antibiotic treatment continue for Sara?
| Wrong Answer | 18 | 54.6 | 14 | 42.4 | |
| Correct Answer | 15 | 45.5 | 19 | 57.6 | ||
How long should antibiotic treatment continue for Sara?
| Wrong Answer | 29 | 87.9 | 20 | 60.6 | |
| Correct Answer | 4 | 12.1 | 13 | 39.4 | ||
| Case 3: Ahmad is a 61-year-old male with a history of asthma, COPD, hypertension, hyperlipidemia, and osteoarthritis. He presents to the hospital’s emergency department with breathlessness from any extra exertion over the last three days. He uses his salbutamol inhaler several times each hour and is not getting relief. Ahmad had increasing sputum purulence, and antibiotics were prescribed. While Ahmad was in the hospital, there was a worldwide shortage of rosuvastatin, and the hospital could not source it anywhere. A decision is made to prescribe simvastatin instead. After one week, Ahmad is feeling much better, and a decision is made to discharge him. | What would be the treatment of choice for Ahmad?
| Wrong Answer | 22 | 66.7 | 22 | 66.7 |
| Correct Answer | 11 | 33.3 | 11 | 33.3 | ||
At what time of day will it have its maximum efficacy?
| Wrong Answer | 22 | 66.7 | 15 | 45.5 | |
| Correct Answer | 11 | 33.3 | 18 | 54.6 | ||
What would not be a recommended medication for Ahmad?
| Wrong Answer | 21 | 63.6 | 16 | 48.5 | |
| Correct Answer | 12 | 36.4 | 17 | 51.5 | ||
| Case 4: Khalid is a 25-year-old male of nomadic origin. He lives a basic lifestyle, including cooking over campfires, bathing in the nearby river, and sleeping in makeshift tents. Several months ago, he had recovered from a high fever and had pain in multiple joints. He has presented again with the same symptoms, experiencing ongoing fatigue and dyspnea. He was diagnosed with rheumatic heart disease and developed atrial fibrillation. Several years have passed, Khalid is managing well. However, he had recently been to the dentist for a tooth extraction. | What would be the initial treatment choice if the goal is to obtain rate control?
| Wrong Answer | 18 | 54.5 | 12 | 36.4 |
| Correct Answer | 15 | 45.5 | 21 | 63.6 | ||
What would be the recommended antibiotic prophylactic?
| Wrong Answer | 28 | 84.8 | 17 | 51.5 | |
| Correct Answer | 5 | 15.2 | 16 | 48.5 | ||
What would your recommendation be if Khalid reported that he experienced an urticaria and bronchospasm reaction to benzylpenicillin in the past?
| Wrong Answer | 18 | 54.5 | 11 | 33.3 | |
| Correct Answer | 15 | 45.5 | 22 | 66.7 | ||
What would your treatment recommendation be if Khalid was having dental impressions and the construction of dentures done?
| Wrong Answer | 19 | 57.6 | 22 | 66.7 | |
| Correct Answer | 14 | 42.4 | 12 | 33.3 | ||
| Case 5: Mona is a 55-year-old woman complaining of diarrhea for the past three days. She had recently returned home from Vietnam, and since then, she has been feeling nauseous and feverish for several days before the diarrhea. She takes metformin 500 mg daily for type II diabetes and warfarin at night for deep vein thrombosis. | What would be the most appropriate management of Mona?
| Wrong Answer | 25 | 75.8 | 16 | 48.5 |
| Correct Answer | 8 | 24.2 | 17 | 51.5 | ||
What would your treatment recommendation be if Margaret had not reported any recent overseas travel?
| Wrong Answer | 24 | 72.7 | 22 | 66.7 | |
| Correct Answer | 9 | 27.3 | 11 | 33.3 | ||
Mona is also taking warfarin; what other advice would you give her at this stage?
| Wrong Answer | 24 | 72.7 | 20 | 60.6 | |
| Correct Answer | 9 | 27.3 | 13 | 39.4 | ||
| Pre/Post | Unconfident | Unsure | Confident | Test Value | p Value | ||
|---|---|---|---|---|---|---|---|
| Total Confidence Level | Confidence Level | Unconfident | 0 (0.00%) | 3 (9.09%) | 2 (6.06%) | Symmetry disagreement Z = 20 | 0.002 * |
| Unsure | 1 (3.03%) | 5 (15.15%) | 17 (51.52%) | ||||
| Confident | 0 (0.00%) | 0 (0.00%) | 5 (15.15%) | ||||
| Total knowledge score | Mean score | Pre-Course | Post-Course | t = 3.540 | <0.001 ** | ||
| 5.36 ± 1.74 | 7.66 ± 2.59 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malli, I.A.; Mohamud, M.S.; Al-Nasser, S. Enhancing Medical Students’ Confidence and Knowledge in Antibiotic Prescription and Administration through Virtual Education: A Quasi-Experimental Study. Antibiotics 2023, 12, 1546. https://doi.org/10.3390/antibiotics12101546
Malli IA, Mohamud MS, Al-Nasser S. Enhancing Medical Students’ Confidence and Knowledge in Antibiotic Prescription and Administration through Virtual Education: A Quasi-Experimental Study. Antibiotics. 2023; 12(10):1546. https://doi.org/10.3390/antibiotics12101546
Chicago/Turabian StyleMalli, Israa Abdullah, Mohamud Salaad Mohamud, and Sami Al-Nasser. 2023. "Enhancing Medical Students’ Confidence and Knowledge in Antibiotic Prescription and Administration through Virtual Education: A Quasi-Experimental Study" Antibiotics 12, no. 10: 1546. https://doi.org/10.3390/antibiotics12101546
APA StyleMalli, I. A., Mohamud, M. S., & Al-Nasser, S. (2023). Enhancing Medical Students’ Confidence and Knowledge in Antibiotic Prescription and Administration through Virtual Education: A Quasi-Experimental Study. Antibiotics, 12(10), 1546. https://doi.org/10.3390/antibiotics12101546






