Secondary and Co-Infections in Hospitalized COVID-19 Patients: A Multicenter Cross-Sectional Study in Malaysia
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Study Design
3.2. Data Source and Data Selection
3.3. Laboratory Confirmation
3.4. Disease Staging and Clinical Management
3.5. Study Variables and Outcome of Interest
3.6. Statistical Analyses
3.7. Study Ethics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Verweij, P.E.; Gangneux, J.-P.; Bassetti, M.; Brüggemann, R.J.; Cornely, O.A.; Koehler, P.; Lass-Flörl, C.; van de Veerdonk, F.L.; Chakrabarti, A.; Hoenigl, M. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e53–e55. [Google Scholar] [CrossRef]
- Duarte-Neto, A.N.; Monteiro, R.A.A.; da Silva, L.F.F.; Malheiros, D.; de Oliveira, E.P.; Theodoro-Filho, J.; Pinho, J.R.R.; Gomes-Gouvêa, M.S.; Salles, A.P.M.; de Oliveira, I.R.S.; et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology 2020, 77, 186–197. [Google Scholar] [CrossRef]
- Kaeuffer, C.; Le Hyaric, C.; Fabacher, T.; Mootien, J.; Dervieux, B.; Ruch, Y.; Hugerot, A.; Zhu, Y.J.; Pointurier, V.; Clere-Jehl, R.; et al. Clinical characteristics and risk factors associated with severe COVID-19: Prospective analysis of 1045 hospitalised cases in North-Eastern France, March 2020. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2020, 25, 2000895. [Google Scholar]
- Adler, H.; Ball, R.; Fisher, M.; Mortimer, K.; Vardhan, M.S. Low rate of bacterial co-infection in patients with COVID-19. Lancet Microbe 2020, 1, e62. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Bamford, C.G. SARS-CoV-2, bacterial co-infections, and AMR: The deadly trio in COVID-19? EMBO Mol. Med. 2020, 12, e12560. [Google Scholar] [CrossRef]
- De Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; De Pauw, I.; Stessel, B.; Dubois, J. Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation. BMC Infect. Dis. 2022, 22, 207. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Xie, J.; Wu, W.; Li, S.; Hu, Y.; Hu, M.; Li, J.; Yang, Y.; Huang, T.; Zheng, K.; Wang, Y. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: A retrospective multicenter study. Intensive Care Med. 2020, 46, 1863–1872. [Google Scholar] [CrossRef]
- Livingston, E.; Bucher, K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA 2020, 323, 1335. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chan, K.-G. Underestimation of co-infections in COVID-19 due to non-discriminatory use of antibiotics. J. Infect. 2020, 81, e29–e30. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Ng, C.F.S.; Seposo, X.T.; Moi, M.L.; Tajudin, M.A.B.A.; Madaniyazi, L.; Sahani, M. Characteristics of COVID-19 epidemic and control measures to curb transmission in Malaysia. Int. J. Infect. Dis. 2020, 101, 409–411. [Google Scholar] [CrossRef]
- Supramanian, R.K.; Sivaratnam, L.; Abd Rahim, A.; Abidin, N.D.I.Z.; Richai, O.; Zakiman, Z.; Taib, S.M.; Soo, L.; Jamalullai, S.H.S.I.; Khirusalleh, M.N.A. Descriptive epidemiology of the first wave of COVID-19 in Petaling District, Malaysia: Focus on asymptomatic transmission. West. Pac. Surveill. Response J. WPSAR 2021, 12, 82. [Google Scholar]
- Hashim, J.H.; Adman, M.A.; Hashim, Z.; Mohd Radi, M.F.; Kwan, S.C. COVID-19 epidemic in Malaysia: Epidemic progression, challenges, and response. Front. Public Health 2021, 9, 560592. [Google Scholar] [CrossRef]
- Ghazali, S.M.; Singh, S.; Zulkifli, A.A.; Cheong, Y.L.; Md Iderus, N.H.; Md Zamri, A.S.S.; Ahmad Jaafar, N.; Lai, C.H.; Wan Mohamed Noor, W.N.; Rusli, N.; et al. COVID-19 in Malaysia: Descriptive Epidemiologic Characteristics of the First Wave. Int. J. Environ. Res. Public Health 2022, 19, 3828. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Leung, V.; Raybardhan, S.; Lo, J.; Kan, T.; Leung, F.; Westwood, D.; Daneman, N.; MacFadden, D.R. Predictors and microbiology of respiratory and bloodstream bacterial infection in patients with COVID-19: Living rapid review update and meta-regression. Clin. Microbiol. Infect. 2022, 28, 491–501. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.; Mei, H.; Zheng, H.; Liang, G.; She, X.; Liu, W. Fungal co-infection in COVID-19 patients: Evidence from a systematic review and meta-analysis. Aging 2021, 13, 7745. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Soltani, S.; Zakeri, A.; Zandi, M.; Kesheh, M.M.; Tabibzadeh, A.; Dastranj, M.; Faramarzi, S.; Didehdar, M.; Hafezi, H.; Hosseini, P. The role of bacterial and fungal human respiratory microbiota in COVID-19 patients. BioMed. Res. Int. 2021, 2021, 6670798. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Abbas, A.; Abdukahil, S.A.; Abdulkadir, N.N.; Abe, R.; Abel, L.; Absil, L.; Jabal, K.A.; Zayyad, H.A.; Acharya, S.; Acker, A.; et al. ISARIC-COVID-19 dataset: A Prospective, Standardized, Global Dataset of Patients Hospitalized with COVID-19. Sci. Data 2022, 9, 454. [Google Scholar]
- Sharma, A.; Grover, P. Application of WHONET for the surveillance of antimicrobial resistance. Indian J. Med. Microbiol. 2004, 22, 115–118. [Google Scholar] [CrossRef]
- Cong, W.; Stuart, B.; AIhusein, N.; Liu, B.; Tang, Y.; Wang, H.; Wang, Y.; Manchundiya, A.; Lambert, H. Antibiotic Use and Bacterial Infection in COVID-19 Patients in the Second Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2022, 11, 991. [Google Scholar] [CrossRef]
- Chen, X.; Liao, B.; Cheng, L.; Peng, X.; Xu, X.; Li, Y.; Hu, T.; Li, J.; Zhou, X.; Ren, B. The microbial coinfection in COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 7777–7785. [Google Scholar] [CrossRef]
- Murphy, W.; Foley, M.; Doherty, C.; Tierney, G.; Kinsella, A.; Salami, A.; Cadden, E.; Coakley, P. Screening platelet concentrates for bacterial contamination: Low numbers of bacteria and slow growth in contaminated units mandate an alternative approach to product safety. Vox Sang. 2008, 95, 13–19. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M. Community-acquired pneumonia requiring hospitalization among US adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- Baghdadi, J.D.; Coffey, K.; Adediran, T.; Goodman, K.E.; Pineles, L.; Magder, L.S.; O’Hara, L.M.; Pineles, B.L.; Nadimpalli, G.; Morgan, D. Chemotherapy, Antibiotic use and bacterial infection among inpatients in the first wave of COVID-19: A retrospective cohort study of 64,691 patients. Antimicrob. Agents Chemother. 2021, 65, e0134121. [Google Scholar] [CrossRef]
- Vaillancourt, M.; Jorth, P. The unrecognized threat of secondary bacterial infections with COVID-19. MBio 2020, 11, e01806–e01820. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Nebreda-Mayoral, T.; Miguel-Gómez, M.A.; March-Rosselló, G.A.; Puente-Fuertes, L.; Cantón-Benito, E.; Martínez-García, A.M.; Muñoz-Martín, A.B.; Orduña-Domingo, A. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enfermedades Infecc. Y Microbiol. Clin. 2022, 40, 158–165. [Google Scholar] [CrossRef]
- Said, K.B.; Alsolami, A.; Moussa, S.; Alfouzan, F.; Bashir, A.I.; Rashidi, M.; Aborans, R.; Taha, T.E.; Almansour, H.; Alazmi, M. COVID-19 clinical profiles and fatality rates in hospitalized patients reveal case aggravation and selective co-infection by limited gram-negative bacteria. Int. J. Environ. Res. Public Health 2022, 19, 5270. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Ghaddar, N.; El Roz, A.; Ghssein, G.; Ibrahim, J.-N. Emergence of Vulvovaginal Candidiasis among Lebanese Pregnant Women: Prevalence, Risk Factors, and Species Distribution. Infect. Dis. Obstet. Gynecol. 2019, 2019, 5016810. [Google Scholar] [CrossRef]
- Silva, D.L.; Lima, C.M.; Magalhães, V.C.R.; Baltazar, L.M.; Peres, N.T.A.; Caligiorne, R.B.; Moura, A.S.; Fereguetti, T.; Martins, J.C.; Rabelo, L.F.; et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J. Hosp. Infect. 2021, 113, 145–154. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Wu, J.; Li, Y.; Zhou, X.; Li, X.; Chen, H.; Guo, M.; Chen, S.; Sun, F.; et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 1958–1964. [Google Scholar] [CrossRef]
- Feng, Y.; Ling, Y.; Bai, T.; Xie, Y.; Huang, J.; Li, J.; Xiong, W.; Yang, D.; Chen, R.; Lu, F. COVID-19 with different severities: A multicenter study of clinical features. Am. J. Respir. Crit. Care Med. 2020, 201, 1380–1388. [Google Scholar] [CrossRef]
- Ministry of Health Malaysia. National Antimicrobial Guideline 2019. Pharm. Serv. Program 2019, 64, 509–519. [Google Scholar]
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book and Prevention of Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2022; pp. 5–19. [Google Scholar]
- Sokhn, E.S.; Salami, A.; El Roz, A.; Salloum, L.; Bahmad, H.F.; Ghssein, G. Antimicrobial susceptibilities and laboratory profiles of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates as agents of urinary tract infection in Lebanon: Paving the way for better diagnostics. Med. Sci. 2020, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Hendaus, M.A.; Jomha, F.A. COVID-19 induced superimposed bacterial infection. J. Biomol. Struct. Dyn. 2021, 39, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]


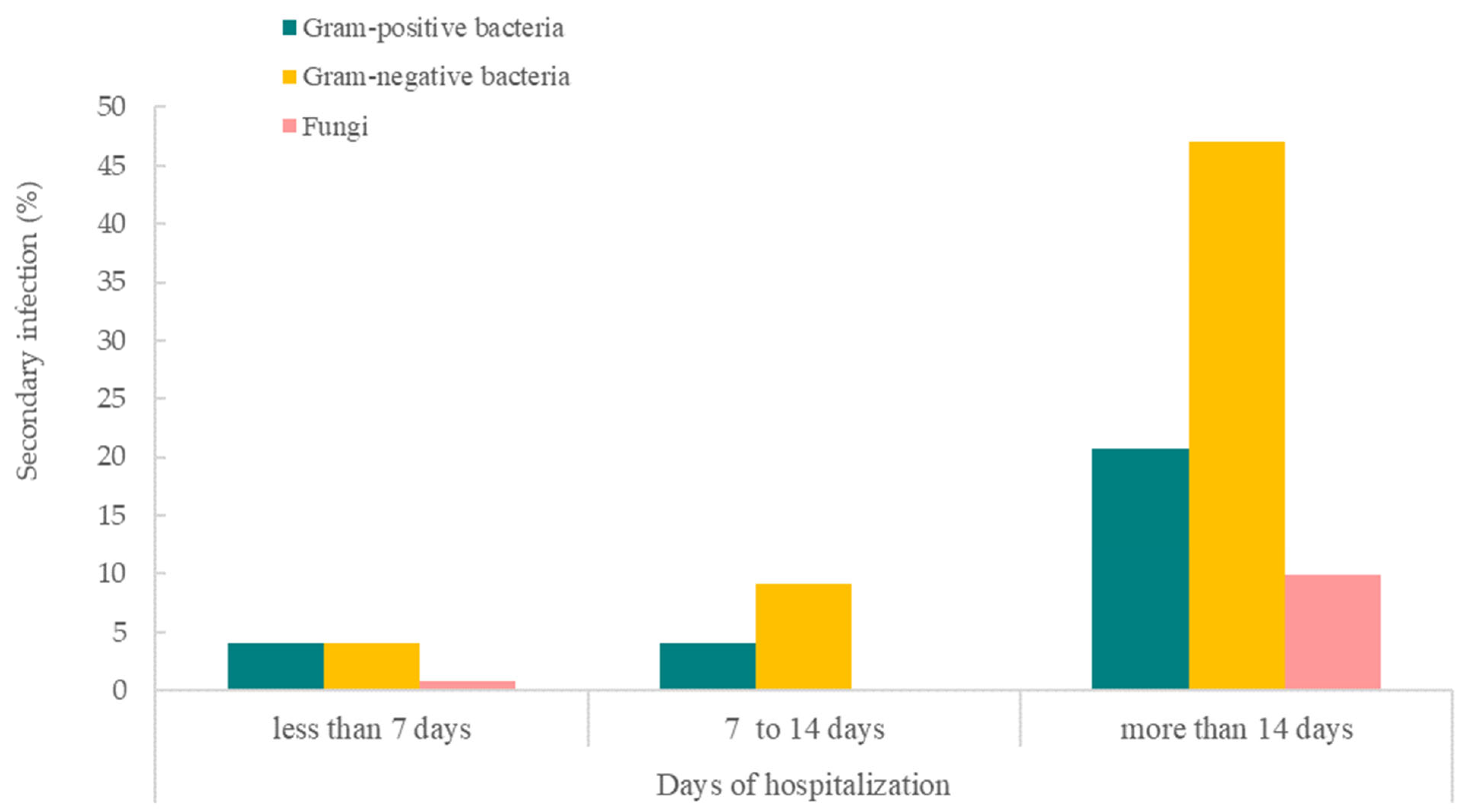

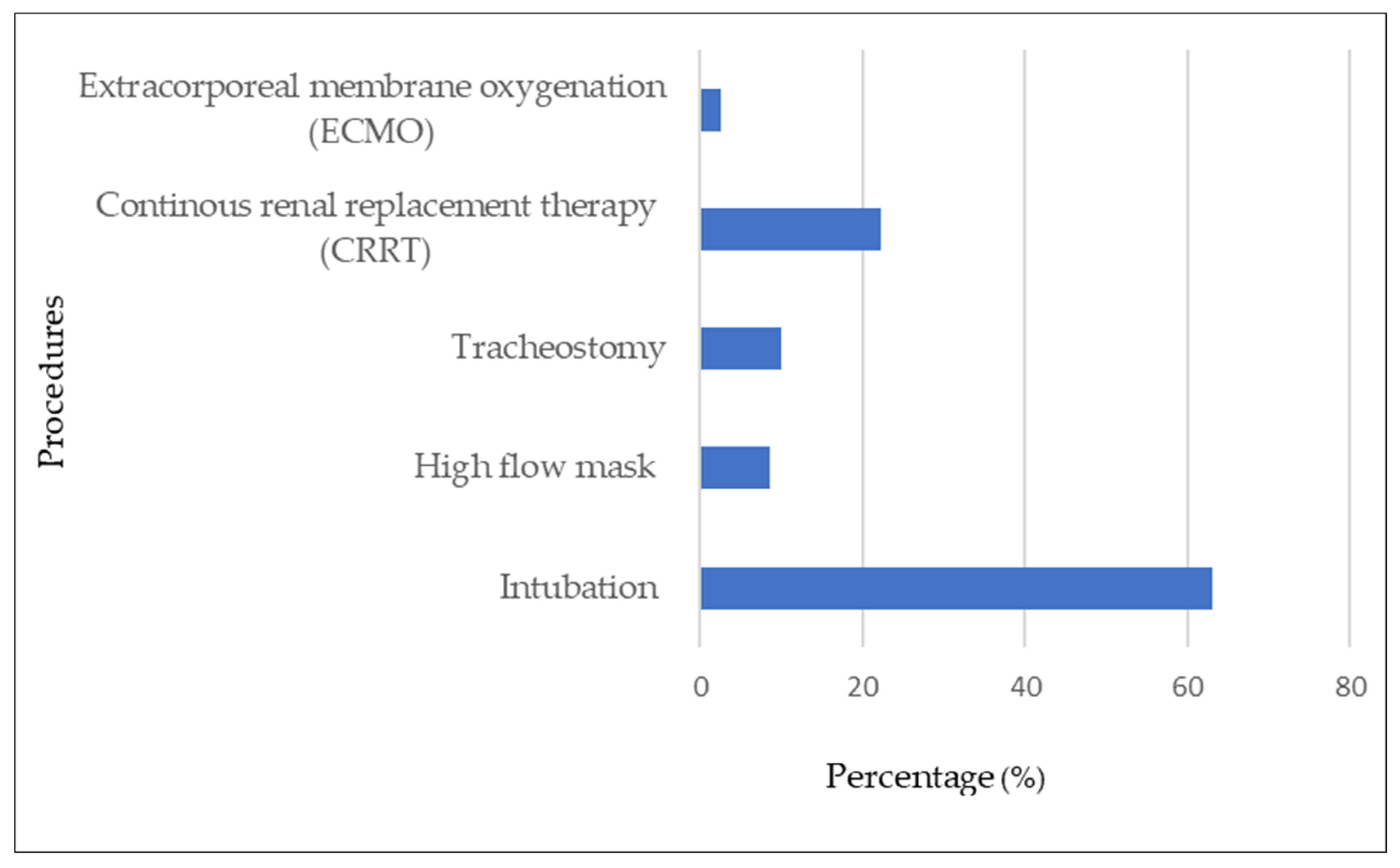
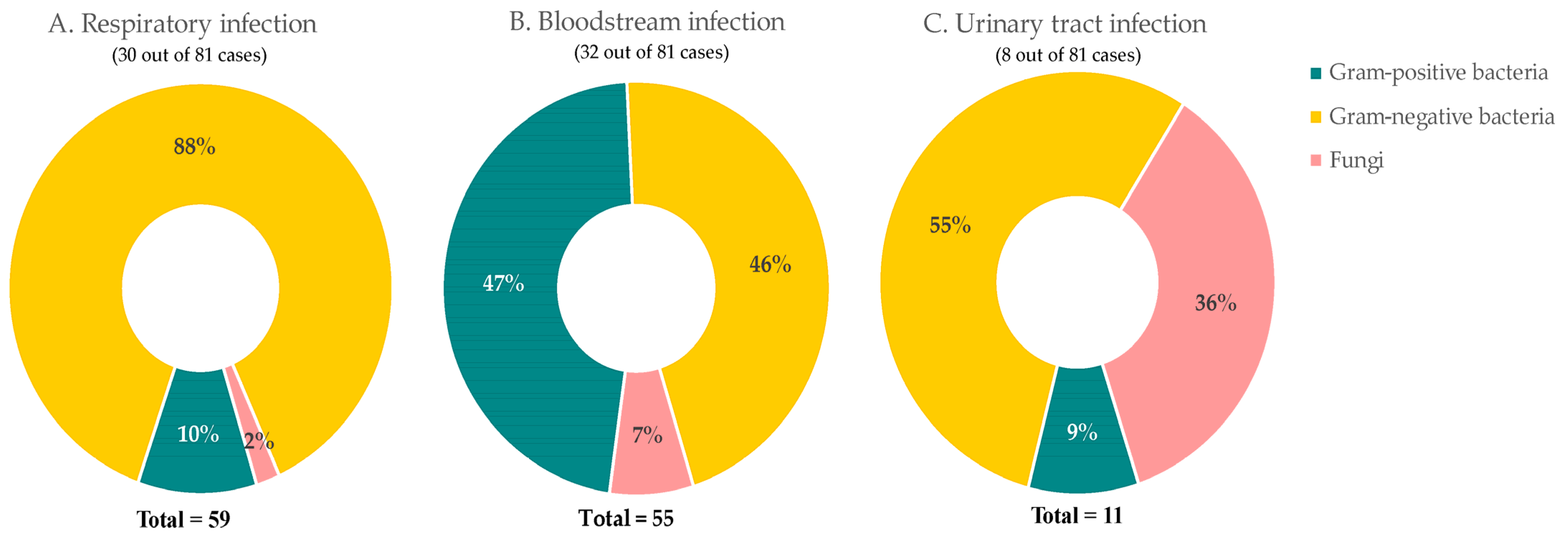
| Variables | With Bacterial/ Fungal Infection | Without Bacterial/ Fungal Infection | p-Value | |
|---|---|---|---|---|
| n = 81 (2.23%) | n = 3451 (97.7%) | |||
| Demography | ||||
| Age group (years) | <0.001 a | |||
| <31 | 12 (0.7) | 1618 (99.3) | ||
| 31–50 | 13 (1.2) | 1030 (98.8) | ||
| 51–70 | 49 (6.4) | 715 (93.6) | ||
| 71+ | 7 (7.4) | 88 (92.6) | ||
| Gender | 0.947 a | |||
| Female | 21 (2.3) | 906 (97.7) | ||
| Male | 60 (2.3) | 2545 (97.7) | ||
| Race | <0.001 b | |||
| Malay | 66 (3.1) | 2072 (96.9) | ||
| Chinese | 3 (1.5) | 191 (98.5) | ||
| Indian | 4 (3.4) | 113 (96.6) | ||
| Others (Sabahan, Sarawakian, Orang Asli, foreigners) | 8 (0.7) | 1075 (99.3) | ||
| Exposure and medical history | ||||
| History of travel | 0.064 a | |||
| Yes | 19 (1.6) | 1148 (98.4) | ||
| No | 62 (2.6) | 2303 (97.4) | ||
| Co-morbidities | <0.001 a | |||
| Bronchial asthma | 5 (4.5) | 107 (95.5) | ||
| Hypertension | 46 (8.4) | 502 (91.6) | ||
| Chronic kidney disease | 17 (23.6) | 55 (76.4) | ||
| Malignancy | 1 (6.3) | 15 (93.8) | ||
| Chronic hematological disorder | 1 (14.3) | 6 (85.7) | ||
| Obesity | 3 (11.1) | 24 (88.9) | ||
| Diabetes mellitus | 39 (10.7) | 326 (89.3) | ||
| Smoking | 7 (1.9) | 364 (98.1) | ||
| History of taking medications | 39 (6.3) | 585 (93.8) | ||
| Clinical features | ||||
| Symptoms | <0.001 a | |||
| Fever | 52 (5.3) | 929 (94.7) | ||
| Cough | 48 (4.4) | 1037 (95.6) | ||
| Runny nose | 9 (2.5) | 348 (97.5) | ||
| Shortness of breath | 22 (11.6) | 168 (88.4) | ||
| Nausea/vomiting | 5 (8.2) | 56 (91.8) | ||
| Diarrhea | 15 (7.9) | 176 (92.1) | ||
| Skin rashes | 1 (16.7) | 5 (83.3) | ||
| Respiratory rate (per minute) | <0.001 b | |||
| <20 | 52 (1.6) | 3262 (98.4) | ||
| >20 | 29 (13.3) | 189 (86.7) | ||
| Temperature on admission (°C) | <0.001 a | |||
| <37.5 | 57 (1.7) | 3218 (98.3) | ||
| >37.5 | 24 (9.3) | 233 (90.7) | ||
| Chest X-ray findings | 0.001 a | |||
| Abnormal findings | 38 (3.6) | 1017 (96.4) | ||
| Normal findings | 43 (1.7) | 2434 (98.3) | ||
| Systolic blood pressure (mmHg) | 0.008 a | |||
| <140 | 50 (2.2) | 2174 (97.8) | ||
| >140 | 31 (4.0) | 737 (96.0) | ||
| Diastolic blood pressure (mmHg) | 0.964 a | |||
| <90 | 72 (2.3) | 3062 (97.7) | ||
| >90 | 9 (2.3) | 389 (97.7) | ||
| Complications | <0.001 b | |||
| Seizure | 1 (20.0) | 4 (80.0) | ||
| Stroke | 3 (50.0) | 3 (50.0) | ||
| Congestive heart failure | 4 (22.2) | 14 (77.8) | ||
| Cardiac arrhythmia | 11 (37.9) | 18 (62.1) | ||
| Cardiac arrest | 7 (28.0) | 18 (72.0) | ||
| Disseminated intravascular coagulopathy | 5 (41.7) | 7 (58.3) | ||
| Acute kidney injury | 47 (27.6) | 123 (72.4) | ||
| Gastrointestinal hemorrhage | 7 (46.7) | 8 (53.3) | ||
| Liver dysfunction | 26 (8.4) | 285 (91.6) | ||
| Acute respiratory distress syndrome | 39 (37.5) | 65 (62.5) | ||
| Hospitalization, staging, and outcome | ||||
| COVID-19 stage | <0.001 a | |||
| 1 | 18 (1.0) | 1814 (99.0) | ||
| 2 | 15 (1.4) | 1033 (98.6) | ||
| 3 | 5 (1.1) | 461 (98.9) | ||
| 4 | 21 (15.3) | 116 (84.7) | ||
| 5 | 22 (44.9) | 27 (55.1) | ||
| Duration of hospitalization (days) | 0.003 a | |||
| <7 | 10 (1.1) | 941 (98.9) | ||
| >7 | 71 (2.8) | 2510 (97.2) | ||
| Outcome | <0.001 b | |||
| Death | 22 (40.7) | 32 (59.3) | ||
| Discharged alive | 59 (1.7) | 3419 (98.3) | ||
| Laboratory and radiology investigations | ||||
| C-reactive protein (CRP) (mg/L) | <0.001 a | |||
| <10 | 48 (1.5) | 3212 (98.5) | ||
| 10+ | 33 (12.1) | 239 (87.9) | ||
| Hemoglobin (g/dL) | <0.001 a | |||
| <12 | 32 (7.1) | 417 (92.9) | ||
| >12 | 49 (1.6) | 3034 (98.4) | ||
| Platelet count (×109/L) | 0.006 b | |||
| <450 | 74 (2.2) | 3362 (97.8) | ||
| >450 | 7 (7.3) | 89 (92.7) | ||
| Total white count (×109/L) | <0.001 a | |||
| <11 | 61 (1.9) | 3135 (98.1) | ||
| >11 | 20 (6.0) | 316 (94.0) | ||
| Neutrophil count (×109/L) | <0.001 a | |||
| <8 | 59 (1.8) | 3184 (98.2) | ||
| >8 | 22 (7.6) | 267 (92.4) | ||
| Lymphocyte count (×109/L) | >0.999 b | |||
| <4 | 78 (2.3) | 3286 (97.7) | ||
| >4 | 3 (1.8) | 165 (98.2) | ||
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Odd Ratio (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | ||
| Age, years | 1.06 (1.04–1.07) | <0.001 ‡ | 1.03 (1.01–1.05) | 0.003 ‡ | |
| Gender | Female | reference | |||
| Male | 1.02 (0.62–1.68) | 0.95 | |||
| Co-morbidity | Asthma | 2.06 (0.82–5.19) | 0.13 | ||
| Chronic kidney disease | 16.40 (9.02–29.81) | <0.001 ‡ | 3.76 (1.92–7.38) | <0.001 ‡ | |
| Hypertension | 7.72 (4.93–12.11) | <0.001 ‡ | 2.10 (1.12–3.91) | 0.02 ‡ | |
| Neoplasm | 2.86 (0.37–21.94) | 0.31 | |||
| Chronic hematology disease | 7.18 (0.86–60.31) | 0.07 | 14.55 (1.64–129.01) | 0.02 ‡ | |
| Obesity | 5.49 (1.62–18.62) | 0.006 ‡ | |||
| Diabetes mellitus | 8.93 (5.69–14.02) | <0.001 ‡ | 2.69 (1.52–4.79) | 0.001 ‡ | |
| Smoking | 0.80 (0.37–1.76) | 0.58 | |||
| History of taking medications | 4.55 (2.92–7.10) | <0.001 ‡ | |||
| Days of hospitalization | Less than 7 days of hospitalization | reference | |||
| More than 7 days of hospitalization | 2.66 (1.37–5.18) | 0.004 ‡ | |||
| Symptoms | Fever | 4.87 (3.07–7.71) | <0.001 ‡ | 2.21 (1.29–3.80) | 0.004 ‡ |
| Cough | 3.39 (2.16–5.31) | <0.001 ‡ | |||
| Runny nose | 1.12 (0.56–2.25) | 0.76 | |||
| Shortness of breath | 7.29 (4.36–12.18) | <0.001 ‡ | |||
| Vomiting/nausea | 3.99 (1.55–10.24) | 0.004 ‡ | |||
| Diarrhea | 4.23 (2.37–7.56) | <0.001 ‡ | |||
| Skin rash | 8.62 (1.00–74.59) | 0.05 | |||
| Complications | Acute respiratory distress syndrome | 48.37 (29.33–79.77) | <0.001 ‡ | 7.72 (3.94–15.12) | <0.001 ‡ |
| Stroke/cerebrovascular accident | 44.20 (8.78–222.47) | <0.001 ‡ | |||
| Congestive heart failure | 12.75 (4.10–39.63) | <0.001 ‡ | |||
| Cardiac arrhythmia | 29.97 (13.65–65.81) | <0.001 ‡ | |||
| Cardiac arrest | 18.04 (7.31–44.50) | <0.001 ‡ | |||
| DIVC | 32.37 (10.05–104.28) | <0.001 ‡ | |||
| Acute renal failure | 37.40 (23.22–60.24) | <0.001 ‡ | 9.64 (5.17–17.99) | <0.001 ‡ | |
| Gastrointestinal hemorrhage | 40.71 (14.39–115.20) | <0.001 ‡ | |||
| Liver dysfunction | 5.25 (3.24–8.50) | <0.001 ‡ | |||
| Outcome | Discharged alive | reference | |||
| Death | 39.84 (21.85–72.65) | <0.001 ‡ | |||
| Stage of COVID-19 | 1 Asymptomatic | reference | |||
| 2 Symptomatic but no pneumonia | 1.46 (0.73–2.92) | 0.28 | |||
| 3 Pneumonia without hypoxia | 1.09 (0.40–2.96) | 0.86 | |||
| 4 Pneumonia with oxygen therapy | 18.24 (9.46–35.19) | <0.001 ‡ | |||
| 5 Critically ill | 82.12 (39.59–170.34) | <0.001 ‡ | |||
| Chest X-ray | Normal | reference | |||
| Abnormal | 2.12 (1.36–3.29) | 0.001 ‡ | |||
| Systolic blood BP | <140 | reference | reference | ||
| (mmHg) | >140 | 2.28 (1.45–3.60) | <0.001 ‡ | 2.02 (1.18–3.44) | 0.01 ‡ |
| Diastolic BP | <90 | reference | |||
| (mmHg) | >90 | 0.98 (0.49–1.98) | 0.96 | ||
| Respiratory rate | <20 | reference | |||
| (per minute) | >20 | 9.63 (5.97–15.51) | <0.001 ‡ | ||
| Lab Investigations | Unit | With Secondary and/or Co-Infections | Without Secondary and/or Co-Infections | Total | Z Score * | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| n | Mean Rank | n | Mean Rank | |||||
| WBC count | (109/L) | 80 | 1870.79 | 3310 | 1691.26 | 3390 | −1.621 | 0.105 |
| Hemoglobin | (g/dL) | 80 | 977.44 | 3310 | 1712.85 | 3390 | −6.642 | <0.001 ‡ |
| Platelets | (109/L) | 80 | 1433.19 | 3310 | 1701.84 | 3390 | −2.426 | 0.015 ‡ |
| Neutrophil count | (109/L) | 79 | 2030.37 | 3268 | 1665.39 | 3347 | −3.317 | <.001 ‡ |
| Lymphocyte count | (109/L) | 79 | 926.57 | 3301 | 1708.78 | 3380 | −7.043 | <.001 ‡ |
| PCT | (ng/mL) | 18 | 35.72 | 34 | 21.62 | 52 | −3.194 | 0.001 ‡ |
| LDH | (units/L) | 44 | 2113.75 | 2397 | 1204.61 | 2441 | −8.479 | <0.001 ‡ |
| D-dimer | (mg/L) | 14 | 103.29 | 126 | 66.86 | 140 | −3.202 | 0.001 ‡ |
| Ferritin | (µg/L) | 20 | 152.45 | 180 | 94.73 | 200 | −4.231 | <0.001 ‡ |
| CRP | (mg/dL) | 81 | 2296.37 | 3416 | 1736.02 | 3497 | −5.073 | <0.001 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, S.R.; Abdul Hadi, F.S.; Nor Amdan, N.A.; Kamaradin, I.H.; Zabari, N.; Maniam, S.; Sulaiman, N.S.; Ghazali, S.; Seman, Z.; Hashim, R.; et al. Secondary and Co-Infections in Hospitalized COVID-19 Patients: A Multicenter Cross-Sectional Study in Malaysia. Antibiotics 2023, 12, 1547. https://doi.org/10.3390/antibiotics12101547
Ramli SR, Abdul Hadi FS, Nor Amdan NA, Kamaradin IH, Zabari N, Maniam S, Sulaiman NS, Ghazali S, Seman Z, Hashim R, et al. Secondary and Co-Infections in Hospitalized COVID-19 Patients: A Multicenter Cross-Sectional Study in Malaysia. Antibiotics. 2023; 12(10):1547. https://doi.org/10.3390/antibiotics12101547
Chicago/Turabian StyleRamli, Siti Roszilawati, Fashihah Sherina Abdul Hadi, Nur Asyura Nor Amdan, Insyirah Husna Kamaradin, Noraliza Zabari, Saraswathiy Maniam, Nur Suffia Sulaiman, Sumarni Ghazali, Zamtira Seman, Rohaidah Hashim, and et al. 2023. "Secondary and Co-Infections in Hospitalized COVID-19 Patients: A Multicenter Cross-Sectional Study in Malaysia" Antibiotics 12, no. 10: 1547. https://doi.org/10.3390/antibiotics12101547
APA StyleRamli, S. R., Abdul Hadi, F. S., Nor Amdan, N. A., Kamaradin, I. H., Zabari, N., Maniam, S., Sulaiman, N. S., Ghazali, S., Seman, Z., Hashim, R., & Ahmad, N. (2023). Secondary and Co-Infections in Hospitalized COVID-19 Patients: A Multicenter Cross-Sectional Study in Malaysia. Antibiotics, 12(10), 1547. https://doi.org/10.3390/antibiotics12101547






