24/7 Therapeutic Drug Monitoring of Beta-Lactam Antibiotics with CLAM-2000
Abstract
:1. Introduction
2. Results
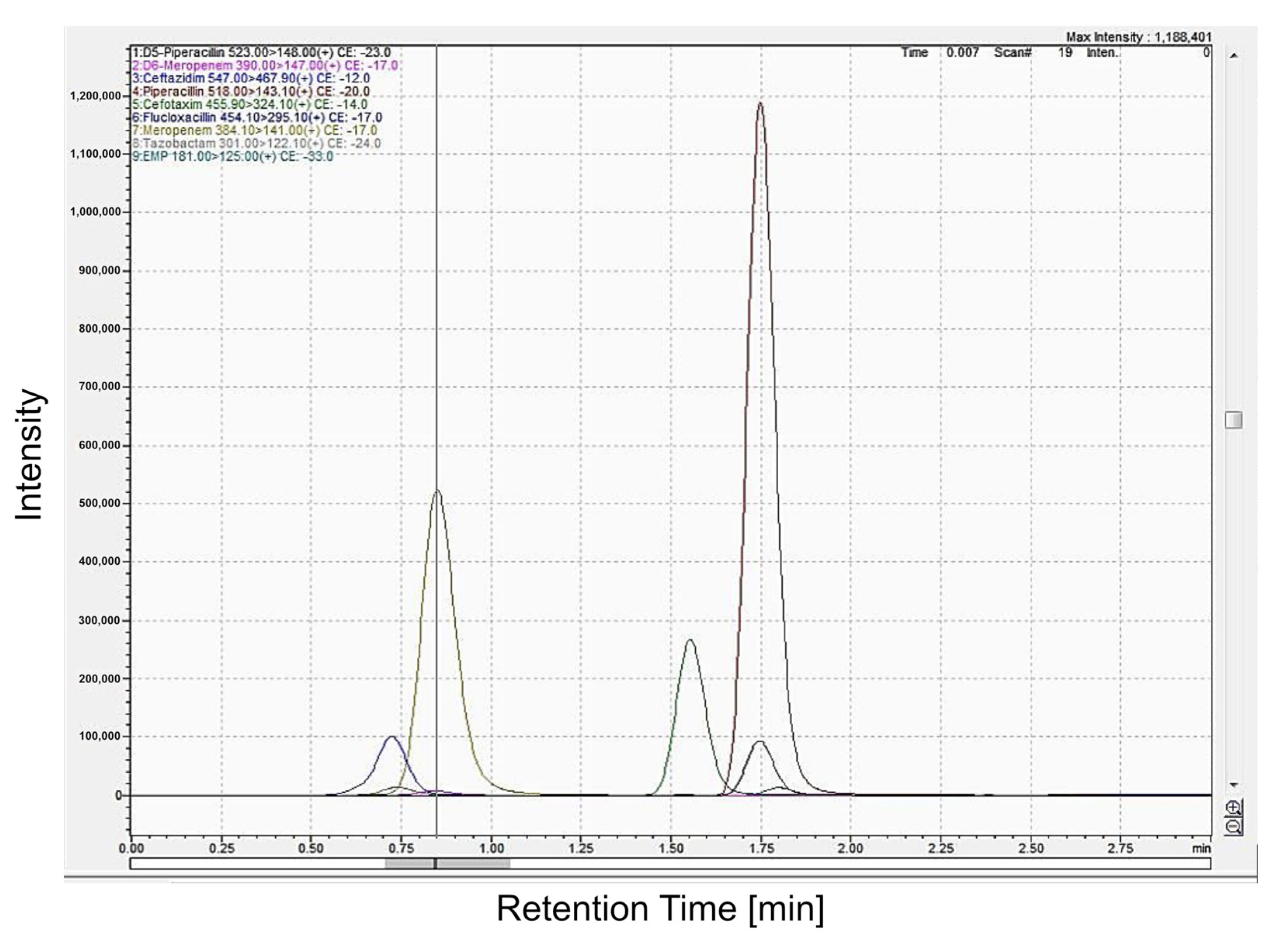
2.1. Validation of HPLC Assays (Chromatography)
2.2. Linearity
2.3. Accuracy and Imprecision
2.4. Stability Studies
2.5. Method Correlation
2.6. Selectivity and Specificity Studies
2.7. Robustness
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Matrix Effects
4.3. Analysis of Beta-Lactam Antibiotics in a 24/7 Environment (CLAM-2000)
4.3.1. Sample Preparation
4.3.2. Separation (Chromatographic Conditions)
4.3.3. Mass Spectrometry
4.3.4. Validation Assay
4.3.5. Accuracy and Precision
4.3.6. Linearity
4.3.7. Stability and Robustness
4.4. Comparison with Routine Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, J.A.; Norris, R.; Paterson, D.L.; Martin, J.H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 2012, 73, 27–36. [Google Scholar] [CrossRef]
- Wong, G.; Sime, F.B.; Lipman, J.; Roberts, J.A. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect. Dis. 2014, 14, 288. [Google Scholar] [CrossRef]
- Wong, G.; Brinkman, A.; Benefield, R.J.; Carlier, M.; De Waele, J.J.; Helali, N.E.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef]
- Forrest, A.; Nix, D.E.; Ballow, C.H.; Goss, T.F.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 1993, 37, 1073–1081. [Google Scholar] [CrossRef]
- Lacy, M.K.; Nicolau, D.P.; Nightingale, C.H.; Quintiliani, R. The pharmacodynamics of aminoglycosides. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1998, 27, 23–27. [Google Scholar] [CrossRef]
- MacGowan, A.P. Pharmacodynamics, pharmacokinetics, and therapeutic drug monitoring of glycopeptides. Ther. Drug Monit. 1998, 20, 473–477. [Google Scholar] [CrossRef]
- Frey, O.R.; Köberer, A.; Röhr, A.C.; Fuchs, T.; Brinkmann, A. Therapeutisches Drug Monitoring (TDM) von Antiinfektiva bei kritisch Kranken. Intensiv-News 2013, 17, 16–18. [Google Scholar]
- Lagler, H.; Zeitlinger, M. Gewebepenetration von Antibiotika: Erreicht die Behandlung den Zielort? Med. Klin.—Intensivmed. Und Notfallmedizin 2014, 109, 175–181. [Google Scholar] [CrossRef]
- Vazquez-Guillamet, C.; Scolari, M.; Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Kollef, M. Using the Number Needed to Treat to Assess Appropriate Antimicrobial Therapy as a Determinant of Outcome in Severe Sepsis and Septic Shock*. Crit. Care Med. 2014, 42, 2342–2349. [Google Scholar] [CrossRef]
- Sime, F.; Roberts, M.S.; Peake, S.L.; Lipman, J.; Roberts, J.A. Does Beta-lactam Pharmacokinetic Variability in Critically Ill Patients Justify Therapeutic Drug Monitoring? A Systematic Review. Ann. Intensive Care 2012, 2, 35. [Google Scholar] [CrossRef]
- Brinkmann, A.; Röhr, A.C.; Köberer, A.; Fuchs, T.; Preisenberger, J.; Krüger, W.A.; Frey, O.R. Therapeutisches Drug Monitoring und individualisierte Dosierung von Antibiotika bei der Sepsis: Modern oder nur “modisch”. Med. Klin.—Intensivmed. Und Notfallmedizin 2016, 113, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; De Waele, J.J.; Verstraete, A.G.; Stove, V. Exploration of the pre-analytical stability of β-lactam antibiotics in plasma and blood—Implications for therapeutic drug monitoring and pharmacokinetic studies. Clin. Chem. Lab. Med. 2015, 53, e227–e230. [Google Scholar] [CrossRef] [PubMed]
- Pinder, N.; Brenner, T.; Swoboda, S.; Weigand, M.A.; Hoppe-Tichy, T. Therapeutic drug monitoring of beta-lactam antibiotics—Influence of sample stability on the analysis of piperacillin, meropenem, ceftazidime and flucloxacillin by HPLC-UV. J. Pharm. Biomed. Anal. 2017, 143, 86–93. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current ß-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Witebolle, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–12. [Google Scholar] [CrossRef]
- Roberts, J.a.; Webb, S.; Paterson, D.; Ho, K.M.; Lipman, J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit. Care Med. 2009, 37, 2071–2078. [Google Scholar] [CrossRef]
- Roberts, J.A.; Taccone, F.S.; Lipman, J. Understanding PK/PD. Intensive Care Med. 2016, 42, 1797–1800. [Google Scholar] [CrossRef]
- Rea, R.S.; Capitano, B.; Bies, R.; Bigos, K.L.; Smith, R.; Lee, H. Suboptimal aminoglycoside dosing in critically ill patients. Ther. Drug Monit. 2008, 30, 674–681. [Google Scholar] [CrossRef]
- Roberts, J.A.; Taccone, F.S.; Udy, A.A.; Vincent, J.L.; Jacobs, F.; Lipman, J. Vancomycin dosing in critically ill patients: Robust methods for improved continuous-infusion regimens. Antimicrob. Agents Chemother. 2011, 55, 2704–2709. [Google Scholar] [CrossRef] [PubMed]
- Van Zanten, A.R.H.; Polderman, K.H.; van Geijlswijk, I.M.; van der Meer, G.Y.G.; Schouten, M.A.; Girbes, A.R.J. Ciprofloxacin pharmacokinetics in critically ill patients: A prospective cohort study. J. Crit. Care 2008, 23, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, S.; Ober, M.C.; Lichtenstern, C.; Saleh, S.; Schwenger, V.; Sonntag, H.G.; Haefeli, W.E.; Hempel, G.; Hoppe-Tichy, T.; Weigand, M.A. Pharmacokinetics of linezolid in septic patients with and without extended dialysis. Eur. J. Clin. Pharmacol. 2010, 66, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Implications of augmented renal clearance in critically ill patients. Nat. Rev. Nephrol. 2011, 7, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Streit, F.; Perl, T.; Schulze, M.H.; Binder, L. Personalised beta-lactam therapy: Basic principles and practical approach. LaboratoriumsMedizin 2016, 40, 385–397. [Google Scholar] [CrossRef]
- Chiriac, U.; Richter, D.; Frey, O.R.; Röhr, A.C.; Helbig, S.; Hagel, S.; Liebchen, U.; Weigand, M.A.; Brinkmann, A. Software-and TDM-Guided Dosing of Meropenem Promises High Rates of Target Attainment in Critically Ill Patients. Antibiotics 2023, 12, 1112. [Google Scholar] [CrossRef]
- Scharf, C.; Paal, M.; Schroeder, I.; Vogeser, M.; Draenert, R.; Irlbeck, M.; Zoller, M.; Liebchen, U. Therapeutic drug monitoring of meropenem and piperacillin in critical illness—Experience and recommendations from one year in routine clinical practice. Antibiotics 2020, 9, 131. [Google Scholar] [CrossRef]
- Plasse, J.C.; Chabloz, C.; Terrier, A.; Bellon, G. To the editor: Is it safe to administer a continuous infusion of ceftazidime (Fortum®) prepared for 24 hours in cystic fibrosis (CF) patients? Pediatr. Pulmonol. 2002, 33, 232–233. [Google Scholar] [CrossRef]
- Prescott, W.A.; Gentile, A.E.; Nagel, J.L.; Pettit, R.S. Continuous-infusion antipseudomonal beta-lactam therapy in patients with cystic fibrosis. P T Group 2011, 36, 723–740. [Google Scholar]
- European Medicines Agency. Guideline on bioanalytical method validation. EMA Guidel. 2012, 44, 865–868. [Google Scholar]
- Blecka, L.J.; Jackson, G.J. Immunoassays in therapeutic drug monitoring. Clin. Lab. Med. 1987, 7, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, S.E.; McWhinney, B.C.; Lipman, J.; Roberts, J.A.; Ungerer, J.P.J. A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 907, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Stove, V.; Roberts, J.A.; Velde, E.V.D.; Waele, J.J.D.; Verstraete, A.G. Quantification of seven beta-lactam antibiotics and two beta-lactamase inhibitors in human plasma using UPLC-MS/MS method. Int. J. Antimicrob. Agents 2012, 40, 416–422. [Google Scholar] [CrossRef]
- Kumar, P.R.; Dinesh, S.R.; Rini, R. Lcms-a Review and a Recent Update. J. Pharm. Pharm. Sci. 2016, 5, 377–391. [Google Scholar]
- McWhinney, B.C.; Wallis, S.C.; Hillister, T.; Roberts, J.A.; Lipman, J.; Ungerer, J.P.J. Analysis of 12 beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.S.L.; Steppe, M.; Schapoval, E.E.S. Validation of HPLC and UV spectrophotometric methods for the determination of meropenem in pharmaceutical dosage form. J. Pharm. Biomed. Anal. 2003, 33, 947–954. [Google Scholar] [CrossRef]
- Ohmori, T.; Suzuki, A.; Niwa, T.; Ushikoshi, H.; Shirai, K.; Yoshida, S.; Ogura, S.; Itoh, Y. Simultaneous determination of eight β-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1038–1042. [Google Scholar] [CrossRef]
- Zander, J.; Maier, B.; Suhr, A.; Zoller, M.; Frey, L.; Teupser, D.; Vogeser, M. Quantification of piperacillin, tazobactam, cefepime, meropenem, ciprofloxacin and linezolid in serum using an isotope dilution UHPLC-MS/MS method with semi-automated sample preparation. Clin. Chem. Lab. Med. 2015, 53, 781–791. [Google Scholar] [CrossRef]
- Sörgel, F.; Höhl, R.; Glaser, R.; Stelzer, C.; Munz, M.; Vormittag, M.; Kinzig, M.; Bulitta, J.; Landersdorfer, C.; Junger, A.; et al. Pharmakokinetik und Pharmakodynamik von Antibiotika in der Intensivmedizin. Med. Klin.—Intensivmed. Und Notfallmedizin 2016, 112, 11–23. [Google Scholar] [CrossRef]
- Carlier, M.; Stove, V.; De Waele, J.J.; Verstraete, A.G. Ultrafast quantification of β-lactam antibiotics in human plasma using UPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 978–979, 89–94. [Google Scholar] [CrossRef]
- Zhang, M.; Moore, G.A.; Everts, R.; Begg, E.J. Determination of Total and Free Concentrations of Flucloxacillin and Cefazolin in Human Plasma by Liquid Chromatography/Tandem Mass Spectrometry. J. Anal. Bioanal. Tech. 2014, 5, 182. [Google Scholar] [CrossRef]
- Garcia-Capdevila, L.; López-Calull, C.; Arroyo, C.; Moral, M.a.; Mangues, M.a.; Bonal, J. Determination of imipenem in plasma by high-performance liquid chromatography for pharmacokinetic studies in patients. J. Chromatogr. B Biomed. Sci. Appl. 1997, 692, 127–132. [Google Scholar] [CrossRef]
- Ahsman, M.J.; Wildschut, E.D.; Tibboel, D.; Mathot, R.A. Microanalysis of beta-lactam antibiotics and vancomycin in plasma for pharmacokinetic studies in neonates. Antimicrob. Agents Chemother. 2009, 53, 75–80. [Google Scholar] [CrossRef]
- Pickering, M.; Brown, S. Quantification and validation of HPLC-UV and LC-MS assays for therapeutic drug monitoring of ertapenem in human plasma. Biomed. Chromatogr. 2013, 27, 568–574. [Google Scholar] [CrossRef]
- Verdier, M.C.; Tribut, O.; Tattevin, P.; Le Tulzo, Y.; Michelet, C.; Bentue-Ferrer, D. Simultaneous Determination of 12 beta-Lactam Antibiotics in Human Plasma by High-Performance Liquid Chromatography with UV Detection: Application to Therapeutic Drug Monitoring. Antimicrob. Agents Chemother. 2011, 55, 4873–4879. [Google Scholar] [CrossRef]
- Zander, J.; Maier, B.; Zoller, M.; Döbbeler, G.; Frey, L.; Teupser, D.; Vogeser, M. Effects of biobanking conditions on six antibiotic substances in human serum assessed by a novel evaluation protocol. Clin. Chem. Lab. Med. 2016, 54, 265–274. [Google Scholar] [CrossRef]
- Rigo-Bonnin, R.; Cobo-Sacristan, S.; Padulles, A.; Ribera, A.; Arbiol-Roca, A.; Murillo, O.; Sabater-Riera, J.; Alia, P. Measurement of ceftazidime concentration in human plasma by ultra-performance liquid chromatography-tandem mass spectrometry. Application to critically ill patients and patients with osteoarticular infections. Biomed. Chromatogr. 2016, 30, 410–418. [Google Scholar] [CrossRef]
- FDA—Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health and Human Services: Washington, DC, USA, 2001. Available online: http://www.fda.gov/cder/Guidance/4252fnl.pdf (accessed on 28 September 2023).
| Analyte | Y | R2 | Range (mg/L) |
|---|---|---|---|
| Ceftazidime | 1.0086x − 0.7149 | 0.9992 | 0.5–156 |
| Cefotaxime | 0.9888x − 0.4574 | 0.9961 | 0.5–156 |
| Flucloxacillin | 1.0374x + 0.7133 | 0.9987 | 0.5–156 |
| Meropenem | 1.0033x + 0.2383 | 0.9994 | 0.5–62.5 |
| Piperacillin | 0.9758x + 2.0205 | 0.9932 | 0.5–156 |
| Sample | Concentration (mg/L) | Nominal Value (mg/L) | Concentration Found (mg/L) (Mean ± SD) | Imprecision CV (%) | Bias d (%) |
|---|---|---|---|---|---|
| Ceftazidime | |||||
| LLOQ | 0.35 | 0.35 | 0.3 ± 0.04 | 11.7 | 3.9 |
| QC 1 | 10 | 10 | 10.5 ± 1.0 | 9.5 | −4.5 |
| QC 2 | 25 | 25 | 25.5 ± 2.1 | 8.03 | −1.9 |
| QC 3 | 50 | 51 | 49 ± 4 | 8.2 | 3.9 |
| QC 4 | 150 | 191 | 174.6 ± 17.9 | 10.3 | −8.6 |
| Cefotaxime | |||||
| LLOQ | 0.5 | 0.5 | 0.5 ± 0.03 | 6.1 | 3.5 |
| QC 1 | 10 | 8.7 | 9 ± 0.3 | 3.1 | −3.2 |
| QC 2 | 25 | 20 | 20.3 ± 0.8 | 3.7 | −1.6 |
| QC 3 | 50 | 46 | 47.4 ± 2.2 | 4.6 | −3.1 |
| QC 4 | 150 | 163 | 161.4 ± 9.4 | 5.8 | −1 |
| Flucloxacillin | |||||
| LLOQ | 0.5 | 0.5 | 0.5 ± 0.04 | 7.9 | 5.8 |
| QC 1 | 10 | 9 | 8.9 ± 0.5 | 5.2 | 1.7 |
| QC 2 | 25 | 25 | 24.3 ± 1.6 | 6.6 | 2.9 |
| QC 3 | 50 | 46 | 46.4 ± 2.4 | 5.2 | −0.9 |
| QC 4 | 150 | -- | -- | -- | -- |
| Meropenem | |||||
| LLOQ | 0.5 | 0.5 | 0.5 ± 0.02 | 4.2 | 0.9 |
| QC 1 | 10 | 10 | 10.2 ± 0.5 | 4.6 | −2 |
| QC 2 | 25 | 25 | 24.1 ± 1.1 | 4.6 | 3.5 |
| QC 3 | 50 | 50 | 51.9 ± 2.5 | 4.9 | −3.8 |
| QC 4 | 150 | 160 | 162.2 ± 3.9 | 2.4 | 1.4 |
| Piperacillin | |||||
| LLOQ | 0.5 | 0.5 | 0.5 ± 0.02 | 3.8 | 4.8 |
| QC 1 | 10 | 12 | 11.5 ± 0.5 | 4.6 | 4 |
| QC 2 | 25 | 28 | 28 ± 0.7 | 2.5 | −0.1 |
| QC 3 | 50 | 55 | 56± 1.6 | 2.8 | −1.8 |
| QC 4 | 150 | 150 | 142.8 ± 2.3 | 1.6 | −4.8 |
| Sample | Concentration (mg/L) | Nominal Value (mg/L) | Concentration Found (mg/L) (Mean ± SD) | Imprecision CV (%) | Bias d (%) |
|---|---|---|---|---|---|
| Ceftazidime | |||||
| QC 1 | 10 | 10 | 10.5 ± 0.8 | 7.2 | 4.6 |
| QC 2 | 25 | 25 | 26.7 ± 1.7 | 6.3 | 6.9 |
| QC 3 | 50 | 51 | 49.9 ± 4.8 | 9.7 | 2.2 |
| QC 4 | 150 | 191 | 179.2 ± 13.1 | 7.3 | 6.2 |
| Cefotaxime | |||||
| QC 1 | 10 | 8.7 | 8.8 ± 0.1 | 1.6 | 0.6 |
| QC 2 | 25 | 20 | 19.7 ± 0.7 | 3.8 | 1.5 |
| QC 3 | 50 | 46 | 46.7 ± 0.9 | 2 | 1.4 |
| QC 4 | 150 | 163 | 164.8 ± 6.2 | 3.8 | 1.1 |
| Flucloxacillin | |||||
| QC 1 | 10 | 10 | 9 ± 0.4 | 4.04 | 1 |
| QC 2 | 25 | 25 | 24.9 ± 1.5 | 6.03 | 0.5 |
| QC 3 | 50 | 46 | 47.2 ± 2.5 | 5.3 | 2.6 |
| QC 4 | 150 | -- | -- | -- | -- |
| Meropenem | |||||
| QC 1 | 10 | 10 | 10.1 ± 0.3 | 2.8 | 0.5 |
| QC 2 | 25 | 23 | 22.9 ± 0.6 | 2.5 | 0.3 |
| QC 3 | 50 | 50 | 50.6 ± 2 | 3.9 | 1.2 |
| QC 4 | 150 | 160 | 169.2 ± 5.1 | 3 | 5.8 |
| Piperacillin | |||||
| QC 1 | 10 | 10 | 10.5 ± 0.3 | 3.2 | 5.2 |
| QC 2 | 25 | 28 | 25.7 ± 1.1 | 4.2 | 8.1 |
| QC 3 | 50 | 55 | 51 ± 1.1 | 2.2 | 5 |
| QC 4 | 150 | 180 | 179.4 ± 8.8 | 7.3 | 0.4 |
| Analyte | c (mg/L) | CV (%) | d (%) |
|---|---|---|---|
| Ceftazidime | 10 | 9.5 | 4.5 |
| 25 | 8.0 | 1.9 | |
| 50 | 8.2 | 3.9 | |
| Cefotaxime | 9 | 3.1 | 1.6 |
| 20 | 3.7 | 1.6 | |
| 46 | 4.6 | 3.9 | |
| Flucloxacillin | 9 | 5.2 | 1.7 |
| 25 | 6.6 | 2.9 | |
| 46 | 5.2 | 0.9 | |
| Meropenem | 10 | 4.6 | 2.0 |
| 25 | 4.6 | 3.5 | |
| 50 | 4.9 | 3.8 | |
| Piperacillin | 12 | 4.6 | 4.0 |
| 28 | 2.5 | 0.1 | |
| 55 | 2.8 | 1.8 |
| Analyte | n | R | Slope (b) | Intercept (a) | Range MSMS | Range CLAM-2000 |
|---|---|---|---|---|---|---|
| Ceftazidime | 39 | 0.994 | 0.993 | −0.605 | 3.1–187 | 2.9–196 |
| Cefotaxime | 19 | 0.994 | 0.959 | 0.956 | 4.6–54 | 4.6–52 |
| Flucloxacillin | 20 | 0.981 | 0.924 | −0.269 | 9.8–47.9 | 8.3–49.6 |
| Meropenem | 30 | 0.981 | 1.058 | −9.41 | 5.4–155 | 4.8–190 |
| Piperacillin | 36 | 0.983 | 1.076 | −5.03 | 29.1–343 | 22–350.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khromov, T.; Dihazi, G.H.; Brockmeyer, P.; Fischer, A.; Streit, F. 24/7 Therapeutic Drug Monitoring of Beta-Lactam Antibiotics with CLAM-2000. Antibiotics 2023, 12, 1526. https://doi.org/10.3390/antibiotics12101526
Khromov T, Dihazi GH, Brockmeyer P, Fischer A, Streit F. 24/7 Therapeutic Drug Monitoring of Beta-Lactam Antibiotics with CLAM-2000. Antibiotics. 2023; 12(10):1526. https://doi.org/10.3390/antibiotics12101526
Chicago/Turabian StyleKhromov, Tatjana, Gry Helene Dihazi, Phillipp Brockmeyer, Andreas Fischer, and Frank Streit. 2023. "24/7 Therapeutic Drug Monitoring of Beta-Lactam Antibiotics with CLAM-2000" Antibiotics 12, no. 10: 1526. https://doi.org/10.3390/antibiotics12101526
APA StyleKhromov, T., Dihazi, G. H., Brockmeyer, P., Fischer, A., & Streit, F. (2023). 24/7 Therapeutic Drug Monitoring of Beta-Lactam Antibiotics with CLAM-2000. Antibiotics, 12(10), 1526. https://doi.org/10.3390/antibiotics12101526






