Sugar-Based Monoester Surfactants: Synthetic Methodologies, Properties, and Biological Activities
Abstract
1. Introduction
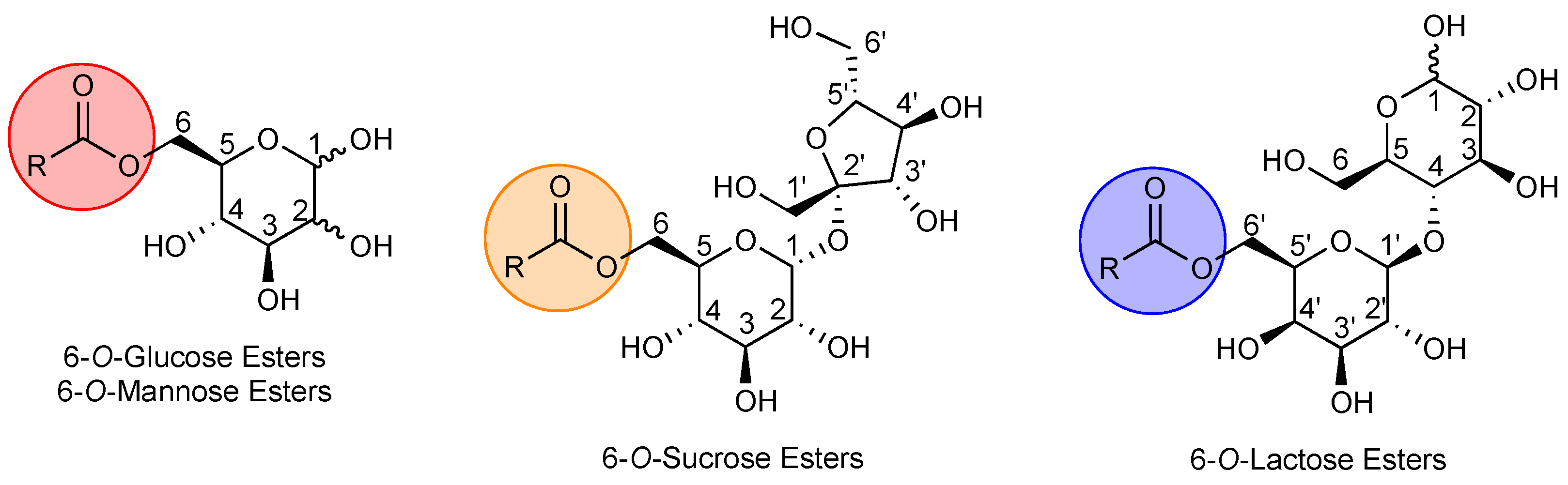
2. Synthesis of Sugar-Based Esters
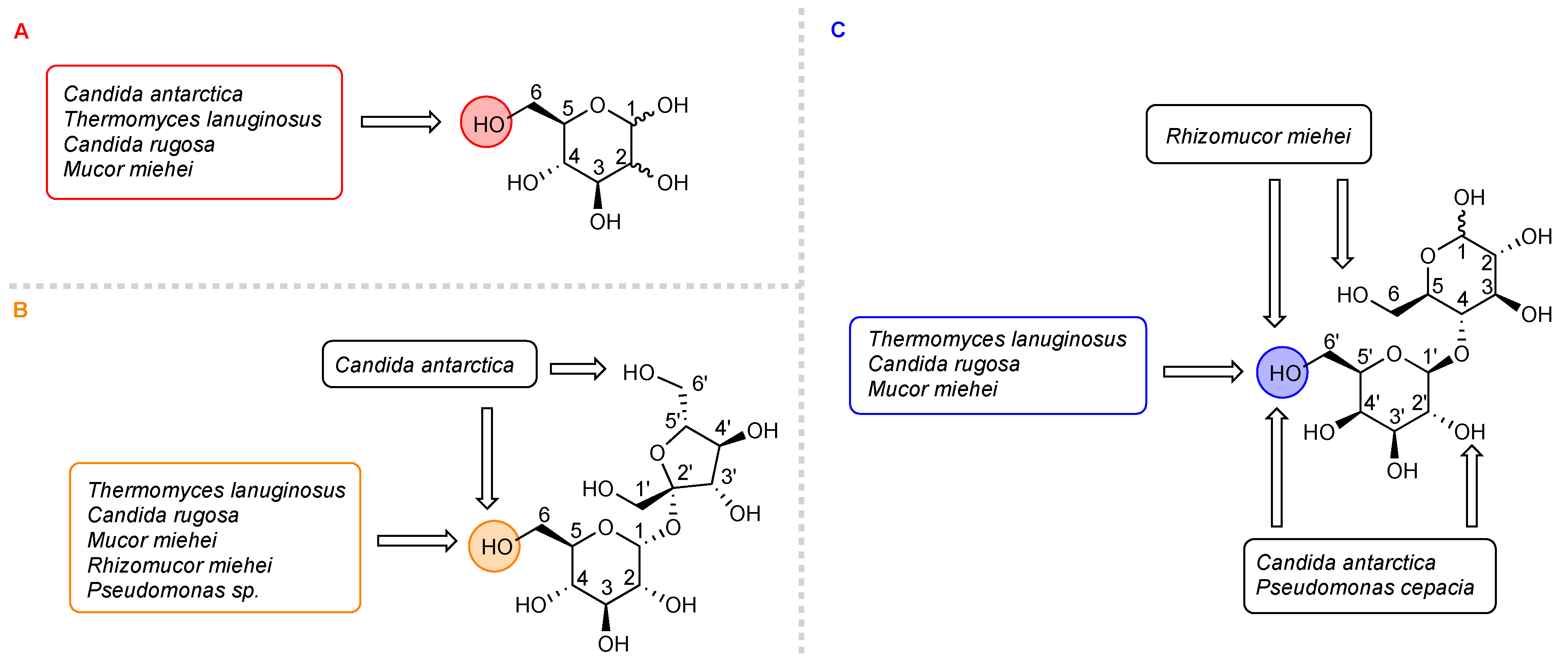
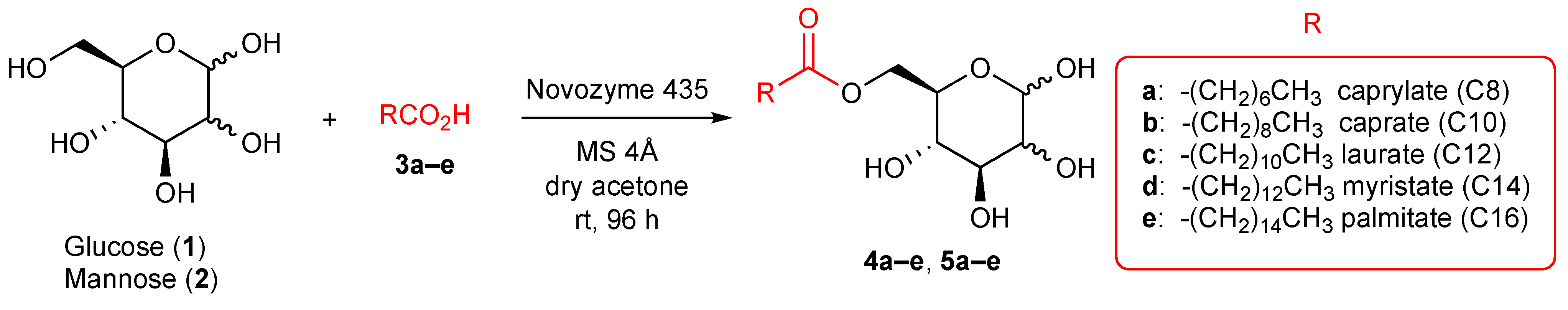
2.1. Synthesis of 6-O-Glucose Esters and 6-O-Mannose Esters
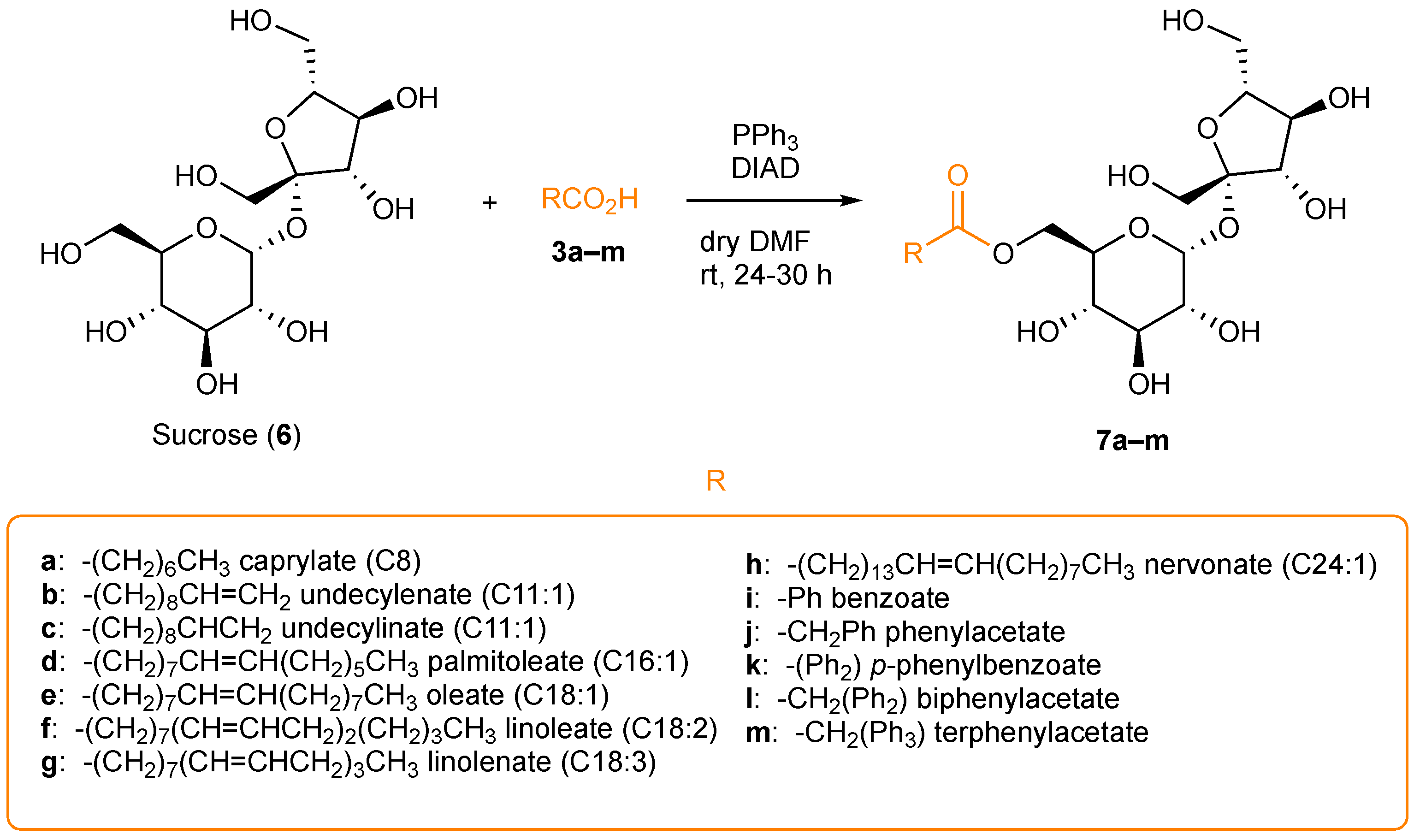
2.2. Synthesis of 6-O-Sucrose Esters
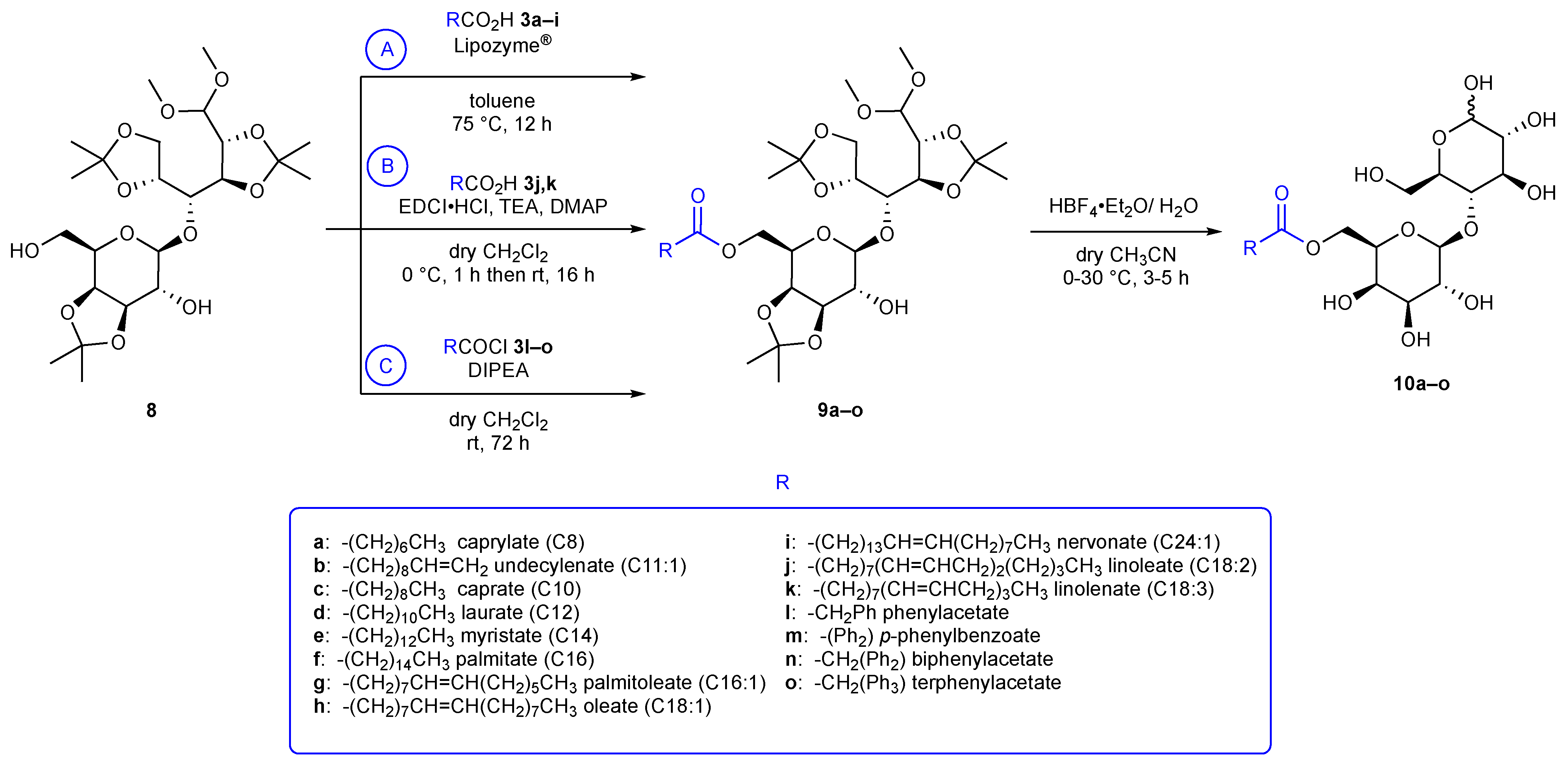
2.3. Synthesis of 6′-O-Lactose Esters
3. Surface Active Properties and Critical Micelle Concentration (CMC) Determination
4. Foaming and Emulsifying Properties
5. Sugar Esters as Permeability-Enhancing Compounds
6. Antimicrobial Activity
| SBEs | MIC (µg/mL) | Microorganisms | Reference | |
|---|---|---|---|---|
| Bacteria | Fungi | |||
| Monosaccharides | ||||
| Glucose C8 | 256 or >256 | E. coli O157:H7 ATCC 35150, K. pneumoniae ATCC 13833, P. aeruginosa ATCC 9027, S. enteritidis ATCC 13076, E. faecalis ATCC 29212, L. monocytogenes ATCC 7644, S. aureus ATCC 43387 | C. albicans ATCC 10231 | [23] |
| Glucose C10 | 256 | |||
| Glucose C12 | >256 | |||
| Glucose C14 | >256 | |||
| Glucose C16 | >256 | |||
| Mannose C8 | >256 | |||
| Mannose C10 | 256 | |||
| Mannose C12 | 256 | |||
| Mannose C14 | 256 or >256 | |||
| Mannose C16 | 256 or >256 | |||
| Sucrose | ||||
| Sucrose C12 | 128–1024 | S. aureus, MRSA and P. aeruginosa strains | T. mentagrophytes, T. rubrum, T. violaceum, E. floccosum, C. albicans ATCC 10231 | [46] |
| Sucrose C8 | 1024 or >1024 | E. faecalis ATCC 29212, E. coli O157:H7 ATCC 35150, S. aureus ATCC 29213 and ATCC 43300, K. pneumoniae ATCC 13883, L. monocytogenes ATCC 7644, S. enteritidis ATCC 13076, P. aeruginosa ATCC 9027 | A. fumigatus IDRAH01, A. niger ATCC 9642, Fusarium spp., C. albicans ATCC 10231 | [33] |
| Sucrose C11:1 | 512 to >1024 | |||
| Sucrose undecylinate | 1024 or >1024 | |||
| Sucrose C16:1 | 16 to >1024 | |||
| Sucrose C18:1 | 16 to >1024 | |||
| Sucrose C18:2 | 32 to >1024 | |||
| Sucrose C18:3 | 128 to >1024 | |||
| Sucrose C24:1 | 1024 or >1024 | |||
| Sucrose benzoate | 256 to >1024 | |||
| Sucrose phenylacetate | 256 to >1024 | |||
| Sucrose p-phenylbenzoate | 1024 or >1024 | |||
| Sucrose p-biphenylacetate | 1024 or >1024 | |||
| Sucrose p-terphenylacetate | >1024 | |||
| Lactose | ||||
| Lactose C8 | 256 | E. coli O157:H7 ATCC 35150, K. pneumoniae ATCC 13833, P. aeruginosa ATCC 9027, S. enteritidis ATCC 13076, E. faecalis ATCC 29212, L. monocytogenes ATCC 7644, S. aureus ATCC 43387 | C. albicans ATCC 10231 | [23] |
| Lactose C10 | 128–256 | |||
| Lactose C12 | 256 or >256 | |||
| Lactose C14 | 256 or >256 | |||
| Lactose C16 | 256 or >256 | |||
| Lactose phenylacetate | 256 or >256 | |||
| Lactose p-phenylbenzoate | 256 | |||
| Lactose p-biphenylacetate | 256 | |||
| Lactose p-terphenylacetate | 256 | |||
| Lactose C16:1 Lactose C24:1 | 64–128 | E. coli O157:H7 ATCC 35150, L. monocytogenes ATCC 7644, S. enteritidis ATCC 13076, E. faecalis ATCC 29212, S. aureus ATCC 43387, P. aeruginosa ATCC 9027, Y. enterocolitica ATCC 27729 | C. albicans ATCC 10231 | [42] |
| Lactose C18:1 | 128 | [44] | ||
| Lactose C11:1 | 512 to >1024 | S. aureus, MRSA and P. aeruginosa strains | T. mentagrophytes, T. rubrum, T. violaceum, E. floccosum, C. albicans ATCC 10231 | [78] |
| Lactose C18:2 Lactose C18:3 | 128 to >1024 | [46] | ||
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Makkar, R.S.; Rockne, K.J. Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 2003, 22, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Baker, I.J.A.; Matthews, B.; Suares, H.; Krodkiewska, I.; Furlong, D.N.; Grieser, F.; Drummond, C.I. Sugar fatty acid ester surfactants: Structure and ultimate aerobic biodegradability. J Surfactants Deterg. 2000, 3, 1–11. [Google Scholar] [CrossRef]
- Tracy, P.; Dasgupta, D.; More, S. Challenges and opportunities for production of C5 sugar fatty acid esters (SFAEs) from renewable resources. Ind. Crops Prod. 2023, 193, 116170. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, W.; Lu, Y.; Wang, Y.; Chen, X.; Bai, S.; Zhao, Y.; He, T.; Lao, F.; Shang, Y.; et al. Naturally occurring cinnamic acid sugar ester derivatives. Molecules 2016, 21, 1402. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouz-Chaambouni, S.; Ghribi, D. Glycolipid biosurfactants, main classes, functional properties and related potential applications in environmental biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Teng, Y.; Stewart, S.G.; Hai, Y.W.; Li, X.; Banwell, M.G.; Lan, P. Sucrose fatty acid esters: Synthesis, emulsifying capacities, biological activities and structure-property profiles. Crit. Rev. Food Sci. Nutr. 2020, 61, 3297–3317. [Google Scholar] [CrossRef]
- Staroń, J.; Dąbrowski, J.M.; Cichoń, E.; Guzik, M. Lactose esters: Synthesis and biotechnological applications. Crit. Rev. Biotechnol. 2018, 38, 245–258. [Google Scholar] [CrossRef]
- Plat, T.; Linhardt, R.J. Syntheses and applications of sucrose-based esters. J. Surfactant Deterg. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- AlFindee, M.N.; Zhang, Q.; Subedi, Y.P.; Shrestha, J.P.; Kawasaki, Y.; Grilley, M.; Takemoto, J.Y.; Chang, C.W.T. One-step synthesis of carbohydrate esters as antibacterial and antifungal agents. Bioorg. Med. Chem. 2018, 26, 765–774. [Google Scholar] [CrossRef]
- Semproli, R.; Robescu, M.S.; Sangiorgio, S.; Pargoletti, E.; Bavaro, T.; Rabuffetti, M.; Cappelletti, G.; Speranza, G.; Ubiali, D. From lactose to alkyl galactoside fatty acid esters as non-ionic biosurfactants: A two-step enzymatic approach to cheese whey valorization. ChemPlusChem 2023, 88, e202200331. [Google Scholar] [CrossRef] [PubMed]
- Verboni, M.; Lucarini, S.; Duranti, A. 6’-O-Lactose ester surfactants as an innovative opportunity in the pharmaceutical field: From synthetic methods to biological applications. Pharmaceuticals 2021, 14, 1306. [Google Scholar] [CrossRef] [PubMed]
- Markets and Markets Sucrose Esters Market Size, Share and Forecasts up to 2025 Industry Statistics & Trends. Available online: https://www.marketsandmarkets.com/Market-Reports/sucrose-esters-market-191170937.html (accessed on 18 April 2023).
- Grüninger, J.; Delavault, A.; Ochsenreither, K. Enzymatic glycolipid surfactant synthesis from renewables. Process Biochem. 2019, 87, 45–54. [Google Scholar] [CrossRef]
- Koumba Ibinga, S.K.; Fabre, J.F.; Bikanga, R.; Mouloungui, Z. Atypical reaction media and organized systems for the synthesis of low-substitution sugar esters. Front. Chem. 2019, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Cruces, M.A.; Plou, F.J.; Pastor, E.; Fuentes, G.; Bernabé, M.; Parra, J.L.; Ballesteros, A. Chemical versus enzymatic catalysis for the regioselective synthesis of sucrose esters of fatty acids. Stud. Surf. Sci. Catal. 2000, 130, 509–514. [Google Scholar] [CrossRef]
- Queneau, Y.; Chambert, S.; Besset, C.; Cheaib, R. Recent progress in the synthesis of carbohydrate-based amphiphilic materials: The examples of sucrose and isomaltulose. Carbohydr. Res. 2008, 343, 1999–2009. [Google Scholar] [CrossRef]
- Alvarez, E.; Villa, R.; Nieto, S.; Donaire, A.; Garcìa-Verduco, E.; Luis, S.V.; Lozano, P. The suitability of lipases for the synthesis of bioactive compounds with cosmeceutical applications. Mini-Rev. Org. Chem. 2021, 18, 515–528. [Google Scholar] [CrossRef]
- Kobayashi, T. Lipase-catalyzed syntheses of sugar esters in non-aqueous media. Biotechnol. Lett. 2011, 33, 1911–1919. [Google Scholar] [CrossRef]
- Coelho, A.L.S.; Orlandelli, R.C. Immobilized microbial lipases in the food industry: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1689–1703. [Google Scholar] [CrossRef]
- Treichel, H.; de Oliveira, D.; Mazutti, M.A.; Di Luccio, M.; Oliveira, J.V. A review on microbial lipases production. Food Bioprocess Technol. 2010, 3, 182–196. [Google Scholar] [CrossRef]
- Arcos, J.A.; Bernabé, M.; Otero, C. Quantitative enzymatic production of 6-O-acylglucose esters. Biotechnol. Bioeng. 1998, 57, 505–509. [Google Scholar] [CrossRef]
- Campana, R.; Merli, A.; Verboni, M.; Biondo, F.; Favi, G.; Duranti, A.; Lucarini, S. Synthesis and evaluation of saccharide-based aliphatic and aromatic esters as antimicrobial and antibiofilm agents. Pharmaceuticals 2019, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Zago, E.; Joly, N.; Chaveriat, L.; Lequart, V.; Martin, P. Enzymatic synthesis of amphiphilic carbohydrate esters: Influence of physicochemical and biochemical parameters. Biotechnol. Rep. 2021, 30, e00631. [Google Scholar] [CrossRef]
- Liang, M.Y.; Banwell, M.G.; Wang, Y.; Lan, P. Effect of variations in the fatty acid residue of lactose monoesters on their emulsifying properties and biological activities. J. Agric. Food Chem. 2018, 66, 12594–12603. [Google Scholar] [CrossRef] [PubMed]
- Arcens, D.E.; Grau, S.; Grelier, H.; Cramail, F.; Peruch, F. 6-O-glucose palmitate synthesis with lipase: Investigation of some key parameters. Mol. Catal. 2018, 460, 63–68. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Z.-L. Enzymatic synthesis of sugar fatty acid esters in ionic liquids. Catal. Sci. Technol. 2012, 2, 1767–1775. [Google Scholar] [CrossRef]
- Shin, D.W.; Mai, N.L.; Bae, S.W.; Koo, Y.M. Enhanced lipase-catalyzed synthesis of sugar fatty acid esters using supersaturated sugar solution in ionic liquids. Enzyme Microb. Technol. 2019, 126, 18–23. [Google Scholar] [CrossRef]
- Zhao, K.-H.; Cai, Y.-Z.; Lin, X.-S.; Xiong, J.; Halling, P.; Yang, Z. Enzymatic synthesis of glucose-based fatty acid esters in bisolvent systems containing ionic liquids or deep eutectic solvents. Molecules 2016, 21, 1294. [Google Scholar] [CrossRef]
- Jumina, J.; Mutmainah, M.; Purwono, B.; Pranowo, D.; Kurniawan, Y.S.; Syah, Y.S. Antibacterial and Antifungal Activity of Three Monosaccharide Monomyristate Derivatives. Molecules 2019, 24, 3692. [Google Scholar] [CrossRef]
- Molinier, V.; Fitremann, J.; Bouchu, A.; Queneau, Y. Sucrose esterification under Mitsunobu conditions: Evidence for the formation of 6-O-acyl-3′,6′-anhydrosucrose besides mono and diesters of fatty acids. Tetrahedron Asymmetry 2004, 15, 1753–1762. [Google Scholar] [CrossRef]
- Verboni, M.; Perinelli, D.R.; Qiu, C.Y.; Tiboni, M.; Aluigi, A.; Lucarini, S.; Lam, J.K.W.; Duranti, A. Synthesis and properties of sucrose- and lactose-based aromatic ester surfactants as potential drugs permeability enhancers. Pharmaceuticals 2023, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Verboni, M.; Sisti, M.; Campana, R.; Benedetti, S.; Palma, F.; Potenza, L.; Lucarini, S.; Duranti, A. Synthesis and biological evaluation of 6-O-sucrose monoester glycolipids as possible new antifungal agents. Pharmaceuticals 2023, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.P.; Liang, M.Y.; Ma, Y.R.; White, L.V.; Banwell, M.G.; Teng, Y.; Lan, P. Enzymatic synthesis of an homologous series of long- and very long-chain sucrose esters and evaluation of their emulsifying and biological properties. Food Hydrocoll. 2022, 124, 107149. [Google Scholar] [CrossRef]
- Shao, S.Y.; Shi, Y.G.; Wu, Y.; Bian, L.Q.; Zhu, Y.J.; Huang, X.Y.; Pan, Y.; Zeng, L.Y.; Zhang, R.R. Lipase-catalyzed synthesis of sucrose monolaurate and its antibacterial property and mode of action against four pathogenic bacteria. Molecules 2018, 23, 1118. [Google Scholar] [CrossRef] [PubMed]
- Habulin, M.; Sabeder, S.; Knez, Z. Enzymatic synthesis of sugar fatty acid esters in organic solvent and in supercritical carbon dioxide and their antimicrobial activity. J. Supercrit. Fluids 2008, 45, 338–345. [Google Scholar] [CrossRef]
- Sasayama, T.; Kanezawa, A.; Hiromori, K.; Takahashi, A.; Shibasaki-Kitakawa, N. Controlling reaction selectivity for sugar fatty acid ester synthesis by using resins with different basicities. Food Chem. 2021, 340, 128100. [Google Scholar] [CrossRef]
- Xie, M.F.; White, L.V.; Banwell, M.G.; Wang, Y.; Lan, P. Solvent-free synthesis of high-purity sucrose fatty acid monoesters and a comparison of their properties with those of their commercial counterparts. ACS Food Sci. Technol. 2021, 1, 1550–1560. [Google Scholar] [CrossRef]
- Thévenet, S.; Descotes, G.; Bouchu, A.; Queneau, Y. Hydrophobic effect driven esterification of sucrose in aqueous medium. J. Carb. Chem. 1997, 16, 691–696. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Yang, J. Regioselective formation of 6-O-acylsucroses and 6,3′-di-O-acylsucroses via the stannylene acetal method. Carbohydr. Res. 2007, 342, 2657–2663. [Google Scholar] [CrossRef]
- Sarney, D.B.; Kapeller, H.; Fregapane, G.; Vulfson, E.N. Chemo-enzymatic synthesis of disaccharide fatty acid esters. J. Am. Oil Chem. Soc. 1994, 71, 711–714. [Google Scholar] [CrossRef]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; Vllasaliu, D.; Casettari, L. Unsaturated fatty acids lactose esters: Cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.; Campana, M.; Stolnik, S.; Lam, J.; Casettari, L.; Duranti, A.; et al. Synthesis, structure–activity relationships and in vitro toxicity profile of lactose-based fatty acid monoesters as possible drug permeability enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Lucarini, S.; Fagioli, L.; Campana, R.; Vllasaliu, D.; Duranti, A.; Casettari, L. Lactose oleate as new biocompatible surfactant for pharmaceutical applications. Eur. J. Pharm. Biopharm. 2018, 124, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P. Lipases in lipophilization reactions. Biotechnol. Adv. 2007, 25, 515–536. [Google Scholar] [CrossRef]
- Tiboni, M.; Elmowafy, E.; El-Derany, M.O.; Benedetti, S.; Campana, R.; Verboni, M.; Potenza, L.; Palma, F.; Citterio, B.; Sisti, M.; et al. A combination of sugar esters and chitosan to promote in vivo wound care. Int. J. Pharm. 2022, 616, 121508. [Google Scholar] [CrossRef]
- Zaidan, U.H.; Abdul Rahman, M.B.; Othman, S.S.; Basri, M.; Abdulmalek, E.; Abdul Rahman, R.N.Z.R.; Salleh, A.B. Biocatalytic production of lactose ester catalysed by mica-based immobilised lipase. Food Chem. 2012, 131, 199–205. [Google Scholar] [CrossRef]
- Liang, M.Y.; Chen, Y.; Banwell, M.G.; Wang, Y.; Lan, P. Enzymatic preparation of a homologous series of long-chain 6-o-acylglucose esters and their evaluation as emulsifiers. J. Agric. Food Chem. 2018, 66, 3949–3956. [Google Scholar] [CrossRef]
- Enayati, M.; Gong, Y.; Abbaspourrad, A. Synthesis of lactose lauryl ester in organic solvents using aluminosilicate zeolite as a catalyst. Food Chem. 2019, 279, 401–407. [Google Scholar] [CrossRef]
- Karami, M.; Faraji, A.R.; Saremnezhad, S.; Soltani, M. Synthesis and characterization of a lactose-based biosurfactant by a novel nanodendritic catalyst and evaluating its efficacy as an emulsifier in a food emulsion system. RSC Adv. 2022, 12, 32280–32296. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant self-assembling and critical micelle concentration: One approach fits all? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F. Comparative study of surface-active properties and antimicrobial activities of disaccharide monoesters. PLoS ONE 2014, 9, e114845. [Google Scholar] [CrossRef]
- McCartney, F.; Perinelli, D.R.; Tiboni, M.; Cavanagh, R.; Lucarini, S.; Palmieri, G.F.; Casettari, L.; Brayden, D.J. Permeability-enhancing effects of three laurate-disaccharide monoesters across isolated rat intestinal mucosae. Int. J. Pharm. 2021, 601, 120593. [Google Scholar] [CrossRef]
- Ferrer, M.; Comelles, F.; Plou, F.J.; Cruces, M.A.; Fuentes, G.; Parra, J.L.; Ballesteros, A. Comparative surface activities of di- and trisaccharide fatty acid esters. Langmuir 2002, 18, 667–673. [Google Scholar] [CrossRef]
- An, D.; Zhang, X.; Liang, F.; Xian, M.; Feng, D.; Ye, Z. Synthesis, surface properties of glucosyl esters from renewable materials for use as biosurfactants. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 257–264. [Google Scholar] [CrossRef]
- Coppola, L.; Gordano, A.; Procopio, A.; Sindona, G. Phase equilibria and physical-chemical properties of sugar-based surfactants in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2002, 196, 175–187. [Google Scholar] [CrossRef]
- Watanabe, Y.; Miyawaki, Y.; Adachi, S.; Nakanishi, K.; Matsuno, R. Continuous production of acyl mannoses by immobilized lipase using a packed-bed reactor and their surfactant properties. Biochem. Eng. J. 2001, 8, 213–216. [Google Scholar] [CrossRef]
- Favrelle, A.; Boyère, C.; Laurent, P.; Broze, G.; Blecker, C.; Paquot, M.; Jérôme, C.; Debuigne, A. Enzymatic synthesis and surface active properties of novel hemifluorinated mannose esters. Carbohydr. Res. 2011, 346, 1161–1164. [Google Scholar] [CrossRef]
- Ren, K.; Lamsal, B.P. Synthesis of some glucose-fatty acid esters by lipase from Candida antarctica and their emulsion functions. Food Chem. 2017, 214, 556–563. [Google Scholar] [CrossRef]
- Soultani, S.; Ognier, S.; Engasser, J.M.; Ghoul, M. Comparative study of some surface active properties of fructose esters and commercial sucrose esters. Colloids Surf. A Physicochem. Eng. Asp. 2003, 227, 35–44. [Google Scholar] [CrossRef]
- Fernandes, R.N.; Simiqueli, A.A.; Vidigal, M.C.T.R.; Minim, V.P.R.; Minim, L.A. Kinetic stability of the oil-in-water emulsions and dynamic interfacial properties of mixtures of sucrose esters and polysaccharides. Food Chem. 2021, 357, 129693. [Google Scholar] [CrossRef]
- Maher, S.; Brayden, D.; Casettari, L.; Illum, L.; Maher, S.; Brayden, D.J.; Casettari, L.; Illum, L. Application of permeation enhancers in oral delivery of macromolecules: An update. Pharmaceutics 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef]
- Szuts, A.; Szabó-Révész, P. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef]
- Kürti, L.; Veszelka, S.; Bocsik, A.; Dung, N.T.K.; Ózsvári, B.; Puskás, L.G.; Kittel, Á.; Szabó-Révész, P.; Deli, M.A. The effect of sucrose esters on a culture model of the nasal barrier. Toxicol. In Vitro 2012, 26, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.; Hellinger, É.; Pilbat, A.-M.; Kittel, Á.; Török, Z.; Füredi, A.; Szakács, G.; Veszelka, S.; Sipos, P.; Ózsvári, B.; et al. Sucrose esters increase drug penetration, but do not inhibit p-glycoprotein in caco-2 intestinal epithelial cells. J. Pharm. Sci. 2014, 103, 3107–3119. [Google Scholar] [CrossRef]
- Glynn, A.; Igra, A.M.; Sand, S.; Ilbäck, N.G.; Hellenäs, K.E.; Rosén, J.; Aspenström-Fagerlund, B. Are additive effects of dietary surfactants on intestinal tight junction integrity an overlooked human health risk?—A mixture study on Caco-2 monolayers. Food Chem. Toxicol. 2017, 106, 314–323. [Google Scholar] [CrossRef]
- Maher, S.; Heade, J.; McCartney, F.; Waters, S.; Bleiel, S.B.; Brayden, D.J. Effects of surfactant-based permeation enhancers on mannitol permeability, histology, and electrogenic ion transport responses in excised rat colonic mucosae. Int. J. Pharm. 2018, 539, 11–22. [Google Scholar] [CrossRef]
- Alama, T.; Katayama, H.; Hirai, S.; Ono, S.; Kajiyama, A.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Enhanced oral delivery of alendronate by sucrose fatty acids esters in rats and their absorption-enhancing mechanisms. Int. J. Pharm. 2016, 515, 476–489. [Google Scholar] [CrossRef]
- Yamamoto, A.; Katsumi, H.; Kusamori, K.; Sakane, T. Improvement of intestinal absorption of poorly absorbable drugs by various sugar esters. Yakugaku Zasshi 2014, 134, 47–53. [Google Scholar] [CrossRef]
- Onishi, H.; Imura, Y.; Uchida, M.; Machida, Y. Enhancement potential of sucrose laurate (L-1695) on intestinal absorption of water-soluble high molecular weight compounds. Curr. Drug Deliv. 2012, 9, 487–494. [Google Scholar] [CrossRef] [PubMed]
- McCartney, F.; Rosa, M.; Brayden, D.J. Evaluation of sucrose laurate as an intestinal permeation enhancer for macromolecules: Ex vivo and in vivo studies. Pharmaceutics 2019, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020, 98, 442–442A. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 2 May 2023).
- Datt, N.; Poonuru, R.R.; Yadav, P.K. Development and characterization of griseofulvin loaded nanostructured lipid carrier gel for treating dermatophytosis. Food Hydrocoll. Health 2022, 2, 100074. [Google Scholar] [CrossRef]
- Lee, S.M.; Sandhu, G.; Walsh, M.K. Growth inhibitory properties of lactose fatty acid esters. Saudi J. Biol. Sci. 2017, 24, 1483–1488. [Google Scholar] [CrossRef][Green Version]
- Verboni, M.; Benedetti, S.; Campana, R.; Palma, F.; Potenza, L.; Sisti, M.; Duranti, A.; Lucarini, S. Synthesis and biological characterization of the new glycolipid lactose undecylenate (URB1418). Pharmaceuticals 2022, 15, 456. [Google Scholar] [CrossRef]
- Ferrer, M.; Soliveri, J.; Plou, F.J.; López-Cortés, N.; Reyes-Duarte, D.; Christensen, M.; Copa-Patiño, J.L.; Ballesteros, A. Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antarctica B, and their antimicrobial properties. Enzyme Microb. Technol. 2005, 36, 391–398. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Hao, T.; Li, S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015, 187, 370–377. [Google Scholar] [CrossRef]
- Tabisz, Ł.; Piotrowicz, Z.; Dąbrowska, M.; Dobrowolska, A.; Czaczyk, K.; Nowak, I.; Łęska, B. Sweet surfactants I: Fatty acid esters of sucralose. Food Chem. 2021, 358, 129827. [Google Scholar] [CrossRef]
- Karlová, T.; Poláková, L.; Šmidrkal, J.; Filip, V. Antimicrobial effects of fatty acid fructose esters. Czech J. Food Sci 2010, 28, 146–149. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Stewart, P.S. Biofilms strike back. Nat. Biotechnol. 2005, 23, 1378–1379. [Google Scholar] [CrossRef] [PubMed]
- Monds, R.D.; O’Toole, G.A. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends Microbiol. 2009, 17, 73–87. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verboni, M.; Perinelli, D.R.; Buono, A.; Campana, R.; Sisti, M.; Duranti, A.; Lucarini, S. Sugar-Based Monoester Surfactants: Synthetic Methodologies, Properties, and Biological Activities. Antibiotics 2023, 12, 1500. https://doi.org/10.3390/antibiotics12101500
Verboni M, Perinelli DR, Buono A, Campana R, Sisti M, Duranti A, Lucarini S. Sugar-Based Monoester Surfactants: Synthetic Methodologies, Properties, and Biological Activities. Antibiotics. 2023; 12(10):1500. https://doi.org/10.3390/antibiotics12101500
Chicago/Turabian StyleVerboni, Michele, Diego Romano Perinelli, Alessandro Buono, Raffaella Campana, Maurizio Sisti, Andrea Duranti, and Simone Lucarini. 2023. "Sugar-Based Monoester Surfactants: Synthetic Methodologies, Properties, and Biological Activities" Antibiotics 12, no. 10: 1500. https://doi.org/10.3390/antibiotics12101500
APA StyleVerboni, M., Perinelli, D. R., Buono, A., Campana, R., Sisti, M., Duranti, A., & Lucarini, S. (2023). Sugar-Based Monoester Surfactants: Synthetic Methodologies, Properties, and Biological Activities. Antibiotics, 12(10), 1500. https://doi.org/10.3390/antibiotics12101500








