Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Reduces Vibrio Populations and Increases Body Weight of European Seabass (Dicentrarchus labrax) Juveniles
Abstract
1. Introduction
2. Results
2.1. Effect of Feeding Diet on European Seabass Juvenile Growth Performance
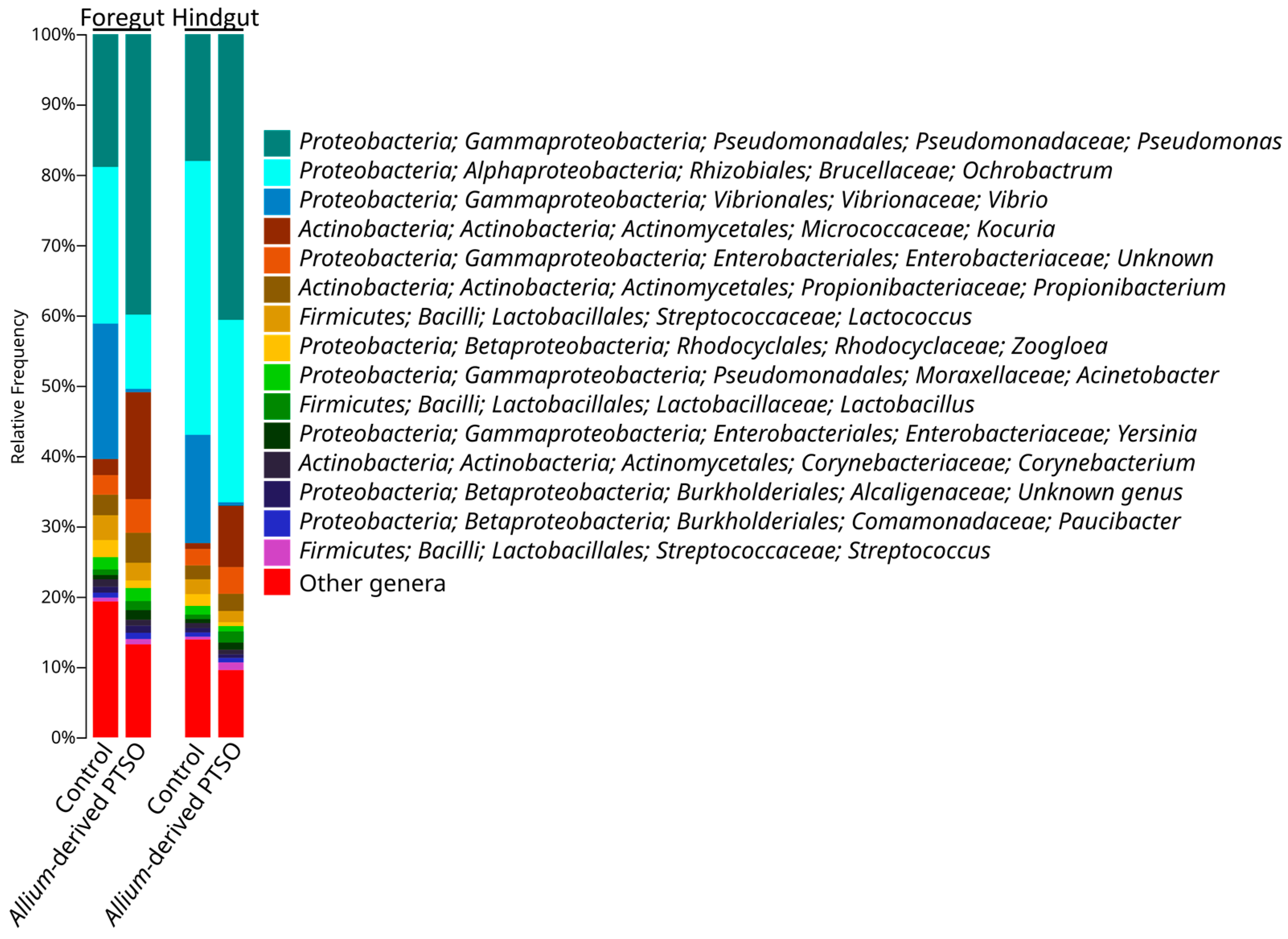
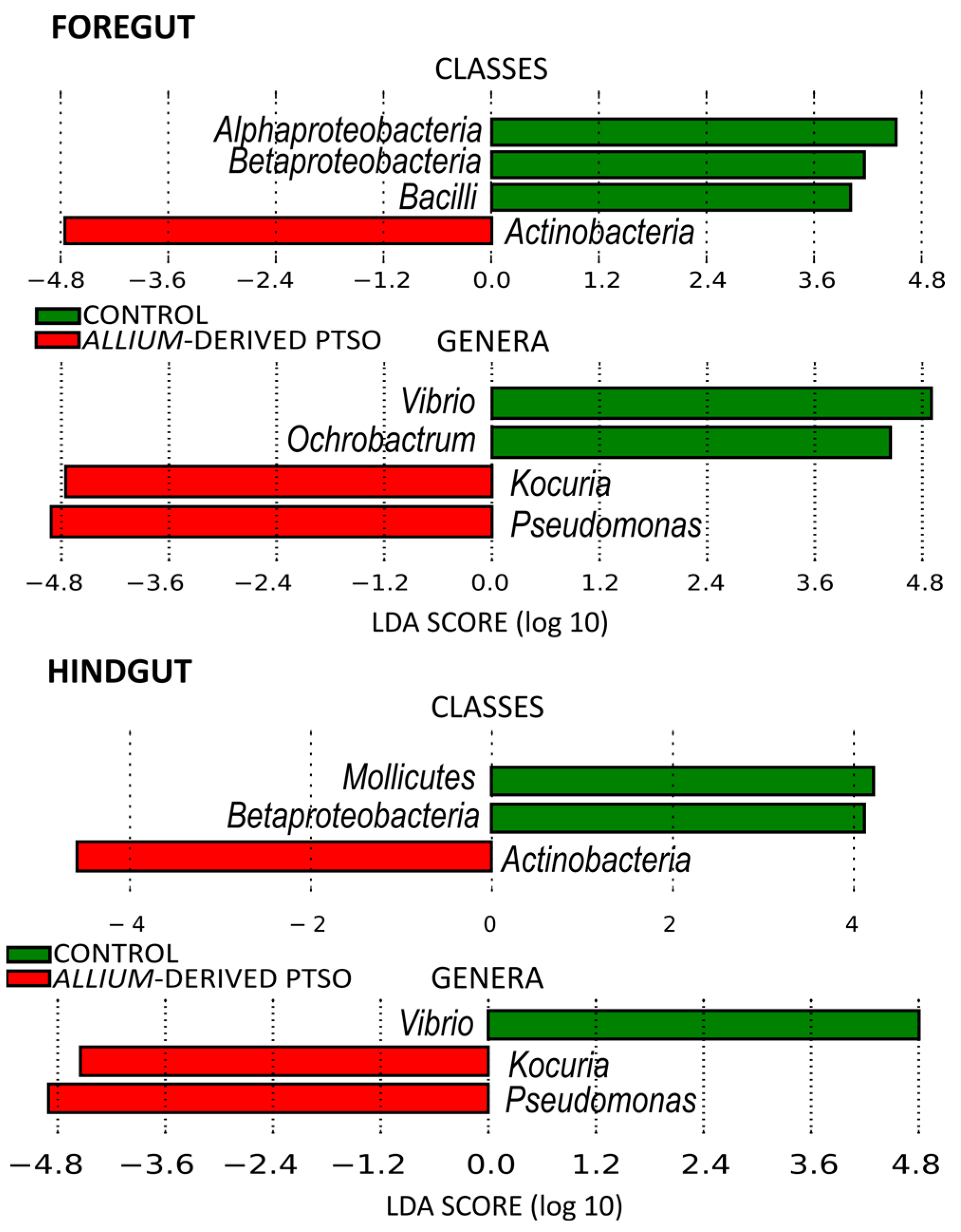
2.2. Bacterial Community Composition
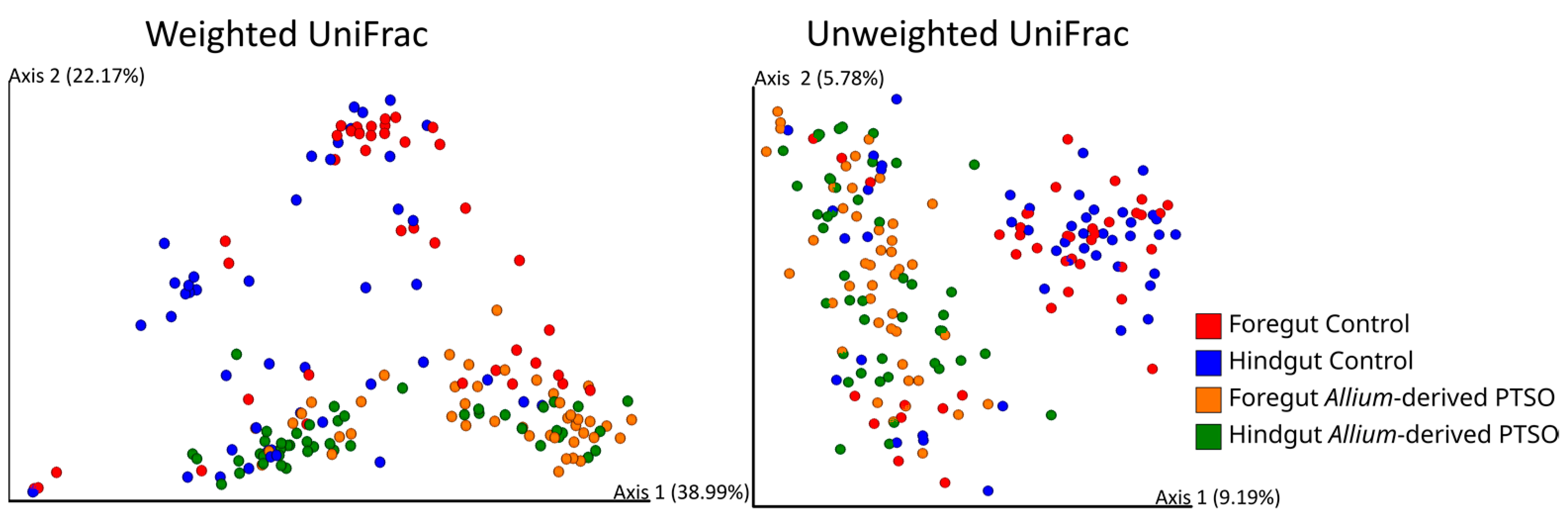
2.3. Effect of Feeding Diet on Alpha and Beta Diversity
3. Discussion
4. Materials and Methods
4.1. Animals, Experimental Design and Fish Sampling
4.2. DNA Extraction
4.3. V6-V8 16S rRNA Gene Amplification and High-Throughput Sequencing
4.4. Sequences Processing and Data Analysis
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). 2019. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 13 December 2022).
- Merino, G.; Barange, M.; Blanchard, J.L.; Harle, J.; Holmes, R.; Allen, I.; Allison, E.H.; Badjeck, M.C.; Dulvy, N.K.; Holt, J.; et al. Can Marine Fisheries and Aquaculture Meet Fish Demand from a Growing Human Population in a Changing Climate? Glob. Environ. Change 2012, 22, 795–806. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut Microbiota Metagenomics in Aquaculture: Factors Influencing Gut Microbiome and Its Physiological Role in Fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Resende, J.A.; Silva, V.L.; Fontes, C.O.; Souza-Filho, J.A.; Lopes, T.; De Oliveira, R.; Coelho, C.M.; César, D.E.; Diniz, C.G. Multidrug-Resistance and Toxic Metal Tolerance of Medically Important Bacteria Isolated from an Aquaculture System. Microbes Environ. 2012, 27, 449–455. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic Use in Aquaculture, Policies and Regulation, Health and Environmental Risks: A Review of the Top 15 Major Producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- He, S.; Wang, Q.; Li, S.; Ran, C.; Guo, X.; Zhang, Z.; Zhou, Z. Antibiotic Growth Promoter Olaquindox Increases Pathogen Susceptibility in Fish by Inducing Gut Microbiota Dysbiosis. Sci. China Life Sci. 2017, 60, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Cañada-Cañada, F.; La Peña, A.M.D.; Espinosa-Mansilla, A. Analysis of Antibiotics in Fish Samples. Anal. Bioanal. Chem. 2009, 395, 987–1008. [Google Scholar] [CrossRef] [PubMed]
- Commision, E. Regulation (EU) 2019/4 of the European Parliament and of the Council of 11 December 2018 on the Manufacture, Placing on the Market and Use of Medicated Feed, Amending Regulation (EC) No 183/2005 of the European Parliament and of the Council and Repealing. Off. J. Eur. Union 2018, 62, L:2019:004:TOC. [Google Scholar]
- Maron, D.F.; Smith, T.J.S.; Nachman, K.E. Restrictions on Antimicrobial Use in Food Animal Production: An International Regulatory and Economic Survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drugs Administration. FDA Reminds Retail Establishments of Upcoming Changes to the Use of Antibiotics in Food Animals; U.S. Food and Drugs Administration: Silver Spring, MD, USA, 2016.
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial Roles of Feed Additives as Immunostimulants in Aquaculture: A Review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Mora-Sánchez, B.; Balcázar, J.L. Biological Approaches for Disease Control in Aquaculture: Advantages, Limitations and Challenges. Trends Microbiol. 2018, 26, 896–903. [Google Scholar] [CrossRef]
- Sapsuha, Y.; Suprijatna, E.; Kismiati, S.; Sugiharto, S. Combination of probiotic and phythobiotic as an alternative for antibiotic growth promoter for broilers chickens-a review. Livest. Res. Rural. Dev. 2021, 33, 49. [Google Scholar]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of Phytogenic Products as Feed Additives for Swine and Poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef]
- Vidanarachchi, J.; Sims, I.M.; Iji, P. Phytobiotics: Alternatives to Antibiotic Growth Promoters in Monogastric Animal Feed. Recent Adv. Anim. Nutr. Aust. 2005, 15, 131–144. [Google Scholar]
- Gheisar, M.M.; Kim, I.H. Phytobiotics in Poultry and Swine Nutrition—A Review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar] [CrossRef]
- Hai, N. Van The Use of Medicinal Plants as Immunostimulants in Aquaculture: A Review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Bulfon, C.; Volpatti, D.; Galeotti, M. Current Research on the Use of Plant-Derived Products in Farmed Fish. Aquac. Res. 2015, 46, 513–551. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial Properties of Allium Species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Yin, Y.; Ma, X. The Nutritional Applications of Garlic (Allium sativum) as Natural Feed Additives in Animals. PeerJ 2021, 9, e11934. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Arroyo-Manzanares, N.; Gil, L.; García-Campaña, A.M. Use of Onion Extract as a Dairy Cattle Feed Supplement: Monitoring Propyl Propane Thiosulfonate as a Marker of Its Effect on Milk Attributes. J. Agric. Food Chem. 2017, 65, 793–799. [Google Scholar] [CrossRef]
- Zhu, Z.; Hang, S.; Zhu, H.; Zhong, S.; Mao, S.; Zhu, W. Effects of Garlic Oil on Milk Fatty Acid Profile and Lipogenesis-Related Gene Expression in Mammary Gland of Dairy Goats. J. Sci. Food Agric. 2013, 93, 560–567. [Google Scholar] [CrossRef]
- Sánchez, C.J.; Martínez-Miró, S.; Ariza, J.J.; Madrid, J.; Orengo, J.; Aguinaga, M.A.; Baños, A.; Hernández, F. Effect of Alliaceae Extract Supplementation on Performance and Intestinal Microbiota of Growing-Finishing Pig. Animals 2020, 10, 1557. [Google Scholar] [CrossRef]
- Zivkovic, V.; Stankovic, B.; Radovic, C.; Gogic, M.; Stanojkovic, A.; Obradovic, S.; Stojiljkovic, N. Garlic as Alternative for Antibiotics in Diet for Growing Pigs. Biotechnol. Anim. Husb. 2019, 35, 281–287. [Google Scholar] [CrossRef]
- Omer, H.A.A.; Ahmed, S.M.; Abdel-Magid, S.S.; El-Mallah, G.M.H.; Bakr, A.A.; Fattah, M.M.A. Nutritional Impact of Inclusion of Garlic (Allium sativum) and/or Onion (Allium cepa L.) Powder in Laying Hens’ Diets on Their Performance, Egg Quality, and Some Blood Constituents. Bull. Natl. Res. Cent. 2019, 43, 23. [Google Scholar] [CrossRef]
- Abad, P.; Arroyo-Manzanares, N.; Ariza, J.J.; Baños, A.; García-Campaña, A.M. Effect of Allium Extract Supplementation on Egg Quality, Productivity, and Intestinal Microbiota of Laying Hens. Animals 2021, 11, 41. [Google Scholar] [CrossRef]
- Ismail, I.E.; Alagawany, M.; Taha, A.E.; Puvača, N.; Laudadio, V.; Tufarelli, V. Effect of Dietary Supplementation of Garlic Powder and Phenyl Acetic Acid on Productive Performance, Blood Haematology, Immunity and Antioxidant Status of Broiler Chickens. Asian-Australas. J. Anim. Sci. 2020, 34, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.E.; Al-Khalaifah, H.S.; Mohamed, W.A.M.; Gharib, H.S.A.; Osman, A.; Al-Gabri, N.A.; Amer, S.A. Effects of Phenolic-Rich Onion (Allium cepa L.) Extract on the Growth Performance, Behavior, Intestinal Histology, Amino Acid Digestibility, Antioxidant Activity, and the Immune Status of Broiler Chickens. Front. Vet. Sci. 2020, 7, 728. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Gutiérrez, R.; Lago-Lestón, A.; Vargas-Albores, F.; Cicala, F.; Martínez-Porchas, M. Exploring the Garlic (Allium sativum) Properties for Fish Aquaculture. Fish Physiol. Biochem. 2021, 47, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Akrami, R.; Gharaei, A.; Mansour, M.R.; Galeshi, A. Effects of Dietary Onion (Allium cepa) Powder on Growth, Innate Immune Response and Hemato–Biochemical Parameters of Beluga (Huso Huso Linnaeus, 1754) Juvenile. Fish Shellfish. Immunol. 2015, 45, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Gao, Y. Review of the Application of Garlic, Allium sativum, in Aquaculture. J. World Aquac. Soc. 2012, 43, 447–458. [Google Scholar] [CrossRef]
- Büyükdeveci, M.E.; Balcázar, J.L.; Demirkale, İ.; Dikel, S. Effects of Garlic-Supplemented Diet on Growth Performance and Intestinal Microbiota of Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2018, 486, 170–174. [Google Scholar] [CrossRef]
- Zare, M.; Tran, H.Q.; Prokešová, M.; Stejskal, V. Effects of Garlic Allium sativum Powder on Nutrient Digestibility, Haematology, and Immune and Stress Responses in Eurasian Perch Perca fluviatilis Juveniles. Animals 2021, 11, 2735. [Google Scholar] [CrossRef]
- Motlagh, H.A.; Safari, O.; Selahvarzi, Y.; Baghalian, A.; Kia, E. Non-Specific Immunity Promotion in Response to Garlic Extract Supplemented Diets in Female Guppy (Poecilia reticulata). Fish Shellfish. Immunol. 2020, 97, 96–99. [Google Scholar] [CrossRef]
- Guillamón, E.; Andreo-Martínez, P.; Mut-Salud, N.; Fonollá, J.; Baños, A. Beneficial Effects of Organosulfur Compounds from Allium cepa on Gut Health: A Systematic Review. Foods 2021, 10, 1680. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Baños-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In Vitro Antibacterial Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate Derived from Allium spp. Against Gram-Negative and Gram-Positive Multidrug-Resistant Bacteria Isolated from Human Samples. BioMed Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Gil-Martinez, L.; Ariza-Romero, J.J.; Maroto-Tello, A.; Baños-Arjona, A.; Gutierrez-Fernandez, J. Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals 2021, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved Resistance to Eimeria acervulina Infection in Chickens Due to Dietary Supplementation with Garlic Metabolites. Br. J. Nutr. 2013, 109, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Aranda-Olmedo, I.; Rubio, L.A. Garlic Derivative PTS-O Modulates Intestinal Microbiota Composition and Improves Digestibility in Growing Broiler Chickens. Anim. Feed. Sci. Technol. 2013, 181, 87–92. [Google Scholar] [CrossRef]
- Rabelo-Ruiz, M.; Ariza-Romero, J.J.; Zurita-González, M.J.; Martín-Platero, A.M.; Baños, A.; Maqueda, M.; Valdivia, E.; Martínez-Bueno, M.; Peralta-Sánchez, J.M. Allium-Based Phytobiotic Enhances Egg Production in Laying Hens through Microbial Composition Changes in Ileum and Cecum. Animals 2021, 11, 448. [Google Scholar] [CrossRef]
- Rabelo-Ruiz, M.; Teso-Pérez, C.; Peralta-Sánchez, J.M.; Ariza, J.J.; Martín-Platero, A.M.; Casabuena-Rincón, Ó.; Vázquez-Chas, P.; Guillamón, E.; Aguinaga-Casañas, M.A.; Maqueda, M.; et al. Allium Extract Implements Weaned Piglet’s Productive Parameters by Modulating Distal Gut Microbiota. Antibiotics 2021, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Peinado, M.J.; Aranda-Olmedo, I.; Abecia, L.; Suárez-Pereira, E.; Melle, C.O.; Fernández, J.M.G.; Rubio, L.A. Effects of Feed Additives on Ileal Mucosa-Associated Microbiota Composition of Broiler Chickens. J. Anim. Sci. 2015, 93, 3410–3420. [Google Scholar] [CrossRef]
- Vezza, T.; Garrido-Mesa, J.; Diez-Echave, P.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; García, F.; Sánchez, M.; Toral, M.; Romero, M.; Duarte, J.; et al. Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Attenuates Metabolic Alterations in Mice Fed a High-Fat Diet through Its Anti-Inflammatory and Prebiotic Properties. Nutrients 2021, 13, 2595. [Google Scholar] [CrossRef]
- Lira, A.C.; Prieto, A.I.; Baños, A.; Guillamón, E.; Moyano, R.; Jos, A.; Cameán, A.M. Safety Assessment of Propyl-Propane-Thiosulfonate (PTSO): 90-Days Oral Subchronic Toxicity Study in Rats. Food Chem. Toxicol. 2020, 144, 111612. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Feijoo, C.G.; Navarrete, P. Antibiotics in Aquaculture—Use, Abuse and Alternatives. In Health and Environment in Aquaculture; IntechOpen Limited: London, UK, 2012. [Google Scholar]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of Plant Extracts in Fish Aquaculture as an Alternative to Chemotherapy: Current Status and Future Perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Kothari, D.; Lee, W.-D.; Niu, K.-M.; Kim, S.-K. The Genus Allium as Poultry Feed Additive: A Review. Animals 2019, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Peinado, M.J.; Ruiz, R.; Echavarri, A.; Rubio, L.A. Garlic Derivative Propyl Propane Thiosulfonate Is Effective against Broiler Enteropathogens in Vivo. Poult. Sci. 2012, 91, 2148–2157. [Google Scholar] [CrossRef]
- Rubio, L.A.; Peinado, M.J.; Ruiz, R.; Suárez-Pereira, E.; Mellet, C.O.; Fernández, J.M.G. Correlations between Changes in Intestinal Microbiota Composition and Performance Parameters in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2015, 99, 418–423. [Google Scholar] [CrossRef]
- Rabelo-Ruiz, M.; Newman-Portela, A.M.; Peralta-Sánchez, J.M.; Martín-Platero, A.M.; Agraso, M.d.M.; Bermúdez, L.; Aguinaga, M.A.; Baños, A.; Maqueda, M.; Valdivia, E.; et al. Beneficial Shifts in the Gut Bacterial Community of Gilthead Seabream (Sparus aurata) Juveniles Supplemented with Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO). Animals 2022, 12, 1821. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.M.; El-Bahr, S.M.; Al-Khamees, S. Influence of Dietary Garlic (Allium sativum) and/or Ascorbic Acid on Performance, Feed Utilization, Body Composition and Hemato-Biochemical Parameters of Juvenile Asian Sea Bass (Lates calcarifer). Animals 2020, 10, 2396. [Google Scholar] [CrossRef] [PubMed]
- Talpur, A.D.; Ikhwanuddin, M. Dietary Effects of Garlic (Allium sativum) on Haemato-Immunological Parameters, Survival, Growth, and Disease Resistance against Vibrio Harveyi Infection in Asian Sea Bass, Lates Calcarifer (Bloch). Aquaculture 2012, 364–365, 6–12. [Google Scholar] [CrossRef]
- Motlagh, H.A.; Paolucci, M.; Bami, M.L.; Safari, O. Sexual Parameters, Digestive Enzyme Activities, and Growth Performance of Guppy (Poecilia reticulata) Fed Garlic (Allium sativum) Extract Supplemented Diets. J. World Aquac. Soc. 2020, 51, 1087–1097. [Google Scholar] [CrossRef]
- Nya, E.J.; Dawood, Z.; Austin, B. The Garlic Component, Allicin, Prevents Disease Caused by Aeromonas Hydrophila in Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2010, 33, 293–300. [Google Scholar] [CrossRef]
- Bae, Y.; Koo, B.; Lee, S.; Mo, J.; Oh, K.; Mo, I.P. Bacterial Diversity and Its Relationship to Growth Performance of Broilers. Korean J. Vet. Res. 2017, 57, 159–167. [Google Scholar] [CrossRef]
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut Microbiome Diversity and High-Fibre Intake Are Related to Lower Long-Term Weight Gain. Int. J. Obes. 2017, 41, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Q.; Ringø, E.; Wu, X.; He, Y.; Yang, D. The Influence of Weight and Gender on Intestinal Bacterial Community of Wild Largemouth Bronze Gudgeon (Coreius guichenoti, 1874). BMC Microbiol. 2016, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Betiku, O.C.; Yeoman, C.J.; Gaylord, T.G.; Americus, B.; Olivo, S.; Duff, G.C.; Sealey, W.M. Water System Is a Controlling Variable Modulating Bacterial Diversity of Gastrointestinal Tract and Performance in Rainbow Trout. PLoS ONE 2018, 13, e0195967. [Google Scholar] [CrossRef] [PubMed]
- Fadel, A.; Mabrok, M.; Aly, S. Epizootics of Pseudomonas Anguilliseptica among Cultured Seabream (Sparus aurata) Populations: Control and Treatment Strategies. Microb. Pathog. 2018, 121, 1–8. [Google Scholar] [CrossRef]
- Wiklund, T. Pseudomonas Anguilliseptica Infection as a Threat to Wild and Farmed Fish in the Baltic Sea. Microbiol. Aust. 2016, 37, 135. [Google Scholar] [CrossRef]
- Altinok, I.; Kayis, S.; Capkin, E. Pseudomonas Putida Infection in Rainbow Trout. Aquaculture 2006, 261, 850–855. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Sukumaran, V. Effects of Dietary Supplementation of Potential Probiotic Pseudomonas Aeruginosa VSG-2 on the Innate Immunity and Disease Resistance of Tropical Freshwater Fish, Labeo Rohita. Fish Shellfish. Immunol. 2012, 32, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Melchiorsen, J.; Spanggaard, B.; Huber, I.; Nielsen, T.F. Inhibition of Vibrio Anguillarum by Pseudomonas Fluorescens AH2, a Possible Probiotic Treatment of Fish. Appl. Environ. Microbiol. 1999, 65, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.; Eissa, A.E.; Hanna, M.; Okada, M.A. Identifying Some Pathogenic Vibrio/Photobacterium Species during Mass Mortalities of Cultured Gilthead Seabream (Sparus aurata) and European Seabass (Dicentrarchus labrax) from Some Egyptian Coastal Provinces. Int. J. Vet. Sci. Med. 2013, 1, 87–95. [Google Scholar] [CrossRef]
- Mohamad, N.; Amal, M.N.A.; Yasin, I.S.M.; Saad, M.Z.; Nasruddin, N.S.; Al-saari, N.; Mino, S.; Sawabe, T. Vibriosis in Cultured Marine Fishes: A Review. Aquaculture 2019, 512, 734289. [Google Scholar] [CrossRef]
- Stratev, D.; Zhelyazkov, G.; Noundou, X.S.; Krause, R.W.M. Beneficial Effects of Medicinal Plants in Fish Diseases. Aquac. Int. 2018, 26, 289–308. [Google Scholar] [CrossRef]
- Natasya-Ain, R.; Eirna-Liza, N.; Jasmin, M.Y.; Karim, M. Antibacterial Activity of Garlic Extracts on Fish Pathogenic Bacteria. J. Environ. Biol. 2018, 39, 808–812. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Moreno, F.J.; Baños, A.; Nuñez, C.; Guillamón, E.; Cameán, A.M. Acute Toxicological Studies of the Main Organosulfur Compound Derived from Allium Sp. Intended to Be Used in Active Food Packaging. Food Chem. Toxicol. 2015, 82, 1–11. [Google Scholar] [CrossRef]
- Abad, P.; Arroyo-Manzanares, N.; García-Campaña, A.M. A Rapid and Simple UHPLC-ESI-MS/MS Method for the Screening of Propyl Propane Thiosulfonate, a New Additive for Animal Feed. Anal. Methods 2016, 8, 3730–3739. [Google Scholar] [CrossRef]
- Martín-Platero, A.M.; Valdivia, E.; Maqueda, M.; Martínez-Bueno, M. Fast, Convenient, and Economical Method for Isolating Genomic DNA from Lactic Acid Bacteria Using a Modification of the Protein “Salting-out” Procedure. Anal. Biochem. 2007, 366, 102–104. [Google Scholar] [CrossRef]
- Comeau, A.M.; Li, W.K.W.; Tremblay, J.-É.; Carmack, E.C.; Lovejoy, C. Arctic Ocean Microbial Community Structure before and after the 2007 Record Sea Ice Minimum. PLoS ONE 2011, 6, e27492. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Xu, Z.Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; McDonald, D.; Gonzalez, A.; Navas-Molina, J.A.; Jiang, L.; Xu, Z.Z.; Winker, K.; Kado, D.M.; Orwoll, E.; Manary, M.; et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. mSystems 2018, 3, e00021-18. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Baeza, Y.; Gonzalez, A.; Smarr, L.; McDonald, D.; Morton, J.T.; Navas-Molina, J.A.; Knight, R. Bringing the Dynamic Microbiome to Life with Animations. Cell Host Microbe 2017, 21, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A Tool for Visualizing High-Throughput Microbial Community Data. GigaScience 2013, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; No 2010/63/EU; European Commission: Brussels, Belgium, 2010; pp. 33–79.


| Control | Allium-Derived PTSO | p | |
|---|---|---|---|
| Body Weight Day 0 (g/fish) | 3.72 ± 0.05 | 3.84 ± 0.02 | 0.110 |
| Body Weight Day 12 (g/fish) | 4.64 ± 0.06 | 4.70 ± 0.04 | 0.438 |
| Body Weight Day 26 (g/fish) | 5.90 ± 0.12 | 5.87 ± 0.09 | 0.818 |
| Body Weight Day 42 (g/fish) | 8.21 ± 0.12 | 8.14 ± 0.08 | 0.640 |
| Body Weight Day 63 (g/fish) | 11.96 ± 0.09 | 12.25 ± 0.10 | 0.089 |
| Body Weight Day 89 (g/fish) | 21.14 ± 0.21 | 22.08 ± 0.08 | 0.013 |
| Alpha Diversity Index | Explanatory Variables | D.f | F | p |
|---|---|---|---|---|
| Diet | 1177 | 83.97 | <0.001 | |
| Chao1 Index | Gut Region | 1177 | 0.02 | 0.899 |
| Diet*Gut Region | 1177 | 1.52 | 0.220 | |
| Diet | 1177 | 79.90 | <0.001 | |
| Faith PD | Gut Region | 1177 | 0.02 | 0.903 |
| Diet*Gut Region | 1177 | 0.36 | 0.547 | |
| Diet | 1177 | 95.10 | <0.001 | |
| OTUs Richness | Gut Region | 1177 | 0.21 | 0.652 |
| Diet*Gut Region | 1177 | 0.55 | 0.459 | |
| Diet | 1177 | 6.51 | 0.012 | |
| Shannon Diversity Index | Gut Region | 1177 | 15.23 | <0.001 |
| Diet*Gut Region | 1177 | 0.96 | 0.330 |
| β-Diversity Distance Matrix | Explanatory Variables | D.f | Pseudo-F | p |
|---|---|---|---|---|
| Diet | 1177 | 31.51 | 0.001 | |
| Weighted UniFrac | Gut Region | 1177 | 14.00 | 0.001 |
| Diet*Gut Region | 1177 | 0.98 | 0.409 | |
| Diet | 1177 | 8.89 | 0.001 | |
| Unweighted UniFrac | Gut Region | 1177 | 1.05 | 0.325 |
| Diet*Gut Region | 1177 | 0.94 | 0.595 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo-Ruiz, M.; Peralta-Sánchez, J.M.; Martín-Platero, A.M.; Ruiz, A.J.; Agraso, M.d.M.; Bermúdez, L.; Ariza, J.J.; Baños, A.; Valdivia, E.; Martínez-Bueno, M. Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Reduces Vibrio Populations and Increases Body Weight of European Seabass (Dicentrarchus labrax) Juveniles. Antibiotics 2023, 12, 134. https://doi.org/10.3390/antibiotics12010134
Rabelo-Ruiz M, Peralta-Sánchez JM, Martín-Platero AM, Ruiz AJ, Agraso MdM, Bermúdez L, Ariza JJ, Baños A, Valdivia E, Martínez-Bueno M. Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Reduces Vibrio Populations and Increases Body Weight of European Seabass (Dicentrarchus labrax) Juveniles. Antibiotics. 2023; 12(1):134. https://doi.org/10.3390/antibiotics12010134
Chicago/Turabian StyleRabelo-Ruiz, Miguel, Juan Manuel Peralta-Sánchez, Antonio Manuel Martín-Platero, Ana J. Ruiz, María del Mar Agraso, Laura Bermúdez, Juan José Ariza, Alberto Baños, Eva Valdivia, and Manuel Martínez-Bueno. 2023. "Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Reduces Vibrio Populations and Increases Body Weight of European Seabass (Dicentrarchus labrax) Juveniles" Antibiotics 12, no. 1: 134. https://doi.org/10.3390/antibiotics12010134
APA StyleRabelo-Ruiz, M., Peralta-Sánchez, J. M., Martín-Platero, A. M., Ruiz, A. J., Agraso, M. d. M., Bermúdez, L., Ariza, J. J., Baños, A., Valdivia, E., & Martínez-Bueno, M. (2023). Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Reduces Vibrio Populations and Increases Body Weight of European Seabass (Dicentrarchus labrax) Juveniles. Antibiotics, 12(1), 134. https://doi.org/10.3390/antibiotics12010134






