Pre-Referral Microbiology in Long Bone Infection: What Can It Tell Us?
Abstract
1. Introduction
2. Results
2.1. Demographics and Sampling Information
2.2. Pre-Referral Microbiology Versus Intra-Operative Sampling
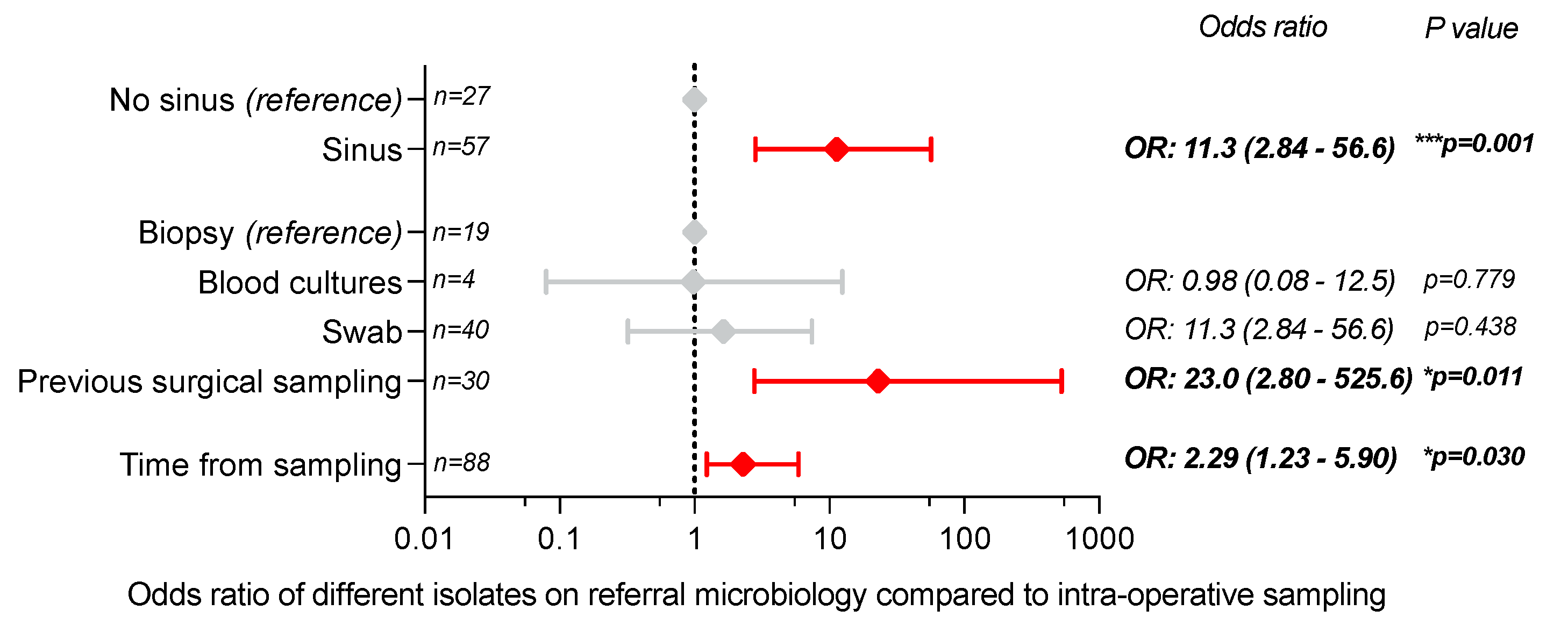
2.3. Factors Predicting Different Pre-Referral Microbiology from Intra-Operative Sampling Isolates
2.4. Factors Predicting Increased Resistance Patterns on Intra-Operative Sampling
3. Discussion
4. Materials and Methods
4.1. Patient Identification
4.2. Data Acquisition
4.3. Comparison of Pre-Referral Microbiology to Intra-Operative Sampling
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziran, B.H.; Rao, N.; Hall, R.A. A dedicated team approach enhances outcomes of osteomyelitis treatment. Clin. Orthop. Relat. Res. 2003, 414, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Salvana, J.; Rodner, C.; Browner, B.D.; Livingston, K.; Schreiber, J.; Pesanti, E. Chronic osteomyelitis: Results obtained by an integrated team approach to management. Conn. Med. 2005, 69, 195–202. [Google Scholar] [PubMed]
- Walter, N.; Rupp, M.; Baertl, S.; Alt, V. The role of multidisciplinary teams in musculoskeletal infection. Bone Jt. Res. 2022, 11, 6. [Google Scholar] [CrossRef]
- Hotchen, A.J.; Wismayer, M.G.; Robertson-Waters, E.; McDonnell, S.M.; Kendrick, B.; Taylor, A.; Alvand, A.; McNally, M. The Joint-Specific BACH classification: A predictor of outcome in prosthetic joint infection. EClinicalMedicine 2021, 42, 101192. [Google Scholar] [CrossRef] [PubMed]
- Hotchen, A.J.; Dudareva, M.; Corrigan, R.A.; Ferguson, J.Y.; McNally, M.A. Can we predict outcome after treatment of long bone osteomyelitis? A study of patient-reported quality of life stratified with the BACH classification. Bone Jt. J. 2020, 102, 1587–1596. [Google Scholar] [CrossRef]
- Vasoo, S.; Chan, M.; Sendi, P.; Berbari, E. The Value of Ortho-ID Teams in Treating Bone and Joint Infections. J. Bone Jt. Infect. 2019, 4, 295–299. [Google Scholar] [CrossRef]
- Depypere, M.; Kuehl, R.; Metsemakers, W.J.; Senneville, E.; McNally, M.A.; Obremskey, W.T.; Zimmerli, W.; Atkins, B.L.; Trampuz, A. Recommendations for Systemic Antimicrobial Therapy in Fracture-Related Infection: A Consensus from an International Expert Group. J. Orthop. Trauma. 2020, 34, 30. [Google Scholar] [CrossRef]
- Atkins, B.L.; Athanasou, N.; Deeks, J.J.; Crook, D.W.; Simpson, H.; Peto, T.E.; McLardy-Smith, P.; Berendt, A.R.; Group, T.O.; The OSIRIS Collaborative Study Group. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J. Clin. Microbiol. 1998, 36, 2932–2939. [Google Scholar] [CrossRef]
- Dudareva, M.; Barrett, L.K.; Morgenstern, M.; Atkins, B.L.; Brent, A.J.; McNally, M.A. Providing an Evidence Base for Tissue Sampling and Culture Interpretation in Suspected Fracture-Related Infection. J. Bone Jt. Surg. Am. 2021, 103, 977–983. [Google Scholar] [CrossRef]
- Atkins, B.L.; Bowler, I.C. The diagnosis of large joint sepsis. J. Hosp. Infect. 1998, 40, 263–274. [Google Scholar] [CrossRef]
- Minassian, A.M.; Newnham, R.; Kalimeris, E.; Bejon, P.; Atkins, B.L.; Bowler, I.C. Use of an automated blood culture system (BD BACTECTM) for diagnosis of prosthetic joint infections: Easy and fast. BMC Infect. Dis. 2014, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Alexander, M.; Bruce, S.; O’Connell, M.; Beecroft, S.; McNally, M. A retrospective cohort study comparing clinical outcomes and healthcare resource utilisation in patients undergoing surgery for osteomyelitis in England: A case for reorganising orthopaedic infection services. J. Bone Jt. Infect. 2021, 6, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Dudareva, M.; Vicentine, M.; Hotchen, A.; McNally, M. Microbiology of recurrent bone and joint infections demonstrates both microbial persistence and replacement. In Proceedings of the European Bone and Joint Infection Society (EBJIS) Meeting, Graz, Austria, 8–10 September 2022. [Google Scholar]
- Dudareva, M.; Hotchen, A.J.; Ferguson, J.; Hodgson, S.; Scarborough, M.; Atkins, B.L.; McNally, M.A. The microbiology of chronic osteomyelitis: Changes over ten years. J. Infect. 2019, 79, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, G.M.; Dibas, M.; Dung, N.M.; Alkhebairy, A.A.; Mahmoud, M.H.; Ibrahim, M.H.; Abd Elhady, N.R.; Sayed, A.M.; Gehad, A.S.; Abdelrahman, A.S.; et al. Concordance of bone and non-bone specimens in microbiological diagnosis of osteomyelitis: A systematic review and meta-analysis. J. Infect. Public Health 2020, 13, 1682–1693. [Google Scholar] [CrossRef]
- Govaert, G.A.; Kuehl, R.; Atkins, B.L.; Trampuz, A.; Morgenstern, M.; Obremskey, W.T.; Verhofstad, M.H.; McNally, M.A.; Metsemakers, W.J. Diagnosing Fracture-Related Infection: Current Concepts and Recommendations. J. Orthop. Trauma 2020, 34, 8–17. [Google Scholar] [CrossRef]
- Sigmund, I.K.; McNally, M.A. Diagnosis of bone and joint infections. Orthop. Trauma 2019, 33, 144–152. [Google Scholar] [CrossRef]
- Kendal, A.R.; Ferguson, J.Y.; Wong, T.H.N.; Atkins, B.L.; NcNally, M. Osteomyelitis—Symptoms, diagnosis and treatment|BMJ Best Practice US. Available online: https://bestpractice.bmj.com/topics/en-us/354 (accessed on 24 November 2022).
- Bernard, L.; Uçkay, I.; Vuagnat, A.; Assal, M.; Stern, R.; Rohner, P.; Hoffmeyer, P. Two consecutive deep sinus tract cultures predict the pathogen of osteomyelitis. Int. J. Infect. Dis. 2010, 14, e390–e393. [Google Scholar] [CrossRef]
- Ulug, M.; Ayaz, C.; Celen, M.K.; Geyik, M.F.; Hosoglu, S.; Necmioglu, S. Are sinus-track cultures reliable for identifying the causative agent in chronic osteomyelitis? Arch. Orthop. Trauma Surg. 2009, 129, 1565–1570. [Google Scholar] [CrossRef]
- Soomro, S.; Siddiqi, M.A.; Taufiq, I. Diagnostic value of sinus tract culture versus intraoperative bone culture in patients with chronic osteomyelitis. J. Pak. Med. Assoc. 2016, 66 (Suppl. S3), S109–S111. [Google Scholar]
- Zuluaga, A.F.; Galvis, W.; Jaimes, F.; Vesga, O. Lack of microbiological concordance between bone and non-bone specimens in chronic osteomyelitis: An observational study. BMC Infect. Dis. 2002, 2, 8. [Google Scholar] [CrossRef]
- Mackowiak, P.A.; Smith, J.W.; Jones, S.R. Diagnostic value of sinus-tract cultures in chronic osteomyelitis. JAMA 1978, 239, 2772–2775. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.A.; Sliepen, J.; Dudareva, M.; IJpma, F.F.; Govaert, G.; Atkins, B.L.; Rentenaar, R.; Wouthuyzen-Bakker, M.; McNally, M. Causative Pathogens Do Not Differ between Early, Delayed or Late Fracture-Related Infections. Antibiotics 2022, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Baertl, S.; Walter, N.; Engelstaedter, U.; Ehrenschwender, M.; Hitzenbichler, F.; Alt, V.; Rupp, M. What Is the Most Effective Empirical Antibiotic Treatment for Early, Delayed, and Late Fracture-Related Infections? Antibiotics 2022, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Sliepen, J.; Onsea, J.; Debaveye, Y.; Govaert, G.A.; IJpma, F.F.; Zimmerli, W.; Metsemakers, W.J. The Microbiological Etiology of Fracture-Related Infection. Front. Cell. Infect. Microbiol. 2022, 12, 934485. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, J.; Corrigan, R.A.; Dudareva, M.; Wouthuyzen-Bakker, M.; Rentenaar, R.J.; Atkins, B.L.; Govaert, G.A.; McNally, M.A.; IJpma, F.F. Does the Use of Local Antibiotics Affect Clinical Outcome of Patients with Fracture-Related Infection? Antibiotics 2022, 11, 1330. [Google Scholar] [CrossRef]
- Li, H.K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]



| Age (Mean, SD) | Mean 51.2 Years (SD 17.3 Years) | |
|---|---|---|
| Site of infection | ||
| Upper limb | 30 | |
| Lower limb | 111 | |
| Aetiology of infection | ||
| Fracture related infection – closed | 45 (31.9%) | |
| Fracture related infection – open | 40 (28.4%) | |
| Haematogenous | 26 (18.4%) | |
| Contiguous focus | 15 (10.6%) | |
| Post-orthopaedic procedure (non-fracture) | 15 (10.6%) | |
| Pre-referral microbiology not available | 69 | |
| Pre-referral microbiology available (n = 88) * | ||
| Swab | 40 | |
| Previous surgical sampling | 25 | |
| Biopsy | 19 | |
| Blood culture | 4 | |
| Time from pre-referral microbiology to surgery (n = 88) * | median 0.8 years (IQR 0.49–1.53 years) | |
| JS-BACH Classification of pre-referral microbiology | ||
| No referral information (Ax) | 69 | |
| Ax/A1 | 62 | |
| A2/A3 (MDR+) | 10 | |
| JS-BACH Classification from intra-operative sampling | ||
| Ax/A1 | 113 | |
| A2/A3 (MDR+) | 28 | |
| Previous debridement for osteomyelitis | ||
| Yes | 41 | |
| No | 100 | |
| Growth from Referral Microbiology Samples | Growth Reported from Intra-Operative Sampling | Number | |
|---|---|---|---|
| ‘Complete match’ | |||
| Yes | Same as referral microbiology | 16 (18.2%) | |
| No | No growth | 6 (6.8%) | |
| ‘Partial match’ | |||
| Yes | Same as referral microbiology with additional isolates | 8 (9.1%) | |
| ‘Non-match’ | |||
| No | Significant growth | 6 (6.8%) | |
| Yes | Isolates not present on referral microbiology samples | 52 (59.1%) | |
| Intra-Operative Classification | ||||||
|---|---|---|---|---|---|---|
| Total | Ax/A1 | A2/A3 (MDR+) | Odds Ratio | 95% CI | ||
| Referral microbiology | ||||||
| Not available (Ax) | 69 | 58 (84.1%) | 11 (15.9%) | - | ||
| Ax/A1 | 61 | 47 (77.1%) | 14 (22.9%) | 1.2 | 0.44–3.2 | |
| A2/A3 | 11 | 8 (72.7%) | 3 (27.3%) | 1.4 | 0.26–5.6 | |
| Previous debridement | ||||||
| No (reference) | 99 | 84 (84.8%) | 15 (15.2%) | - | ||
| Yes | 42 | 29 (69.0%) | 13 (31.0%) | 3.6 | 1.5–8.7 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotchen, A.J.; Corrigan, R.A.; Dudareva, M.; Bernard, A.; Ferguson, J.; Atkins, B.L.; McNally, M. Pre-Referral Microbiology in Long Bone Infection: What Can It Tell Us? Antibiotics 2023, 12, 13. https://doi.org/10.3390/antibiotics12010013
Hotchen AJ, Corrigan RA, Dudareva M, Bernard A, Ferguson J, Atkins BL, McNally M. Pre-Referral Microbiology in Long Bone Infection: What Can It Tell Us? Antibiotics. 2023; 12(1):13. https://doi.org/10.3390/antibiotics12010013
Chicago/Turabian StyleHotchen, Andrew J., Ruth A. Corrigan, Maria Dudareva, Andrew Bernard, Jamie Ferguson, Bridget L. Atkins, and Martin McNally. 2023. "Pre-Referral Microbiology in Long Bone Infection: What Can It Tell Us?" Antibiotics 12, no. 1: 13. https://doi.org/10.3390/antibiotics12010013
APA StyleHotchen, A. J., Corrigan, R. A., Dudareva, M., Bernard, A., Ferguson, J., Atkins, B. L., & McNally, M. (2023). Pre-Referral Microbiology in Long Bone Infection: What Can It Tell Us? Antibiotics, 12(1), 13. https://doi.org/10.3390/antibiotics12010013






