Homocysteine and Inflammatory Cytokines in the Clinical Assessment of Infection in Venous Leg Ulcers
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Patients and Samples
4.2. Microbiology
4.3. Biofilm Production
4.4. High-Sensitivity C-Reactive Protein (CRP) Assay
4.5. Wound Fluid Sampling and Cytokines Analysis
4.6. Biofilm Imaging
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kolluri, R.; Lugli, M.; Villalba, L.; Varcoe, R.; Maleti, O.; Gallardo, F.; Black, S.; Forgues, F.; Lichtenberg, M.; Hinahara, J.; et al. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc. Med. 2022, 27, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Leren, L.; Johansen, E.; Eide, H.; Falk, R.S.; Juvet, L.K.; Ljoså, T.M. Pain in persons with chronic venous leg ulcers: A systematic review and meta-analysis. Int. Wound J. 2020, 17, 66–484. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Scharffetter-Kochanek, K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen. 2005, 13, 452–461. [Google Scholar] [CrossRef]
- Chen, W.Y.; Rogers, A.A. Recent insights into the causes of chronic leg ulceration in venous diseases and implications on other types of chronic wounds. Wound Repair Regen. 2007, 15, 434–449. [Google Scholar] [CrossRef]
- Wallace, H.J.; Stacey, M.C. Levels of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in chronic venous leg ulcers–correlations to healing status. J. Investig. Dermatol. 1998, 110, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Tarnuzzer, R.W.; Schultz, G.S. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996, 4, 321–325. [Google Scholar] [CrossRef]
- Trengove, N.J.; Bielefeldt-Ohmann, H.; Stacey, M.C. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000, 8, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Peschen, M.; Grenz, H.; Grothe, C.; Schopf, E.; Vanscheidt, W. Patterns of epidermal growth factor receptor, basic fibroblast growth factor, and transforming growth factor-beta3 expression in skin with chronic venous insufficiency. Eur. J. Dermatol. 1998, 8, 334–338. [Google Scholar] [PubMed]
- Tian, Y.W.; Stacey, M.C. Cytokines and growth factors in keratinocytes and sweat glands in chronic venous leg ulcers: An immunohistochemical study. Wound Repair Regen. 2003, 11, 316–325. [Google Scholar] [CrossRef]
- Gohel, M.S.; Windhaber, R.A.; Tarlton, J.F.; Whyman, M.R.; Poskitt, K.R. The relationship between cytokine concentrations and wound healing in chronic venous ulceration. J. Vasc. Surg. 2008, 48, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Esse, R.; Barroso, M.; Tavares de Almeida, I.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Martin, J.J. Homocysteine. Int. J. Biochem. Cell Biol. 2000, 32, 385–389. [Google Scholar] [CrossRef]
- Dawson, H.; Collins, G.; Pyle, R.; Deep-Dixit, V.; Taub, D.D. The immunoregulatory effects of homocysteine and its intermediates on T-lymphocyte function. Mech. Ageing Dev. 2004, 125, 107–110. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevention of Cardiovascular Disease Guidelines for Assessment and Management of Cardiovascular Risk WHO Library Cataloguing-in-Publication Data; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Debreceni, L. Homocysteine: A risk factor for atherosclerosis. Orv. Hetil. 2001, 142, 1439–1444. [Google Scholar] [PubMed]
- Kalita, J.; Kumar, G.; Bansal, V.; Misra, U.K. Relationship of homocysteine with other risk factors and outcome of ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 364–367. [Google Scholar] [CrossRef]
- Wierzbicki, A.S. Homocysteine and cardiovascular disease: A review of the evidence. Diab. Vasc. Dis. Res. 2007, 4, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Attinger, C.; Wolcott, R. Clinically addressing biofilm in chronic wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Gillings, M.R.; Kjelleberg, S.; Manefield, M.J. Secondary Effects of Antibiotics on Microbial Biofilms. Front. Microbiol. 2020, 11, 2109. [Google Scholar] [CrossRef]
- Penesyan, A.; Nagy, S.S.; Kjelleberg, S.; Gillings, M.R.; Paulsen, I.T. Rapid microevolution of biofilm cells in response to antibiotics. NPJ Biofilms Microbiomes 2019, 5, 34. [Google Scholar] [CrossRef]
- Bui, U.T.; Finlayson, K.; Edwards, H. The diagnosis of infection in chronic leg ulcers: A narrative review on clinical practice. Int. Wound J. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Pugliese, D.J. Infection in Venous Leg Ulcers: Considerations for Optimal Management in the Elderly. Drugs Aging 2016, 33, 87–96. [Google Scholar] [CrossRef]
- Garcia, T.F.; Borges, E.L.; Junho, T.O.C.; Spira, J.A.O. Microbiological profile of leg ulcer infections: Review study. Rev. Bras. Enferm. 2021, 74, e20190763. [Google Scholar] [CrossRef]
- Cooper, R.A.; Ameen, H.; Price, P.; McCulloch, D.A.; Harding, K.G. A clinical investigation into the microbiological status of ’locally infected’ leg ulcers. Int. Wound J. 2009, 6, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kirketerp-Møller, K.; Jensen, P.Ø.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Heggers, J.P. Bacterial quantification of open wounds. Mil. Med. 1969, 134, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Misic, A.M.; Gardner, S.E.; Grice, E.A. The wound microbiome: Modern approaches to examining the role of microorganisms in impaired chronic wound healing. Adv. Wound Care 2014, 3, 502–510. [Google Scholar] [CrossRef]
- Gardner, S.E.; Hillis, S.L.; Heilmann, K.; Segre, J.A.; Grice, E.A. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes 2013, 62, 923–930. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Sun, Y.; Dowd, S.E. Comparison of culture and molecular identification of bacteria in chronic wounds. Int. J. Mol. Sci. 2012, 13, 2535–2550. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Scales, B.S.; Huffnagle, G.B. The microbiome in wound repair and tissue fibrosis. J. Pathol. 2013, 229, 323–331. [Google Scholar] [CrossRef]
- Wolcott, R.; Dowd, S. The role of biofilms: Are we hitting the right target? Plast Reconstr. Surg. 2011, 127, 28S–35S. [Google Scholar] [CrossRef]
- Dunyach-Remy, C.; Salipante, F.; Lavigne, J.P.; Brunaud, M.; Demattei, C.; Yahiaoui-Martinez, A.; Bastide, S.; Palayer, C.; Sotto, A.; Gélis, A. Pressure ulcers microbiota dynamics and wound evolution. Sci. Rep. 2021, 11, 18506. [Google Scholar] [CrossRef]
- Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Smith, E.; Rhoads, D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE 2008, 3, e3326. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P. The potential significance of biofilms in wounds. Wounds 2004, 16, 234–240. [Google Scholar]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.A.; Barbul, A. Bacterial biofilms in wounds. Wound Repair Regen. 2008, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- International Wound Infection Institute. Wound Infection in Clinical Practice: Principles of Best Practice; International Wound Infection Institute: London, UK, 2016. [Google Scholar]
- James, G.A.; Swogger, E.; Wolcott, R.; deLancey Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Tuttle, M.S. Association Between Microbial Bioburden and Healing Outcomes in Venous Leg Ulcers: A Review of the Evidence. Adv. Wound Care 2015, 4, 1–11. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Farulla, I.; Prignano, G.; Gallo, M.T.; Vespaziani, M.; Cavallo, I.; Sperduti, I.; Pontone, M.; Bordignon, V.; Cilli, L.; et al. Biofilm is a Major Virulence Determinant in Bacterial Colonization of Chronic Skin Ulcers Independently from the Multidrug-Resistant Phenotype. Int. J. Mol. Sci. 2017, 18, 1077. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Beidler, S.K.; Douillet, C.D.; Berndt, D.F.; Keagy, B.A.; Rich, P.B.; Marston, W.A. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J. Vasc. Surg. 2009, 49, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Ambrosch, A.; Lobmann, R.; Pott, A.; Preissler, J. Interleukin-6 concentrations in wound fluids rather than serological markers are useful in assessing bacterial triggers of ulcer inflammation. Int. Wound J. 2008, 5, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mues, N.; Chu, H.W. Out-Smarting the Host: Bacteria Maneuvering the Immune Response to Favor Their Survival. Front. Immunol. 2020, 11, 819. [Google Scholar] [CrossRef]
- Duell, B.L.; Tan, C.K.; Carey, A.J.; Wu, F.; Cripps, A.W.; Ulett, G.C. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol. Med. Microbiol. 2012, 64, 295–313. [Google Scholar] [CrossRef]
- Mege, J.L.; Meghari, S.; Honstettre, A.; Capo, C.; Raoult, D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect. Dis. 2006, 6, 557–569. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2006, 2, 66–77. [Google Scholar] [CrossRef]
- Kingsley, A.; Jones, V. Diagnosing wound infection: The use of C-reactive protein. Wounds UK 2008, 4, 32–46. [Google Scholar]
- Hogervorst, E.; Ribeiro, H.M.; Molyneux, A.; Budge, M.; Smith, A.D. Plasma homocysteine levels, cerebrovascular risk factors, and cerebral white matter changes (leu-koaraiosis) in patients with Alzheimer disease. Arch. Neurol. 2002, 59, 787–793. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Tang, M.X.; Shea, S.; Miller, J.; Green, R.; Mayeux, R. Plasma homocysteine levels and risk of Alzheimer disease. Neurology 2004, 62, 1972–1976. [Google Scholar] [CrossRef]
- Lawrence de Koning, A.B.; Werstuck, G.H.; Zhou, J.; Austin, R.C. Hyperhomocysteinemia and its role in the development of atherosclerosis. Clin. Biochem. 2003, 36, 431–441. [Google Scholar] [CrossRef]
- González, R.; Pedro, T.; Real, J.T.; Martínez-Hervás, S.; Abellán, M.R.; Lorente, R.; Priego, A.; Catalá, M.; Chaves, F.J.; Ascaso, J.F.; et al. Plasma homocysteine levels are associated with ulceration of the foot in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2010, 26, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, X.; Wang, T.; Yang, X.; Fan, L. Elevated serum homocysteine levels are associated with the development of chronic venous ulcers. Vasc. Med. 2022, 27, 358–364. [Google Scholar] [CrossRef]
- de Franciscis, S.; De Sarro, G.; Longo, P.; Buffone, G.; Molinari, V.; Stillitano, D.M.; Gallelli, L.; Serra, R. Hyperhomocysteinaemia and chronic venous ulcers. Int. Wound J. 2015, 12, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Martinelli, N.; Olivieri, O.; Pizzolo, F.; Friso, S.; Faccini, G.; Bozzini, C.; Tenuti, I.; Lotto, V.; Villa, G.; et al. Hyperhomocysteinemia and mortality after coronary artery bypass grafting. PLoS ONE 2006, 1, e83. [Google Scholar] [CrossRef]
- Nagai, Y.; Tasaki, H.; Takatsu, H.; Nihei, S.; Yamashita, K.; Toyokawa, T.; Nakashima, Y. Homocysteine inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2001, 281, 726–731. [Google Scholar] [CrossRef]
- Shastry, S.; Tyagi, N.; Hayden, M.R.; Tyagi, S.C. Proteomic analysis of homocysteine inhibition of microvascular endothelial cell angiogenesis. Cell Mol. Biol. (Noisy-le-Grand) 2004, 50, 931–937. [Google Scholar]
- Papatheodorou, L.; Weiss, N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid. Redox Signal. 2007, 9, 1941–1958. [Google Scholar] [CrossRef]
- Duan, K.; Dammel, C.; Stein, J.; Rabin, H.; Surette, M.G. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 2003, 50, 1477–1491. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Song, C.; Zhang, Y.; Wang, Z.; Liu, Z.; Wei, H.; Yu, J. Autoinducer-2 facilitates Pseudomonas aeruginosa PAO1 pathogenicity in vitro and in vivo. Front. Microbiol. 2017, 8, 1944. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, Z.; Fu, Y.; Ai, Q.; Dong, Y.; Yu, J. Autoinducer-2 regulates Pseudomonas aeruginosa PAO1 biofilm formation and virulence production in a dose-dependent manner. BMC Microbiol. 2015, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, F.S.; Raček, T.; Wobser, D.; Puchalka, J.; Rabener, E.M.; Reiger, M.; Hendrickx, A.P.A.; Diederich, A.-K.; Jung, K.; Klein, C.; et al. Phage-mediated dispersal of biofilm and distribution of bacterial virulence genes is induced by quorum sensing. PLoS Pathog. 2015, 11, e1004653. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Zenilman, J.M.; Ms, J.H.M.; Shirtliff, M.; Agostinho, A.; James, G.; Stewart, P.; Mongodin, E.F.; Rao, D.; Rickard, A.H.; et al. The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds. Wound Repair Regen. 2011, 19, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg. Clin. N. Am. 1997, 77, 637–650. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Davies, B.J. The microbiology of infected and noninfected leg ulcers. Int. J. Dermatoly 1999, 38, 573–578. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Toma, L.; Prignano, G.; Pelagalli, L.; Police, A.; Cavallotti, C.; Torelli, R.; Sanguinetti, M.; Ensoli, F. Misidentification of Streptococcus uberis as a human pathogen: A case report and literature review. Int. J. Infect. Dis. 2015, 33, 79–81. [Google Scholar] [CrossRef]
- Sivori, F.; Cavallo, I.; Kovacs, D.; Guembe, M.; Sperduti, I.; Truglio, M.; Pasqua, M.; Prignano, G.; Mastrofrancesco, A.; Toma, L.; et al. Role of Extracellular DNA in Dalbavancin Activity against Methicillin-Resistant Staphylococcus aureus (MRSA) Biofilms in Patients with Skin and Soft Tissue Infections. Microbiol. Spectr. 2022, 10, e0035122. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Marchesi, F.; Cavallo, I.; Toma, L.; Sivori, F.; Papa, E.; Spadea, A.; Cafarella, G.; Terrenato, I.; Prignano, G.; et al. The Impact of Bacterial Biofilms on End-Organ Disease and Mortality in Patients with Hematologic Malignancies Developing a Bloodstream Infection. Microbiol. Spectr. 2021, 9, e0055021. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Sivori, F.; Marchesi, F.; Prignano, G.; Pimpinelli, F.; Sperduti, I.; Pelagalli, L.; Di Salvo, F.; Celesti, I.; et al. Biofilm Production by Carbapenem-Resistant Klebsiella pneumoniae Significantly Increases the Risk of Death in Oncological Patients. Front. Cell. Infect. Microbiol. 2020, 10, 561741. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Toma, L.; Provot, C.; Ascenzioni, F.; Sperduti, I.; Prignano, G.; Gallo, M.T.; Pimpinelli, F.; Bordignon, V.; Bernardi, T.; et al. Development of an in vitro Assay, Based on the BioFilm Ring Test®, for Rapid Profiling of Biofilm-Growing Bacteria. Front. Microbiol. 2016, 7, 1429. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, M.; Beckert, S.; Joos, T.O.; Königsrainer, A.; Schneiderhan-Marra, N.; Löffler, M.W. Superficial wound swabbing: A novel method of sampling and processing wound fluid for subsequent immunoassay analysis in diabetic foot ulcerations. Diabetes Care 2012, 35, 2113–2120. [Google Scholar] [CrossRef] [PubMed][Green Version]
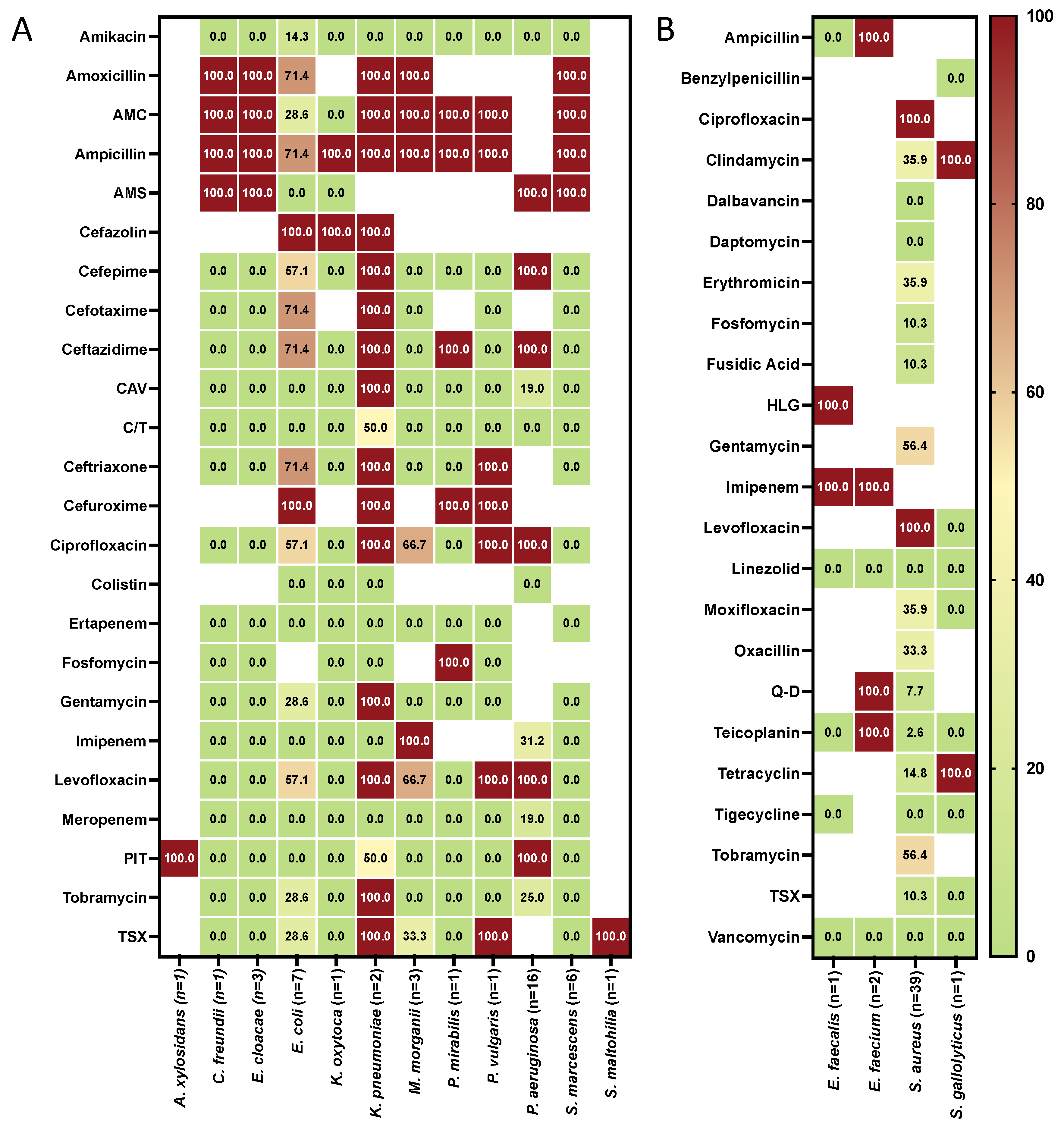
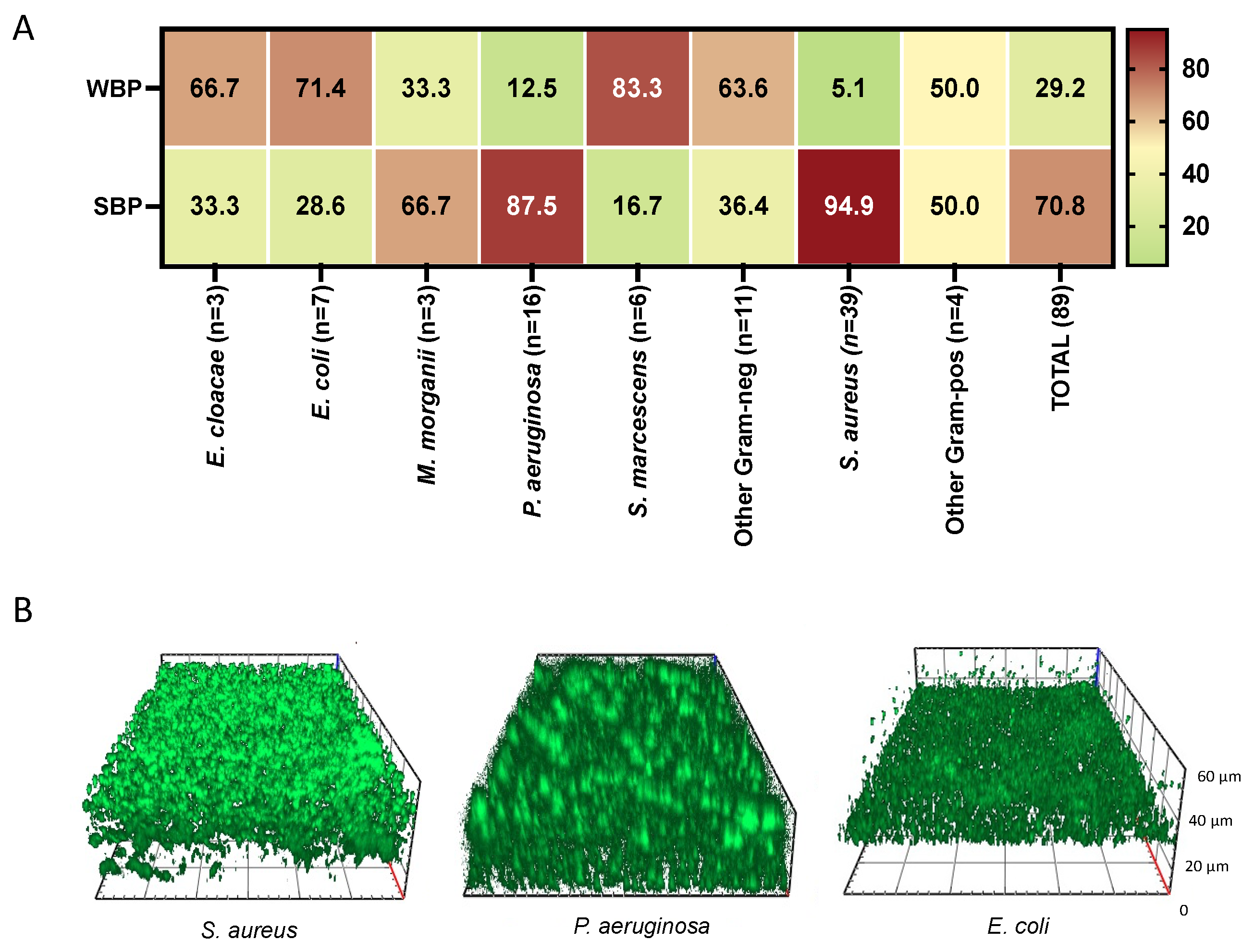
| Clinical Data | NVU (N 45) | IVU (N 56) | p |
|---|---|---|---|
| Sex (Male/Female) (%) | 38/62 | 42/58 | 0.79 |
| Age (Years) (Median-Range) | 81 (41–97) | 76.5 (28–97) | 0.35 |
| BMI (Kg/m2) (Median-Range) | 26.7 (21.0–40.2) | 25.2 (20.2–42.5) | 0.78 |
| Diabetic (%) | N = 6 (13.3) | N = 11 (20.4) | 0.46 |
| Duration of ulcer (months) (Median-Range) | 6.8 (3–48) | 4 (3–48) | 0.72 |
| Size of ulcer (cm2) (Median-Range) | 8.6 (1.5–53) | 28 (3–140) | <0.0001 |
| Depth of ulcer (% Grade 3) | N = 2 (4.4) | N = 17 (30.3) | 0.0008 |
| HHcy (% of positive subjects) | N = 7 (15.6) | N = 33 (58.9) | <0.0001 |
| CRP (% of positive subjects) | N = 28 (62.2) | N = 38 (67.9) | 0.58 |
| IFN-(pg/mL) (Median-Range) | 1.44 × 101 (1.11 × 100–3.76 × 102) | 1.78 × 100 (1.02 × 100–8.21 × 101) | 0.03 |
| IL-1(pg/mL) (Median-Range) | 7.49 × 104 (3.73 × 103–6.99 × 105) | 7.53 × 104 (3.95 × 104–4.93 × 105) | 0.80 |
| IL-6 (pg/mL) (Median-Range) | 1.84 × 104 (4.71 × 102–8.28 × 104) | 1.75 × 105 (6.94 × 104–3.31 × 105) | 0.0003 |
| IL-8 (pg/mL) (Median-Range) | 8.21 × 104 (2.07 × 104–8.94 × 105) | 1.30 × 105 (3.89 × 104–1.86 × 106) | 0.23 |
| IL-10 (pg/mL) (Median-Range) | 7.36 × 101 (4.61 × 100–3.12 × 102) | 1.42 × 102 (6.26 × 101–6.02 × 102) | 0.02 |
| IL-17A (pg/mL) (Median-Range) | 6.46 × 101 (2.38 × 100–2.01 × 102) | 9.50 × 103 (5.49 × 101–7.84 × 103) | <0.0001 |
| IL-22 (pg/mL) (Median-Range) | 2.72 × 102 (1.05 × 100–8.34 × 102) | 2.47 × 102 (1.03 × 100–1.67 × 104) | 0.14 |
| TNF-(pg/mL) (Median-Range) | 3.75 × 102 (8.68 × 101–1.81 × 103) | 1.59 × 103 (3.94 × 102–9.38 × 103) | 0.0002 |
| Strain | N | % |
|---|---|---|
| Staphylococcus aureus | 39 | 43.8 |
| Pseudomonas aeruginosa | 16 | 18.0 |
| Escherichia coli | 7 | 7.9 |
| Serratia marcescens | 6 | 6.7 |
| Enterobacter cloacae | 3 | 3.4 |
| Morganella morganii | 3 | 3.4 |
| Enterococcus faecium | 2 | 2.3 |
| Klebsiella pneumoniae | 2 | 2.3 |
| Shewanella algae | 2 | 2.3 |
| Achromobacter xylosoxidans | 1 | 1.1 |
| Bordetella trematum | 1 | 1.1 |
| Citrobacter freundii | 1 | 1.1 |
| Enterococcus faecalis | 1 | 1.1 |
| Klebsiella oxytoca | 1 | 1.1 |
| Proteus mirabilis | 1 | 1.1 |
| Proteus vulgaris | 1 | 1.1 |
| Stenotrophomnas maltophilia | 1 | 1.1 |
| Streptococcus gallolyticus | 1 | 1.1 |
| Total | 89 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallo, I.; Lesnoni La Parola, I.; Sivori, F.; Toma, L.; Koudriavtseva, T.; Sperduti, I.; Kovacs, D.; D’Agosto, G.; Trento, E.; Cameli, N.; et al. Homocysteine and Inflammatory Cytokines in the Clinical Assessment of Infection in Venous Leg Ulcers. Antibiotics 2022, 11, 1268. https://doi.org/10.3390/antibiotics11091268
Cavallo I, Lesnoni La Parola I, Sivori F, Toma L, Koudriavtseva T, Sperduti I, Kovacs D, D’Agosto G, Trento E, Cameli N, et al. Homocysteine and Inflammatory Cytokines in the Clinical Assessment of Infection in Venous Leg Ulcers. Antibiotics. 2022; 11(9):1268. https://doi.org/10.3390/antibiotics11091268
Chicago/Turabian StyleCavallo, Ilaria, Ilaria Lesnoni La Parola, Francesca Sivori, Luigi Toma, Tatiana Koudriavtseva, Isabella Sperduti, Daniela Kovacs, Giovanna D’Agosto, Elisabetta Trento, Norma Cameli, and et al. 2022. "Homocysteine and Inflammatory Cytokines in the Clinical Assessment of Infection in Venous Leg Ulcers" Antibiotics 11, no. 9: 1268. https://doi.org/10.3390/antibiotics11091268
APA StyleCavallo, I., Lesnoni La Parola, I., Sivori, F., Toma, L., Koudriavtseva, T., Sperduti, I., Kovacs, D., D’Agosto, G., Trento, E., Cameli, N., Mussi, A., Latini, A., Morrone, A., Pimpinelli, F., & Di Domenico, E. G. (2022). Homocysteine and Inflammatory Cytokines in the Clinical Assessment of Infection in Venous Leg Ulcers. Antibiotics, 11(9), 1268. https://doi.org/10.3390/antibiotics11091268








