Abstract
Introduction. Knowledge of local and regional antimicrobial resistance (AMR) is crucial in clinical decision-making, especially with critically ill patients. The aim of this study was to investigate the rate and pattern of infections in valvular heart disease patients admitted to the intensive care unit (ICU) at the Salam Centre for Cardiac Surgery in Khartoum, Sudan (run by EMERGENCY NGO). Methods. This is a retrospective, observational study from a single, large international referral centre (part of a Regional Programme), which enrolled patients admitted to the ICU between 1 January and 31 December 2019. Data collected for each patient included demographic data, operating theatre/ICU data and microbiological cultures. Results. Over the study period, 611 patients were enrolled (elective surgery n = 491, urgent surgery n = 34 and urgent medical care n = 86). The infection rate was 14.2% and turned out to be higher in medical than in surgical patients (25.6% vs. 12.4%; p = 0.002; OR = 2.43) and higher in those undergoing urgent surgery than those undergoing elective (29.4% vs. 11.2%; p = 0.004; OR = 3.3). Infection was related to (a) SOFA score (p < 0.001), (b) ICU length of stay (p < 0.001) and (c) days from ICU admission to OT (p = 0.003). A significant relationship between the type of admission (elective, urgent surgery or medical) and the presence of infections was found (p < 0.001). The mortality rate was higher among infected patients (infected vs. infection-free: 10.3% vs. 2.1%; p < 0.001; OR = 5.38; 95% CI: 2.16–13.4; p < 0.001). Conclusions. Hospital-acquired infections remain a relevant preventable cause of mortality in our particular population.
1. Introduction
Patients in the cardiac surgery intensive care unit (ICU) are particularly vulnerable to post-operative infection, considering the underlying chronic disease, prolonged and complex surgical techniques used and exposure to life-saving invasive procedures [1]. Although many studies have focused on the topic, scientific papers documenting the extent of ICU-acquired infection in low-income areas are sparse.
Particularly in East African countries, where surveillance capacity is minimal, reducing the impact of infections remains an unsolved challenge. Moreover, despite a wide range of risk factors contributing to the occurrence of infections in critically ill patients that have been described in developed countries [2,3,4,5], to the best of our knowledge, little information is available on this topic in cardiac surgery ICUs in low-income settings.
The aim of our study is to assess the epidemiology and clinical impact of infections, together with their risk factors, in patients admitted to the ICU of an international referral centre for cardiac surgery in Sudan.
2. Methods
2.1. Study Design, Patient Selection and End Points
This is a retrospective observational study carried out from 1 January to 31 December 2019, which enrolled patients admitted to a cardiac surgery ICU in East Africa (Khartoum, Sudan). In the given study period, for each consecutive patient admitted to the ICU, a study sheet was filled in when the patient was discharged. Patients re-admitted to the ICU were excluded from our analysis.
Patients were divided on the basis of the different types of hospital admission:
- (1)
- Elective: For clinically stable patients scheduled for cardiac surgery. Ideally, these patients are meant to be admitted on a ward level a few days before undergoing surgery in order to perform a standard pre-operative assessment including laboratory exams, ECG and heart ultrasound. Following surgery, they are initially referred to the ICU and then to the sub-ICU/ward for post-operative care.
- (2)
- Urgent: For patients requiring urgent care as a result of a precipitating condition. These patients receive medical care and, when appropriate, some of them also undergo urgent surgery. In relation to their clinical conditions, at hospital admission, patients are referred either to the ICU, sub-ICU or ward.
Therefore, patients were finally grouped as (1) elective surgery, (2) urgent surgery or (3) urgent medical care.
The primary outcome was to evaluate the rate of hospital-acquired infection in the ICU.
Secondary outcomes were the identification of (1) pathogens and site of infection, (2) risk factors associated with infection occurrence and (3) ICU crude mortality rate and ICU infection-associated mortality rate.
Moreover, we compared medical and surgical patients, and, in the latter group, we compared patients undergoing elective surgery with those treated urgently.
2.2. Study Setting
The Salam Centre for Cardiac Surgery in Khartoum, Sudan, is a cardiac hospital run by an Italian NGO (EMERGENCY NGO) that provides high-quality care for child and adult patients with underlying heart conditions [6]. Its services are entirely free of charge and provided not only to locals but also patients coming from other parts of Sudan, other African countries and even Iraq and Afghanistan (through the Regional Programme).
The facility includes a surgical block with three operating theatres (OTs), a 15-bed intensive care unit (ICU), a 16-bed sub-ICU and a 32-bed ward.
Patients admitted to the centre are mainly suffering from valvular heart disease (VHD) (most often rheumatic heart disease, RHD) or congenital heart disease (CHD).
Most of the procedures performed at our centre take the form of open-heart surgery with cardiopulmonary bypass.
2.3. Definitions
Infection severity was determined using a sequential organ failure assessment (SOFA) score in the first 24 h from infection onset.
The length of hospital and ICU stay were calculated as the number of days from the date of admission to the date of discharge or death. Re-admissions to the ICU were ruled out.
Patients were divided into infected and not infected on the basis of the presence or absence of at least one infectious event (defined as at least one positive microbiological isolate together with clinical signs of infection). Patients that did not develop any infections were defined as “censored”.
Infections were classified according to the following categories: Ventilator-associated pneumonia (VAP), urinary tract infection (UTI), abdominal infections and bloodstream infection (BSI).
Multi-drug resistance (MDR) definition was in line with the Centers for Disease Control and Prevention’s guidelines.
2.4. Microbiological Studies
Microbial isolates were collected in cases of fever or suspicion of infection and were classified as infection or contamination in accordance with clinical judgment. The site of specimen collection was specified (respiratory tract, blood, CVC tip, urine, tissue fluids or swab).
Identification of microbial strains was based accordingly on local laboratory techniques. The Kirby–Bauer disk diffusion test was used for antimicrobial susceptibility testing.
2.5. Statistical Analysis
We performed an explanatory analysis of all patients separately by groups: Categorical data are presented as absolute frequencies and percentages and continuous data as the median with the minimum and maximum range and the mean with standard deviation (SD). Categorical variables were compared using the Chi-Square test, whereas continuous variables were compared using the Mann–Whitney U test for means and the Mood median test for medians. We evaluated, by explanatory analysis, frequencies of specimens positive for infection with attention to antibiotic resistance. We obtained overall bacterial prevalence in our population and observed infection prevalence by the site of the specimen, not considering bacteria found more than once at the same site. We investigated any difference between infected and non-infected patients in terms of frequencies, central tendency and variability measures of variables we believed could be associated with infection. We tested differences using the Chi-Square and Mann–Whitney U tests. Then, we evaluated risks by the odds ratio (OR). In order to define the best predictors for multiple logistic regression, we evaluated correlations among risk factors and univariate logistic regressions AIC, pseudo R2 and increases in OR per unit increase in risk variable regression, as well as the number of not available (NA) observations. We considered optimal pseudo R2 values between 0.4 and 0.2. Moreover, we explored, by a Pearson correlation test, whether significant predictors were significantly and highly or moderately correlated with each other [7]. We evaluated multi-collinearity by the variance inflation factor (VIF). Moreover, we analysed the multivariate regression model by splitting patients into training (which contain 80% of the observations) and test datasets, stratified by the presence or absence of infection. Predictive properties of the model were evaluated by sensitivity (SE), specificity (SP) and positive and negative predictive values (PPV and NPP); the optimal threshold for maximising both SE and SP was defined over the training dataset. Kaplan–Meier curves highlight differences between groups in terms of time free from infection, with attention paid to the mean time spent in the hospital without infection. Curves for days free from infection were difference-tested using the log-rank test. All test results were evaluated considering α = 0.05; normality was assessed by the Shapiro–Wilk test. Missing data were not transformed, and in order to compute specific statistics, patients who did not report the specific information were excluded.
2.6. Ethics
The study was carried out in accordance with the Helsinki Declaration. Ethical approval was not required because the study was based on a retrospective analysis of data collected for diagnostic and clinical purposes by the medical staff and stored in a deidentified manner. The study complies with the indications of the STROBE Statement checklist.
3. Results
3.1. Description of Study Population
During the study period, 680 cases of ICU admissions were recorded. With re-admissions having been ruled out, a total of 611 patients were enrolled in the study. Of them, 491 were post-elective surgery patients and 34 were urgent surgery patients, whereas the remaining 86 admissions were for urgent medical care. Demographic characteristics and clinical data of the study population, together with a comparison of surgical and medical groups, can be found in Table 1. A comparison of elective and urgent patients is given in Table 2.

Table 1.
Overall analysis of study population and comparison of surgical and medical groups.

Table 2.
Comparison of surgical patients: Elective vs. urgent surgery.
3.2. Outcomes Evaluation
3.2.1. Infection Prevalence
The overall infection prevalence was 14.2% and turned out to be higher in medical than in surgical patients (25.6% vs. 12.4%; p = 0.002; OR = 2.43; 95% CI: 1.4–4.21; p = 0.001). However, in comparison to patients scheduled for elective surgery, those undergoing urgent surgery had a significantly higher prevalence of infection (29.4% vs. 11.2%; p = 0.004; OR = 3.3; 95% CI: 1.5–7.27; p = 0.002).
3.2.2. Microbiological and Clinical Characteristics of Infections
Out of 611 ICU patients enrolled, 14.2% (n = 87) had at least one positive microbiological culture. A total of 156 samples were found positive for microbial growth; the prevalence of different microbial isolates at the site of specimen collection and by different types of hospital admission are summarised in Figure 1.
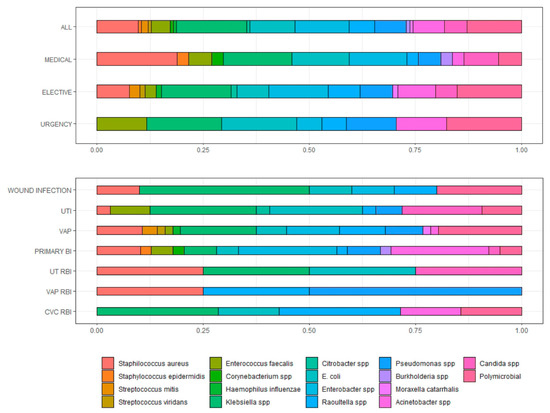
Figure 1.
Prevalence of microbial isolates from different specimen sites and different types of hospital admission. UTI = urinary tract infection; VAP = ventilatory associated pneumonia; BSI = bloodstream infection; RBI = related blood infection.
Among Gram-positive isolates, out of 23 staphylococci isolated, 28.5% (n = 4, all S. aureus) were cefoxitin-resistant. Among all Gram-negative isolates where an antibiogram was available (n = 110), 32.73% (n = 36) were carbapenem-resistant. In particular, out of 11 Acinetobacter baumannii isolates with a complete antibiogram available, 2 (18.2%) were carbapenem-resistant, whereas the remaining were sensitive. Concerning Pseudomonas aeruginosa, 6 (50%) out of 12 isolates were carbapenem-resistant. Among Klebsiella pneumoniae isolates with a complete antibiogram (n = 27), 13 (48.15%) were carbapenem-resistant.
Among the 87 patients with one or more positive microbiological cultures, all had clinical signs of infection. Table 3 shows the frequency of microbiological isolates and the prevalence of different infections in the different groups.

Table 3.
Microbiological isolate and type of infections in different groups.
3.2.3. Risk Factors Associated with Development of Infections
Infection was related to (a) the degree of organ failures assessed by SOFA score (p < 0.001), (b) the number of days spent in the ICU (p < 0.001) and (c) days from ICU admission to OT (p = 0.003). We evaluated risks associated with these variables: Per unit increase in risk variables, the risk of infection increases by (a) 1.44 (95% CI 1.32–1.59; p < 0.001), (b) 1.56 (95% CI 1.41–1.71, p < 0.001), (c) and 1.39 (95% CI 1.02–1.89, p < 0.001), respectively.
Finally, we observed a significant association between the type of admission (elective, urgent surgery or medical care) and the presence of infection (p < 0.001): In particular, the risk in the medical group is 2.43 (95% CI: 1.4–4.21; p = 0.001) times higher than in surgical patients. Among the surgical group, patients treated urgently had a 3.30 (95% CI: 1.5–7.27; p = 0.0018) times higher risk than elective patients.
Among people who underwent surgery, reopening and delayed chest closure were significantly associated with infection (both p < 0.001): Those who underwent reopening and who had delayed chest closure had a significantly higher risk of infection: OR = 5.11 (95% CI: 2.11–12.37, p < 0.001) and OR = 10.98 (95% CI: 3.37–35.73; p < 0.001), respectively. A consistent difference in the average time spent in ECC between infected and non-infected was also observed (120 min vs. 94.8 min; p = 0.0015). Moreover, per minute of ECC, the risk of infection increases by 1.01 (95% CI: 1.005–1.015; p < 0.001). All clinically relevant variables were evaluated in terms of central tendencies, in which we found significant differences between infected and non-infected. We built univariate logistic regressions, the results of which are summarised in Table S1. Pearson correlations between relevant variables are summarised in Figure S1.
Multiple logistic regression was performed. We modelled the risk of infection by three explanatory variables (days spent in the ICU, different types of admission if surgical or medical and SOFA score); OR and statistics are summarised in Table 4, while Figure 2 gives the logistic regression line with 95% CI. The ROC curve can be found in Figure S2.

Table 4.
Multiple logistic regression on risk of infection in the ICU.
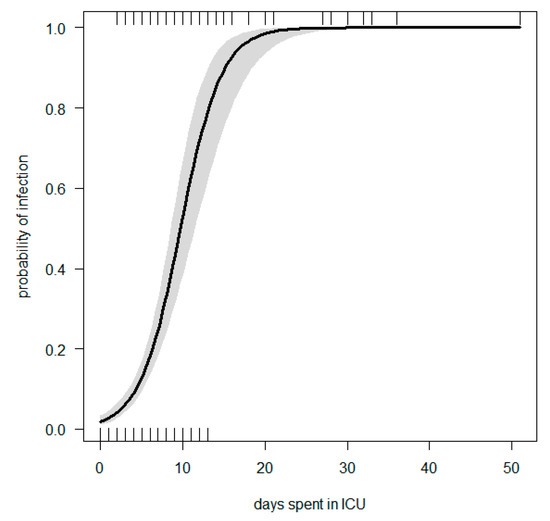
Figure 2.
Logistic regression line: Probability of infection vs. days spent in ICU, corrected by group of admission and SOFA score.
The chance of being infection-free during the time spent in the hospital is shown in the Kaplan–Meier curves given in Figure 3a, together with the frequency distribution for censored patients over time in Figure 3b. The log-rank test shows significant differences between curves for days free from infection with a p-value of < 0.001. Censored patients had a mean time at risk of infection (correspondent to the time they spent in hospital) of 10.6 days (±9.3) in elective patients, 9.6 days (±4.7) in urgent patients and, finally, 6.8 days (±11.1) in medical patients, on average. Time spent at the hospital (Figure 3b) for non-infected patients shows a log-normal distribution just for urgent patients (p = 0.166), while elective and medical time–frequency distributions are not statistically log-normal, with p-values of <0.001. Infected patients spent, on average, 17.7 days at the hospital (±14.6); more specifically, elective patients spent 18 days (±11.2), medical patients spent 14.6 days (±11.6) and urgent patients spent 22.5 days (±30.5). Time spent at the hospital shows a log-normal distribution for elective and medical patients (p-value = 0.858 and 0.135), while days of hospitalisation are not log-normal for urgent patients (0.041).
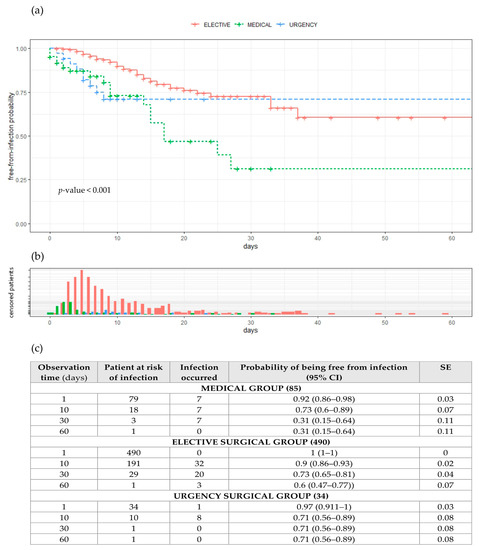
Figure 3.
(a) Probabilities of being free from infection during time spent in hospital, (b) frequency distribution for “censored” patients over time, (c) observation time, patients at risk of infection, no. of infections that occurred, probability of being free from infection together with 95% confidence interval and standard error. Abbreviations: CI = confidential intervals; SE = standard error.
3.2.4. Mortality
The ICU crude mortality rate was 3.3%. This was significantly higher in infected patients (infected vs. infection-free: 10.3% vs. 2.1%; p < 0.001; OR = 5.38; 95% CI: 2.16–13.4; p < 0.001). The ICU crude mortality rate was higher in medical than in surgical patients (13.9% vs. 1.5%; p < 0.001; OR = 10.4; 95% CI: 4.146–26.486; p < 0.001). Although not significant, the ICU crude mortality rate was higher in urgent than in elective patients (2.9% vs. 1.4%; p = 1).
4. Discussion
Severe infections and sepsis are major factors in patients’ clinical outcomes, in particular those with underlying cardiovascular diseases [8]. Investigating nosocomial infection epidemiology and predictive factors in infection is crucial in guiding clinical decisions and has an impact on morbidity and mortality, especially in settings with limited resources where complications are not always affordable.
In our study, the overall incidence of infection during ICU stay was 14.2%, with significant differences between medical and surgical patients (25.6% vs. 12.4%). These latest data can be explained by considering that part of the medical group consisted of an extremely fragile population affected by end-stage heart failure with heavy contraindications to cardiac surgery. Indeed, the SOFA score was remarkably higher (6 vs. 4; p < 0.001) in the medical than in the surgical group. Moreover, the infection rate for medical patients overlapped with that for the group undergoing urgent surgery (29.4%) and was significantly higher than for those undergoing elective surgery. Primary bloodstream infections (BSIs) are significantly more frequent in the medical group, as expected in cases of consistent microbial translocation, with this being more common in patients with end-stage cardiovascular disease [9].
On the other hand, VAP was rather more common in the surgical group. This observation is in line with what is to be expected in patients undergoing cardiac surgery, in which the respiratory dynamics are functionally impaired following sternotomy, which is consistent with previous studies [10,11,12].
The higher frequency of infectious events recorded in the group undergoing urgent surgery compared to the elective group can be explained not only by the more critical condition of these patients (as indicated by the SOFA score) but also by the higher number of devices used and longer exposure time to these medical aids (CVC, Foley) [13,14,15,16]. Moreover, surgical patients treated urgently spent more time in extracorporeal circulation (ECC) and continuous veno-venous haemofiltration (CVVH) than patients treated with elective surgery: These are two well-known risk factors for hospital-acquired infection [8,17,18,19].
Overall, logistic regression analysis showed that the risk of infection increased for each day spent in ICU, for more severe SOFA scores and in patients with more compromised underlying disease. In fact, medical patients in our cohort had a higher risk of infection due to their fragile condition, which prevented them from receiving surgery.
From a microbiological point of view, a large number of infections observed were due to pathogens included in the family of Enterobacteriaceae. This can be explained by considering that intestinal ischaemia reperfusion that occurs during cardiac surgery and in end-stage cardiovascular diseases will induce a systemic inflammatory reaction and may cause intestinal flora translocation [20]. Interestingly, among Enterobacteriaceae isolated in our cohort, 40.7% were carbapenem-resistant. Moreover, for Acinetobacter, which caused the majority of BSI, microbial translocation can be recognised as the main pathological mechanism. However, in contrast to what has been reported in European countries, in our study, carbapenems-resistant isolates were observed in only 25% of cases. The problem of antimicrobial resistance (AMR) represents a global public health issue that is particularly relevant in developing countries [21]. In fact, AMR has multiple and varied causes [22], namely (1) unreasonable prescription and/or self-prescription of antibiotics in an area with a high burden of infection diseases; (2) poor socioeconomic conditions; (3) weak product regulation, oversight or quality control and (4) limited capacity for microbiology testing and lack of local and national surveillance in low-income areas. This problem becomes particularly crucial in hospitalised patients, especially those that are critically ill. AMR is a major cause of morbidity and mortality in the ICU [23].
It is well reckoned that ICU mortality attributable to infectious diseases is considerably higher in developing countries (DCs) (14% in North Africa vs. 6% in high-income countries) [24]. Interestingly, the overall ICU mortality rate observed at our cardiac surgery centre was 3.3%. This figure is comparable to those recorded in high-income countries [3,25]. Taking into consideration overall challenges related to this specific setting (patients’ backgrounds, limited local resources), we found these data quite interesting, as they offer positive feedback on the high standard of care delivered at our centre. Moreover, we found a consistent difference in mortality between medical and surgical patients (12% vs. 8%; p < 0.001). In this case, this observation can be explained by the fact that some of the medical patients were end-stage heart failure cases urgently admitted following decompensation but without further surgical options.
Our study, conducted in a difficult setting, has several limitations, which must necessarily be taken into account when interpreting the results: First of all, the hospital had a microbiology laboratory with limited resources. Secondly, it is important to underline the unavailability of detailed information on the antibiotic treatments carried out, which made it impossible to take this aspect into account in the statistical analysis. Furthermore, the therapeutic options available for difficult-to-treat infections supported by multidrug-resistant germs were limited by the availability of the carbapenem class. Finally, our study population is unique, with the rate of patients suffering from RHD being so high in comparison to the global cardiac surgery dataset. Indeed, despite rising rates of CVD and atherosclerosis in DCs, RHD still remains one of the most common cardiovascular diseases in this setting [26,27,28]. Moreover, in contrast with the Western experience of cardiac surgery, our centre is mostly dealing with young and severely malnourished patients.
Despite those limitations, to the best of our knowledge, this is the first study to provide insight into the ICU cardiac surgery patient population, infection epidemiology and outcomes from a highly specialised hospital in a low-income country in North-East Africa, contributing to the implementation of research on this topic.
5. Conclusions
Cardiac surgery procedures require high-standard ICUs, which are still scarce in DCs, mainly as a result of a shortage of medical expertise and public infrastructure [29]. However, the presence of those few highly specialised facilities introduced a new “variable” in the management of critical cardiac surgery patients living in low-income areas. In fact, the availability of state-of-the-art treatments could not only have an impact on clinical outcomes, but also influence the ecology in hospitals and therefore the epidemiology of possible nosocomial infections associated with cardiac surgery in critically ill patients. However, limited data are available on the impact of high-resource centres delivering cardiac surgery in Africa, and there is a growing need for studies that provide information on the topic [30,31].
6. Recommendation
The availability of facilities capable of delivering high standards of care in developing countries requires a strong epidemiological surveillance: policy makers should consider this new reality in infection control programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11091227/s1, Table S1. Descriptive statistics among infected and non-infected patients: Wilcoxon-Mann-Whitney p-values, increase in OR and model characteristics. Figure S1. Correlations among significant risk factors, ordered by clusters, all the correlation tests are significant with α < 0.05. Figure S2. Multiple logistic regression: ROC curve.
Author Contributions
O.S. devised the study and contributed to its design and data interpretation, as well as drafted and revised the document. D.R.S.S., E.G. and M.B.S. collected the data. G.P., M.L. and G.C. supervised data acquisition and contributed to the critical revision of the document for medical content. S.F. participated in its writing, data analysis and revision, and designed the figure. G.P., M.L., M.C., G.d.E, G.C. and M.L. made substantial contributions to the conception and design of the study, developed the theoretical framework and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be shared upon request.
Acknowledgments
The authors wish to thank all the national and international staff who have taken part in the EMERGENCY NGO’s project in Sudan. A special thanks to George Cowie (EMERGENCY UK) for editing and language revision. In loving memory of Gino Strada (founder of EMERGENCY in 1994).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gelijns, A.C.; Moskowitz, A.J.; Acker, M.A.; Argenziano, M.; Geller, N.L.; Puskas, J.D.; Perrault, L.P.; Smith, P.K.; Kron, I.L.; Michler, R.E.; et al. Management Practices and Major Infections After Cardiac Surgery. J. Am. Coll. Cardiol. 2014, 64, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Toeg, H.; French, D.; Gilbert, S.; Rubens, F. Incidence of sternal wound infection after tracheostomy in patients undergoing cardiac surgery: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2017, 153, 1394–1400.e7. [Google Scholar] [CrossRef]
- Massart, N.; Mansour, A.; Ross, J.T.; Piau, C.; Verhoye, J.P.; Tattevin, P.; Nesseler, N. Mortality due to hospital-acquired infection after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2020, 163, 2131–2140.e3. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Wang, Y.; Li, J.; Wang, J.; Wang, S.; Tian, Y.; Liu, J.; Diao, X.; Zhao, W. Establishment and Validation of a Nomogram to Predict Hospital-Acquired Infection in Elderly Patients After Cardiac Surgery. Clin. Interv. Aging. 2022, 17, 141–150. [Google Scholar] [CrossRef]
- He, S.; Chen, B.; Li, W.; Yan, J.; Chen, L.; Wang, X.; Xiao, Y. Ventilator-associated pneumonia after cardiac surgery: A meta-analysis and systematic review. J. Thorac. Cardiovasc. Surg. 2014, 148, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://salamcentre.emergency.it/ (accessed on 30 July 2022).
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Russo, A.; Carriero, G.; Farcomeni, A.; Ceccarelli, G.; Tritapepe, L.; Venditti, M. Role of oral nystatin prophylaxis in cardiac surgery with prolonged extracorporeal circulation. Mycoses 2017, 60, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Kirsop, J.; Tang, W.H. Exploring the Microbiome in Heart Failure. Curr. Heart Fail Rep. 2016, 13, 103–109. [Google Scholar] [CrossRef]
- Wang, D.S.; Huang, X.F.; Wang, H.F.; Le, S.; Du, X.L. Clinical risk score for postoperative pneumonia following heart valve surgery. Chin. Med. J. 2021, 134, 2447–2456. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.; Wang, H.; Le, S.; Yang, H.; Wang, F.; Du, X. Risk factors for postoperative pneumonia after cardiac surgery: A prediction model. J. Thorac. Dis. 2021, 13, 2351–2362. [Google Scholar] [CrossRef]
- Bouza, E.; Pérez, A.; Muñoz, P.; Jesús Pérez, M.; Rincón, C.; Sánchez, C.; Martín-Rabadán, P.; Riesgo, M.; Cardiovascular Infection Study Group. Ventilator-associated pneumonia after heart surgery: A prospective analysis and the value of surveillance. Crit. Care Med. 2003, 31, 1964–1970. [Google Scholar] [CrossRef]
- Xu, F.; Li, W.; Zhang, C.; Cao, R. Performance of Sequential Organ Failure Assessment and Simplified Acute Physiology Score II for Post-Cardiac Surgery Patients in Intensive Care Unit. Front. Cardiovasc. Med. 2021, 8, 774935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, F.; Gong, S.; Liao, B.; Liu, H.; Yuan, J.; Yu, D.; Qin, H.; Wu, M.; Dong, S. Validity of SOFA score as a prognostic tool for critically ill elderly patients with acute infective endocarditis. Rev. Cardiovasc. Med. 2021, 22, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Pitiriga, V.; Kanellopoulos, P.; Bakalis, I.; Kampos, E.; Sagris, I.; Saroglou, G.; Tsakris, A. Central venous catheter-related bloodstream infection and colonization: The impact of insertion site and distribution of multidrug-resistant pathogens. Antimicrob. Resist. Infect. Control. 2020, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Toor, H.; Farr, S.; Savla, P.; Kashyap, S.; Wang, S.; Miulli, D.E. Prevalence of Central Line-Associated Bloodstream Infections (CLABSI) in Intensive Care and Medical-Surgical Units. Cureus 2022, 14, e22809. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, G.; Cheng, Z.; Mei, C.; Liao, X.; Li, J.; Yuan, Y. Analysis of Nosocomial Infections in Post-Cardiac Surgery Extracorporeal Membrane Oxygenation Support Therapy. Heart Surg. Forum. 2018, 21, E387–E391. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Jia, M.; Du, Z.; Hou, X. Extracorporeal Membrane Oxygenation-Related Nosocomial Infection after Cardiac Surgery in Adult Patients. Braz. J. Cardiovasc. Surg. 2021, 36, 743–751. [Google Scholar] [CrossRef]
- Parienti, J.J.; Dugué, A.E.; Daurel, C.; Mira, J.P.; Mégarbane, B.; Mermel, L.A.; Daubin, C.; du Cheyron, D. Members of the Cathedia Study Group. Continuous renal replacement therapy may increase the risk of catheter infection. Clin. J. Am. Soc. Nephrol. 2010, 5, 1489–1496. [Google Scholar] [CrossRef]
- Ding, W.; Liu, J.; Zhou, X.; Miao, Q.; Zheng, H.; Zhou, B.; Dou, G.; Tong, Y.; Long, Y.; Su, L. Clinical Multi-Omics Study on the Gut Microbiota in Critically Ill Patients After Cardiovascular Surgery Combined With Cardiopulmonary Bypass With or Without Sepsis (MUL-GM-CSCPB Study): A Prospective Study Protocol. Front. Med. 2020, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Byarugaba, D.K. A view on antimicrobial resistance in developing countries and responsible risk factors. Int. J. Antimicrob. Agents. 2004, 24, 105–110. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.K.; Fowler, R.A.; Bhagwanjee, S.; Rubenfeld, G.D. Critical care and the global burden of critical illness in adults. Lancet 2010, 376, 1339–1346. [Google Scholar] [CrossRef]
- Zante, B.; Reichenspurner, H.; Kubik, M.; Kluge, S.; Schefold, J.C.; Pfortmueller, C.A. Base excess is superior to lactate-levels in prediction of ICU mortality after cardiac surgery. PLoS ONE 2018, 13, e0205309. [Google Scholar]
- Watson, G.; Jallow, B.; Le Doare, K.; Pushparajah, K.; Anderson, S.T. Acute rheumatic fever and rheumatic heart disease in resource-limited settings. Arch. Dis. Child. 2015, 100, 370–375. [Google Scholar] [CrossRef]
- Kumar, R.K.; Tandon, R. Rheumatic fever & rheumatic heart disease: The last 50 years. Indian J. Med. Res. 2013, 137, 643–658. [Google Scholar]
- Seckeler, M.D.; Hoke, T.R. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin. Epidemiol. 2011, 3, 67–84. [Google Scholar] [CrossRef]
- Mirabel, M.; Grimaldi, A.; Freers, J.; Jouven, X.; Marijon, E. Access to cardiac surgery in sub-Saharan Africa. Lancet 2015, 385, 606. [Google Scholar] [CrossRef]
- Chavez-Lindell, T.; Kikwe, B.; Gikonyo, A.; Odoi, A. Patient characteristics and cardiac surgical outcomes at a tertiary care hospital in Kenya, 2008–2017: A retrospective study. PeerJ 2021, 9, e11191. [Google Scholar] [CrossRef]
- Yangni-Angate, K.H.; Meneas, C.; Diby, F.; Diomande, M.; Adoubi, A.; Tanauh, Y. Cardiac surgery in Africa: A thirty-five year experience on open heart surgery in Cote d’Ivoire. Cardiovasc. Diagn. Ther. 2016, 6 (Suppl 1), S44–S63. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).