Abstract
Sepsis is one of the leading causes of hospitalization and death among hemodialysis patients. Infections due to multidrug-resistant organisms (MDROs) are common among these patients, but empiric broad-spectrum coverage for every septic patient is associated with unfavorable outcomes. A retrospective case–control study was conducted at Shamir Medical Center, Israel (July 2016–April 2020), to determine predictors of MDRO infections among septic (per SEPSIS-3) ambulatory adult hemodialysis patients with permanent dialysis access (i.e., fistula, graft, or tunneled Perm-A-Cath). MDROs were determined according to established definitions. Least Absolute Shrinkage and Selection Operator (LASSO) regression was used to construct a prediction score and determine its performance. Of 509 patients, 225 (44%) had microbiologically confirmed infection, and 79 patients (35% of 225) had MDROs. The eventual independent predictors of MDRO infections were Perm-A-Cath access (vs. fistula or graft, aOR = 3, CI-95% = 2.1–4.2) and recent hospitalization in the previous three months (aOR = 2.3, CI-95% = 1.6–3.3). The score to predict MDRO sepsis with the highest performances contained seven parameters and displayed an area under the receiver operating characteristic curve (ROC AUC) of 0.74. This study could aid in defining a group of hemodialysis patients for which empiric broad-spectrum agents could be safely avoided.
1. Introduction
Sepsis is one of the leading causes of hospitalization and death among chronic hemodialysis patients [1]. The high incidence of infections among this population is related to immunosuppressive and immunomodulatory states and conditions, constant access to a large blood vessel, and extensive and constant exposures to healthcare settings, environments, and procedures [1,2].
Infections due to multidrug-resistant organisms (MDROs) were defined by the World Health Organization (WHO) as one of the current greatest challenges in Medicine [3]. MDRO infections are relatively prevalent among dialysis patients [4], since these patients are subjected to high colonization pressure (facilitating “patient-to-patient transmission” of MDROs) and high selective pressure (facilitating “emergence of resistance” among the patient’s own non-MDRO susceptible strains) [4]. Delay in administration of appropriate antimicrobial therapy (DAAT) is common in MDRO infections [5] and is the strongest independent modifiable predictor of mortality in septic shock [5]. Therefore, it is important to avoid DAAT among certain hemodialysis septic patients suspected of having MDRO infection while avoiding usage of overly broad-spectrum coverage among hemodialysis patients at lower risk for MDROs [4]. Our study aim was to explore the epidemiology of MDRO sepsis among chronic ambulatory hemodialysis patients.
2. Results
There were 509 chronic ambulatory hemodialysis patients with sepsis upon admission to Shamir Medical Center (SMC) during the study period. Most patients were elderly (i.e., 71%, mean age 71 ± 13 years), and 67% were male. One-hundred and sixty-six patients (33%) were already flagged as known MDRO carriers in the previous 24 months. The median dialysis vintage (i.e., duration) was 2 years (range 0–31 years). With regard to the infectious syndromes of acute sepsis, 169 patients (33%) had respiratory infections, and 163 (32%) had primary bloodstream infections (i.e., BSI). The median length of stay in the hospital was eight days (IQR 4–13 days). Sixty-four patients (13%) died in the hospital, and 125 (25%) died within 90 days.
The microbiological diagnosis was confirmed for 225 (44%) patients (Table 1). Staphylococcus aureus was the commonest pathogen (17.8%), followed by Escherichia coli (16%) and Pseudomonas aeruginosa (9.8%). MDROs were documented in 79 patients (35% of microbiologically confirmed cases), and the commonest MDROs were third-generation-cephalosporin-non-susceptible Enterobacterales (i.e., extended-spectrum beta-lactamase (ESBL) and/or blaAmpC-hyperproducing strains, 16%), Pseudomonas aeruginosa (10%), and methicillin-resistant S. aureus (i.e., MRSA, 5%).

Table 1.
Chronic ambulatory hemodialysis patients with microbiologically confirmed sepsis upon admission to Shamir Medical Center (July 2016–April 2020).
Empiric usage of inappropriate agents (i.e., either an overly broad or narrow spectrum, or completely “wrong” therapy) was documented among 105 patients, i.e., 47% of the patients with microbiologically confirmed infection.
Next, we analyzed the predictors of MDRO infections. In univariable analyses (Table 2), there were multiple parameters associated with MDRO sepsis. Although demographic features (age, sex, and place of residence) did not differ between groups, there were multiple other significant statistical associations and potential predictors of MDRO sepsis, i.e., Perm-A-Cath access (vs. fistula or graft), certain background comorbidities (e.g., peripheral arterial disease and chronic skin ulcers), recent healthcare-associated exposures (i.e., hospitalizations, procedures, devices, and antibiotics), and certain acute illness indices (e.g., tachycardia at presentation, fatal McCabe score, low serum albumin, and admission to intensive care unit). The portion of patients with MDRO sepsis who were treated with appropriate antibiotics (per in vitro report) in the first 48 h (73%) was significantly lower in comparison to patients with non-MDRO sepsis (86%; OR = 0.4, p = 0.02) and experienced significantly worse outcomes (Table 2).

Table 2.
Univariable analysis of septic hemodialysis adult patients with multidrug-resistant organism (MDRO) infections versus non-MDRO infections.
In a multivariable model, the only independent significant predictors of MDRO sepsis remained Perm-A-Cath access (vs. fistula or graft, aOR = 3, CI-95% = 2.1–4.2) and recent hospitalization in the previous three months (aOR = 2.3, CI-95% = 1.6–3.3). The negative predictive value (NPV), if neither one of these parameters was present, was 91%.
The score to predict MDRO sepsis with the highest performance contained seven parameters: (1) tachycardia (2 points), (2) antibiotic use in the previous 3 months (2 points), (3) known MDRO carriage in the past two years (3 points), (4) chronic skin ulcers (3 points), (5) admission to ICU (4 points), (6) skilled nursing care at home (4 points), and (7) Perm-A-Cath access (4 points). A cutoff of 6 points yielded 75% sensitivity, 60% specificity, 31% positive predictive value (PPV), and 91% NPV, with a ROC AUC of 0.74 (Figure 1).
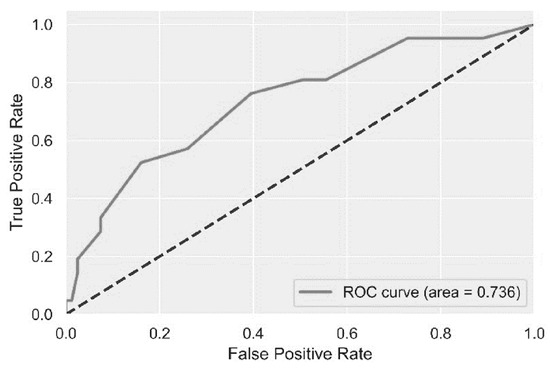
Figure 1.
A prediction score for multidrug-resistant organism (MDRO) infection among chronic ambulatory adult hemodialysis patients (Shamir Medical Center, July 2016–April 2020).
This plot demonstrates the receiver operating characteristic curve analysis (ROC) for the prediction performance of the score to predict multidrug-resistant organism infection among chronic hemodialysis patients.
There were certain outcomes among survivors that were significantly worse among MDRO patients, including elongated length of stay (LOS; 10 vs. 7 days, p = 0.001) and functional status deterioration following the index event (23% vs. 11%, OR = 2.5, p = 0.005). The in-hospital mortality rate was higher as well (though insignificantly) among patients with MDRO infections (19% vs. 11%, p = 0.06). In separate multivariable models, one for each outcome parameter, MDRO remained significantly associated with elongated LOS (i.e., LOS > 10 days) following the infection (aOR = 2, CI-95% = 1.1–3.6, p = 0.03), but not with functional status deterioration nor with in-hospital mortality.
3. Discussion
In this study, among a cohort of 509 patients, MDROs were documented among 79 patients, i.e., 35% of the 225 patients with microbiologically confirmed infection. Moreover, one of every three patients was already a known MDRO carrier upon admission. This indicates a large burden of MDROs, implying that MDROs are endemic among chronic ambulatory hemodialysis patients admitted with acute sepsis to SMC. This theoretically justifies the current practices of empiric administration of broad-spectrum agents for hemodialysis patients presenting with acute sepsis to an acute-care facility. Nonetheless, we were able to isolate a group of hemodialysis patients in whom overly broad-spectrum (and frequently toxic) agents could be safely avoided. Despite the endemicity of MDROs at SMC, 99 of 146 (68%) patients with microbiologically confirmed susceptible organism (non-MDRO) sepsis had empirically received an overly broad-spectrum agent (Table 2). The use of overly broad-spectrum antibiotics for every septic patient further increases the selective pressure among this population and increases the rates of adverse events, which are frequently elevated among patients with reduced kidney function who receive broad-spectrum non-beta-lactam regimens [15].
In a multivariable model, the only independent significant predictors of MDROs were Perm-A-Cath access and recent acute-care hospitalization (in the past 3 months). If neither one of these parameters was present, the negative predictive value (NPV) for having an MDRO infection was as high as 91%. Among this group of chronic ambulatory hemodialysis patients, empiric broad-spectrum coverage could be safely avoided among patients with non-life-threatening infections. Next, we developed a prediction score to further improve MDRO prediction performance, but while the NPV of the seven-component score was the same (i.e., 91%), the overall ROC AUC was only 0.74. Therefore, specifically at SMC, the absence of the two independent predictors of MDRO infection (i.e., no Perm-A-Cath access and no recent hospitalizations) should guide the empiric management to avoid overly broad-spectrum agents among certain patients, and this should not be based on the incorporation of a complicated score with difficult assimilation challenges among busy healthcare workers.
Our study has limitations associated with its retrospective chart-review design from a single center. The conclusions of this study could not be generalized to additional facilities and centers without conducting internal validations. However, as opposed to previous MDRO epidemiology analyses executed among ambulatory hemodialysis patients [16], this analysis included not only patients with BSI but all septic patients (per SEPSIS-III definition) [17] while also attributing offending pathogens to non-BSI infectious syndromes, determined by a single senior Infectious Disease specialist. This design better reflects the spectrum and complexities of decisions that practitioners face while attending a hemodialysis patient with acute sepsis.
To conclude, at SMC, MDRO infections are prevalent among chronic ambulatory hemodialysis patients, and it is a safe routine to empirically administer broad-spectrum agents to the majority of septic patients. However, we were able to identify a group of patients in whom overly broad-spectrum coverage (i.e., frequently more toxic, less bactericidal, and more expensive alternatives) could be safely avoided. This could lead to improved outcomes among these individuals and could result, over time, in beneficial institutional ecological impacts.
4. Materials and Methods
This was a retrospective cohort study at Shamir Medical Center (SMC, July 2016–April 2020), Israel. The ambulatory hemodialysis department at SMC hosts ~200 chronic hemodialysis inpatients and outpatients at any given time. The institutional ethics committee at SMC had approved the study prior to its initiation. Adult patients (>18 years) on chronic hemodialysis with acute sepsis [17] upon admission to SMC’s emergency room (ER) were enrolled. Patients on acute dialysis, patients with nosocomial sepsis, and patients with a non-tunneled temporary line (i.e., no arteriovenous fistula/graft or tunneled line (e.g., Perm-A-Cath)) were all excluded. Patients could be included more than once, but only if the septic episodes were separated by more than one month, and only if the patient presented with a different infectious syndrome. The clinical infectious syndrome was determined according to established guidelines [18]. We captured cultures that were obtained from sterile sites (e.g., blood) or cultures that were obtained from non-sterile sites but matched the patient’s infectious syndrome (e.g., respiratory cultures among patients with pneumonia and urine cultures among patients with urinary tract infections).
MDROs were defined in accordance with established definitions [19]: (1) methicillin-resistant Staphylococcus aureus (MRSA); (2) ampicillin-resistant enterococci; (3) penicillin- or ceftriaxone-non-susceptible Streptococcus pneumoniae; (4) P. aeruginosa; (5) A. baumannii; (6) Enterobacterales non-susceptible to one or more 3rd-generation cephalosporins (e.g., ceftriaxone or ceftazidime); and (7) fluoroquinolone-resistant Campylobacter jejuni. Extensively drug-resistant organisms (XDROs) included: (1) vancomycin-resistant enterococci (VRE); (2) heterogeneous vancomycin-intermediate S. aureus (hVISA) or S. aureus with MIC ≥ 2 to vancomycin; (3) carbapenem-resistant Enterobacterales (i.e., CRE, evidence of carbapenemase production and/or meropenem MIC ≥ 2); (4) carbapenem-resistant A. baumannii (CRAB); (5) carbapenem-resistant P. aeruginosa (CRPA); and (6) intrinsically carbapenem-non-susceptible Gram-negatives (e.g., Stenotrophomonas maltophilia). Microbiological processing was in accordance with Clinical and Laboratory Standards Institute (CLSI) criteria and definitions [20].
Predictors of MDRO sepsis were queried by logistic regression. We randomized the cohort into two groups: data from 80% of the patients were used in order to generate and create the prediction score, and data from 20% of the patients were used in order to validate the prediction score that was generated. A prediction score of MDRO sepsis was developed using Least Absolute Shrinkage and Selection Operator (LASSO) regression. In short, the L1-penalty was used to determine the model with the best performance while penalizing the sum of absolute values of the coefficients. The coefficients for the least useful variables for prediction were driven to 0 and were excluded from further analysis. The Python “scikit-learn” machine learning library was used in order to perform the LASSO regression. The remaining variables were used in the multivariable logistic regression. The variables that were significant were used in order to construct the final predictive score. The score was then evaluated using a validation cohort that was not used in the development of the score.
Author Contributions
Conceptualization, S.Z.-I. and D.M.; methodology, S.Z.-I. and D.M.; validation, S.Z.-I., Y.E. and D.M.; formal analysis, S.Z.-I., M.S. and D.M.; investigation, S.Z.-I., Y.E., R.W.S., D.Z.Y., A.S., A.K. and D.M.; resources, D.Z.Y.; data curation, S.Z.-I.; writing—original draft preparation, S.Z.-I. and D.M.; writing—review and editing S.Z.-I. and D.M.; supervision, D.M.; project administration, S.Z.-I. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Shamir Medical Center (protocol number 0224-18-ASF, on 21 September 2018).
Informed Consent Statement
Patient consent was waived due to the retrospective cohort chart-review-based design of the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of Immune Dysfunction in End-stage Renal Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Allon, M. Treatment Guidelines for Dialysis Catheter–Related Bacteremia: An Update. Am. J. Kidney Dis. 2009, 54, 13–17. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 13 August 2017).
- Rteil, A.; Kazma, J.M.; El Sawda, J.; Gharamti, A.; Koubar, S.H.; Kanafani, Z.A. Clinical characteristics, risk factors and microbiology of infections in patients receiving chronic hemodialysis. J. Infect. Public Health 2020, 13, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Shani, V.; Muchtar, E.; Kariv, G.; Robenshtok, E.; Leibovici, L. Systematic Review and Meta-Analysis of the Efficacy of Appropriate Empiric Antibiotic Therapy for Sepsis. Antimicrob. Agents Chemother. 2010, 54, 4851–4863. [Google Scholar] [CrossRef] [PubMed]
- National Healthcare Safety Network (NHSN). Patient Safety Component Manual; National Healthcare Safety Network (NHSN): Atlanta, GA, USA, 2021. [Google Scholar]
- Acute Clostridioides difficile infection. In Israeli Ministry of Health set of Clinical and Microbiological Guidelines; Israeli Ministry of Health: Jerusalem, Izrael, 2012.
- Marchaim, D.; Gottesman, T.; Schwartz, O.; Korem, M.; Maor, Y.; Rahav, G.; Karplus, R.; Lazarovitch, T.; Braun, E.; Sprecher, H.; et al. National Multicenter Study of Predictors and Outcomes of Bacteremia upon Hospital Admission Caused by Enterobacteriaceae Producing Extended-Spectrum β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 5099–5104. [Google Scholar] [CrossRef] [PubMed]
- Edemekong, P.F.; Bomgaars, D.L.; Sukumaran, S.; Levy, S.B. Activities of Daily Living; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; Bassetti, M.; De Rosa, F.G.; Spanu, T.; Di Meco, E.; Losito, A.R.; Parisini, A.; Pagani, N.; Cauda, R. Identifying Patients Harboring Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae on Hospital Admission: Derivation and Validation of a Scoring System. Antimicrob. Agents Chemother. 2011, 55, 3485–3490. [Google Scholar] [CrossRef] [PubMed]
- Bion, J.F.; Edlin, S.A.; Ramsay, G.; McCabe, S.; Ledingham, I.M. Validation of a prognostic score in critically ill patients undergoing transport. BMJ 1985, 291, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Ko, W.-C.; von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G.; Klugman, K.P.; Bonomo, R.A.; et al. International prospective study of Klebsiella pneumoniae bacteremia: Implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Intern. Med. 2004, 140, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The index of Adl: A standardized measure of biological and phychological funcation. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, S.J.; De Vriese, A. Vancomycin Dosing in Patients on Intermittent Hemodialysis. Semin. Dial. 2011, 24, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Golestaneh, L.; Allon, M.; Abreo, K.; Mokrzycki, M.H. Prevention of Bloodstream Infections in Patients Undergoing Hemodialysis. Clin. J. Am. Soc. Nephrol. 2019, 15, 132–151. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- (CDC) CfDCaP. Types of Healthcare-Associated Infections 2016. Available online: https://www.cdc.gov/hai/infectiontypes.html (accessed on 12 September 2022).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 12 September 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).