Abstract
Bacterial antibiotic resistance is rapidly growing globally and poses a severe health threat as the number of multidrug resistant (MDR) and extensively drug-resistant (XDR) bacteria increases. The observed resistance is partially due to natural evolution and to a large extent is attributed to antibiotic misuse and overuse. As the rate of antibiotic resistance increases, it is crucial to develop new drugs to address the emergence of MDR and XDR pathogens. A variety of strategies are employed to address issues pertaining to bacterial antibiotic resistance and these strategies include: (1) the anti-virulence approach, which ultimately targets virulence factors instead of killing the bacterium, (2) employing antimicrobial peptides that target key proteins for bacterial survival and, (3) phage therapy, which uses bacteriophages to treat infectious diseases. In this review, we take a renewed look at a group of ESKAPE pathogens which are known to cause nosocomial infections and are able to escape the bactericidal actions of antibiotics by reducing the efficacy of several known antibiotics. We discuss previously observed escape mechanisms and new possible therapeutic measures to combat these pathogens and further suggest caseinolytic proteins (Clp) as possible therapeutic targets to combat ESKAPE pathogens. These proteins have displayed unmatched significance in bacterial growth, viability and virulence upon chronic infection and under stressful conditions. Furthermore, several studies have showed promising results with targeting Clp proteins in bacterial species, such as Mycobacterium tuberculosis, Staphylococcus aureus and Bacillus subtilis.
1. Introduction
The ESKAPE pathogens are a group of pathogens that pose a global health threat due to their ability to evade antibiotic biocidal effects. This group of pathogens is composed of both Gram-positive and Gram-negative bacterial species, namely: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter (Figure 1) [1,2,3]. ESKAPE pathogens are mainly responsible for nosocomial infections and these infections are defined as hospital-acquired infections (HAIs) that affect patients within 48 h of admission, 3 days of discharge or 30 days of an operation [4]. HAIs present challenges in care delivery and no institution in any country seems to have solved this challenging situation. As a result, ESKAPE pathogens are responsible for more than 40% of infections in intensive care units (ICU) and pose an economic burden, especially in low- and middle-income countries [5,6]. Over the years, an increasing number of pathogens have been reported to be antibiotic resistant as a result of the misuse and overuse of antibiotics globally [1,7]. ESKAPE pathogens exhibit drug resistance via numerous mechanisms, such as using enzymes to irreversibly cleave and therefore inactivate the drug, modifying the drug-binding site, decreasing drug permeability or increasing drug efflux to decrease drug accumulation, or by the production of biofilms (Table 1) [3,8]. The emergence of antibiotic-resistant pathogens renders drugs that were initially used to combat bacteria to be redundant and ineffective, therefore allowing bacteria to grow in the presence of high antibiotic concentrations [1,7]. Subsequently, it is important to find alternative targets to inhibit the growth and spread of pathogens [2,3,9]. One such target is a group of proteins referred to as caseinolytic proteins, which are found in a number of organisms and play an important role in maintaining protein homeostasis in the cell [9].
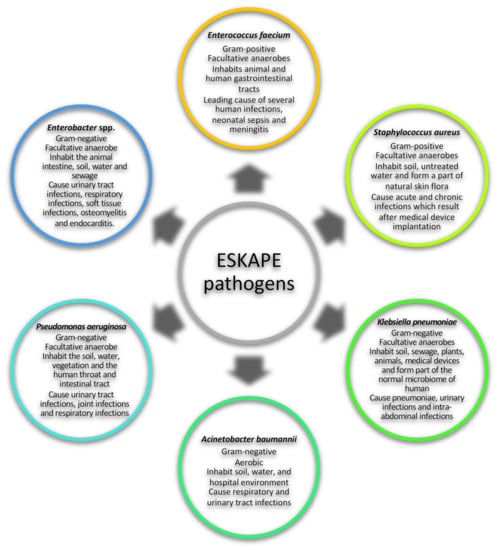
Figure 1.
General characteristics ESKAPE pathogens. The ESKAPE group of pathogens consists of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter [1,2,3,10,11,12,13,14,15,16,17,18,19,20,21].

Table 1.
Resistance strategies used by ESKAPE pathogens to escape antibiotics.
Antivirulence drugs that do not necessarily kill the bacterial cells, but prevent bacterial pathogenesis by targeting virulence traits in bacteria, can be used to combat the emergence of antibiotic-resistant pathogens [27,28]. The use of antivirulence strategies to kill pathogens is advantageous as it results in less evolutionary pressure, thus reducing the development of resistant strains [27]. In this approach, the anti-ESKAPE drug administered would interfere with bacterial virulence factors instead of growth pathways to cure disease, thus leading to the development of new strategies for the prevention and control of infections [7,29].
2. Caseinolytic Proteins: Classification, Function and Structure
Caseinolytic (Clp) proteins are found in bacteria, fungi, in the mitochondria of eukaryotes and in the chloroplast of plants [9]. Microorganisms use Clp proteins, which function as a complex of catalytic (ClpP) and regulatory subunits (further referred to as Clp ATPases), to perform a variety of vital roles in the cell, such as protein homeostasis and cellular stress response (Table 2). An imbalance in protein quality and quantity control due to cellular stress, such as heat shock, the presence of antibiotics or a change in pH leads to a build-up of proteins in the cell, which results in cell death [9]. In such stressful conditions, it is important for the cell to remove these unfolded proteins for cellular function and growth to continue [30].

Table 2.
Caseinolytic proteins identified across various species, exhibiting diverse functions.
Additionally, it has been found that Clp proteins play an important role in the pathogenecity and virulence of several pathogens [42,43,44]. Several studies have linked ClpP to one of the mechanisms S. aureus uses to evade phagocytosis, which is one of the defence mechanisms that the host uses to fight S. aureus infection. For example, Frees et al. [42] established that S. aureus mutants lacking ClpX (Clp ATPase) or ClpP exhibited decreased virulence in a murine skin abscess model [42,43]. ClpP regulates the agr locus, which is responsible for the production of effectors, such as the haemolytic factor α-hemolysin. This effector generates small pores in the phagocytotic cells, enabling the phagocytosed bacterium to escape from the immune cells into the host system, thus enhancing the bacterium’s virulence [42,43,44]. Furthermore, this model also showed that the activity of ClpX and ClpP was crucial for the iron-regulated surface determinant system. This system is important for S. aureus iron uptake, which is essential for the pathogen to survive in the host [45]. This phenomena of ClpP regulating proteins to escape phagocytosis during an immune response is also observed in other bacteria, such as Listeria monocytogenes. This pathogenic bacteria causes listeriosis and it contains both ClpP1 and ClpP2 [46]. Although the functional significance of ClpP1 is unknown, L. monocytogenes mutants lacking ClpP2 were found to be susceptible to the activity of host macrophages, as these bacteria lacked their usual haemolytic abilities [47]. Finally, ClpP in Pseudomonas aeruginosa regulates the expression of alginate, an exopolysaccharide that protects the pathogen [48]. Furthermore, the importance of Clp proteins in bacterial virulence has also been demostrated in Streptococcus pneumonia, where strains lacking the clpP gene were found to lose their ability to invade lung tissues and colonise the nasopharynx [49]. Therefore, considering the importance of Clp proteins in the survival of pathogens in various environments, these proteins could be targeted for the development of potential drugs to inhibit their growth.
2.1. Catalytic Subunit—ClpP
Most bacteria contain a single ClpP catalytic subunit with an exception of a few, such as Mycobacterium tuberculosis, Mycobacterium smegmatis, Listeria monocytogenes, Chlamydia trachomatis and Pseudomonas aeruginosa, which have been found to contain two ClpP homologs that are referred to as ClpP1 and ClpP2 [50,51,52,53,54,55]. ClpP is a serine protease composed of two heptameric rings, which forms a barrel-shaped structure referred to as a tetradecamer, and this tetradecamer may be formed by just one ClpP or a mixture of ClpP1 and ClpP2 homo- or hetero-tetradecamers. The ClpP tetradecamer encloses a protease active site with a catalytic triad consisting of three amino acids, namely serine, histidine and aspartic acid [56,57]. ClpP can adopt two conformations, that is: the closed/inactive and open/active conformation (Figure 2). In the closed conformation, the cavity for protein substrates to enter the proteolysis chamber is closed and the catalytic triad is misaligned. Therefore, protein hydrolysis is blocked. In this closed conformation, ClpP only functions as a peptidase, degrading only short peptides [36]. In order for ClpP to adopt an active conformation, it needs to be bound to a Clp ATPase (the regulatory subunit of a Clp protein) [36]. Clp ATPases bind to either one or both ends of ClpP; this binding results in conformational changes, which leads to the opening of the cavity for substrates to access the active site and in the alignment of the catalytic triad residues [43]. Subsequently, ClpP degrades damaged proteins, which are translocated into its chamber via Clp ATPases [36,56].
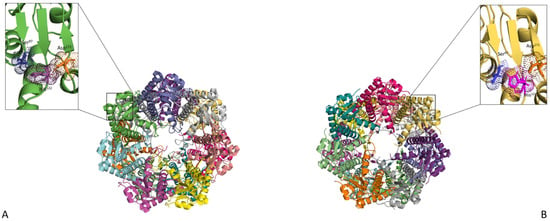
Figure 2.
ClpP in its unbound and bound state. (A) Top view of the unbound ClpP tetradecamer (PDB—1YG6). (B) Top view of the active form of ClpP (PDB—5E0S). The Ser97, His122 and Asp171 catalytic residues are shown up-close and coloured orange, pink and blue, respectively. Upon Clp ATPase binding, ClpP assumes an opened/active conformation resulting in the ordering of the axial pore (represented by grey dotted lines) and the alignment of the catalytic residues. The structures were visualised using PyMol [58].
2.2. Regulatory Subunits—Clp ATPases
Clp ATPase proteins belong to a protein superfamily referred to as AAA+ proteins (ATPases associated with diverse cellular activities) [9]. The AAA+ ATPases have an AAA+ unfoldase/disaggregase, which recognises specific substrates and uses energy generated from ATP hydrolysis to contribute to functions, such as protein quality control, the degradation of transcriptional regulators, DNA replication and repair and cytoskeleton regulation, among other things [36]. The hallmark of the AAA family is a 200–250 amino acid ATP-binding domain that contains Walker A and Walker B motifs (Figure 3) [59]. The canonical Walker A forms the floor of the nucleotide-binding pocket (binds the ATP phosphate) and Walker B forms a loop that overlays the pockets and positions cations (binds metals and plays a role in ATP catalysis) [30,56]. Additionally, Clp ATPases have the unique ability to promote the resolubilisation of protein aggregates and are therefore grouped into the heat shock protein (Hsp) 100 family [9,30].
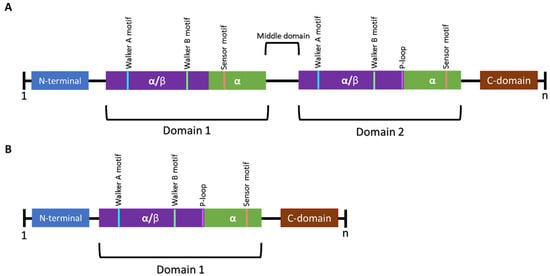
Figure 3.
Schematic representation of the general structural features of Class I and Class II Clp ATPases of prokaryotes. (A) Class I Clp ATPases contain two nucleotide-binding domains (NBD) referred to as domain 1 and domain 2. A middle domain has been identified to be present in certain Clp ATPases and plays a role in separating the two NBDs. (B) Class II Clp ATPases contain one NBD. Each domain contains Walker A and Walker B motifs. Certain Clp ATPases contain a P-loop, a tripeptide (represented in pink) required for interaction with ClpP. n represents the number of amino acids in the sequence [60,61].
Clp ATPases are grouped into two classes (class I and II) based on the number of NBDs they contain. ClpA, ClpB, ClpC, ClpD, ClpE, ClpK and ClpL are class I members and contain two NBDs, whereas ClpM, ClpN, ClpX and ClpY have been identified as being class II members and contain one NBD (Figure 3) [39,56,62]. The Clp ATPases are further divided into two subfamilies, namely the ClpA and ClpB/Hsp104 subfamily based on the presence or absence of the tripeptide sequence [I-G-F/L] required for ClpP interaction, respectively (Figure 4) [36]. The ClpA subfamily forms hexameric complexes which bind and unfold proteins before translocating unfolded proteins to the ClpP proteolytic chamber for final degradation [9,36]. It is believed that the ClpB/Hsp104 subfamily functions as chaperones under stressful conditions to prevent protein unfolding or to assist in protein disaggregation as they lack the ClpP-binding motif [62,63]. Aligned with this chaperone activity, ClpB/Hsp104 have been reported to interact with the DnaK system to mediate protein unfolding and reactivation [39,62]. Members of both the ClpA and ClpB/Hsp104 subfamily have been identified in ESKAPE pathogens (Table 3).
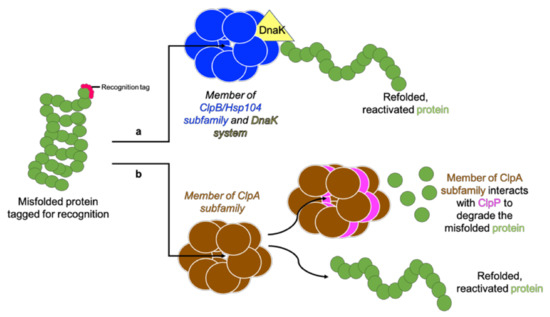
Figure 4.
Two Clp ATPase subfamilies. A tagged protein is recognised by a Clp ATPase. (a) Members of the ClpB/Hsp104 subfamily lack the tripeptide for ClpP interaction and function along with the DnaK system to unfold and refold the protein into its functional conformation. (b) Members of the ClpA subfamily contain the tripeptide for ClpP interaction and therefore redirect proteins which cannot be unfolded and reactivated to ClpP for degradation. Adapted from Maurizi and Xia [61].

Table 3.
Caseinolytic proteins identified in ESKAPE pathogens.
3. Caseinolytic Proteins Targeted in ESKAPE Pathogens
To date, very few Clp proteins from ESKAPE pathogens have been identified (Table 3) and there are a limited number of drugs which are currently under investigation (Table 4). The current drugs being studied inhibit Clp proteins in one of three ways: firstly, they interfere with proteolytic activity by inhibiting ClpP; secondly, they interfere with ATPase activity by inhibiting or enhancing the activity of Clp ATPases; and lastly, they disrupt the ClpP and Clp ATPase complex [43,72,73]. Interestingly, of the drugs being studied, only five target S. aureus, which is a member of the ESKAPE pathogens and most of the other drugs target mainly M. tuberculosis (Table 4).

Table 4.
Compounds developed as potential targets for Clp ATPases.
Drugs that have been developed to target the proteolytic subunit (ClpP) of Clp proteins in S. aureus include D2, E3 and G2 compounds (Table 4) which irreversibly inhibit the proteolytic activity of ClpP, therefore resulting in the build-up of damaged proteins in the cell and eventually leading to cell death [43]. Based on the current literature, ClpP associates with the ClpA subfamily to ultimately degrade proteins that cannot be unfolded and/or reactivated. Subsequently, targeting ClpP would prevent the formation of the ClpP-ClpA subfamily complex, thus impairing cell protein homeostasis [9].
According to our knowledge, only one drug candidate, Armeniaspirols, targeting Clp ATPases belonging to the ClpB subfamily from B. subtilis has been reported. This drug candidate targets Clp ATPase (ClpY) when complexed to the proteolytic subunit (ClpQ) and is reported to disrupt the regulation of proteins essential for cell division, such as MreB and FtsZ [78]. On the other hand, the ClpA subfamily has a number of drug candidates being investigated, however most of these drug candidates target Mycobacterium tuberculosis, ClpC1 [Table 4]. The structures of lassomycin, cyclomarin A, rufomycin and ecumicin are show in Table 4 and these anti-TB peptides target the Clp ATPase subunit of Clp proteins, specifically the ClpC1 N-terminal domain (ClpC1-NTD) [16,43,74,76]. The three-dimensional structure of ClpC1-NTD complexed with some of these anti-TB peptides show that (a) their mode of action varies, (b) some may share a binding pocket (e.g., rufomycin cannot bind in the presence of ecumicin) and (c) the number of binding site/s on ClpC1-NTD varies per anti-TB peptide (Figure 5) [77,81,82]. For example, the crystal structure of ClpC1-NTD complexed with ecumicin or rufomycin showed that one rufomycin molecule binds to ClpC1-NTD, whereas two ecumicin molecules bind to ClpC1-NTD (Figure 5). The extent to which these anti-TB peptides impact the Clp ATPase is not similar across all of them, suggesting a difference in their mode of action. Biochemical studies have shown that ecumicin and lassomycin increase the ATPase activity of ClpC1, whereas rufomycin has no significant effect on ClpC1 ATPase activity [76,82,83,84]. Structural studies suggest that enhanced ClpC1 ATPase activity stimulated by ecumicin may be due to the conformational changes on the N-terminal domain and D1 interface, thereby improving ATP access for ATP hydrolysis in the nucleotide-binding domain [82]. As much as lassomycin binding to ClpC1 results in the overactivation of ClpC1 ATPase activity and therefore uncontrolled unfoldase activity [76], in the absence of ClpC1-NTD-lassomycin complex, it is unclear whether the binding of lassomycin to ClpC1 induces structural changes which are similar to those seen for ecumicin binding.
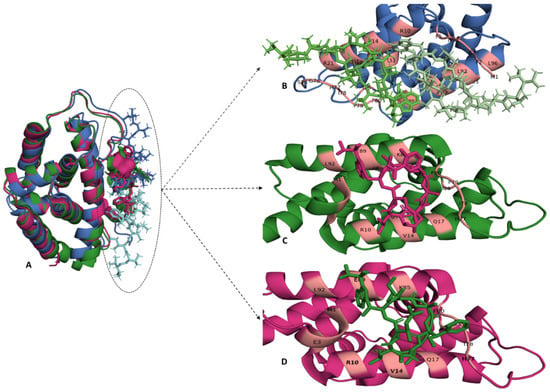
Figure 5.
The binding site of Ecumicin, Cyclomarin-A and Rufomycin in the ClpC1 N-terminal domain. (A) The crystal structure of ClpC1-NTD-Ecumicin (PDB-6pbs), ClpC1-NTD-Rufomycin (PDB-6cn8) and ClpC1-NTD-Cyclomarin A (PDB-3wdc) are shown in blue, green and pink, respectively, and were superimposed to compare the local environment of the three ligands. (B) The binding of two ecumicin molecules (ecumicin 1-light green, ecumicin 2-dark green) per ClpC1 N-terminal domain shows that these molecules bind adjacent to each other. The binding stochiometric ratio of rufomycin (pink) or cyclomarin A (green) to ClpC1 N-terminal domain is 1:1, represented in (C) and (D), respectively. The binding sites for these anti-TB peptides are predominantly located on residues in α-helices 1 and 5 and the loop region connecting α-helices 4 and 5. The interacting residues are coloured light pink on their respective secondary structural elements. The structures and interactions were visualised using PyMol [58].
Given that lassomycin binds to an essential domain in Clp ATPases, it was interesting to observe that this compound is species specific, and is inactive against S. aureus, Bacillus anthracis and K. pneumoniae. One would expect functioning of Clp ATPases across different species to be impaired given that it targets a conserved region [30,76]. Keeping in mind the inability of designed compounds, such as lassomycin, to inhibit Clp ATPases across species, it may be worthwhile to design compounds to target specific motifs on Clp ATPases, ClpP and the ClpP/Clp–ATPase complex interface. It is unlikely that these compounds will be species- or class-specific but rather target Clp ATPases across species. This strategy will be advantageous since pathogens usually contain more than one Clp isoform, thus ensuring the complete inhibition of Clp activity in the pathogens [85]. There are a number of conserved motifs that could be targeted in Clp ATPases, these include the N-terminal domain, the extended N-terminal domain, the middle domain, the nucleotide-binding domain(s), the zinc-binding motif and the C-terminal domain [16,57]. These domains are responsible for a variety of functions. For example, the extended N-terminal domain of ClpB is important for the growth of bacteria at high temperatures in the absence of DnaK, whereas the extended N- terminal has been found to be important for homodimer formation in Caenorhabditis elegans ABCB6/HMT-1, suggesting that the N-terminal extension may be essential for Clp ATPase assembly and stability [86,87]. The primary function of the N-terminal domain is to stabilise Clp ATPases, therefore targeting the N-terminal domain would destabilise the Clp ATPases and lead to the loss of protein function [88].
The nucleotide-binding domain(s) and subsequently the Walker A and Walker B motifs are important drug targets, since drug candidates targeting this site would inhibit the ability of the Clp ATPase to hydrolyse ATP and consequently, the unfoldase activity of Clp ATPases will be abolished. We have noted a slight difference in the amino acid sequences of the NBDs between class I and class II, therefore it is important that drug-design studies are cognisant of this observation to ensure that the designed drugs are not class-specific. Another potential drug target is the middle domain and zinc-binding motif. The middle domain is implicated in protein stability and interdomain communication between NBD1 and NBD2. Taking into account, the importance of this domain in terms of protein stability, it is anticipated that targeting the middle domain would potentially destabilise the protein [89,90]. However, compounds developed against this region would need optimisation, given that the length of the middle domain differs across species and this domain is not observed to be in present in all Clp ATPases. The zinc-binding domain is present in ClpX and ClpK. The role of this domain in unknown in ClpK, however it is positioned in the N-terminal domain of ClpX and plays a role in substrate recognition [16,57,61]. Therefore, essentially compounds targeting the zinc-binding domain could inhibit substrate recognition and would theoretically lead to the build-up of damaged proteins in the cell.
4. Conclusions
ESKAPE pathogens are resistant to a number of antibiotics and pose a major threat to patients in the nosocomial environment, therefore it is important to develop alternative methods to target and control their spread. Clp proteins, which are essential for both virulence and survival of the pathogen, are emerging as potential drug targets. Therefore, these proteins have been studied in various organisms and a number of drug candidates are being investigated to target these proteins. As much as bioinformatic studies have shown that Clp proteins evolve to diversify their response to stressful environmental factors, certain key motifs and domains remain conserved. These conserved domains and motifs provide a potential site for the development of drug candidates to target Clp ATPases across species. Further studies could investigate the distribution of Clp proteins in ESKAPE pathogens and develop drug candidates that could reduce the impact of ESKAPE pathogens.
Author Contributions
Conceptualisation, T.K. and I.A.; writing—original draft preparation, T.M. and T.K.; writing—review and editing, Q.M., I.A. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
Tehrim Motiwala would like to thank the Department of Science and Technology-National Research Foundation (DST-NRF), South Africa for Innovation Master’s Scholarships with Grant Number 123132 and 141266. Thandeka Khoza also thanks the National Research Foundation Grant number 121275, South African Medical Research Council-SIR grant and the University of KwaZulu-Natal for their research grants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Ramsamy, Y.; Essack, S.Y.; Sartorius, B.; Patel, M.; Mlisana, K.P. Antibiotic resistance trends of ESKAPE pathogens in Kwazulu-Natal, South Africa: A five-year retrospective analysis. Afr. J. Lab. Med. 2018, 7, 887. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Inweregbu, K.; Dave, J.; Pittard, A. Nosocomial infections. Contin. Educ. Anaesth. Crit. Care Pain 2005, 5, 14–17. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Bin Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Progress and Challenges in Implementing the Research on ESKAPE Pathogens. Infect. Control Hosp. Epidemiol. 2010, 31 (Suppl. 1), S7–S10. [Google Scholar] [CrossRef]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; Bardanca, M.G.; Ambroa, A.; López, M.; Bou, G.; Tomás, M. Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases. Antibiotics 2020, 9, 65. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- AhYoung, A.P.; Koehl, A.; Cascio, D.; Egea, P.F. Structural mapping of the C lp B ATP ases of Plasmodium falciparum: Targeting protein folding and secretion for antimalarial drug design. Protein Sci. 2015, 24, 1508–1520. [Google Scholar] [CrossRef]
- Higaki, S.; Morohashi, M.; Yamagishi, T. Isolation of Enterococcus species from infectious skin lesions. Drugs Under Exp. Clin. Res. 2002, 28, 91–93. [Google Scholar]
- Kafil, H.S.; Asgharzadeh, M. Vancomycin-resistant enteroccus faecium and enterococcus faecalis isolated from education hospital of iran. Maedica 2014, 9, 323–327. [Google Scholar]
- Giacometti, A.; Cirioni, O.; Schimizzi, A.M.; Del Prete, M.S.; Barchiesi, F.; D’Errico, M.M.; Petrelli, E.; Scalise, G. Epidemiology and Microbiology of Surgical Wound Infections. J. Clin. Microbiol. 2000, 38, 918–922. [Google Scholar] [CrossRef] [Green Version]
- Calfee, D.P. Recent advances in the understanding and management of Klebsiella pneumoniae. F1000Research 2017, 6, 1760. [Google Scholar] [CrossRef]
- Martin, R.M.; Bachman, M.A. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Bojer, M.S.; Struve, C.; Ingmer, H.; Hansen, D.S.; Krogfelt, K.A. Heat Resistance Mediated by a New Plasmid Encoded Clp ATPase, ClpK, as a Possible Novel Mechanism for Nosocomial Persistence of Klebsiella pneumoniae. PLoS ONE 2010, 5, e15467. [Google Scholar] [CrossRef]
- Nakonieczna, J.; Woźniak, A.; Pierański, M.K.; Rapacka-Zdonczyk, A.; Ogonowska, P.; Grinholc, M. Photoinactivation of ESKAPE pathogens: Overview of novel therapeutic strategy. Future Med. Chem. 2019, 11, 443–461. [Google Scholar] [CrossRef]
- Llaca-Díaz, J.M.; Mendoza-Olazarán, S.; Camacho-Ortiz, A.; Flores, S.; Garza-González, E. One-Year Surveillance of ESKAPE Pathogens in an Intensive Care Unit of Monterrey, Mexico. Chemotherapy 2012, 58, 475–481. [Google Scholar] [CrossRef]
- Breidenstein, E.B.; de la Fuente-Núñez, C.; Hancock, R.E. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef]
- Driscoll, J.A.; Brody, S.L.; Kollef, M.H. The Epidemiology, Pathogenesis and Treatment of Pseudomonas aeruginosa Infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Xie, G.; Traglia, G.M.; Johnson, S.L.; Davenport, K.W.; van Duin, D.; Ramazani, A.; Perez, F.; Jacobs, M.R.; Sherratt, D.J.; et al. Whole-Genome Comparative Analysis of Two Carbapenem-Resistant ST-258 Klebsiella pneumoniae Strains Isolated during a North-Eastern Ohio Outbreak: Differences within the High Heterogeneity Zones. Genome Biol. Evol. 2016, 8, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.; Obi, L.; Morobe, I.; Bisi-Johnson, M. Molecular Characteristics and Antibiotic Resistance Profiles of Klebsiella Isolates in Mthatha, Eastern Cape Province, South Africa. Int. J. Microbiol. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, M.J.; Ruiz, J. Current trends in epidemiology and antimicrobial resistance in intensive care units. J. Emerg. Crit. Care Med. 2019, 3, 5. [Google Scholar] [CrossRef]
- Kidd, T.J.; Mills, G.; Sá-Pessoa, J.; Dumigan, A.; Frank, C.G.; Insua, J.L.; Ingram, R.; Hobley, L.; Bengoechea, J.A. A Klebsiella pneumoniae antibiotic resistance mechanism that subdues host defences and promotes virulence. EMBO Mol. Med. 2017, 9, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-Virulence Strategies To Combat Bacteria-Mediated Disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- Mühlen, S.; Dersch, P. Anti-Virulence Strategies to Target Bacterial Infections. In How to Overcome the Antibiotic Crisis; Springer: Cham, Switzerland, 2015; pp. 147–183. [Google Scholar]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different Drugs for Bad Bugs: Antivirulence Strategies in the Age of Antibiotic Resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef]
- Schirmer, E.C.; Glover, J.R.; Singer, M.A.; Lindquist, S. HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 1996, 21, 289–296. [Google Scholar] [CrossRef]
- Moreno-Cinos, C.; Goossens, K.; Salado, I.G.; Van Der Veken, P.; De Winter, H.; Augustyns, K. ClpP Protease, a Promising Antimicrobial Target. Int. J. Mol. Sci. 2019, 20, 2232. [Google Scholar] [CrossRef]
- Ingmer, H.; Vogensen, F.K.; Hammer, K.; Kilstrup, M. Disruption and Analysis of the clpB, clpC, and clpE Genes in Lactococcus lactis: ClpE, a New Clp Family in Gram-Positive Bacteria. J. Bacteriol. 1999, 181, 2075–2083. [Google Scholar] [CrossRef]
- Lee, S.; Sowa, M.E.; Watanabe, Y.-H.; Sigler, P.B.; Chiu, W.; Yoshida, M.; Tsai, F.T. The Structure of ClpB: A Molecular Chaperone that Rescues Proteins from an Aggregated State. Cell 2003, 115, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Pietrosiuk, A.; Lenherr, E.D.; Falk, S.; Bönemann, G.; Kopp, J.; Zentgraf, H.; Sinning, I.; Mogk, A. Molecular Basis for the Unique Role of the AAA+ Chaperone ClpV in Type VI Protein Secretion. J. Biol. Chem. 2011, 286, 30010–30021. [Google Scholar] [CrossRef]
- Zheng, B.; Halperin, T.; Hruskova-Heidingsfeldova, O.; Adam, Z.; Clarke, A.K. Characterization of chloroplast Clp proteins in Arabidopsis: Localization, tissue specificity and stress responses. Physiol. Plant. 2002, 114, 92–101. [Google Scholar] [CrossRef]
- Capestany, C.A.; Tribble, G.D.; Maeda, K.; Demuth, D.R.; Lamont, R.J. Role of the Clp system in stress tolerance, biofilm formation, and intracellular invasion in Porphyromonas gingivalis. J. Bacteriol. 2008, 190, 1436–1446. [Google Scholar] [CrossRef]
- Nair, S.; Milohanic, E.; Berche, P. ClpC ATPase Is Required for Cell Adhesion and Invasion of Listeria monocytogenes. Infect. Immun. 2000, 68, 7061–7068. [Google Scholar] [CrossRef]
- Bojer, M.S.; Struve, C.; Ingmer, H.; Krogfelt, K.A. ClpP-dependent and-independent activities encoded by the polycistronic clpK-encoding locus contribute to heat shock survival in Klebsiella pneumoniae. Res. Microbiol. 2013, 164, 205–210. [Google Scholar] [CrossRef]
- Park, S.-S.; Kwon, H.-Y.; Tran, T.D.-H.; Choi, M.-H.; Jung, S.-H.; Lee, S.; Briles, D.E.; Rhee, D.-K. ClpL is a chaperone without auxiliary factors. FEBS J. 2015, 282, 1352–1367. [Google Scholar] [CrossRef]
- Thibault, G.; Tsitrin, Y.; Davidson, T.; Gribun, A.; Houry, W.A. Large nucleotide-dependent movement of the N-terminal domain of the ClpX chaperone. EMBO J. 2006, 25, 3367–3376. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.-H. Protective Roles of Cytosolic and Plastidal Proteasomes on Abiotic Stress and Pathogen Invasion. Plants 2020, 9, 832. [Google Scholar] [CrossRef]
- Frees, D.; Qazi, S.N.A.; Hill, P.; Ingmer, H. Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol. Microbiol. 2003, 48, 1565–1578. [Google Scholar] [CrossRef]
- Bhandari, V.; Wong, K.S.; Zhou, J.L.; Mabanglo, M.F.; Batey, R.A.; Houry, W.A. The Role of ClpP Protease in Bacterial Pathogenesis and Human Diseases. ACS Chem. Biol. 2018, 13, 1413–1425. [Google Scholar] [CrossRef]
- Silbergleit, M.; Vasquez, A.A.; Miller, C.J.; Sun, J.; Kato, I. Chapter Five-Oral and intestinal bacterial exotoxins: Potential linked to carcinogenesis. In Progress in Molecular Biology and Translational Science; Sun, J., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 171, pp. 131–193. [Google Scholar]
- Farrand, A.J.; Reniere, M.L.; Ingmer, H.; Frees, D.; Skaar, E.P. Regulation of Host Hemoglobin Binding by the Staphylococcus aureus Clp Proteolytic System. J. Bacteriol. 2013, 195, 5041–5050. [Google Scholar] [CrossRef]
- Fsihi, H.; Steffen, P.; Cossart, P. CHAPTER 16-Listeria monocytogenes. In Principles of Bacterial Pathogenesis; Groisman, E.A., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 751–803. [Google Scholar]
- Gaillot, O.; Pellegrini, E.; Bregenholt, S.; Nair, S.; Berche, P. The ClpP serine protease is essential for the intracellular parasitism and virulence of Listeria monocytogenes. Mol. Microbiol. 2002, 35, 1286–1294. [Google Scholar] [CrossRef]
- Qiu, D.; Eisinger, V.M.; Head, N.E.; Pier, G.B.; Yu, H.D. ClpXP proteases positively regulate alginate overexpression and mucoid conversion in Pseudomonas aeruginosa. Microbiology 2008, 154, 2119–2130. [Google Scholar] [CrossRef]
- Kwon, H.-Y.; Ogunniyi, A.D.; Choi, M.-H.; Pyo, S.-N.; Rhee, D.-K.; Paton, J.C. The ClpP Protease of Streptococcus pneumoniae Modulates Virulence Gene Expression and Protects against Fatal Pneumococcal Challenge. Infect. Immun. 2004, 72, 5646–5653. [Google Scholar] [CrossRef]
- Schmitz, K.R.; Carney, D.W.; Sello, J.K.; Sauer, R.T. Crystal structure of Mycobacterium tuberculosis ClpP1P2 suggests a model for peptidase activation by AAA+ partner binding and substrate delivery. Proc. Natl. Acad. Sci. USA 2014, 111, E4587–E4595. [Google Scholar] [CrossRef]
- Raju, R.M.; Unnikrishnan, M.; Rubin, D.H.F.; Krishnamoorthy, V.; Kandror, O.; Akopian, T.N.; Goldberg, A.L.; Rubin, E.J. Mycobacterium tuberculosis ClpP1 and ClpP2 Function Together in Protein Degradation and Are Required for Viability in vitro and During Infection. PLoS Pathog. 2012, 8, e1002511. [Google Scholar] [CrossRef]
- Dahmen, M.; Vielberg, M.-T.; Groll, M.; Sieber, S.A. Structure and Mechanism of the Caseinolytic Protease ClpP1/2 Heterocomplex from Listeria monocytogenes. Angew. Chem. Int. Ed. 2015, 54, 3598–3602. [Google Scholar] [CrossRef]
- Nagpal, J.; Paxman, J.; Zammit, J.E.; Alhuwaider, A.; Truscott, K.N.; Heras, B.; Dougan, D.A. Molecular and structural insights into an asymmetric proteolytic complex (ClpP1P2) from Mycobacterium smegmatis. Sci. Rep. 2019, 9, 18019. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Malik, I.T.; Thomy, D.; Henrichfreise, B.; Sass, P. The functional ClpXP protease of Chlamydia trachomatis requires distinct clpP genes from separate genetic loci. Sci. Rep. 2019, 9, 14129. [Google Scholar] [CrossRef] [PubMed]
- Mawla, G.D.; Hall, B.M.; Cárcamo-Oyarce, G.; Grant, R.A.; Zhang, J.J.; Kardon, J.R.; Ribbeck, K.; Sauer, R.T.; Baker, T.A. ClpP1P2 peptidase activity promotes biofilm formation in Pseudomonas aeruginosa. Mol. Microbiol. 2020, 115, 1094–1109. [Google Scholar] [CrossRef] [PubMed]
- Brötz-Oesterhelt, H.; Sass, P. Bacterial caseinolytic proteases as novel targets for antibacterial treatment. Int. J. Med. Microbiol. 2014, 304, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Chaudhary, H.; Marsee, J.D. Phylogenetic analysis predicts structural divergence for proteobacterial ClpC proteins. J. Struct. Biol. 2018, 201, 52–62. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System. 2020. Available online: https://www.schrodinger.com/products/pymol (accessed on 18 August 2022).
- Hanson, P.I.; Whiteheart, S.W. AAA+ proteins: Have engine, will work. Nat. Rev. Mol. Cell Biol. 2005, 6, 519–529. [Google Scholar] [CrossRef]
- Baker, T.A.; Sauer, R.T. ClpXP, an ATP-powered unfolding and protein-degradation machine. Biochim. Biophys. Acta 2012, 1823, 15–28. [Google Scholar] [CrossRef]
- Maurizi, M.R.; Xia, D. Protein binding and disruption by Clp/Hsp100 chaperones. Structure 2004, 12, 175–183. [Google Scholar] [CrossRef]
- Sauer, R.T.; Baker, T.A. AAA+ Proteases: ATP-Fueled Machines of Protein Destruction. Annu. Rev. Biochem. 2011, 80, 587–612. [Google Scholar] [CrossRef]
- Frees, D.; Gerth, U.; Ingmer, H. Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of Staphylococcus aureus. Int. J. Med. Microbiol. 2014, 304, 142–149. [Google Scholar] [CrossRef]
- Leigh, R.J.; McKenna, C.; McWade, R.; Lynch, B.; Walsh, F. Comparative genomics and pangenomics of vancomycin resistant and susceptible Enterococcus faecium from Irish hospitals across 20 years. BioRxiv 2021. [Google Scholar] [CrossRef]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020, 10, 3937. [Google Scholar] [CrossRef]
- Chatterjee, I.; Becker, P.; Grundmeier, M.; Bischoff, M.; Somerville, G.A.; Peters, G.; Sinha, B.; Harraghy, N.; Proctor, R.A.; Herrmann, M. Staphylococcus aureus ClpC Is Required for Stress Resistance, Aconitase Activity, Growth Recovery, and Death. J. Bacteriol. 2005, 187, 4488–4496. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, S.B.; Bojer, M.S.; Boll, E.J.; Martin, Y.; Helmersen, K.; Skogstad, M.; Struve, C. Heat-resistant, extended-spectrum β-lactamase-producing Klebsiella pneumoniae in endoscope-mediated outbreak. J. Hosp. Infect. 2016, 93, 57–62. [Google Scholar] [CrossRef]
- Belisario, J.; Lee, H.; Luknauth, H.; Rigel, N.; Martinez, L. Acinetobacter baumannii Strains Deficient in the Clp Chaperone-Protease Genes Have Reduced Virulence in a Murine Model of Pneumonia. Pathogens 2021, 10, 204. [Google Scholar] [CrossRef]
- Wang, N.; Ozer, E.; Mandel, M.; Hauser, A.R. Genome-Wide Identification of Acinetobacter baumannii Genes Necessary for Persistence in the Lung. mBio 2014, 5, e01163-14. [Google Scholar] [CrossRef]
- Boyd, A.; Chakrabarty, A.M. Pseudomonas aeruginosa biofilms: Role of the alginate exopolysaccharide. J. Ind. Microbiol. Biotechnol. 1995, 15, 162–168. [Google Scholar] [CrossRef]
- Lee, C.; Franke, K.B.; Kamal, S.M.; Kim, H.; Lünsdorf, H.; Jäger, J.; Nimtz, M.; Trček, J.; Jänsch, L.; Bukau, B.; et al. Stand-alone ClpG disaggregase confers superior heat tolerance to bacteria. Proc. Natl. Acad. Sci. USA 2017, 115, E273–E282. [Google Scholar] [CrossRef]
- Kirstein, J.; Hoffmann, A.; Lilie, H.; Schmidt, R.; Rübsamen-Waigmann, H.; Brötz-Oesterhelt, H.; Mogk, A.; Turgay, K. The antibiotic ADEP reprogrammes ClpP, switching it from a regulated to an uncontrolled protease. EMBO Mol. Med. 2009, 1, 37–49. [Google Scholar] [CrossRef]
- Lee, B.-G.; Park, E.Y.; Lee, K.-E.; Jeon, H.; Sung, K.H.; Paulsen, H.; Rübsamen-Schaeff, H.; Brötz-Oesterhelt, H.; Song, H.K. Structures of ClpP in complex with acyldepsipeptide antibiotics reveal its activation mechanism. Nat. Struct. Mol. Biol. 2010, 17, 471–478. [Google Scholar] [CrossRef]
- Fetzer, C.; Korotkov, V.S.; Thänert, R.; Lee, K.M.; Neuenschwander, M.; Von Kries, J.P.; Medina, E.; Sieber, S.A. A Chemical Disruptor of the ClpX Chaperone Complex Attenuates the Virulence of Multidrug-Resistant Staphylococcus aureus. Angew. Chem. Int. Ed. 2017, 56, 15746–15750. [Google Scholar] [CrossRef]
- Gao, W.; Kim, J.-Y.; Anderson, J.R.; Akopian, T.; Hong, S.; Jin, Y.-Y.; Kandror, O.; Kim, J.-W.; Lee, I.-A.; Lee, S.-Y.; et al. The Cyclic Peptide Ecumicin Targeting ClpC1 Is Active against Mycobacterium tuberculosis In Vivo. Antimicrob. Agents Chemother. 2015, 59, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Gavrish, E.; Sit, C.S.; Cao, S.; Kandror, O.; Spoering, A.; Peoples, A.; Ling, L.; Fetterman, A.; Hughes, D.; Bissell, A.; et al. Lassomycin, a Ribosomally Synthesized Cyclic Peptide, Kills Mycobacterium tuberculosis by Targeting the ATP-Dependent Protease ClpC1P1P2. Chem. Biol. 2014, 21, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, N.M.; Lee, H.; Choules, M.P.; Pauli, G.F.; Phansalkar, R.; Anderson, J.R.; Gao, W.; Ren, J.; Santarsiero, B.D.; Lee, H.; et al. High-Resolution Structure of ClpC1-Rufomycin and Ligand Binding Studies Provide a Framework to Design and Optimize Anti-Tuberculosis Leads. ACS Infect. Dis. 2019, 5, 829–840. [Google Scholar] [CrossRef]
- Labana, P.; Dornan, M.H.; Lafrenière, M.; Czarny, T.L.; Brown, E.D.; Pezacki, J.P.; Boddy, C.N. Armeniaspirols inhibit the AAA+ proteases ClpXP and ClpYQ leading to cell division arrest in Gram-positive bacteria. Cell Chem. Biol. 2021, 28, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, C.; Korotkov, V.S.; Sieber, S.A. Hydantoin analogs inhibit the fully assembled ClpXP protease without affecting the individual peptidase and chaperone domains. Org. Biomol. Chem. 2019, 17, 7124–7127. [Google Scholar] [CrossRef]
- Cherinka, B.; Andrews, B.H.; Sánchez-Gallego, J.; Brownstein, J.; Argudo-Fernández, M.D.C.; Blanton, M.; Bundy, K.; Jones, A.; Masters, K.; Law, D.R.; et al. Marvin: A Tool Kit for Streamlined Access and Visualization of the SDSS-IV MaNGA Data Set. Astron. J. 2019, 158, 74. [Google Scholar] [CrossRef]
- Vasudevan, D.; Rao, S.P.S.; Noble, C.G. Structural Basis of Mycobacterial Inhibition by Cyclomarin A. J. Biol. Chem. 2013, 288, 30883–30891. [Google Scholar] [CrossRef]
- Wolf, N.M.; Lee, H.; Zagal, D.; Nam, J.-W.; Oh, D.-C.; Lee, H.; Suh, J.-W.; Pauli, G.F.; Cho, S.; Abad-Zapatero, C. Structure of the N-terminal domain of ClpC1 in complex with the antituberculosis natural product ecumicin reveals unique binding interactions. Acta Crystallogr. Sect. D Struct. Biol. 2020, 76, 458–471. [Google Scholar] [CrossRef]
- Choules, M.P.; Wolf, N.M.; Lee, H.; Anderson, J.R.; Grzelak, E.M.; Wang, Y.; Ma, R.; Gao, W.; McAlpine, J.B.; Jin, Y.-Y.; et al. Rufomycin Targets ClpC1 Proteolysis in Mycobacterium tuberculosis and M. abscessus. Antimicrob. Agents Chemother. 2019, 63, e02204-18. [Google Scholar] [CrossRef]
- Kazmaier, U.; Junk, L. Recent Developments on the Synthesis and Bioactivity of Ilamycins/Rufomycins and Cyclomarins, Marine Cyclopeptides That Demonstrate Anti-Malaria and Anti-Tuberculosis Activity. Mar. Drugs 2021, 19, 446. [Google Scholar] [CrossRef]
- Hall, B.M.; Breidenstein, E.B.M.; de la Fuente-Núñez, C.; Reffuveille, F.; Mawla, G.D.; Hancock, R.E.W.; Baker, T.A. Two Isoforms of Clp Peptidase in Pseudomonas aeruginosa Control Distinct Aspects of Cellular Physiology. J. Bacteriol. 2017, 199. [Google Scholar] [CrossRef] [PubMed]
- Zolkiewski, M.; Zhang, T.; Nagy, M. Aggregate reactivation mediated by the Hsp100 chaperones. Arch. Biochem. Biophys. 2012, 520, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Sharma, A.K.; Vatamaniuk, O.K. N-Terminal Extension and C-Terminal Domains Are Required for ABCB6/HMT-1 Protein Interactions, Function in Cadmium Detoxification, and Localization to the Endosomal-Recycling System in Caenorhabditis elegans. Front. Physiol. 2018, 9, 885. [Google Scholar] [CrossRef]
- Lo, J.H.; Baker, T.A.; Sauer, R.T. Characterization of the N-terminal repeat domain of Escherichia coli ClpA—A class I Clp/HSP100 ATPase. Protein Sci. 2001, 10, 551–559. [Google Scholar] [CrossRef]
- Kedzierska, S.; Akoev, V.; Barnett, A.M.E.; Zolkiewski, M. Structure and Function of the Middle Domain of ClpB from Escherichia coli. Biochemistry 2003, 42, 14242–14248. [Google Scholar] [CrossRef]
- Cashikar, A.G.; Schirmer, E.C.; Hattendorf, D.A.; Glover, J.R.; Ramakrishnan, M.S.; Ware, D.M.; Lindquist, S.L. Defining a Pathway of Communication from the C-Terminal Peptide Binding Domain to the N-Terminal ATPase Domain in a AAA Protein. Mol. Cell 2002, 9, 751–760. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).