Designed Multifunctional Peptides for Intracellular Targets
Abstract
:1. Introduction
2. Sequence-Based Servers for Predicting Peptide Activity and Proposed Ranking Methods
3. Under-Appreciated Versatility of Penetratins
3.1. The Evolutionary Depth of Homeobox Domains and Penetratin-like Cryptides in the Animalia Kingdom
3.2. The Penetratin-like Cryptides from Other Kingdoms
3.3. The Translocation Function of Homeobox Proteins, Homeobox, Penetratin, and Penetratin-like Peptides
3.4. Penetratin Sequence Optimization and Possible Applications
3.5. Multifunctional or Hybrid Penetratin-like Peptides
| No. | Peptide/Gene/Origin * | Extended TriPaxB or Reverse Penetratin/Sequence Number * | DNA- Bind. ** | CPP $ | Anti- Microbial ‡ | Anti- Cancer $$ | Anti- Viral & | Anti- Fungal ⁋ | Anti-Inflamm. Activity § | Hemo- lytic ¥ | Toxicity/Score † |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P02833/D. melanogaster penetratin/E | RQIKIWFQNRRMKWKK /339–254/pAntp | + | 0.998/H | 0.97/0.42 | 0.812/0.985 | 1.0/0.70/0.77 | 0.28/0.95 | 0.57/0.68 | 0.94/+ | −0.66 |
| 2 | Rev. VV-pen. [136]/E | KKWKMRRNQFWVKVQR | + | 0.956/H | 0.96/0.53 | 0.649/0.981 | 0.10/0.44/0.28 | 0.15/0.68 | 0.52/0.66 | 0.19/− | −0.81 |
| 3 | TriPaxB penetratin | RVVQVWFQNQRAKLKK | + | 0.807/L | 0.74/0.42 | 0.036/0.971 | 0.00/0.40/0.01 | 0.22/0.21 | 0.52/0.61 | 0.02/− | −1.42 |
| 4 | A0A7M5V8Y3/N/A /Clytia hemisphaerica | GLSVRVVQVWFQNQRAKLKKIQKK/227–250 | + | 0.642/L | 0.96/0.32 | 0.189/0.980 | 0.44/0.47/0.82 | 0.56/0.59 | 0.65/0.62 | 0.03/− | −1.45 |
| 5 | T2M9B9/UP &&/Hydra vulgaris | GLSVRVVQVWFQNQRAKLKKLHRK/227–250 and 108–131 | + | 0.761/L | 0.93/0.37 | 0.065/0.983 | 0.66/0.46/0.97 | 0.58/0.45 | 0.66/0.61 | 0.03/− | −1.16 |
| 6 | A0A1S0TPC1/UP &&/ Loa loa | NLSVRVVQVWFQNQRAKLKKIQRK/118–141 | + | 0.715/L | 0.91/0.29 | 0.049/0.769 | 0.21/0.57/1.0 | 0.28/0.59 | 0.67/0.63 | 0.04/− | −1.47 |
| 7 | PexNC-TriPaxB-I/DJ | GIGK-RVVQVWFQNQRAKLKK-ILKK | + | 0.731/L | 0.99/0.67 | 0.968/0.980 | 0.93/0.75/1.0 | 0.97/0.59 | 0.61/0.58 | 0.09/− | −1.51 |
| 8 | PexShort-TriPaxB-II (PexT)/DJ | GIGKLKKAKKFGKKILKK-G- RVVQVWFQNQRAKLKK | + | 0.792/L | 1.0/0.98 | 0.995/0.951 | 0.98/0.76/0.52 | 1.0/0.61 | 0.64/0.58 | 0.13/− | −1.17 |
| 9 | PexNC-rev. VV-pen./DJ | GIGK-G-KKWKMRRNQFWVKVQR-ILKK | + | 0.849/H | 1.0/0.58 | 0.919/0.982 | 1.0/0.65/0.95 | 0.94/0.93 | 0.55/0.67 | 0.26/− | −1.17 |
| 10 | TriPaxB–antifungal BP16 [144]/DJ | RVVQVWFQNQRAKLKK-G- KKLFKKILKKL | + | 0.816/L | 0.98/0.95 | 0.992/0.981 | 1.0/0.70/0.93 | 0.98/0.54 | 0.54/0.64 | 0.62/+ | −1.46 |
| 11 | Anti-cancer-I-Rev. VV-pen. [136]/E | PPLSQETFS- KKWKMRRNQFWVKVQRG | + | 0.503/H | 0.41/0.53 | NACP/NACP | 0.40/0.90/1.0 | 0.15/0.05 | 0.62/0.62 | 0.13/− | −1.09 |
| 12 | pAntp-TPR [145]/E | RQIKIWFQNRRMKWKK- KAYARIGNSYFK | + | 0.834/H | 0.91/0.50 | 0.923/0.939 | 1.0/0.80/0.54 | 0.83/0.65 | 0.59/0.62 | 0.91/+ | −1.09 |
| 13 | Rev.-VV-pen. [136]-TPR/DJ | KKWKMRRNQFWVKVQR-G- KAYARIGNSYFK | + | 0.766/H | 0.83/0.48 | 0.766/0.952 | 1.0/0.79/1.0 | 0.87/0.40 | 0.60/0.63 | 0.59/+ | −1.13 |
| 14 | Rev. amoeba (Filasterea) pen. with added N-term-Arg/DJ | RRQKARRNQFWIRIVRR | + | 0.958/H | 1.0/0.46 | 0.110/0.984 | 0.01/0.27/0.73 | 0.44/0.71 | 0.59/0.62 | 0.07/− | −0.58 |
| 15 | Rev. R-am.pen.-tLyP-1 [146]/DJ | RRQKARRNQFWIRIVRR- CGIKRTK | + | 0.962/H | 0.98/0.51 | 0.259/0.984 | 0.78/0.91/0.93 | 0.88/0.43 | 0.68/0.62 | 0.02/− | −0.88 |
| 16 | Optimal penetratin (o-pen P14 [56]/E | RKKRWFRRRRPKWKK | + | 0.992/H | 1.0/1.0 | 0.767/0.978 | 0.97/0.57/1.0 | 0.32/1.0 | 0.47/0.56 | 0.02/− | −0.89 |
| 17 | Rev. opt. penetratin (r-o-p)/DJ | KKWKPRRRRFWRKKR | + | 0.992/H | 1.0/0.99 | 0.767/0.980 | 1.0/0.92/1.0 | 0.32/1.0 | 0.48/0.49 | 0.01/− | −1.11 |
| 18 | Rev.opt.pen. (r-o-p)-tLyP-1 [146]/DJ | KKWKPRRRRFWRKKR- CGIKRTK | + | 0.987/H | 0.94/1.0 | 0.854/0.979 | 1.0/0.82/0.59 | 0.71/0.96 | 0.65/0.68 | 0.006/− | −1.30 |
| 19 | Fusion peptide 46 [139]/E | GSRAHSSHLKSKKGQSTSRH- KKWKMRRNQFWVKVQRG | + | 0.741/L | 0.76/0.87 | 0.61/0.65 | 0.98/0.48/0.15 | 0.81/0.44 | 0.67/0.59 | ND | −0.89 |
| 20 | Rev.opt.pen. (r-o-p A8I15)-tLyP-1 [146]-analog1/DJ | KKWKPRRARFWRKKI- CGIKRTK | + | 0.987/H | 0.96/0.993 | 0.972/0.98 | 1.0/0.8183/0.77 | 0.89/0.922 | 0.6674/0.647/ 1.397 | 0.007/− | −1.39 |
| 21 | Rev.opt.pen. A8I15— tLyPA3-1-analog2/DJ | KKWKPRRARFWRKKI- CGAKRTK | + | 0.985/H | 0.97/0.99 | 0.943/0.981 | 1.0/0.78/0.27 | 0.86/0.96 | 0.66/0.66/ 1.56083 | 0.006/− | −1.22 |
| 22 | Rev. optimized penetratin analog/DJ | GKRIGKKWKPRRRRFWRK | + | 0.991/H | 1.0/1.0 | 0.944/0.979 | 1.0/0.96/0.95 | 0.59/1.0 | 0.61/0.61 | 0.003/− | −1.26 |
| No. | Peptide or Parent Protein/ Gene/Origin/Reference * | CPP Constructs/Sequence Number * | CPP | Anti-Microbial | Anti-Cancer | Anti-Viral | Anti-Fungal | Anti-Inflamm. | Hemo- lytic | Toxicity/ Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PenArg (Bahnsen-2013 [55])/E | RQIRIWFQNRRMRWRR | 0.99/H | 0.99/0.57 | 0.32/0.98 | 1.0/0.7/0.4 | 0.24/0.96 | 0.60/0.66 | 0.94 | −1.12 |
| 2 | DiR6WF OLQ14316.1/ S. microadriaticum | RRRRRRWFRRRRRRWFRKI /603–621 DiR6WF | 0.99/H | 1.00/0.97 | 0.92/0.91 | 1.0/0.3/1.0 | 0.43/0.82 | 0.57/0.59 | 0.68 | −0.97 |
| 3 | WFR8 from CellPPD $ scan of DiR6WF/DJ | RRWFRRRRRR | 0.99/H | 1.00/0.99 | 0.95/0.98 | 0.9/0.6/0.9 | 0.42/ND | 0.53/0.53 | 0.21 | −0.92 |
| 4 | Reverse WFR8 (R8FW)/DJ | RRRRRRFWRR | 0.99/H | 1.00/0.89 | 0.95/0.98 | 0.9/0.4/0.9 | 0.42/ND | 0.56/0.53 | 0.08 | −0.93 |
| 5 | Ribos.-hom.-pept. (RHP)-pAntp/ [54]/E | YKWYYRGAA- RQIKIWFQNRRMKWKK | 0.90/H | 0.74/0.46 | 0.95/0.98 | 1.0/0.9/0.8 | 0.49/0.68 | 0.64/0.62 | 0.97 | −0.63 |
| 6 | HK2-WFR8 [147]/DJ | MIASHLLAYFFTELN-GG- RRWFRRRRRR | 0.80/H | 0.62/0.19 | 0.15/0.99 | 1.0/0.8/1.0 | 0.17/0.45 | 0.62/0.59 | 0.30 | −1.28 |
| 7 | RHP [54] -WFR8/DJ | YKWYYRGAA-RRWFRRRRRR | 0.97/H | 1.0/0.97 | 0.85/0.98 | 1.0/0.8/1.0 | 0.63/0.93 | 0.60/0.63 | 0.12 | −1.10 |
| 8 | RtLyp-1-G-VV-pen. &/DJ | RCGNKRTR-G- RQVKVWFQNRRMKWKK | 0.94/H | 0.78/0.49 | 0.12/0.98 | 1.0/0.5/1.0 | 0.57/0.83 | 0.58/0.61 | 0.24 | −0.67 |
| 9 | L-K6V1 temporin 1CEb [112]-GG-WFR8/DJ | IKKIVSKIKKLLK-GG-RRWFRRRRRR | 0.97/H | 0.98/1.00 | 0.97/0.98 | 1.0/1.0/1.0 | 0.97/1.00 | 0.54/0.67/1.0796 | 0.17 | −1.35 |
| 10 | CAMEL [148]-WFR8/DJ | KWKLFKKIGAVLKVL- RRWFRRRRRR | 0.96/H | 1.00/1.00 | 0.81/0.98 | 1.0/1.0/1.0 | 0.82/1.00 | 0.61/0.66 | 0.98 | −1.33 |
| 11 | Rev. WFR8–CAMEL [148]/DJ | RRRRRRFWRR-GG- KWKLFKKIGAVLKVL | 0.96/H | 1.00/1.00 | 0.73/0.98 | 1.0/0.9/1.0 | 0.92/0.98 | 0.60/0.63 | 0.71 | −1.33 |
| 12 | [R4, R10]-chensinin-1b [149]- WFR8/DJ | VWRRWRRFWRR-GG- RRWFRRRRRR | 0.99/H | 1.00/0.95 | 0.93/0.98 | 1.0/0.7/0.1 | 0.41/0.99 | 0.58/0.71 | 0.72 | −1.02 |
| 13 | ZY4 [150]-GG-WFR8/DJ | VCKRWKKWKRKWKKWCV-GG- RRWFRRRRRR | 0.98/H | 0.99/1.00 | 0.91/0.98 | 1.0/0.9/0.8 | 0.40/1.00 | 0.53/0.68 | 0.60 | −0.50 |
| 14 | Puroindoline [151]-WFR8/DJ | FPVTWRWWKWWKG-G- RRWFRRRRRR | 0.99/H | 1.00/1.00 | 0.87/0.98 | 1.0/0.9/1.0 | 0.21/0.99 | 0.61/0.66 | 0.85 | −0.99 |
| 15 | Rev. WFR8—puroindoline/DJ | RRRRRRFWRR-GG- FPVTWRWWKWWKG | 0.98/H | 1.00/0.95 | 0.85/0.98 | 1.0/0.9/1.0 | 0.23/0.99 | 0.61/0.62 | 0.49 | −1.01 |
| 16 | Novispirin [152] -WFR8/DJ | KNLRIIRKGIHIIKKY-GG- RRWFRRRRRR | 0.95/H | 1.00/1.00 | 0.95/0.98 | 1.0/1.0/1.0 | 0.94/0.99 | 0.63/0.62 | 0.53 | −1.14 |
| 17 | BP33 antifungal [144]/E | LKLFKKILKVL | 0.85/H | 0.84/1.00 | 1.0/0.98 | 1.0/0.5/1.0 | 0.98/1.00 | 0.48/0.65 | 0.57 | −1.30 |
| 18 | BP33 antif. [144]-pAntp/DJ | LKLFKKILKVL-G- RQIKIWFQNRRMKWKK | 0.86/H | 1.00/0.92 | 0.98/0.98 | 1.0/1.0/1.0 | 0.98/1.00 | 0.54/0.66 | 1.0 | −1.09 |
| 19 | TriPaxB-antifungal-BP33 [144]-with-GGG-tag/DJ | RVVQVWFQNQRAKLKK- LKLFKKILKVL-GGG | 0.62/H | 0.96/0.95 | 0.84/0.84 | 0.9/0.9/1.0 | 0.98/0.23 | 0.64/0.65 | 0.63 | −1.58 |
| 20 | rWFR8-antif-BP16 [144]/DJ | RRRRRRFWRR-GG- KKLFKKILKKL | 0.982/H | 1.00/1.00 | 0.97/0.98 | 1.0/0.7/1.0 | 0.90/1.00 | 0.57/0.68 | 0.57 | −1.40 |
| 21 | T2R1 [88]-WFR8/DJ | RHHWRRYARIGFRAVRTVIGK-G- RRWFRRRRRR | 0.901/H | 1.00/1.00 | 0.73/0.97 | 1.0/1.0/0.9 | 0.70/0.90 | 0.71/0.64 | 0.30 | −1.18 |
| 22 | WFR8-DiPGLa-H [153]/DJ | RRRRRRFWRR-G- KIAKVALKALKIAKVALKAL | 0.970/H | 1.00/1.00 | 0.57/0.97 | 1.0/0.9/1.0 | 0.91/0.99 | 0.64/0.66 | 0.80 | −1.09 |
| 23 | WFR8-TPR [145] with G4 link/DJ | RRWFRRRRRR-GGGG- KAYARIGNSYFK | 0.893/H | 1.00/0.73 | 0.71/0.98 | 1.0/0.6/1.0 | 0.90/0.76 | 0.59/0.57 | 0.16 | −1.38 |
| 24 | GV1001 vaccine [154]-WFR8/DJ | EARPALLTSRLRFIPK-GG- RRWFRRRRRR | 0.951/H | 1.00/0.59 | 0.95/0.98 | 1.0/1.0/1.0 | 0.66/0.95 | 0.69/0.74 | 0.05 | −1.35 |
| 25 | BP100 [155]-WFR8/DJ | KKLFKKILKYL-GG- RRWFRRRRRR | 0.981/H | 1.00/1.00 | 0.45/0.98 | 1.0/1.0/1.0 | 0.92/0.97 | 0.60/0.69 | 0.71 | −1.39 |
| 26 | RWBP100 [156]-WFR8/DJ | RRLFRRILRWL-GG- RRWFRRRRRR | 0.994/H | 1.00/0.97 | 0.84/0.98 | 1.0/0.8/1.0 | 0.53/0.99 | 0.61/0.70 | 0.65 | −1.23 |
| 27 | Mitochondrial targeting [157]- WFR8/DJ | KLLNLISKLF-GGG-RRWFRRRRRR | 0.938/L | 1.00/0.98 | 0.43/0.98 | 1.0/0.9/0.8 | 0.81/0.99 | 0.62/0.67 | 0.82 | −1.31 |
| 28 | Nosangiotide [158]-WFR8/DJ | RKKTFKEVANAVKISA-GG- RRWFRRRRRR | 0.917/H | 0.98/0.96 | 0.25/0.97 | 0.9/0.9/0.9 | 0.79/0.95 | 0.67/0.58 | 0.08 | −1.09 |
| 29 | Buforin [159] -WFR8/DJ | TRSSRAGLQFPVGRVHRLLRK- GGG-RRWFRRRRRR | 0.945/H | 0.99/0.87 | 0.05/0.98 | 1.0/0.9/0.4 | 0.91/0.99 | 0.68/0.60 | 0.04 | −0.89 |
| 30 | Buforin-BR2 [160]/E | RAGLQFPVGRLLRRLLR | 0.879/L | 1.00/0.71 | 0.42/0.98 | 0.8/0.9/1.0 | 0.20/1.00 | 0.53/0.63 | 0.01 | −1.12 |
| 31 | BR2-WFR8/DJ | RAGLQFPVGRLLRRLLR-GG- RRWFRRRRRR | 0.960/H | 1.00/0.97 | 0.43/0.98 | 1.0/0.9/1.0 | 0.68/1.00 | 0.53/0.63 | 0.25 | −1.23 |
| 32 | WFR8-Zp3a [161]/DJ | RRWFRRRRRR-GIKAKIGIKIKK | 0.98/H | 0.99/1.00 | 0.84/0.98 | 1.0/0.8/1.0 | 0.89/0.98 | 0.53/0.66 | 0.07 | −1.25 |
| 33 | RHP [54]-rev. WFR8/DJ | YKWYYRGAA-RRRRRRFWRR | 0.97/H | 1.00/0.80 | 0.85/0.98 | 1.0/0.9/1.0 | 0.63/0.95 | 0.61/0.62 | 0.04 | −1.03 |
| 34 | T2R3G3/DJ | RRRHHWRRYARIGFRAVRTVIGK-GGG | 0.87/H | 0.99/0.84 | 0.85/0.97 | 1.0/0.9/1.0 | 0.85/0.54 | 0.67/0.66 | 0.06 | −1.19 |
| 35 | Temporin-asparagutin analog1/DJ | IKKIVSKILKLLKV-G-RRWFRRRRRR | 0.96/H | 0.998/1.00 | 0.96/0.98 | 1.0/1.0/1.0 | 0.96/1.0 | 0.60/0.71/1.625 | 0.76 | −1.47 |
| 36 | Temporin-asparagutin analog2/DJ | IKKIVSKIRKLLK-GG-RRWFRSRRRR | 0.96/H | 0.92/0.99 | 0.96/0.98 | 1.0/1.0/1.0 | 0.97/0.99 | 0.62/0.66/1.5 | 0.18 | −1.30 |
| 37 | Temporin-asparagutin analog3/DJ | VKKIVSKIRKLLK-GG-RRWFRSRRRR | 0.97/H | 0.92/0.99 | 0.95/0.98 | 1.0/1.0/1.0 | 0.96/0.99 | 0.63/0.64/1.72 | 0.13 | −1.27 |
| 38 | Novispirin [152]-WFR8-analog1/DJ | KNLRLIRKGIHIILKY-GG-RRWFLRRRRR | 0.938/H | 1.0/1.0 | 0.768/ 0.981 | 1.0/0.9854/1.0 | 0.96/0.995 | 0.5814/0.648/1.5622 | 0.551 | −1.17 |
| 39 | Temporin-1CEb [162]/E | ILPILSLIGGLLGK | 0.453 | 0.789/1.0 | 0.991/ 0.984 | 0.101/0.083/0.68 | 0.98/0.97 | 0.47/0.62 | 0.959 | −1.08 |
| 40 | L-K6V1-Temporin-1CEb [112]/E | IKKIVSKIKKLLK | 0.880/L | 0.930/1.0 | 1.0/0.982 | 0.991/0.589/0.40 | 0.87/1.0 | 0.53/0.64 | 0.009 | −1.20 |
| 41 | T2R1 [88]/E | RHHWRRYARIGFRAVRTVIGK | 0.907/H | 0.973/0.617 | 0.96/0.984 | 0.999/0.901/0.89 | 0.67/0.764 | 0.63/0.63 | 0.017 | −1.06 |
| 42 | Rev. WFR8-hinge-aurein 1.2 [3]/DJ | RRRRRRFWRR-GGGPPK- GLFDIIKKIAESF | 0.817/H | 0.941/0.994 | 0.897/0.916 | 1.0/0.874/1.0 | 0.94/0.988 | 0.609/0.575 | 0.082 | −1.02 |
| 43 | SVS-1 [163]/E | KVKVKVKVDPLPTKVKVKVK | 0.817/L | 0.96/0.746 | 0.962/0.973 | 0.0/0.4/0.344 | 0.48/0.978 | 0.43/0.41 | 0.003 | −0.84 |
| 44 | HPRP-A1-TAT [6,164]/E | FKKLKLFSKLWNW-KRKKRQRRR | 0.975/H | 0.997/0.997 | 0.527/0.984 | 1.0/0.928/0.972 | 0.69/0.987 | 0.586/0.669 | 0.043 | −0.98 |
| 45 | Beclin-1-R11 [165]/E | TNVFNATFEIWHDGEFGT- RRRRRRRRRRR | 0.814/H | 0.987/0.034 | 0.516/0.846 | 0.83/0.834/0.36 | 0.26/0.268 | 0.565/0.552 | 0.005 | −0.92 |
| 46 | Mapegin [88]/E | KIGKKILKALKGALKELA | 0.707/H | 0.588/1.0 | 1.0/0.982 | 0.783/0.589/0.988 | 0.98/1.0 | 0.59/0.67/1.55745 | 0.079 | −1.32 |
| 47 | MAP [166]/E | KLALKLALKALKAALKLA | 0.998/H | 0.794/1.0 | 0.979/0.986 | 0.345/0.096/0.918 | 0.42/1.0 | 0.54/0.73/0.69540 | 0.973 | −1.13 |
| 48 | Mapegin-TAT/DJ | KIGKKILKALKGALKELA- GRKKRRQRRRPPQ | 0.878/H | 0.929/0.997 | 0.764/0.981 | 0.998/0.975/0.506 | 0.96/1.0 | 0.65/0.65/1.52487 | 0.026 | −1.04 |
| 49 | Mapegin-a1-TAT/DJ | KIGKKILKALKLALKLLA- GRKKRRQRRRPPQ | 0.958/H | 0.980/1.0 | 0.613/0.983 | 0.998/0.975/0.630 | 0.94/1.0 | 0.67/0.76/2.13612 | 0.717 | −1.09 |
| 50 | Mapegin-a2-TAT/DJ | KITKKILKALKGALKELA- GRKKRRQRRRMPQ | 0.881/L | 0.518/0.994 | 0.717/0.942 | 0.998/0.93/0.972 | 0.97/0.996 | 0.68/0.65/1.53687 | 0.077 | −1.81 |
| No. | Peptide Name/Ref. | Extended CPP at the N or C-terminal * | CPP | Anti-Microbial | Anti-Cancer | Anti-Viral | Anti-Fungal | Anti-Inflamm. | Hemo-lytic | Toxicity/ Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | KW [168]/E | KRKRWHW | 0.99/H | 1.00/ND | 0.98/0.98 | 0.8/0.4/1.0 | 0.38/ND | 0.62/0.54 | 0.01 | −0.93 |
| 2 | Ribosomal-homing- peptide (RHP)-KW [54]/DJ | YKWYYRGAA-KRKRWHW | 0.93/H | 0.97/1.00 | 0.99/0.98 | 1.0/0.9/0.8 | 0.62/0.74 | 0.51/0.61 | 0.02 | −0.58 |
| 3 | L-K6V1 temp [112]-KW/DJ | IKKIVSKIKKLLK-GG-KRKRWHW | 0.89/H | 0.98/1.00 | 1.0/0.98 | 1.0/1.0/0.2 | 0.95/1.00 | 0.59/0.66 | 0.02 | −1.35 |
| 4 | CAMEL [148]-KW/DJ | KWKLFKKIGAVLKVL-KRKRWHW | 0.92/H | 1.00/1.00 | 0.99/0.98 | 1.0/1.0/1.0 | 0.78/0.99 | 0.61/0.67 | 0.94 | −0.90 |
| 5 | R2-chensenin [149]-KW/DJ | VWRRWRRFWRR-GG-KRKRWHW | 0.99/H | 1.00/0.99 | 0.95/0.98 | 1.0/0.9/1.0 | 0.27/1.00 | 0.66/0.70 | 0.15 | −1.03 |
| 6 | ZY4 [150]-KW/DJ | VCKRWKKWKRKWKKWCV-GG- KRKRWHW | 0.95/H | 1.00/1.00 | 0.99/0.98 | 1.0/0.9/1.0 | 0.34/1.00 | 0.58/0.68 | 0.23 | −0.36 |
| 7 | Puroindoline [151]-KW/DJ | FPVTWRWWKWWKG-G-KRKRWHW | 0.88/H | 1.00/0.99 | 0.98/0.98 | 1.0/0.9/1.0 | 0.46/0.99 | 0.64/0.65 | 0.67 | −0.83 |
| 8 | Novispirin [152]-KW/DJ | KNLRIIRKGIHIIKKY-GG-KRKRWHW | 0.90/L | 0.98/1.00 | 0.99/0.98 | 1.0/1.0/1.0 | 0.96/1.00 | 0.63/0.63 | 0.43 | −0.90 |
| 9 | BP33 [144]-KW/DJ | LKLFKKILKVL-G-KRKRWHW | 0.93/H | 1.00/1.00 | 0.99/0.98 | 1.0/1.0/1.0 | 0.92/1.00 | 0.62/0.70 | 0.82 | −1.19 |
| 10 | T2R1 [88]-KW/DJ | RHHWRRYARIGFRAVRTVIGK- KRKRWHW | 0.94/H | 0.99/0.94 | 0.92/0.98 | 1.0/1.0/0.8 | 0.71/0.86 | 0.66/0.62 | 0.12 | −1.09 |
| 11 | DiPGLa-H [153]-KW peptide/DJ | KIAKVALKALKIAKVALKAL- KRKRWHW | 0.92/L | 0.98/1.00 | 0.99/0.98 | 1.0/1.0/1.0 | 0.97/0.99 | 0.49/0.63 | 0.94 | −0.92 |
| 12 | Neoepitope4-WFR8 [169]/DJ | VLSHGSFVM-GG-RRWFRRRRRR | 0.89/H | 0.89/0.93 | 0.59/0.98 | 1.0/0.8/0.1 | 0.62/0.76 | 0.62/0.62 | 0.43 | −1.22 |
| 13 | WFR8 -tumor homing [170]/DJ | RRWFRRRRRR-GG-IFLLWQR | 0.99/H | 1.00/0.78 | 0.48/0.98 | 1.0/0.5/0.8 | 0.46/0.96 | 0.63/0.63 | 0.07 | −1.26 |
| 14 | BP100 [155]-KW/DJ | KKLFKKILKYL-GG-KRKRWHW | 0.93/H | 1.00/1.00 | 1.0/0.98 | 1.0/1.0/1.0 | 0.94/0.99 | 0.58/0.65 | 0.61 | −1.35 |
| 15 | Mitoch. target. [157]-KW/DJ | KLLNLISKLF-GGG-KRKRWHW | 0.80/L | 0.97/1.00 | 0.74/0.98 | 1.0/1.0/0.9 | 0.91/0.98 | 0.63/0.67 | 0.41 | −1.29 |
| 16 | Nosangiotide [158]-KW/DJ | RKKTFKEVANAVKISA-GG-KRKRWHW | 0.69/L | 0.85/0.93 | 0.87/0.97 | 0.5/0.9/0.9 | 0.88/0.84 | 0.69/0.59 | 0.01 | −1.03 |
| 17 | Adepantin-1A [88]-WFR8/DJ | GIKKAVGKALKGLKGLLKALGES -GG-RRWFRRRRRR | 0.80/L | 1.00/0.99 | 0.95/0.98 | 1.0/1.0/1.0 | 0.98/1.00 | 0.60/0.66/1.30566 | 0.66 | −1.46 |
| 18 | WFR8-adepantin-1A/DJ | RRWFRRRRRR- GIKKAVGKALKGLKGLLKALGES | 0.86/L | 1.00/1.00 | 0.95/0.96 | 1.0/1.0/1.0 | 0.97/0.99 | 0.62/0.62/1.36028 | 0.63 | −1.56 |
| 19 | KW-pexiganan-L18 [88]/DJ | KRKRWHW- GIGKFLKKAKKFGKAFVLILKK | 0.87/H | 0.99/1.00 | 1.0/0.98 | 1.0/0.9/1.0 | 0.99/0.99 | 0.53/0.64 | 0.81 | −1.04 |
| 20 | RtLyp-1-flexampin [114]/DJ | RCGNKRTR- GIKKWVKGVAKGVAKDLAKKIL | 0.59/L | 0.92/1.00 | 1.0/0.97 | 1.0/1.0/1.0 | 1.00/1.00 | 0.44/0.63 | 0.68 | −0.74 |
| 21 | Zyk-1- [88]-WFR8/DJ | GIGREIIKKIIKKIGKKIGRII -GG-RRWFRRRRRR | 0.89/H | 1.00/1.00 | 0.99/0.98 | 1.0/1.0/1.0 | 0.96/0.99 | 0.60/0.66 | 0.88 | −1.18 |
| 22 | MG2-bombesin [171]/E | GIGKFLHSAKKFGKAFVGEIMNS-GG- QRLGNQWAVGHLM | 0.30 | 0.83/0.85 | 0.86/0.54 | 1.0/0.9/0.9 | 0.97/0.41 | 0.53/0.55 | ND | −0.97 |
| 23 | MG2-pAntp [172]/E | GIGKFLHSAKKFGKAFVGEIMNS-GG- KKWKMRRNQFWVKVQRG | 0.52/L | 0.95/1.00 | 0.93/0.81 | 1.0/1.0/1.0 | 0.99/0.81 | 0.56/0.52 | ND | −0.68 |
| 24 | DP1 [173]/E | RRQRRTSKLMKR-GG- KLAKLAKKLAKLAK | 0.95/L | 0.84/1.00 | 0.91/0.98 | 0.7/0.7/0.2 | 0.95/0.55 | 0.50/0.65 | 0.03 | −0.36 |
| 25 | KW-BMAP-18 [174]/DJ | KRKRWHW-GGLRSLGRKILRAWKKYG | 0.90/H | 1.00/1.00 | 0.98/0.98 | 1.0/1.0/1.0 | 0.88/0.98 | 0.58/0.68/1.3653 | 0.09 | −1.01 |
| 26 | Chrysophin-1-KW [175]/DJ | FFGWLIKGAIHAGKAIHGLI-GG- KRKRWHW | 0.59/L | 0.99/1.00 | 0.98/0.98 | 1.0/1.0/1.0 | 0.96/0.97 | 0.52/0.55 | 0.97 | −1.03 |
| 27 | KW-mastoparan [176]/DJ | KRKRWHW-GG-INLKALAALAKKIL | 0.90/L | 0.87/1.00 | 0.75/0.98 | 1.0/0.9/1.0 | 0.92/0.96 | 0.62/0.66 | 0.42 | −1.11 |
| 28 | KW-pleuricidin [177]/DJ | KRKRWHW- GWGSFFKKAAHVGKHVGKAALTHYL | 0.66/L | 0.89/1.00 | 0.99/0.98 | 1.0/0.9/0.9 | 0.96/1.00 | 0.60/0.65 | 0.37 | −0.95 |
| 29 | MTD [178]/E | RRRRRRRRGRQ-KLLNLISKLF | 0.98/H | 0.28/0.60 | 0.58/0.96 | 1.0/0.6/0.9 | 0.73/0.97 | 0.67/0.70 | 0.06 | −1.06 |
| 30 | L-K6V1 temp [112]-KW-analog/DJ | IKKIVSKIRKLLKR-G-KRKRWHW | 0.95/H | 0.98/1.00 | 1.0/0.98 | 1.0/1.0/0.8 | 0.92/1.0 | 0.65/0.66/1.678 | 0.07 | −1.12 |
| 31 | T2R1 [88]-KW-analog1/DJ | RHHWRRYARIGFRAVRSVIGK- KTKRWHW | 0.92/H | 0.93/0.94 | 0.98/0.98 | 1.0/1.0/1.0 | 0.69/0.97 | 0.66/0.62/1.36406 | 0.03 | −1.09 |
| 32 | T2R1 [88]-KW-analog2/DJ | RHHWRRLARIGFRAVRSVIGK- KTKRWHW | 0.93/H | 0.96/0.87 | 0.97/0.98 | 1.0/1.0/1.0 | 0.60/0.97 | 0.67/0.62/1.5722 | 0.05 | −1.30 |
| 33 | KW-BMAP-18 [174]-analog1/DJ | KRKRWHW-GGLRSLGRKLLRAWKKYG | 0.91/H | 1.00/0.99 | 0.96/0.98 | 1.0/1.0/1.0 | 0.84/0.97 | 0.63/0.71/1.62236 | 0.08 | −1.05 |
| 34 | BP100 [155]-KW-analog/DJ | LKLFKKILKYLN-G-KRKRWHW | 0.93/H | 0.996/0.999 | 0.966/0.981 | 1.0/0.961/1.0 | 0.87/1.0 | 0.635/0.687/1.80571 | 0.894 | −1.32 |
| 35 | Zyk-1 [88]-WFR8-analog/DJ | GIGLEIVKKIILKIGKKIGRII-GG- RRWFRRRRRR | 0.83/L | 0.999/0.998 | 0.986/0.977 | 1.0/0.985/0.964 | 0.98/0.99 | 0.60/0.614/1.599 | 0.938 | −1.33 |
| 36 | KW-BMAP-18 [174]-analog2/DJ | KRKRWHW-GGLASLGRKLLRAWKKYG | 0.85/H | 0.988/0.986 | 0.951/0.982 | 1.0/0.971/1.0 | 0.85/0.97 | 0.684/0.708/1.85648 | 0.399 | −1.09 |
| 37 | R8FW-GGGPPKG- temp [112] R9R14/DJ | RRRRRRFWRR-GGGPPKG- IKKIVSKIRKLLKR | 0.95/H | 0.997/1.0 | 0.954/0.968 | 1.0/0.958/0.822 | 0.97/0.99 | 0.71/0.60/1.25133 | 0.032 | −1.18 |
| 38 | R8FW-GGEPPKG- temp [112] R9R14/DJ | RRRRRRFWRR-GGEPPKG- IKKIVSKIRKLLKR | 0.94/H | 0.998/0.988 | 0.926/0.973 | 1.0/0.96/0.996 | 0.96/0.944 | 0.70/0.596/1.51442 | 0.028 | −1.20 |
| 39 | R7A5FW-GGEPPKG temp [112]/DJ | RRRRARFWRR-GGEPPKG- IKKIVSKIRKLLKR | 0.92/H | 0.998/0.968 | 0.916/0.973 | 1.0/0.9654/0.95 | 0.97/0.927 | 0.72/0.597/1.80807 | 0.010 | −1.25 |
| 40 | L-K6V1 temp. [112]-GGEPPKG-KW/DJ | IKKIVSKIKKLLK-GGEPPKG- KRKRWHW | 0.72/L | 0.971/0.986 | 0.996/0.955 | 0.994/0.985/0.97 | 0.94/0.839 | 0.50/0.624 | 0.007 | −1.15 |
| 41 | R8FW-GGGPPKG-IDR-1002 [9]/DJ | RRRRRRFWRR-GGGPPKG- VQRWLIVWRIRK | 0.97/H | 1.0/0.944 | 0.181/0.979 | 1.0/0.862/0.854 | 0.71/0.40 | 0.633/0.606 | 0.064 | −1.17 |
| 42 | R8FW-GGGPPKG-IDR-1018 [9]/DJ | RRRRRRFWRR-GGGPPKG- VRLIVAVRIWRR | 0.95/H | 1.0/0.978 | 0.344/0.980 | 1.0/0.863/0.982 | 0.79/0.33 | 0.612/0.602 | 0.039 | −1.17 |
| 43 | R8FW-GGGPPKG- IDR-1018-R6/DJ | RRRRRRFWRR-GGGPPKG- VRLIVRVRIWRR | 0.96/H | 1.0/0.929 | 0.406/0.980 | 1.0/0.832/0.354 | 0.81/0.59 | 0.626/0.605 | 0.027 | −1.21 |
| 44 | R8FW-GGEPPKG-IDR-1018-R6/DJ | RRRRRRFWRR-GGEPPKG- VRLIVRVRIWRR | 0.96/H | 1.0/0.702 | 0.356/0.983 | 1.0/0.854/0.99 | 0.78/0.21 | 0.612/0.607/1.16498 | 0.026 | −1.23 |
| 45 | R8FW-GGEPPKG-IDR-1018-L1R6/DJ | RRRRRRFWRR-GGEPPKG- LRLIVRVRIWRR | 0.96/H | 1.0/0.939 | 0.277/0.983 | 1.0/0.86/0.04 | 0.77/0.338 | 0.623/0.622/1.43256 | 0.025 | −1.20 |
| 46 | Pexiganan-L18 [88]/E | GIGKFLKKAKKFGKAFVLILKK | 0.75/L | 0.997/1.0 | 1.0/0.976 | 0.46/0.299/0.22 | 1.0/1.0 | 0.598/0.661 | 0.892 | −0.94 |
| 47 | Flexampin [114]/E | GIKKWVKGVAKGVAKDLAKKIL | 0.56/L | 0.990/1.0 | 1.0/0.977 | 0.993/0.937/0.544 | 0.99/1.0 | 0.423/0.531 | 0.817 | −0.78 |
| 48 | Zyk-1 [88]/E | GIGREIIKKIIKKIGKKIGRII | 0.65/L | 0.978/0.998 | 0.998/0.97 | 1.0/0.933/0.946 | 0.88/1.0 | 0.526/0.662 | 0.583 | −0.86 |
| 49 | Adepantin-1A [88]/E | GIKKAVGKALKGLKGLLKALGES | 0.39 | 0.980/1.0 | 1.0/0.977 | 1.0/0.972/0.398 | 0.99/1.0 | 0.554/0.659/1.35587 | 0.17 | −1.51 |
| 50 | Novispirin [152]-KW-analog2/DJ | KNLRIFRKGIHIHKKY-GG- KRKRWHW | 0.903/H | 0.972/0.946 | 0.994/0.983 | 1.0/0.939/0.822 | 0.96/0.989 | 0.5884/0.6 | 0.195 | −1.63 |
| 51 | WFR8-adepantin-1A- analog2/DJ | RRWFRRRRRR- GIKKAVGKALKGLKLLLKALGES | 0.878/L | 0.999/1.0 | 0.923/0.9485 | 1.0/0.987/0.908 | 0.96/0.983 | 0.616/0.622/1.62411 | 0.826 | −1.63 |
| 52 | KW-second-bovine- BMAP-18 [179]/DJ | KRKRWHW-GRFKRFRKKFKKLFKKIS | 0.961/H | 0.999/1.0 | 0.995/0.981 | 1.0/0.845/1.0 | 0.91/1.0 | 0.565/0.698 | 0.176 | −1.09 |
| No. | Parent-Protein/Gene/ Origin/Reference * | Extended CPP at the N or C-terminal * | CPP | Anti-Microbial | Anti-Cancer | Anti-Viral | Anti-Fungal | Anti-Inflamm. | Hemo -lytic | Tox./Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tumor-homing-tLyP-1 peptide [146]/E | CGNKRTR | 0.91/L | 0.00/ND | N/0.88 | N/N/N | ND/ND | 0.38/0.47 | 0.01 | −0.42 |
| 2 | A7RG57 C-term. from N. vectensis | RCGIKRTK | 0.93/L | 0.03/ND | 0.93/0.95 | 0.6/0.5/0.5 | 0.92/ND | 0.47/0.62 | 0.00 | −0.88 |
| 3 | MFC/DJ | RCGNKRFRWHW | 0.94/H | 0.43/0.91 | 0.97/0.98 | 1.0/0.8/1.0 | 0.38/0.98 | 0.47/0.63 | 0.01 | −0.92 |
| 4 | NLS-CE [180]/E | WRFVWMNPKKKRKV | 0.92/H | 0.99/0.54 | 0.46/0.98 | 0.8/0.5/0.8 | 0.13/0.76 | 0.47/0.59 | 0.11 | −1.11 |
| 5 | Zp3a [161]/E | GIKAKIGIKIKK | 0.77/L | 0.94/1.00 | 1.0/0.97 | 0.2/0.1/0.3 | 0.86/1.00 | 0.48/0.63 | 0.03 | −0.68 |
| 6 | Magainin 2 (MG2) [181]/E | GIGKFLHSAKKFGKAFVGEIMNS | 0.22 | 0.95/1.00 | 1.0/0.98 | 1.0/1.0/1.0 | 0.99/0.98 | 0.56/0.55 | 0.83 | −0.58 |
| 7 | MG2-tLyP-1 [146]/DJ | GIGKFLHSAKKFGKAFVGEIMNS-GG- CGNKRTR | 0.26 | 0.88/0.99 | 0.99/0.93 | 1.0/1.0/1.0 | 0.99/0.94 | 0.53/0.52 | 0.76 | −0.35 |
| 8 | MG2-KW [168]/DJ | GIGKFLHSAKKFGKAFVGEIMNS-GG- KRKRWHW | 0.43 | 0.94/1.00 | 0.99/0.96 | 1.0/1.0/1.0 | 0.99/0.92 | 0.57/0.52 | 0.63 | −0.68 |
| 9 | MG2-WFR8/DJ | GIGKFLHSAKKFGKAFVGEIMNS-GG- RRWFRRRRRR | 0.74/L | 0.93/1.00 | 0.95/0.97 | 1.0/1.0/1.0 | 0.98/0.99 | 0.61/0.52 | 0.72 | −0.90 |
| 10 | 9P0-1 [182]/E | GIKKWLHSAKKFGKKFVKKIMNS | 0.72/L | 0.99/1.00 | 1.0/0.98 | 0.8/0.9/1.0 | 0.99/0.98 | 0.61/0.64 | 0.96 | −0.42 |
| 11 | MFC-9P0-1-analog [182]/DJ | RCGNKRFRWHW- GIKKWLHSAKKFGKKFVKKIMNS | 0.76/H | 0.92/1.00 | 1.0/0.93 | 1.0/1.0/0.9 | 0.95/0.96 | 0.63/0.70 | 0.86 | −0.59 |
| 12 | MFC-Zp3a [161]/DJ | RCGNKRFRWHW-GIKAKIGIKIKK | 0.89/H | 0.98/0.99 | 0.98/0.98 | 1.0/0.7/0.9 | 0.97/1.00 | 0.57/0.68 | 0.01 | −1.05 |
| 13 | 9P1-3 [182]/E | GIKKWLHSAKKFPKKFVKKIMNS | 0.73/L | 0.99/1.00 | 1.0/0.98 | 0.9/0.9/0.6 | 0.97/0.98 | 0.63/0.64 | 0.94 | −0.30 |
| 14 | MFC-9P1-3 [182]/DJ | RCGNKRFRWHW- GIKKWLHSAKKFPKKFVKKIMNS | 0.78/H | 0.88/1.00 | 1.0/0.92 | 1.0/1.0/0.8 | 0.93/0.96 | 0.64/0.69 | 0.77 | −0.49 |
| 15 | MFC-PexShort/DJ | RCGNKRFRWHW- GIGKLKKAKKFGKKILKK | 0.86/H | 0.99/1.00 | 1.0/0.98 | 1.0/0.9/1.0 | 0.99/1.00 | 0.52/0.64 | 0.03 | −1.19 |
| 16 | MFC-PexNC/DJ | GIGK-G-RCGNKRFRWHW-ILKK | 0.83/H | 0.99/0.99 | 0.92/0.98 | 1.0/0.5/1.0 | 0.94/0.99 | 0.61/0.61 | 0.01 | −0.65 |
| 17 | MG2-I6V9W12T15I17 [183]/E | GIGKFIHSVKKWGKTFIGEIMNS | 0.26 | 0.97/0.99 | 1.0/0.85 | 1.0/0.9/1.0 | 0.93/0.99 | 0.55/0.57 | 0.93 | −0.64 |
| 18 | tLyP-1-MG2- I6V9W12T15I17 [183]/DJ | CGNKRTR- GIGKFIHSVKKWGKTFIGEIMNS | 0.33 | 0.87/1.00 | 1.0/0.27 | 1.0/0.9/1.0 | 0.95/0.97 | 0.50/0.63 | 0.78 | −0.52 |
| 19 | KW-MG2-I6V9W12T15I17 [183]/DJ | KRKRWHW- GIGKFIHSVKKWGKTFIGEIMNS | 0.46 | 0.80/1.00 | 1.0/0.50 | 1.0/1.0/1.0 | 0.85/0.98 | 0.55/0.64 | 0.57 | −0.75 |
| 20 | WFR8 -MG2- I6V9W12T15I17 [183]/DJ | RRWFRRRRRR- GIGKFIHSVKKWGKTFIGEIMNS | 0.82/H | 0.97/1.00 | 0.97/0.98 | 1.0/1.0/1.0 | 0.91/0.98 | 0.68/0.63 | 0.84 | −1.08 |
| 21 | MG2-Q19 [184]/E | GIGKFLHSAKKFGKAFVGQIMNS | 0.48 | 0.99/1.00 | 1.0/0.98 | 1.0/1.0/1.0 | 1.00/1.00 | 0.54/0.58 | 0.89 | −0.50 |
| 22 | MG2-Q19-tLyP-1 [184]/DJ | GIGKFLHSAKKFGKAFVGQIMNS-GG- CGNKRTR | 0.32 | 0.93/1.00 | 0.97/0.96 | 1.0/1.0/0.9 | 1.00/0.99 | 0.51/0.57 | 0.82 | −0.23 |
| 23 | MG2-Q19-KW [184]/DJ | GIGKFLHSAKKFGKAFVGQIMNS-GG- KRKRWHW | 0.61/L | 0.96/1.00 | 0.99/0.97 | 1.0/1.0/0.5 | 0.99/0.99 | 0.56/0.54 | 0.72 | −0.58 |
| 24 | MG2-Q19-WFR8 [184]/DJ | GIGKFLHSAKKFGKAFVGQIMNS-GG- RRWFRRRRRR | 0.77/L | 0.93/1.00 | 0.94/0.98 | 1.0/1.0/1.0 | 0.99/0.99 | 0.60/0.58 | 0.83 | −0.82 |
| 25 | Max-TI-MG2/DJ && | GIAKFLDSAKKFGKKFVKTIMQL | 0.31 | 0.99/1.00 | 1.0/0.98 | 0.8/0.9/1.0 | 1.00/0.98 | 0.57/0.59 | 0.97 | −0.56 |
| 26 | Max-TI-MG2-tLyP-1/DJ | GIAKFLDSAKKFGKKFVKTIMQL-GG- CGNKRTR | 0.44 | 0.95/1.00 | 1.0/0.99 | 1.0/1.0/1.0 | 1.00/0.87 | 0.61/0.57 | 0.98 | −0.38 |
| 27 | RtLyP-1-Max-TI-MG2/DJ | RCGNKRTR- GIAKFLDSAKKFGKKFVKTIMQL | 0.51/L | 0.86/1.00 | 1.0/0.92 | 1.0/0.9/1.0 | 1.00/0.97 | 0.64/0.63 | 0.86 | −0.41 |
| 28 | Max-TI-MG2-KW/DJ | GIAKFLDSAKKFGKKFVKTIMQL-GG- KRKRWHW | 0.55/L | 0.98/1.00 | 1.0/0.98 | 1.0/1.0/0.5 | 1.00/0.95 | 0.69/0.57 | 0.98 | −0.75 |
| 29 | Max-TI-MG2-WFR8/ DJ | GIAKFLDSAKKFGKKFVKTIMQL-GG- RRWFRRRRRR | 0.77/H | 0.97/1.00 | 0.97/0.98 | 1.0/1.0/1.0 | 0.99/0.97 | 0.64/0.58 | 0.98 | −1.00 |
| 30 | KAF5879953.1 36–47 MFCA | RCNRKRFRWQWK | 0.97/H | 1.00/0.64 | 0.86/0.98 | 0.1/0.6/1.0 | 0.14/0.75 | 0.56/0.66 | 0.01 | −0.43 |
| 31 | tLyp-1-RHP [54]/DJ | CGNKRTR-YKWYYRGAA | 0.78/H | 0.21/0.57 | 0.94/0.96 | 0.8/0.2/0.7 | 0.88/0.21 | 0.47/0.62 | 0.01 | 0.07 |
| 32 | R-tLyP-1-RHP/DJ | RCGNKRTR-YKWYYRGAA | 0.83/L | 0.76/0.65 | 0.87/0.98 | 1.0/0.3/0.9 | 0.89/0.14 | 0.57/0.63 | 0.01 | 0.12 |
| 33 | MFC-RHP/DJ | RCGNKRFRWHW-YKWYYRGAA | 0.84/H | 0.96/0.86 | 0.96/0.98 | 1.0/0.7/0.8 | 0.72/0.46 | 0.64/0.62 | 0.04 | −0.51 |
| 34 | MFC2/DJ | RCGNKRFRWHW-GG-RRAKWRR | 0.97/H | 1.00/0.97 | 0.60/0.98 | 1.0/0.9/1.0 | 0.37/0.91 | 0.64/0.64 | 0.01 | −0.69 |
| 35 | MFC-PexSa/DJ | RCGNKRFRWHW- GIGKLLKRKKFGKKILKK | 0.90/H | 0.99/1.00 | 1.0/0.97 | 1.0/1.0/0.5 | 0.99/1.00 | 0.58/0.65/1.60059 | 0.054 | −1.34 |
| 36 | MFC2-analog/DJ | RCGNKRLIWHW-GG-RRAKTRR | 0.95/H | 0.97/0.93 | 0.22/0.98 | 1.0/0.9/0.9 | 0.50/0.84 | 0.65/0.64/1.61375 | 0.005 | −0.43 |
| 37 | MG2-analog/DJ | GIGKLLKSALKFGKAFVGEIMNS | 0.177 | 0.986/1.0 | 0.998/0.988 | 0.982/0.9643/0.926 | 1.0/0.994 | 0.6163/0.627/1.76783 | 0.98 | −1.28 |
| 38 | WFR8-MG2-analog/DJ | RRWFRRRRRR- GIGKLLKSALKFGKAFVGEIMNS | 0.783/H | 0.99/1.0 | 0.839/0.971 | 1.0/0.99/1.0 | 0.96/0.98 | 0.723/0.646/1.5622 | 0.952 | −1.44 |
| 39 | CA-MA2 [185]/E | KWKLFKKI-P-KFLHSAKKF | 0.895/L | 0.997/1.0 | 1.0/0.98 | 0.983/0.73/0.56 | 0.94/0.996 | 0.62/0.645 | 0.008 | −0.09 |
| 40 | K6L9 [186]/E | LKLLKKLLKKLLKLL | 0.958/H | 0.918/1.0 | 0.996/0.92 | 0.999/0.927/0.71 | 0.13/1.0 | 0.62/0.607 | 0.907 | −1.00 |
| 41 | PR-39 pig P80054 | RRRPRPPYLPRPRPPPFFPPRLPP RIPPGFPPRFPPRFP | 0.760/L | 1.00/1.0 | 0.993/0.92 | 1.0/0.857/0.064 | 0.82/0.965 | 0.50/0.550 | ND | −0.71 |
| 42 | Pyrrhocoricin [187]/E | VDKGSYLPRPTPPRPIYNRN | 0.48 | 0.35/1.0 | 0.12/0.576 | 0.248/0.175/0.064 | 0.20/0.965 | 0.481/0.488 | 0.004 | −1.25 |
| 43 | R8FW-Pyrrhocoricin/DJ | RRWFRRRRRR- GVDKGSYLPRPTPPRPIYNRN | 0.864/L | 0.964/1.0 | 0.41/0.98 | 1.0/0.61/0.984 | 0.80/0.96 | 0.561/0.588 | 0.064 | −1.31 |
| 44 | PR-35/E | RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFP | 0.762/L | 1.0/1.0 | 0.978/0.9198 | 1.0/0.805/0.0 | 0.81/0.925 | 0.512/0.55 | 0.001 | −0.66 |
| 45 | PR-35-analog/DJ | RRRVRPPYLPRVRPQPFFPLRLLKRISPGFPPRFP | 0.821/L | 0.993/0.995 | 0.481/0.854 | 1.0/0.984/0.962 | 0.90/0.919 | 0.637/0.581/2.18407 | 0.012 | −1.44 |
| 46 | CA-MA2-analog1/DJ | KWKLFKKILKLLHSVKKF | 0.895/H | 0.996/1.0 | 1.0/0.9786 | 0.999/0.875/0.184 | 0.96/1.0 | 0.6326/0.735/1.8861 | 0.848 | −0.84 |
| 47 | L-K6V1-temp [112]- revP9 [188]/DJ | IKKIVSKIKKLLK-PPWWRRRRR | 0.972/H | 0.984/1.0 | 0.956/0.983 | 0.998/0.953/0.828 | 0.75/1.0 | 0.591/0.664 | 0.017 | −1.17 |
| 48 | L-K4V1-temp-revP9- analog/DJ | IKKIVSLILKLLK-LPWWRRRRR | 0.959/H | 0.999/1.0 | 0.451/0.982 | 0.999/0.959/1.0 | 0.65/1.0 | 0.74/0.764/1.965 | 0.190 | −1.30 |
| 49 | CA-MA2-analog2/DJ | KWRLFKKI-P-RFLRSARRF | 0.954/H | 1.0/0.948 | 0.977/0.980 | 1.0/0.935/0.758 | 0.87/0.992 | 0.605/0.625 | 0.054 | −1.11 |
| 50 | Sub-5 [189]/E | RRWKIVVIRWRR | 0.932/H | 1.0/0.994 | 0.935/0.975 | 0.784/0.579/0.762 | 0.30/1.0 | 0.516/0.643 | 0.037 | −0.76 |
| 51 | Sub-5-G-nuclear- loc.-signal [190]/DJ | RRWKIVVIRWRR-G-PKKKRKV | 0.973/H | 1.0/0.999 | 0.494/0.984 | 0.999/0.930/0.998 | 0.57/0.993 | 0.656/0.603 | 0.007 | −0.93 |
| 52 | temp V1R9 (analog-3)-Sub-5/DJ | VKKIVSKIRKLLK-GG- RRWKIVVIRWRR | 0.940/H | 0.983/1.0 | 0.972/0.976 | 0.999/0.974/0.916 | 0.92/1.0 | 0.528/0.641/1.49473 | 0.098 | −0.94 |
| Length-Amph-AMP * | Table- Peptide # | CPP | Anti- Microbial & | Anti- Cancer & | Anti- Viral & | Anti- Fung & | Anti- Inflamm. $ | Sum/6 | Rank † | Hemol. Probab. | Tox. Score | Reward Low tox. | Total Score § | Overall Rank | CPP Part |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25-αd-temp V1R9 | T3-37 | 0.97/H | 0.955 | 0.965 | 1.00 | 0.975 | 0.997 | 0.9869 | 1 | 0.130 | −1.27 | 0.570 | 0.92734 | 1st | WFS6R7 |
| 31-αtαd-temp R9R14 | T4-39 | 0.92/H | 0.983 | 0.9445 | 0.972 | 0.949 | 1.0412 | 0.9682 | 6 | 0.010 | −1.25 | 0.620 | 0.91846 | 2nd | WFA5R7 |
| 25-αd-temp R9 | T3-36 | 0.96/H | 0.955 | 0.97 | 1.00 | 0.98 | 0.927 | 0.9670 | 7 | 0.180 | −1.30 | 0.560 | 0.90886 | 3rd | WFS6R7 |
| 31-αtαd-temp R9R14 | T4-38 | 0.94/H | 0.993 | 0.9495 | 0.985 | 0.952 | 0.9368 | 0.9594 | 10 | 0.028 | −1.20 | 0.586 | 0.90606 | 4th | WFR8 |
| 22-αd-temp | T4-30 | 0.95/H | 0.99 | 0.99 | 0.927 | 0.96 | 0.996 | 0.9688 | 5 | 0.070 | −1.12 | 0.525 | 0.90540 | 5th | KW |
| 22-αd-r-o-p A8I15 | T2-20 | 0.987/H | 0.977 | 0.976 | 0.863 | 0.906 | 0.9038 | 0.9353 | 0.007 | −1.39 | 0.692 | 0.90047 | 6th | tLyP-1 | |
| 29-βαd-PexS | T5-35 | 0.90/H | 0.995 | 0.985 | 0.83 | 0.995 | 0.9435 | 0.9414 | 21 | 0.054 | −1.34 | 0.643 | 0.89877 | 7th | MFC |
| 25-αd-temp | T3-9 | 0.97/H | 0.99 | 0.975 | 1.00 | 0.985 | 0.757 | 0.9461 | 19 | 0.170 | −1.35 | 0.590 | 0.89523 | 8th | WFR8 |
| 31-αtαd-temp R9R14 | T4-37 | 0.95/H | 0.9985 | 0.961 | 0.927 | 0.98 | 0.8538 | 0.9450 | 20 | 0.032 | −1.18 | 0.574 | 0.89200 | 9th | WFR8 |
| 22-αtαd-temp | T5-48 | 0.959/H | 1.00 | 0.7156 | 0.986 | 0.825 | 1.1563 | 0.9472 | 18 | 0.190 | −1.30 | 0.555 | 0.89119 | 10th | rP9a |
| 31-αtαd-mapegin-a2 | T3-50 | 0.881/L | 0.756 | 0.8295 | 0.967 | 0.983 | 0.9556 | 0.8953 | 0.077 | −1.81 | 0.867 | 0.89118 | 11th | TATa | |
| 25-αd-BMAP | T4-33 | 0.905/H | 0.991 | 0.9715 | 0.993 | 0.905 | 0.9868 | 0.9567 | 12 | 0.080 | −1.05 | 0.485 | 0.88931 | 12th | KW |
| 35-βtαd- PR-35a | T5-45 | 0.821/L | 0.994 | 0.6675 | 0.982 | 0.910 | 1.134 | 0.9180 | 0.012 | −1.44 | 0.714 | 0.88886 | 13th | whole | |
| 25-αd- temp L9V14 | T3-35 | 0.96/H | 0.999 | 0.97 | 1.00 | 0.98 | 0.978 | 0.9738 | 4 | 0.760 | −1.47 | 0.355 | 0.88540 | 14th | WFR8 |
| 28-αd-T2R1-L7S17-a2 | T4-32 | 0.93/H | 0.914 | 0.974 | 0.997 | 0.785 | 0.9557 | 0.9260 | 0.050 | −1.30 | 0.625 | 0.88300 | 15th | KT2W | |
| 25-αd-BMAP-18 | T4-25 | 0.90/H | 1.00 | 0.98 | 1.00 | 0.930 | 0.875 | 0.9475 | 17 | 0.090 | −1.01 | 0.460 | 0.87787 | 16th | KW |
| 27-αtβd-temp V1R9 | T5-52 | 0.940/H | 0.992 | 0.974 | 0.963 | 0.960 | 0.888 | 0.9528 | 14 | 0.098 | −0.94 | 0.421 | 0.87684 | 17th | Sub 5 |
| 25-βαd-BMAP-a2 | T4-36 | 0.849/H | 0.987 | 0.9665 | 0.990 | 0.912 | 1.0828 | 0.9646 | 8 | 0.399 | −1.09 | 0.346 | 0.87616 | 18th | KW |
| 33-αd-adepantin-1A | T4-18 | 0.86/L | 1.00 | 0.955 | 1.00 | 0.98 | 0.8668 | 0.9436 | 0.630 | −1.56 | 0.465 | 0.87525 | 19th | WFR8 | |
| 25-αtβd-novispirin-a1 | T4-50 | 0.903/H | 0.959 | 0.9885 | 0.920 | 0.975 | 0.5942 | 0.8899 | 0.195 | −1.63 | 0.7175 | 0.87307 | 20th | KW | |
| 33-αd adepantin-1A-L15 | T4-51 | 0.878/L | 0.9995 | 0.9358 | 0.965 | 0.972 | 0.954 | 0.9506 | 16 | 0.826 | −1.63 | 0.402 | 0.87225 | 21 | WFR8 |
| 22-r-o-pA8I15 | T2-21 | 0.985/H | 0.98 | 0.962 | 0.683 | 0.91 | 0.960 | 0.9134 | 0.006 | −1.22 | 0.607 | 0.86963 | 22 | tLyPA3-1 | |
| 20-αβ BP100 | T4-34 | 0.927/H | 0.9975 | 0.9735 | 0.987 | 0.935 | 1.0426 | 0.9772 | 3 | 0.894 | −1.32 | 0.213 | 0.86803 | 23 | KW |
| 19-αβ BP33 | T4-9 | 0.93/H | 1.00 | 0.985 | 1.00 | 0.96 | 1.001 | 0.9793 | 2 | 0.820 | −1.19 | 0.185 | 0.86583 | 25 | KW |
| 28-αtα-novispirin-a1 | T3-38 | 0.94/H | 1.00 | 0.8745 | 0.995 | 0.978 | 0.9305 | 0.9526 | 15 | 0.551 | −1.17 | 0.310 | 0.86073 | 26 | WFL5R7 |
| 35-α adep1a | T4-17 | 0.80/L | 0.995 | 0.965 | 1.00 | 0.99 | 0.8552 | 0.9342 | 0.660 | −1.46 | 0.400 | 0.85789 | 29 | WFR8 | |
| 31-αtα mapegin-a1 | T3-49 | 0.958/H | 0.990 | 0.798 | 0.868 | 0.97 | 1.1887 | 0.9621 | 9 | 0.717 | −1.09 | 0.187 | 0.85127 | 30 | TAT |
| 18-αtα r-o-p-analog | T2-22 | 0.991/H | 1.00 | 0.962 | 0.97 | 0.795 | 0.61 | 0.8879 | 0.003 | −1.26 | 0.629 | 0.85091 | 31 | whole | |
| 25-α-BMAP2-18 | T4-52 | 0.961/H | 0.9995 | 0.988 | 0.948 | 0.955 | 0.6315 | 0.9139 | 0.176 | −1.09 | 0.457 | 0.84862 | 32 | KW | |
| 34-αtα-Zyk1a | T4-35 | 0.833/L | 0.9985 | 0.9815 | 0.983 | 0.987 | 0.9377 | 0.9534 | 13 | 0.938 | −1.33 | 0.196 | 0.84520 | 33 | WFR8 |
4. Design of Cell-Penetrating Multifunctional Peptides
4.1. Advantages of Cell-Penetrating Antimicrobial Peptides
4.2. Potential for Clearing Intracellular Drug-Resistant Bacteria
4.3. Short Cell-Penetrating Peptides and Their Conjugates
4.4. Magainin-2 Analogs Fused to Cell-Penetrating Peptides
4.5. Imperfect and Perfect Activity-Enhancing Palindromes
4.6. Construction of Chimeras Containing Bacterial Pheromones or Ribosomal-Homing Peptide
4.7. The Optimization of Multifunctional Constructs
4.8. Antimicrobial Peptides with Anticancer Activity Fused to Cell-Penetrating Peptides
4.9. Design Examples for Low Toxicity and Multiple Activities
5. Summary Comments about Peptide Constructs
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Henriques, S.T.; Lawrence, N.; Chaousis, S.; Ravipati, A.S.; Cheneval, O.; Benfield, A.H.; Elliott, A.G.; Kavanagh, A.M.; Cooper, M.A.; Chan, L.Y.; et al. Redesigned Spider Peptide with Improved Antimicrobial and Anticancer Properties. ACS Chem. Biol. 2017, 12, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M.; Ericsson, A.C. Antimicrobial Peptides: Potential Application in Liver Cancer. Front. Microbiol. 2019, 10, 1257. [Google Scholar] [CrossRef]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial–Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef]
- Rozek, T.; Wegener, K.T.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis. The solution structure of aurein 1.2. Eur. J. Biochem. 2000, 267, 5330–5341. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, C.B.; Kim, J.M.; Jang, S.A.; Park, I.Y.; Kim, M.S.; Cho, J.H.; Kim, S.C. Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett. 2008, 271, 47–55. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Drayton, M.; Deisinger, J.P.; Ludwig, K.C.; Raheem, N.; Müller, A.; Schneider, T.; Straus, S.K. Host Defense Peptides: Dual Antimicrobial and Immunomodulatory Action. Int. J. Mol. Sci. 2021, 22, 11172. [Google Scholar] [CrossRef]
- Shin, M.K.; Lee, B.; Kim, S.T.; Yoo, J.S.; Sung, J.S. Designing a Novel Functional Peptide With Dual Antimicrobial and Anti-inflammatory Activities via in Silico. Methods Front. Immunol. 2022, 13, 821070. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Hoskin, D.W.; Power Coombs, M.R. Anticancer Activities of Natural and Synthetic Peptides. Adv. Exp. Med. Biol. 2019, 1117, 131–147. [Google Scholar] [CrossRef]
- Ajesh, K.; Sreejith, K. Peptide antibiotics: An alternative and effective antimicrobial strategy to circumvent fungal infections. Peptides 2009, 30, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Duncan, V.M.S.; O’Neil, D.A. Commercialization of antifungal peptides. Fungal Biol. Rev. 2013, 26, 156–165. [Google Scholar] [CrossRef]
- Ciociola, T.; Giovati, L.; Conti, S.; Magliani, W.; Santinoli, C.; Polonelli, L. Natural and synthetic peptides with antifungal activity. Future Med. Chem. 2016, 8, 1413–1433. [Google Scholar] [CrossRef]
- Hoffmann, A.R.; Guha, S.; Wu, E.; Ghimire, J.; Wang, Y.; He, J.; Garry, R.F.; Wimley, W.C. Broadspectrum antiviral entry inhibition by interfacially active peptides. J. Virol. 2020, 94, e01682-20. [Google Scholar] [CrossRef]
- Hollmann, A.; Cardoso, N.P.; Espeche, J.C.; Maffía, P.C. Review of antiviral peptides for use against zoonotic and selected non-zoonotic viruses. Peptides 2021, 142, 170570. [Google Scholar] [CrossRef]
- Henriques, S.T.; Melo, M.N.; Castanho, M.A.R.B. Cell-penetrating peptides and antimicrobial peptides: How different are they? Biochem. J. 2006, 399, 1–7. [Google Scholar] [CrossRef]
- Pärn, K.; Eriste, E.; Langel, Ü. The Antimicrobial and Antiviral Applications of Cell-Penetrating Peptides. Methods Mol. Biol. 2015, 1324, 223–245. [Google Scholar] [CrossRef]
- Brasseur, R.; Divita, G. Happy birthday cell penetrating peptides: Already 20 years. Biochim. Biophys. Acta 2010, 1798, 2177–2181. [Google Scholar] [CrossRef] [Green Version]
- Gallo, M.; Defaus, S.; Andreu, D. 1988-2018, Thirty years of drug smuggling at the nano scale. Challenges and opportunities of cell-penetrating peptides in biomedical research. Arch. Biochem. Biophys. 2019, 661, 74–86. [Google Scholar] [CrossRef]
- Manavalan, B.; Subramaniyam, S.; Shin, T.H.; Kim, M.O.; Lee, G. Machine-learning-based prediction of cell-penetrating peptides and their uptake efficiency with improved accuracy. J. Proteome Res. 2018, 17, 2715–2726. [Google Scholar] [CrossRef]
- Tang, H.; Su, Z.D.; Wei, H.H.; Chen, W.; Lin, H. Prediction of cell-penetrating peptides using feature selection techniques. Biochem. Biophys. Res. Commun. 2016, 477, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3, A database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016, 44, D1094–D1097. [Google Scholar] [CrossRef] [PubMed]
- Burdukiewicz, M.; Sidorczuk, K.; Rafacz, D.; Pietluch, F.; Chilimoniuk, J.; Rödiger, S.; Gagat, P. AmpGram: A proteome screening tool for prediction and design of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 4310. [Google Scholar] [CrossRef] [PubMed]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. ACPred: A computational tool for the prediction and analysis of anticancer peptides. Molecules 2019, 24, 1973. [Google Scholar] [CrossRef]
- Boopathi, V.; Subramaniyam, S.; Malik, A.; Lee, G.; Manavalan, B.; Yang, D.C. mACPpred: A support vector machine-based meta-predictor for identification of anticancer peptides. Int. J. Mol. Sci. 2019, 20, 1964. [Google Scholar] [CrossRef]
- Timmons, P.B.; Hewage, C.H. ENNAVIA is a novel method which employs neural networks for antiviral and anti-coronavirus activity prediction for therapeutic peptides. Brief. Bioinform. 2021, 22, bbab258. [Google Scholar] [CrossRef]
- Chowdhury, A.S.; Reehl, S.M.; Kehn-Hall, K.; Bishop, B.; Webb-Robertson, B.J.M. Better understanding and prediction of antiviral peptides through primary and secondary structure feature importance. Sci. Rep. 2020, 10, 19260. [Google Scholar] [CrossRef]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. Meta-iAVP: A sequence-based meta-predictor for improving the prediction of antiviral peptides using effective feature representation. Int. J. Mol. Sci. 2019, 20, 5743. [Google Scholar] [CrossRef] [Green Version]
- Meher, P.K.; Sahu, T.K.; Saini, V.; Rao, A.R. Predicting antimicrobial peptides with improved accuracy by incorporating the compositional, physico-chemical and structural features into Chou’s general PseAAC. Sci. Rep. 2017, 7, 42362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, L.; Tian, Z.; Zhao, W.; Sun, C.; Zhu, L.; Huang, M.; Guo, G.; Liang, G. Large-Scale Screening of Antifungal Peptides Based on Quantitative Structure-Activity Relationship. ACS Med Chem Lett. 2021, 13, 99–104. [Google Scholar] [CrossRef]
- Manavalan, B.; Shin, T.H.; Kim, M.O.; Lee, G. AIPpred: Sequence-Based Prediction of Anti-inflammatory Peptides Using Random Forest. Front. Pharmacol. 2018, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.S.; Hasan, M.M.; Kurata, H. PreAIP: Computational Prediction of Anti-inflammatory Peptides by Integrating Multiple Complementary Features. Front. Genet. 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, A.K.; Shastri, V.; Madhu, M.K.; Sharma, V.K. Prediction of anti-inflammatory proteins/peptides: An in silico approach. J. Transl. Med. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Gou, Z.; Kuznetsov, I.B. DP-Bind: A web server for sequence-based prediction of DNA-binding residues in DNA-binding proteins. Bioinformatics 2007, 23, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P. Peptide toxicity prediction. Methods Mol. Biol. 2015, 1268, 143–157. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Open Source Drug Discovery Consortium; Raghava, G.P.S. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Usmani, S.S.; Kumar, R.; Bhalla, S.; Kumar, V.; Raghava, G.P.S. In Silico Tools and Databases for Designing Peptide-Based Vaccine and Drugs. Adv. Protein Chem. Struct. Biol. 2018, 112, 221–263. [Google Scholar] [CrossRef]
- Timmons, P.B.; Hewage, C.M. HAPPENN is a novel tool for hemolytic activity prediction for therapeutic peptides which employs neural networks. Sci. Rep. 2020, 10, 10869. [Google Scholar] [CrossRef]
- Wei, L.; Ye, X.; Sakurai, T.; Mu, Z.; Wei, L. ToxIBTL: Prediction of peptide toxicity based on information bottleneck and transfer learning. Bioinformatics 2022, 38, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Juretić, D.; Lučin, A. The preference functions method for predicting protein helical turns with membrane propensity. J. Chem. Inf. Comput. Sci. 1998, 38, 575–585. [Google Scholar] [CrossRef]
- Juretić, D.; Zoranić, L.; Zucić, D. Basic charge clusters and predictions of membrane protein topology. J. Chem. Inf. Comput. Sci. 2002, 42, 620–632. [Google Scholar] [CrossRef]
- Juretić, D.; Vukičević, D.; Ilić, N.; Antcheva, N.; Tossi, A. Computational Design of Highly Selective Antimicrobial Peptides. J. Chem. Inf. Model. 2009, 49, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Juretić, D.; Vukičević, D.; Petrov, D.; Novković, M.; Bojović, V.; Lučić, B.; Ilić, I.; Tossi, A. Knowledge-based computational methods for identifying or designing novel, non-homologous antimicrobial peptides. Eur. Biophys. J. 2011, 40, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Kamech, N.; Vukičević, D.; Ladram, A.; Piesse, C.; Vasseur, J.; Bojović, V.; Simunić, J.; Juretić, D. Improving the selectivity of antimicrobial peptides from anuran skin. J. Chem. Inf. Model. 2012, 52, 3341–3351. [Google Scholar] [CrossRef]
- Etzion-Fuchs, A.; Todd, D.A.; Singh, M. dSPRINT: Predicting DNA, RNA, ion, peptide and smallmolecule interaction sites within protein domains. Nucleic Acids Res. 2021, 49, e78. [Google Scholar] [CrossRef]
- Le Roux, I.; Joliot, A.H.; Bloch-Gallego, E.; Prochiantz, A.; Volovitch, M. Neurotrophic activity of the Antennapedia homeodomain depends on its specific DNA-binding properties. Proc. Natl. Acad. Sci. USA 1993, 90, 9120–9124. [Google Scholar] [CrossRef]
- Christiaens, B.; Symoens, S.; Vanderheyden, S.; Engelborghs, Y.; Joliot, A.; Prochiantz, A.; Vandekerckhove, J.; Rosseneu, M.; Vanloo, B. Tryptophan fluorescence study of the interaction of penetratin peptides with model membranes. Eur. J. Biochem. 2002, 269, 2918–2926. [Google Scholar] [CrossRef]
- Palm, C.; Netzereab, S.; Hällbrink, M. Quantitatively determined uptake of cell-penetrating peptides in non-mammalian cells with an evaluation of degradation and antimicrobial effects. Peptides 2006, 27, 1710–1716. [Google Scholar] [CrossRef]
- Masman, M.F.; Rodriguez, A.M.; Raimondi, M.; Zacchino, S.A.; Luiten, P.G.M.; Somlai, C.; Kortvelyesi, T.; Penke, B.; Enriz, R.D. Penetratin and derivatives acting as antifungal agents. Eur. J. Med. Chem. 2009, 44, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Shin, S.Y. Antimicrobial and cytolytic activities and plausible mode of bactericidal action of the cell penetrating peptide penetratin and its Lys-linked two-stranded peptide. Chem. Biol. Drug Des. 2009, 73, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.D.; Jiao, C.Y.; Aubry, S.; Aussedat, B.; Burlina, F.; Chassaing, G.; Sagan, S. Cell biology meets biophysics to unveil the different mechanisms of penetratin internalization in cells. Biochim. Biophys. Acta 2010, 1798, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Rangel, R.; Guzman-Rojas, L.; le Roux, L.G.; Staquicini, F.I.; Hosoya, H.; Barbu, E.M.; Ozawa, M.G.; Nie, J.; Dunner, K., Jr.; Langley, R.R.; et al. Combinatorial targeting and discovery of ligand-receptors in organelles of mammalian cells. Nat. Commun. 2012, 3, 788. [Google Scholar] [CrossRef] [PubMed]
- Bahnsen, J.S.; Franzyk, H.; Sandberg-Schaal, A.; Nielsen, H.M. Antimicrobial and cell-penetrating properties of penetratin analogs: Effect of sequence and secondary structure. Biochim. Biophys. Acta 2013, 1828, 223–232. [Google Scholar] [CrossRef]
- Kauffman, W.B.; Guha, S.; Wimley, W.C. Synthetic molecular evolution of hybrid cell penetrating peptides. Nat. Commun. 2018, 9, 2568. [Google Scholar] [CrossRef]
- Vale, N.; Duarte, D.; Silva, S.; Correia, A.S.; Costa, B.; Gouveia, M.J.; Ferreira, A. Cell-Penetrating Peptides in Oncologic Pharmacotherapy: A Review. Pharmacol. Res. 2020, 162, 105231. [Google Scholar] [CrossRef]
- Shoari, A.; Tooyserkani, R.; Tahmasebi, M.; Löwik, D.W. Delivery of Various Cargos into Cancer Cells and Tissues via Cell-Penetrating Peptides: A Review of the Last Decade. Pharmaceutics 2021, 13, 1391. [Google Scholar] [CrossRef]
- Bellen, H.J.; Wilson, C.; Gehring, W.J. Dissecting the complexity of the nervous system by enhancer detection. Bioessays 1990, 12, 199–204. [Google Scholar] [CrossRef]
- Quiring, R.; Walldorf, U.; Kloter, U.; Gehring, W.J. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 1994, 265, 785–789. [Google Scholar] [CrossRef]
- Halder, G.; Callaerts, P.; Gehring, W.J. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 1995, 267, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.J.; Ikeo, K. Pax 6, mastering eye morphogenesis and eye evolution. Trends Genet. 1999, 15, 371–377. [Google Scholar] [CrossRef]
- Punzo, C.; Seimiya, M.; Flister, S.; Gehring, W.J.; Plaza, S. Differential interactions of eyeless and twin of eyeless with the sine oculis enhancer. Development 2002, 129, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.J. New Perspectives on Eye Development and the Evolution of Eyes and Photoreceptors. J. Hered. 2005, 96, 171–184. [Google Scholar] [CrossRef]
- Kozmik, Z. The role of Pax genes in eye evolution. Brain Res. Bull. 2008, 75, 335–339. [Google Scholar] [CrossRef]
- Gehring, W.J. The evolution of vision. WIREs Dev. Biol. 2014, 3, 1–40. [Google Scholar] [CrossRef]
- Chi, N.; Epstein, J.A. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002, 18, 41–47. [Google Scholar] [CrossRef]
- Gruschus, J.M.; Tsao, D.H.H.; Wang, L.H.; Nirenberg, M.; Ferretti, J.A. The three-dimensional structure of the vnd/NK-2 homeodomain-DNA complex by NMR spectroscopy. J. Mol. Biol. 1999, 289, 529–545. [Google Scholar] [CrossRef]
- Birrane, G.; Soni, A.; Ladias, J.A. Structural Basis for DNA Recognition by the Human PAX3 Homeodomain. Biochemistry 2009, 48, 1148–1155. [Google Scholar] [CrossRef]
- Gehring, W.J.; Qian, Y.Q.; Billeter, M.; Furukubo-Tokunaga, K.; Schier, A.F.; Resendez-Perez, D.; Affolter, M.; Otting, G.; Wüthrich, K. Homeodomain-DNA recognition. Cell 1994, 78, 211–223. [Google Scholar] [CrossRef]
- Dupont, E.; Prochiantz, A.; Joliot, A. Penetratin Story: An Overview. In Cell-Penetrating Peptides; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 683, pp. 21–29. [Google Scholar] [CrossRef]
- Holland, P.W. Evolution of homeobox genes. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Arslan, D.; Legendre, M.; Seltzer, V.; Abergel, C.; Claverie, J.-M. Distant Mimivirus relative with a larger genome highlights the fundamental features of Megaviridae. Proc. Natl. Acad. Sci. USA 2011, 108, 17486–17491. [Google Scholar] [CrossRef] [PubMed]
- Jeudy, S.; Bertaux, L.; Alempic, J.-M.; Lartigue, A.; Legendre, M.; Belmudes, L.; Santini, S.; Philippe, N.; Beucher, L.; Biondi, E.G.; et al. Exploration of the propagation of transpovirons within Mimiviridae reveals a unique example of commensalism in the viral world. ISME J. 2020, 14, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Havrilak, J.A.; Al-Shaer, L.; Baban, N.; Akinci, N.; Layden, M.J. Characterization of the dynamics and variability of neuronal subtype responses during growth, degrowth, and regeneration of Nematostella vectensis. BMC Biol. 2021, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Leach, W.B.; Reitzel, A.M. Decoupling behavioral and transcriptional responses to color in an eyeless cnidarian. BMC Genom. 2020, 21, 361. [Google Scholar] [CrossRef]
- Kaniewska, P.; Alon, S.; Karako-Lampert, S.; Hoegh-Guldberg, O.; Levy, O. Signaling cascades and the importance of moonlight in coral broadcast mass spawning. eLife 2015, 4, e09991. [Google Scholar] [CrossRef]
- Sharma, S.; Wang, W.; Stolfi, A. Single-cell transcriptome profiling of the Ciona larval brain. Dev. Biol. 2019, 448, 226–236. [Google Scholar] [CrossRef]
- Oonuma, K.; Tanaka, M.; Nishitsuji, K.; Kato, Y.; Shimai, K.; Kusakabe, T.G. Revised lineage of larval photoreceptor cells in Ciona reveals archetypal collaboration between neural tube and neural crest in sensory organ formation. Dev. Biol. 2016, 420, 178–185. [Google Scholar] [CrossRef]
- Kusakabe, T.; Kusakabe, R.; Kawakami, I.; Satou, Y.; Satoh, N.; Tsuda, M. Ci-opsin1, a vertebrate-type opsin gene, expressed in the larval ocellus of the ascidian Ciona intestinalis. FEBS Lett. 2001, 506, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Hadrys, T.; DeSalle, R.; Sagasser, S.; Fischer, N.; Schierwater, B. The Trichoplax PaxB gene: A putative Proto-PaxA/B/C gene predating the origin of nerve and sensory cells. Mol. Biol. Evol. 2005, 22, 1569–1578. [Google Scholar] [CrossRef]
- Monteiro, A.S.; Schierwater, B.; Dellaporta, S.L.; Holland, P.W.H. A low diversity of ANTP class homeobox genes in Placozoa. Evol. Dev. 2006, 8, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Larroux, C.; Lu, D.R.; Mohanty, K.; Chapman, J.; Degnan, B.M.; Rokhsar, D.S. Early evolution of the LIM homeobox gene family. BMC Biol. 2010, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Sebé-Pedrós, A.; de Mendoza, A.; Lang, B.F.; Degnan, B.M.; Ruiz-Trillo, I. Unexpected repertoire of metazoan transcription factors in the unicellular holozoan Capsaspora owczarzaki. Mol. Biol. Evol. 2011, 28, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Simunić, J.; Petrov, D.; Bouceba, T.; Kamech, N.; Benincasa, M.; Juretić, D. Trichoplaxin—A new membrane-active antimicrobial peptide from placozoan cDNA. Biochim. Biophys. Acta 2014, 1838, 1430–1438. [Google Scholar] [CrossRef]
- Majzoub, K.; Wrensch, F.; Baumert, T.F. The Innate Antiviral Response in Animals: An Evolutionary Perspective from Flagellates to Humans. Viruses 2019, 11, 758. [Google Scholar] [CrossRef]
- Kamm, K.; Schierwater, B.; DeSalle, R. Innate immunity in the simplest animals–placozoans. BMC Genom. 2019, 20, 5. [Google Scholar] [CrossRef]
- Juretić, D.; Golemac, A.; Strand, D.E.; Chung, K.; Ilić, N.; Goić-Barišić, I.; Pellay, F.-X. The spectrum of design solutions for improving the activity-selectivity product of peptide antibiotics against multidrug-resistant bacteria and prostate cancer PC-3 cells. Molecules 2020, 25, 3526. [Google Scholar] [CrossRef]
- Mayorova, T.D.; Hammar, K.; Jung, J.H.; Aronova, M.A.; Zhang, G.; Winters, C.A.; Reese, T.S.; Smith, C.L. Placozoan fiber cells: Mediators of innate immunity and participants in wound healing. Sci. Rep. 2021, 11, 23343. [Google Scholar] [CrossRef]
- Romanova, D.Y.; Nikitin, M.A.; Shchenkov, S.V.; Moroz, L.L. Expanding of Life Strategies in Placozoa: Insights From Long-Term Culturing of Trichoplax and Hoilungia. Front. Cell Dev. Biol. 2022, 10, 823283. [Google Scholar] [CrossRef]
- Prakash, V.N.; Bull, M.S.; Prakash, M. Motility-induced fracture reveals a ductile-to-brittle crossover in a simple animal’s epithelia. Nat. Phys. 2021, 17, 504–511. [Google Scholar] [CrossRef]
- Martinelli, C.; Spring, J. Expression pattern of the homeobox gene Not in the basal metazoan Trichoplax adhaerens. Gene Expr. Patterns 2004, 4, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Loewenstein, P.M. Autonomous Functional Domains of Chemically Synthesized Human lmmunodeficiency Virus Tat Trans-Activator Protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Grieco, P.; Caraglia, M.; Morelli, G.; Galdiero, M. Exploitation of viral properties for intracellular delivery. J. Pept. Sci. 2014, 20, 468–478. [Google Scholar] [CrossRef]
- van den Berg, A.; Dowdy, S.F. Protein transduction domain delivery of therapeutic macromolecules. Curr. Opin. Biotechnol. 2011, 22, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, W.; Hay, J.G.; Sauthoff, H. Expressed cell-penetrating peptides can induce a bystander effect, but passage through the secretory pathway reduces protein transduction activity. Mol. Ther. 2011, 19, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Behzadipour, Y.; Haddad, M. Decoding the proteome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for cell-penetrating peptides involved in pathogenesis or applicable as drug delivery vectors. Infect. Genet. Evol. 2020, 85, 104474. [Google Scholar] [CrossRef]
- Krüger, D.M.; Neubacher, S.; Grossmann, T.N. Protein-RNA interactions: Structural characteristics and hotspot amino acids. RNA 2018, 24, 1457–1465. [Google Scholar] [CrossRef]
- Brandes, N.; Linial, M. Giant Viruses—Big Surprises. Viruses 2019, 11, 404. [Google Scholar] [CrossRef]
- Bürglin, T.R.; Affolter, M. Homeodomain proteins: An update. Chromosoma 2016, 125, 497–521. [Google Scholar] [CrossRef] [Green Version]
- Rabouille, C. Pathways of Unconventional Protein Secretion. Trends Cell Biol. 2017, 27, 230–240. [Google Scholar] [CrossRef]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.D.; Lyman, E.; Levental, I. Plasma membranes are asymmetric in lipid unsaturation, packing, and protein shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Amblard, I.; Dupont, E.; Alves, I.; Miralvès, J.; Queguiner, I.; Joliot, A. Bidirectional transfer of homeoprotein EN2 across the plasma membrane requires PIP2. J. Cell Sci. 2020, 133, jcs244327. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.R.V.; Fischer, M.J.; Anderson, K.E.; Holdich, J.; Koteci, A.; Balla, T.; Irvine, R.F. PI4P And PI(4,5)P2 Are Essential But Independent Lipid Determinants Of Membrane Identity. Science 2012, 337, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Joliot, A.; Prochiantz, A. Homeoproteins as natural Penetratin cargoes with signaling properties. Adv. Drug Deliv. Rev. 2008, 60, 608–613. [Google Scholar] [CrossRef]
- Dupont, E.; Prochiantz, A.; Joliot, A. Identification of a signal peptide for unconventional secretion. J. Biol. Chem. 2007, 282, 8994–9000. [Google Scholar] [CrossRef]
- Prochiantz, A.; Di Nardo, A.A. Homeoprotein signaling in the developing and adult nervous system. Neuron 2015, 85, 911–925. [Google Scholar] [CrossRef]
- Carlier, L.; Balayssac, S.; Cantrelle, F.-X.; Khemtémourian, L.; Chassaing, G.; Joliot, A.; Lequin, O. Investigation of Homeodomain Membrane Translocation Properties: Insights from the Structure Determination of Engrailed-2 Homeodomain in Aqueous and Membrane-Mimetic Environments. Biophys. J. 2013, 105, 667–678. [Google Scholar] [CrossRef]
- Cardin, A.D.; Weintraub, H.J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 1989, 9, 21–32. [Google Scholar] [CrossRef]
- Cardon, S.; Bolbach, G.; Hervis, Y.P.; Lopin-Bon, C.; Jacquinet, J.; Illien, F.; Walrant, A.; Ravault, D.; He, B.; Molina, L.; et al. A cationic motif in Engrailed-2 homeoprotein controls its internalization via selective cell-surface glycosaminoglycans interactions. bioRxiv 2021. [Google Scholar] [CrossRef]
- Shang, D.; Li, X.; Sun, Y.; Wang, C.; Sun, L.; Wei, S.; Gou, M. Design of Potent, Non-Toxic Antimicrobial Agents Based upon the Structure of the Frog Skin Peptide, Temporin-1CEb from Chinese Brown Frog, Rana chensinensis. Chem. Biol. Drug Des. 2012, 79, 653–662. [Google Scholar] [CrossRef]
- Tünnemann, G.; Cardoso, M.C. Cell-Penetrating Peptides—Uptake, Toxicity, and Applications. In Membrane-Active Peptides: Methods and Results on Structure and Function; Castanho, M.A.R.B., Ed.; International University Line: La Jolla, CA, USA, 2009; Chapter 14; pp. 330–362. [Google Scholar]
- Juretić, D.; Sonavane, Y.; Ilić, N.; Gajski, G.; Goić-Barišić, I.; Tonkić, M.; Kozic, M.; Maravić, A.; Pellay, F.-X.; Zoranić, L. Designed peptide with a flexible central motif from ranatuerins adapts its conformation to bacterial membranes. Biochim. Biophys. Acta 2018, 1860, 2655–2668. [Google Scholar] [CrossRef]
- Kozić, M.; Vukičević, D.; Simunić, J.; Rončević, T.; Antcheva, N.; Tossi, A.; Juretić, D. Predicting the Minimal Inhibitory Concentration for Antimicrobial Peptides with Rana-box Domain. J. Chem. Inf. Model. 2015, 55, 2275–2287. [Google Scholar] [CrossRef]
- Dupont, E.; Prochiantz, A.; Joliot, A. Penetratin Story: An Overview. In Cell-Penetrating Peptides; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2015; Volume 1324, pp. 29–37. [Google Scholar] [CrossRef]
- Religa, T.L.; Johnson, C.M.; Vu, D.M.; Brewer, S.H.; Dyer, R.B.; Fersht, A.R. The helix–turn– helix motif as an ultrafast independently folding domain: The pathway of folding of Engrailed homeodomain. Proc. Natl. Acad. Sci. USA 2007, 104, 9272–9277. [Google Scholar] [CrossRef] [PubMed]
- Joliot, A.H.; Triller, A.; Volovitch, M.; Pernelle, C.; Prochiantz, A. Alpha-2,8-Polysialic acid is the neuronal surface receptor of antennapedia homeobox peptide. New Biol. 1991, 3, 1121–1134. [Google Scholar] [PubMed]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Balayssac, S.; Burlina, F.; Convert, O.; Bolbach, G.; Chassaing, G.; Lequin, O. Comparison of Penetratin and Other Homeodomain-Derived Cell-Penetrating Peptides: Interaction in a Membrane-Mimicking Environment and Cellular Uptake Efficiency. Biochemistry 2006, 45, 1408–1420. [Google Scholar] [CrossRef]
- Polyansky, A.A.; Volynsky, P.E.; Arseniev, A.S.; Efremov, R.G. Adaptation of a Membrane-active Peptide to Heterogeneous Environment. I. Structural Plasticity of the Peptide. J. Phys. Chem. B 2009, 113, 1107–1119. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- Lamazière, A.; Wolf, C.; Lambert, O.; Chassaing, G.; Trugnan, G.; Ayala-Sanmartin, J. The Homeodomain Derived Peptide Penetratin Induces Curvature of Fluid Membrane Domains. PLoS ONE 2008, 3, e1938. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, F.; Pütz, S.; Schwendy, M.; Bonn, M.; Parekh, S.H. Measuring Intracellular Secondary Structure of a Cell-Penetrating Peptide in Situ. Anal. Chem. 2017, 89, 11310–11317. [Google Scholar] [CrossRef] [PubMed]
- Eiríksdóttir, E.; Konate, K.; Langel, Ü.; Divita, G.; Deshayes, S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochim. Biophys. Acta 2010, 1798, 1119–1128. [Google Scholar] [CrossRef]
- Derossi, D.; Calvet, S.; Trembleau, A.; Brunissen, A.; Chassaing, G.; Prochiantz, A. Cell Internalization of the Third Helix of the Antennapedia Homeodomain Is Receptor-independent. J. Biol. Chem. 1996, 271, 18188–18193. [Google Scholar] [CrossRef] [PubMed]
- Duchardt, F.; Fotin-Mleczek, M.; Schwarz, H.; Fischer, R.; Brock, R. A Comprehensive Model for the Cellular Uptake of Cationic Cell-penetrating Peptides. Traffic 2007, 8, 848–866. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Almeida, C.; Lamazière, A.; Filleau, A.; Corvis, Y.; Espeau, P.; Ayala-Sanmartin, J. Membrane re-arrangements and rippled phase stabilisation by the cell penetrating peptide penetratin. Biochim. Biophy. Acta 2016, 1858, 2584–2591. [Google Scholar] [CrossRef]
- Dupont, E.; Prochiantz, A.; Joliot, A. Penetratins; CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zorko, M.; Langel, Ü. Cell-Penetrating Peptides. Methods Mol. Biol. 2022, 2383, 3–32. [Google Scholar] [CrossRef]
- Škrlj, N.; Drevenšek, G.; Hudoklin, S.; Romih, R.; Čurin Šerbec, V.; Dolinar, M. Recombinant Single-Chain Antibody with the Trojan Peptide Penetratin Positioned in the Linker Region Enables Cargo Transfer Across the Blood–Brain Barrier. Appl. Biochem. Biotechnol. 2013, 169, 159–169. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, D.; Singh, J. GLUT-1, An Effective Target To Deliver Brain-Derived Neurotrophic Factor Gene across the Blood Brain Barrier. ACS Chem. Neurosci. 2020, 11, 1620–1633. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, K.; Tai, L.; Liu, Y.; Wei, G.; Lu, W.; Pan, W. Facile Noninvasive Retinal Gene Delivery Enabled by Penetratin. ACS Appl. Mater Interfaces 2016, 8, 19256–19267. [Google Scholar] [CrossRef]
- Vale, N.; Ferreira, A.; Fernandes, I.; Alves, C.; Araújo, M.J.; Mateus, N.; Gomes, P. Gemcitabine anti-proliferative activity significantly enhanced upon conjugation with cell-penetrating peptides. Bioorg Med. Chem. Lett. 2017, 27, 2898–2901. [Google Scholar] [CrossRef]
- Kanovsky, M.; Raffo, A.; Drew, L.; Rosal, R.; Do, T.; Friedman, F.K.; Rubinsteini, P.; Visseri, J.; Robinson, R.; Brandt-Rauf, P.W.; et al. Peptides from the amino terminal mdm-2-binding domain of p53, designed from conformational analysis, are selectively cytotoxic to transformed cells. Proc. Natl. Acad. Sci. USA 2001, 98, 12438–12443. [Google Scholar] [CrossRef]
- Derossi, D.; Chassaing, G.; Prochiantz, A. Trojan peptides: The penetratin system for intracellular delivery. Trends Cell Biol. 1998, 8, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Pincus, M.R.; Brandt-Rauf, P.W.; Fine, R.L.; Michl, J.; Wang, H. NMR Solution Structure of a Peptide from the mdm-2 Binding Domain of the p53 Protein that Is Selectively Cytotoxic to Cancer Cells. Biochemistry 2004, 43, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Selivanova, G.; Iotsova, V.; Okan, I.; Fritsche, M.; Ström, M.; Groner, B.; Grafström, R.C.; Wiman, K.G. Restoration of the growth suppression function of mutant p53 by a synthetic peptide derived from the p53 C-terminal domain. Nat. Med. 1997, 3, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Subekti, D.R.G.; Kamagata, K. The disordered DNA-binding domain of p53 is indispensable for forming an encounter complex to and jumping along DNA. Biochem. Biophys. Res. Commun. 2021, 534, 21–26. [Google Scholar] [CrossRef]
- Bidwell, G.L., 3rd; Raucher, D. Therapeutic peptides for cancer therapy. Part I peptide inhibitors of signal transduction cascades. Expert Opin. Drug Deliv. 2009, 6, 1033–1047. [Google Scholar] [CrossRef]
- Martínez, D.E. Mortality patterns suggest lack of senescence in hydra. Exp. Gerontol. 1998, 33, 217–225. [Google Scholar] [CrossRef]
- Vogg, M.C.; Buzgariu, W.; Suknovic, N.S.; Galliot, B. Cellular, Metabolic, and Developmental Dimensions of Whole-Body Regeneration in Hydra. Cold Spring Harb. Perspect. Biol. 2021, 13, a040725. [Google Scholar] [CrossRef]
- Badosa, E.; Ferré, R.; Francés, J.; Bardají, E.; Feliu, L.; Planas, M.; Montesinos, E. Sporicidal activity of synthetic antifungal undecapeptides and control of Penicillium rot of apples. Appl. Environ. Microbiol. 2009, 75, 5563–5569. [Google Scholar] [CrossRef]
- Horibe, T.; Kohno, M.; Haramoto, M.; Ohara, K.; Kawakami, K. Designed hybrid TPR peptide targeting Hsp90 as a novel anticancer agent. J. Transl. Med. 2011, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Agemy, L.; Kotamraju, V.R.; Braun, G.; Teesalu, T.; Sugahara, K.N.; Hamzah, J.; Ruoslahti, E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene 2012, 31, 3754–3763. [Google Scholar] [CrossRef]
- Woldetsadik, A.D.; Vogel, M.C.; Rabeh, W.M.; Magzoub, M. Hexokinase II–derived cell-penetrating peptide targets mitochondria and triggers apoptosis in cancer cells. FASEB J. 2017, 31, 2168–2184. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Ubach, J.; Boman, A.; Wåhlin, B.; Wade, D.; Merrifield, R.B.; Boman, H.G. Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett. 1992, 296, 190–194. [Google Scholar] [CrossRef]
- Dong, W.; Dong, Z.; Mao, X.; Sun, Y.; Li, F.; Shang, D. Structure-activity analysis and biological studies of chensinin-1b analogues. Acta Biomater. 2016, 37, 59–68. [Google Scholar] [CrossRef]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef]
- Jing, W.; Demcoe, A.R.; Vogel, H.J. Conformation of a bactericidal domain of puroindoline A: Structure and mechanism of action of a 13-residue antimicrobial peptide. J. Bacteriol. 2003, 185, 4938–4947. [Google Scholar] [CrossRef]
- Sawai, M.V.; Waring, A.J.; Kearney, W.R.; McCray, P.B., Jr.; Forsyth, W.R.; Lehrer, R.I.; Tack, B.F. Impact of single-residue mutations on the structure and function of ovispirin/novispirin antimicrobial peptides. Protein Eng. 2002, 15, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rončević, T.; Gajski, G.; Ilić, N.; Goić-Barišić, I.; Tonkić, M.; Zoranić, L.; Simunić, J.; Benincasa, M.; Mijaković, M.; Tossi, A.; et al. PGLa-H tandem-repeat peptides active against multidrug resistant clinical bacterial isolates. Biochim. Biophys. Acta Biomembr. 2017, 1859, 228–237. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, B.R.; Kim, B.K.; Kim, D.W.; Shon, W.J.; Lee, N.R.; Inn, K.S.; Kim, B.J. Heat shock protein-mediated cell penetration and cytosolic delivery of macromolecules by a telomerase-derived peptide vaccine. Biomaterials 2013, 34, 7495–7505. [Google Scholar] [CrossRef] [PubMed]
- Mink, C.; Strandberg, E.; Wadhwani, P.; Melo, M.N.; Reichert, J.; Wacker, I.; Castanho, M.A.R.B.; Ulrich, A.S. Overlapping Properties of the Short Membrane-Active Peptide BP100 With (i) Polycationic TAT and (ii) α-helical Magainin Family Peptides. Front. Cell. Infect. Microbiol. 2021, 11, 609542. [Google Scholar] [CrossRef] [PubMed]
- Torcato, I.M.; Huang, Y.H.; Franquelim, H.G.; Gaspar, D.; Craik, D.J.; Castanho, M.A.R.B.; Henriques, S.T. Design and characterization of novel antimicrobial peptides, R-BP100 and RW-BP100, with activity against Gram-negative and Gram-positive bacteria. Biochim. Biophys. Acta 2013, 1828, 944–955. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, K.; Lim, D.; Jeong, K.; Hong, Y.; Nguyen, V.H.; Kim, T.H.; Ryu, S.; Lim, J.A.; Kim, J.I.; et al. Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PLoS ONE 2014, 9, e80050. [Google Scholar] [CrossRef] [PubMed]
- Howl, J.; Matou-Nasri, S.; West, D.C.; Farquhar, M.; Slaninová, J.; Ostenson, C.-G.; Zorko, M.; Ostlund, P.; Kumar, S.; Langel, U.; et al. Bioportide: An emergent concept of bioactive cell-penetrating peptides. Cell Mol. Life Sci. 2012, 69, 2951–2966. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Yi, K.-S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: The proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef]
- Lim, K.J.; Sung, B.H.; Shin, J.R.; Lee, Y.W.; Kim, D.J.; Yang, S.Y.; Kim, S.C. A Cancer Specific Cell-Penetrating Peptide, BR2, for the Efficient Delivery of an scFv into Cancer Cells. PLoS ONE 2013, 8, e66084. [Google Scholar] [CrossRef]
- Zeng, P.; Cheng, Q.; Xu, J.; Xu, Q.; Xu, Y.; Gao, W.; Wong, K.Y.; Chan, K.F.; Chen, S.; Yi, L. Membrane-disruptive engineered peptide amphiphiles restrain the proliferation of penicillins and cephalosporins resistant Vibrio alginolyticus and Vibrio parahaemolyticus in instant jellyfish. Food Control 2022, 135, 108827. [Google Scholar] [CrossRef]
- Shang, D.; Yu, F.; Li, J.; Zheng, J.; Zhang, L.; Li, Y. Molecular Cloning of cDNAs Encoding Antimicrobial Peptide Precursors from the Skin of the Chinese Brown Frog, Rana chensinensis. Zoolog. Sci. 2009, 26, 220–226. [Google Scholar] [CrossRef]
- Sinthuvanich, C.; Veiga, A.S.; Gupta, K.; Diana Gaspar, D.; Blumenthal, R.; Schneider, J.P. Anticancer β-hairpin peptides: Membrane-induced folding triggers activity. J. Am. Chem. Soc. 2012, 134, 6210–6217. [Google Scholar] [CrossRef]
- Hao, X.; Yan, Q.; Zhao, J.; Wang, W.; Huang, Y.; Chen, Y. TAT Modification of Alpha-Helical Anticancer Peptides to Improve Specificity and Efficacy. PLoS ONE 2015, 10, e0138911. [Google Scholar] [CrossRef]
- Hakata, Y.; Tsuchiya, S.; Michiue, H.; Ohtsuki, T.; Matsui, H.; Miyazawa, M.; Kitamatsu, M. A novel leucine zipper motif-based hybrid peptide delivers a functional peptide cargo inside cells. Chem. Commun. 2015, 51, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Oehlke, J.; Scheller, A.; Wiesner, B.; Krause, E.; Beyermann, M.; Klauschenz, E.; Melzig, M.; Bienert, M. Cellular uptake of an alpha-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim. Biophys. Acta 1998, 1414, 127–139. [Google Scholar] [CrossRef]
- Gautam, A.; Chaudhary, K.; Kumar, R.; Raghava, G.P. Computer-Aided Virtual Screening and Designing of Cell-Penetrating Peptides. Methods Mol. Biol. 2015, 1324, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, C.; Zhang, L.; Xu, X. Design of novel cell penetrating peptides for the delivery of trehalose into mammalian cells. Biochim. Biophys. Acta 2014, 1838, 1911–1920. [Google Scholar] [CrossRef]
- Radvanyi, L.G. Targeting the cancer mutanome of breast cancer. Nat. Med. 2018, 24, 703–704. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Kapoor, P.; Chaudhary, K.; Kumar, R.; Open Source Drug Discovery Consortium; Raghava, G.P.S. Tumor homing peptides as molecular probes for cancer therapeutics, diagnostics and theranostics. Curr. Med. Chem. 2014, 21, 2367–2391. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Wan, L.; Cheng, J.; Lu, X. Penetratin-mediated delivery enhances the antitumor activity of the cationic antimicrobial peptide Magainin II. Cancer Biother. Radiopharm. 2013, 28, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.C.; Mi, Z.; Kim, S.H.; Ng, B.; Robbins, P.D. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001, 61, 7709–7712. [Google Scholar] [PubMed]
- Risso, A.; Braidot, E.; Sordano, M.C.; Vianello, A.; Macrì, F.; Skerlavaj, B.; Zanetti, M.; Gennaro, R.; Bernardi, P. BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol. Cell Biol. 2002, 22, 1926–1935. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lin, L.C.; Tzen, J.T.C.; Chen, J.Y. Characteristics of the antitumor activities in tumor cells and modulation of the inflammatory response in RAW264.7 cells of a novel antimicrobial peptide, chrysophsin-1, from the red sea bream (Chrysophrys major). Peptides 2011, 32, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, D.R.; Gudz, T.I.; Novgorodov, S.A.; Erdahl, W.L. The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J. Biol. Chem. 1995, 270, 4923–4932. [Google Scholar] [CrossRef]
- Cole, A.M.; Weis, P.; Diamond, G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997, 272, 12008–12013. [Google Scholar] [CrossRef]
- Seo, Y.W.; Woo, H.N.; Piya, S.; Moon, A.R.; Oh, J.W.; Yun, C.W.; Kim, K.K.; Min, J.Y.; Jeong, S.Y.; Chung, S.; et al. The Cell Death–Inducing Activity of the Peptide Containing Noxa Mitochondrial-Targeting Domain Is Associated with Calcium Release. Cancer Res. 2009, 69, 8356–8365. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Z.; Peng, H.; Lv, Y.; Feng, Y.; Kang, J.; Lu, N.; Ma, R.; Hou, S.; Sun, W.; et al. Bomidin: An Optimized Antimicrobial Peptide With Broad Antiviral Activity Against Enveloped Viruses. Front. Immunol. 2022, 13, 851642. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Martínez, G.G.R.; Blas, D.M.; Castillo, G.D.A.; Corzo, G.; Castro-Obregon, S.; Del Rio, G.D. Effective Design of Multifunctional Peptides by Combining Compatible Functions. PLoS Comput. Biol. 2016, 12, e1004786. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- Azuma, E.; Choda, N.; Odaki, M.; Yano, Y.; Matsuzaki, K. Improvement of Therapeutic Index by the Combination of Enhanced Peptide Cationicity and Proline Introduction. ACS Infect. Dis. 2020, 6, 2271–2278. [Google Scholar] [CrossRef]
- Dathe, M.; Wieprecht, T.; Nikolenko, H.; Handel, L.; Maloy, W.L.; MacDonald, D.L.; Beyermann, M.; Bienert, M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997, 403, 208–212. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugishita, K.; Harada, M.; Fujii, N.; Miyajima, K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1997, 1327, 119–130. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, S.H.; Yand, S.T.; Park, E.J.; Lee, D.G.; Lee, M.K.; Eom, S.H.; Song, W.K.; Kim, Y.; Hahm, K.-S.; et al. Antibacterial, antitumor and hemolytic activities of α-helical antibiotic peptide, P18 and its analogs. J. Pept. Res. 2001, 58, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, A.; Papo, N.; Shai, Y. In vitro activity and potency of an intravenously injected antimicrobial peptide and its DL amino acid analog in mice infected with bacteria. Antimicrob. Agents Chemother. 2004, 48, 3127–3129. [Google Scholar] [CrossRef]
- Otvos, L., Jr.; Bokonyi, K.; Varga, I.; Otvos, B.I.; Hoffmann, H.; Ertl, H.C.; Wade, J.D.; McManus, A.M.; Craik, D.J.; Bulet, B. Insect peptides with improved protease-resistance protect mice against bacterial infection. Protein Sci. 2000, 9, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, M.; Jiao, P.; Zhang, X.; Yang, G.; Xu, X. Intracellular Paclitaxel Delivery Facilitated by a Dual-Functional CPP with a Hydrophobic Hairpin Tail. ACS Appl. Mater. Interfaces 2021, 13, 4853–4860. [Google Scholar] [CrossRef]
- Hilpert, K.; Volkmer-Engert, R.; Walter, T.; Hancock, R.E. High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 2005, 23, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, C.P.; Künnapuu, K.; Langel, Ü. Cell-penetrating peptides with intracellular organelle targeting. Expert Opin. Drug Deliv. 2017, 14, 245–255. [Google Scholar] [CrossRef]
- Hu, G.; Katuwawala, A.; Wang, K.; Wu, Z.; Ghadermarzi, S.; Gao, J.; Kurgan, L. flDPnn: Accurate intrinsic disorder prediction with putative propensities of disorder functions. Nat. Commun. 2021, 12, 4438. [Google Scholar] [CrossRef]
- Tucker, A.N.; Carlson, T.J.; Sarkar, A. Challenges in Drug Discovery for Intracellular Bacteria. Pathogens 2021, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Rüter, R. Delivery of Antibiotics by Cell-Penetrating Peptides to Kill Intracellular Pathogens. Methods Mol. Biol. 2022, 2383, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Nielsen, E.J.B.; Khafagy, E.-S.; Takeda-Morishita, M. Noninvasive insulin delivery: The great potential of cell-penetrating peptides. Ther. Deliv. 2013, 4, 315–326. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Y.; Yang, K.; Wang, Z. Heterogeneous Strategies to Eliminate Intracellular Bacterial Pathogens. Front. Microbiol. 2020, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.E.G.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Főldes, A.; Székely, E.; Voidăzan, S.T.; Dobreanu, M. Comparison of Six Phenotypic Assays with Reference Methods for Assessing Colistin Resistance in Clinical Isolates of Carbapenemase-Producing Enterobacterales: Challenges and Opportunities. Antibiotics 2022, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Upert, G.; Luther, A.; Obrecht, D.; Ermert, P. Emerging peptide antibiotics with therapeutic potential. Med. Drug Discov. 2021, 9, 100078. [Google Scholar] [CrossRef]
- Röhrig, C.; Huemer, M.; Lorgé, D.; Luterbacher, S.; Phothaworn, P.; Schefer, C.; Sobieraj, A.M.; Zinsli, L.V.; Mairpady Shambat, S.; Leimer, N.; et al. Targeting hidden pathogens: Cell-penetrating enzybiotics eradicate intracellular drug-resistant Staphylococcus aureus. mBio 2020, 11, e00209-20. [Google Scholar] [CrossRef]
- Hu, J.; Chen, C.; Zhang, S.; Zhao, X.; Xu, H.; Zhao, X.; Lu, J.R. Designed antimicrobial and antitumor peptides with high selectivity. Biomacromolecules 2011, 12, 3839–3843. [Google Scholar] [CrossRef]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012, 19, 3794–3804. [Google Scholar] [CrossRef]
- Harris, F.; Dennison, S.R.; Singh, J.; Phoenix, D.A. On the selectivity and efficacy of defense peptides with respect to cancer cells. Med. Res. Rev. 2013, 33, 190–234. [Google Scholar] [CrossRef]
- Freire, J.M.; Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. Shifting gear in antimicrobial and anticancer peptides biophysical studies: From vesicles to cells. J. Pept. Sci. 2015, 21, 178–185. [Google Scholar] [CrossRef]
- Melvin, J.A.; Montelaro, R.C.; Bomberger, J.M. Clinical potential of engineered cationic antimicrobial peptides against drug resistant biofilms. Expert Rev. Anti Infect. Ther. 2016, 14, 989–991. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Sharon, A.J.; Haney, E.F.; Hoskin, D.W.; Bally, M.B.; Franco, O.L.; Corcoran, J.A.; Hancock, R.E.W. Mastoparan is a membranolytic anti-cancer peptide that works synergistically with gemcitabine in a mouse model of mammary carcinoma. Biochim. Biophys. Acta 2016, 1858, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013, 31, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.; Li, Y.; Liew, Y.J.; Baumgarten, S.; Simakov, O.; Wilson, M.C.; Piel, J.; Ashoor, H.; Bougouffa, S.; Bajic, V.B.; et al. Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 2016, 6, 39734. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Jessop, T.C.; Pattabiraman, K.; Pelkey, E.T.; VanDeusen, C.L. An efficient, scalable synthesis of the molecular transporter octaarginine via a segment doubling strategy. Org. Lett. 2001, 3, 3229–3232. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef]
- Song, J.; Tan, H.; Perry, A.J.; Akutsu, T.; Webb, G.I.; Whisstock, J.C.; Pike, R.N. PROSPER: An integrated feature-based tool for predicting protease substrate cleavage sites. PLoS ONE 2012, 7, e50300. [Google Scholar] [CrossRef]
- Futaki, S.; Nakase, I. Cell-Surface Interactions on Arginine-Rich Cell-Penetrating Peptides Allow for Multiplex Modes of Internalization. Acc. Chem. Res. 2017, 50, 2449–2456. [Google Scholar] [CrossRef]
- Benz, C.; Urbaniak, M.D. Organising the cell cycle in the absence of transcriptional control: Dynamic phosphorylation co-ordinates the Trypanosoma brucei cell cycle posttranscriptionally. PLoS Pathog. 2019, 15, e1008129. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, J.K.; Bhat, R. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 2003, 278, 26458–26465. [Google Scholar] [CrossRef]
- Comizzoli, P.; Loi, P.; Patrizio, P.; Hubel, A. Long-term storage of gametes and gonadal tissues at room temperatures: The end of the ice age? J. Assist. Reprod. Genet. 2022, 39, 321–325. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Wang, X.; Becker, D. The DEAD/DEAH box helicase, DDX11, is essential for the survival of advanced melanomas. Mol. Cancer 2012, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Liu, X.; Xu, P.; Hu, Q.; Yu, Y. The Role of Upregulated DDX11 as A Potential Prognostic and Diagnostic Biomarker in Lung Adenocarcinoma. J. Cancer 2019, 10, 4208–4216. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, M.E.; Jang, W.S.; Rha, K.H.; Lee, S.H.; Lee, J.; Ham, W.S. The DEAD/DEAH Box Helicase, DDX11, Is Essential for the Survival of Advanced Clear Cell Renal Cell Carcinoma and Is a Determinant of PARP Inhibitor Sensitivity. Cancers 2021, 13, 2574. [Google Scholar] [CrossRef]
- Brosh, R.M., Jr.; Matson, S.W. History of DNA Helicases. Genes 2020, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Sugihara, K.; Shibata, T.K.; Nakayama, J.; Akama, T.O.; Tamura, N.; Wong, S.-M.; Bobkov, A.A.; Takano, Y.; Ohyama, C.; et al. Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide. Proc. Natl. Acad. Sci. USA 2011, 108, 19587–19592. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, L.; Fu, Y.; Xu, X. Rapid delivery of paclitaxel with an organic solvent-free system based on a novel cell penetrating peptide for suppression of tumor growth. J. Mater. Chem. B 2017, 5, 7768–7774. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, L.; Zhang, L.; Xu, X. Noncovalent interaction-assisted drug delivery system with highly efficient uptake and release of paclitaxel for anticancer therapy. Int. J. Nanomed. 2017, 12, 7039–7051. [Google Scholar] [CrossRef]
- Bobone, S.; Bocchinfuso, G.; Park, Y.; Palleschi, A.; Hahm, K.S.; Stella, L. The importance of being kinked: Role of Pro residues in the selectivity of the helical antimicrobial peptide P5. J. Pept. Sci. 2013, 19, 758–769. [Google Scholar] [CrossRef]
- Takayama, K.; Nakase, I.; Michiue, H.; Takeuchi, T.; Tomizawa, K.; Matsui, H.; Futaki, S. Enhanced intracellular delivery using arginine-rich peptides by the addition of penetration accelerating sequences (Pas). J. Control. Release 2009, 138, 128–133. [Google Scholar] [CrossRef]
- Armstrong, J.S. Mitochondria: A target for cancer therapy. Br. J. Pharmacol. 2006, 147, 239–248. [Google Scholar] [CrossRef]
- Chiara, F.; Castellaro, D.; Marin, O.; Petronilli, V.; Brusilow, W.S.; Juhaszova, M.; Sollott, S.J.; Forte, M.; Bernardi, P.; Rasola, A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE 2008, 3, e1852. [Google Scholar] [CrossRef]
- Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G. The mitochondrial permeability transition pore: Channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol. Rev. 2015, 95, 1111–1155. [Google Scholar] [CrossRef] [PubMed]
- Juretić, D. Bioenergetics: A Bridge Across Life and Universe; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Nederlof, R.; Gürel-Gurevin, E.; Eerbeek, O.; Xie, C.; Deijs, G.S.; Konkel, M.; Hu, J.; Weber, N.C.; Schumacher, C.A.; Baartscheer, A.; et al. Reducing mitochondrial bound hexokinase II mediates transition from non-injurious into injurious ischemia/reperfusion of the intact heart. J. Physiol. Biochem. 2017, 73, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.M.; McMaster, W.R.; Kamysz, E.; Kamysz, W.; Engman, D.M.; McGwire, B.S. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 2006, 62, 1484–1497. [Google Scholar] [CrossRef]
- Seo, Y.W.; Shin, J.N.; Ko, K.H.; Cha, J.H.; Park, J.Y.; Lee, B.R.; Yun, C.W.; Kim, Y.M.; Seol, D.W.; Kim, D.W.; et al. The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53-mediated cell death. J. Biol. Chem. 2003, 278, 48292–48299. [Google Scholar] [CrossRef] [PubMed]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, R.A.; Figueiredo, C.R.; Ferreira, A.K.; Matsuo, A.L.; Massaoka, M.H.; Girola, N.; Auada, A.V.V.; Farias, C.F.; Pasqualoto, K.F.M.; Rodrigues, C.P.; et al. Mastoparan induces apoptosis in B16F10-Nex2 melanoma cells via the intrinsic mitochondrial pathway and displays antitumor activity in vivo. Peptides 2015, 68, 113–119. [Google Scholar] [CrossRef]
- Mi, Z.; Mai, J.; Lu, X.; Robbins, P.D. Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitro and in vivo. Mol. Ther. 2000, 2, 339–347. [Google Scholar] [CrossRef]
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Wan, L.; Cai, H.W.; Li, S.L.; Li, Y.P.; Cheng, J.Q.; Lu, X.F. Enhancement of cytotoxicity of antimicrobial peptide magainin II in tumor cells by bombesin-targeted delivery. Acta Pharmacol. Sin. 2011, 32, 79–88. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Gazdar, A.F.; Chen, H.C.; Johnson, B.E. Antitumor activity of magainin analogues against human lung cancer cell lines. Cancer Res. 1992, 52, 3534–3538. [Google Scholar] [PubMed]
- Baker, M.A.; Maloy, W.L.; Zasloff, M.; Jacob, L.S. Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res. 1993, 53, 3052–3057. [Google Scholar] [PubMed]
- Cruz-Chamorro, L.; Puertollano, M.A.; Puertollano, E.; de Cienfuegos, G.A.; de Pablo, M.A. In vitro biological activities of magainin alone or in combination with nisin. Peptides 2006, 27, 1201–1209. [Google Scholar] [CrossRef]
- Ramos, R.; Moreira, S.; Rodrigues, A.; Gama, M.; Domingues, L. Recombinant expression and purification of the antimicrobial peptide magainin-2. Biotechnol. Prog. 2013, 29, 17–22. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, Y.; Jin, I.; Hahm, K.S.; Lee, H.H.; Moon, Y.H.; Woo, E.R. Structure-antiviral activity relationships of cecropin A-magainin 2 hybrid peptide and its analogues. J. Pept. Sci. 2004, 10, 298–303. [Google Scholar] [CrossRef]
- Jacob, L.; Zasloff, M. Potential therapeutic applications of magainins and other antimicrobial agents of animal origin. Ciba Found. Symp. 1994, 186, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Huertas-Ortiz, K.A.; Leal-Castro, A.L.; Vargas-Casanova, Y.; Parra-Giraldo, C.M.; García-Castañeda, J.E.; Rivera-Monroy, Z.J. Designing Chimeric Peptides: A Powerful Tool for Enhancing Antibacterial Activity. Chem. Biodivers. 2021, 18, e2000885. [Google Scholar] [CrossRef]
- Théolier, J.; Fliss, I.; Jean, J.; Hammami, R. MilkAMP: A comprehensive database of antimicrobial peptides of dairy origin. Dairy Sci. Technol. 2014, 94, 181–193. [Google Scholar] [CrossRef]
- Kobayashi, S.; Chikushi, A.; Tougu, S.; Imura, Y.; Nishida, M.; Yano, Y.; Matsuzaki, K. Membrane Translocation Mechanism of the Antimicrobial Peptide Buforin 2. Biochemistry 2004, 43, 15610–15616. [Google Scholar] [CrossRef]
- Cho, J.H.; Sung, B.H.; Kim, S.C. Buforins: Histone H2A-derived antimicrobial peptides from toad stomach. Biochim. Biophys. Acta 2009, 1788, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.A.; Kim, H.; Lee, J.Y.; Shin, J.R.; Kim, D.J.; Cho, J.H.; Kim, S.C. Mechanism of action and specificity of antimicrobial peptides designed based on buforin IIb. Peptides 2012, 34, 283–289. [Google Scholar] [CrossRef]
- Schibli, D.J.; Hwang, P.M.; Vogel, H.J. The structure of the antimicrobial active center of lactoferricin B bound to sodium dodecyl sulfate micelles. FEBS Lett. 1999, 446, 213–217. [Google Scholar] [CrossRef]
- Strøm, M.B.; Rekdal, Ø.; Svendsen, J.S. The effects of charge and lipophilicity on the antibacterial activity of undecapeptides derived from bovine lactoferricin. J. Peptide Sci. 2002, 7, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.B.; Haug, B.E.; Rekdal, Ø.; Skar, M.L.; Stensen, W.; Svendsen, J.S. Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity. Biochem. Cell Biol. 2002, 80, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef]
- Wender, P.A.; Galliher, W.C.; Goun, E.A.; Jones, L.R.; Pillow, T.H. The design of guanidinium-rich transporters and their internalization mechanisms. Adv. Drug Deliv. Rev. 2008, 60, 452–472. [Google Scholar] [CrossRef] [Green Version]
- Fischer, P.M.; Zhelev, N.Z.; Wang, S.; Melville, J.E.; Fåhraeus, R.; Lane, D.P. Structure-activity relationship of truncated and substituted analogues of the intracellular delivery vector Penetratin. J. Peptide Res. 2000, 55, 163–172. [Google Scholar] [CrossRef]
- Fischer, P.M.; Krausz, E.; Lane, D.P. Cellular delivery of impermeable effector molecules in the form of conjugates with peptides capable of mediating membrane translocation. Bioconjug. Chem. 2001, 12, 825–841. [Google Scholar] [CrossRef]
- Ohno, S. Of palindromes and peptides. Hum. Genet. 1992, 90, 342–345. [Google Scholar] [CrossRef]
- O’Neil, K.T.; Hoess, R.H.; DeGrado, W. F Design of DNA-binding peptides based on the leucine zipper motif. Science 1990, 249, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Keller, W.; König, P.; Richmond, T.J. Crystal Structure of a bZIP/DNA Complex at 2.2 Å: Determinants of DNA Specific Recognition. J. Mol. Biol. 1995, 254, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Suckow, M.; Lopata, M.; Seydel, A.; Kisters-Woike, B.; von Wilcken-Bergmann, B.; Muller-Hill, B. Mutant bZip-DNA complexes with four quasi-identical protein-DNA interfaces. EMBO J. 1996, 15, 598–606. [Google Scholar] [CrossRef]
- Ohlig, S.; Pickhinke, U.; Sirko, S.; Bandari, S.; Hoffmann, D.; Dreier, R.; Farshi, P.; Götz, M.; Grobe, K. An emerging role of Sonic hedgehog shedding as a modulator of heparan sulfate interactions. J. Biol. Chem. 2012, 287, 43708–43719. [Google Scholar] [CrossRef]
- Wallbrecher, R.; Verdurmen, W.P.R.; Schmidt, S.; Bovee-Geurts, P.H.; Broecker, F.; Reinhardt, A.; van Kuppevelt, T.H.; Seeberger, P.H.; Brock, R. The stoichiometry of peptide-heparan sulfate binding as a determinant of uptake efficiency of cell-penetrating peptides. Cell. Mol. Life Sci. 2014, 71, 2717–2729. [Google Scholar] [CrossRef]
- Åmand, H.L.; Rydberg, H.A.; Fornander, L.H.; Lincoln, P.; Nordén, B.; Esbjörner, E.K. Cell surface binding and uptake of arginine- and lysine-rich penetratin peptides in absence and presence of proteoglycans. Biochim. Biophys. Acta 2012, 1818, 2669–2678. [Google Scholar] [CrossRef]
- Ramsey, J.D.; Flynn, N.H. Cell-penetrating peptides transport therapeutics into cells. Pharmacol. Ther. 2015, 154, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Alves, I.D.; Bechara, C.; Walrant, A.; Zaltsman, Y.; Jiao, C.-Y.; Sagan, S. Relationships between Membrane Binding, Affinity and Cell Internalization Efficacy of a Cell-Penetrating Peptide: Penetratin as a Case Study. PLoS ONE 2011, 6, e24096. [Google Scholar] [CrossRef]
- Natarajan, K.; Meyer, M.R.; Jackson, B.M.; Slade, D.; Roberts, C.; Hinnebusch, A.G.; Marton, M.J. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell Biol. 2001, 21, 4347–4368. [Google Scholar] [CrossRef] [PubMed]
- Svensson, S.L.; Pasupuleti, M.; Walse, B.; Malmsten, M.; Mörgelin, M.; Sjögren, C.; Olin, A.I.; Collin, M.; Schmidtchen, A.; Palmer, R.; et al. Midkine and pleiotrophin have bactericidal properties: Preserved antibacterial activity in a family of heparin-binding growth factors during evolution. J. Biol. Chem. 2010, 285, 16105–16115. [Google Scholar] [CrossRef]
- Farshi, P.; Ohlig, S.; Pickhinke, U.; Höing, S.; Jochmann, K.; Lawrence, R.; Dreier, R.; Dierker, T.; Grobe, K. Dual Roles of the Cardin-Weintraub Motif in Multimeric Sonic Hedgehog. J. Biol. Chem. 2011, 286, 23608–23619. [Google Scholar] [CrossRef] [PubMed]
- Farsinejad, S.; Gheisary, Z.; Samani, S.E.; Alizadeh, A.M. Mitochondrial targeted peptides for cancer therapy. Tumor Biol. 2015, 36, 5715–5725. [Google Scholar] [CrossRef] [PubMed]
- Habault, J.; Poyet, J.-L. Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef]
- Eckert, R.; He, J.; Yarbrough, D.K.; Qi, F.; Anderson, M.H.; Shi, W. Targeted killing of Streptococcus mutans by a pheromone-guided “smart” antimicrobial peptide. Antimicrob. Agents Chemother. 2006, 50, 3651–3657. [Google Scholar] [CrossRef]
- Guo, L.; McLean, J.S.; Yang, Y.; Eckert, R.; Kaplan, C.W.; Kyme, P.; Sheikh, O.; Varnum, B.; Lux, R.; Shi, W.; et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 2015, 112, 7569–7574. [Google Scholar] [CrossRef] [PubMed]
- Steinstraesser, L.; Tack, B.F.; Waring, A.J.; Hong, T.; Boo, L.M.; Fan, M.F.; Remick, D.I.; Su, G.L.; Lehrer, R.I.; Wang, S.C. Activity of novispirin G10 against Pseudomonas aeruginosa in vitro and in infected burns. Antimicrob. Agents Chemother. 2002, 46, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Shanker, E.; Morrison, D.A.; Talagas, A.; Nessler, S.; Federle, M.J.; Prehna, G. Pheromone Recognition and Selectivity by ComR Proteins among Streptococcus Species. PloS Pathog. 2016, 12, e1005979. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.C.; Federle, M.J. PptAB Exports Rgg Quorum-Sensing Peptides in Streptococcus. PLoS ONE 2016, 11, e0168461. [Google Scholar] [CrossRef]
- Hidalgo-Grass, C.; Dan-Goor, M.; Maly, A.; Eran, Y.; Kwinn, L.A.; Nizet, V.; Ravins, M.; Jaffe, J.; Peyser, A.; Moses, A.E.; et al. Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet 2004, 363, 696–703. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific α-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Papo, N.; Braunstein, A.; Eshhar, Z.; Shai, Y. Suppression of Human Prostate Tumor Growth in Mice by a Cytolytic D-, L-Amino Acid Peptide: Membrane Lysis, Increased Necrosis, and Inhibition of Prostate Specific Antigen Secretion. Cancer Res. 2004, 64, 5779–5786. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Vasil, A.I.; Vasil, M.L.; Hodges, R.S. “Specificity determinants” improve therapeutic indices of two antimicrobial peptides piscidin 1 and dermaseptin S4 against the Gram-negative pathogens Acinetobacter baumannii and Pseudomonas aeruginosa. Pharmaceuticals 2014, 7, 366–391. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Stolovicki, E.; Hur, D.B.; Shai, Y. Site-Specific Isopeptide Bond Formation: A Powerful Tool for the Generation of Potent and Nontoxic Antimicrobial Peptides. J. Med. Chem. 2022, 65, 5085–5094. [Google Scholar] [CrossRef]
- Kragol, G.; Hoffmann, R.; Chattergoon, M.A.; Lovas, S.; Cudic, M.; Bulet, P.; Condie, B.A.; Rosengren, K.J.; Montaner, L.J.; Otvos, L., Jr. Identification of crucial residues for the antibacterial activity of the proline-rich peptide, pyrrhocoricin. Eur. J. Biochem. 2002, 269, 4226–4437. [Google Scholar] [CrossRef] [PubMed]
- Loveland, A.B.; Svidritskiy, E.; Susorov, D.; Lee, S.; Park, A.; Zvornicanin, S.; Demo, G.; Gao, F.B.; Korostelev, A.A. Ribosome inhibition by C9ORF72-ALS/FTD-associated poly-PR and poly-GR proteins revealed by cryo-EM. Nat. Commun. 2022, 13, 2776. [Google Scholar] [CrossRef] [PubMed]
- Brakel, A.; Krizsan, A.; Itzenga, R.; Kraus, C.N.; Otvos, L., Jr.; Hoffmann, R. Influence of Substitutions in the Binding Motif of Proline-Rich Antimicrobial Peptide ARV-1502 on 70S Ribosome Binding and Antimicrobial Activity. Int. J. Mol. Sci. 2022, 23, 3150. [Google Scholar] [CrossRef]
- Novković, M.; Simunić, J.; Bojović, V.; Tossi, A.; Juretić, D. DADP: The Database of Anuran Defense Peptides. Bioinformatics 2012, 28, 1406–1407. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3, the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G.P. CancerPPD: A database of anticancer peptides and proteins. Nucleic Acids Res. 2015, 43, D837–D843. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef] [PubMed]
- Nijnik, A.; Hancock, R.E.W. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 2009, 16, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, G.; Kim, E.Y.; Shin, S.Y. LL-37-derived membrane-active FK-13 analogs possessing cell selectivity, anti-biofilm activity and synergy with chloramphenicol and anti-inflammatory activity. Biochim. Biophys. Acta Biomembr. 2017, 1859, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.K.; Wang, G.; Coffelt, S.B.; Betancourt, A.M.; Lee, C.W.; Fan, D.; Wu, K.; Yu, J.; Sung, J.J.Y.; Cho, C.H. Emerging Roles of the Host Defense Peptide LL-37 in Human Cancer and its Potential Therapeutic Applications. Int. J. Cancer 2010, 127, 1741–1747. [Google Scholar] [CrossRef]
- Wang, G.; Mishra, B.; Epand, R.F.; Epand, R.M. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta 2014, 1838, 2160–2172. [Google Scholar] [CrossRef]
- Wang, G.; Hanke, M.L.; Mishra, B.; Lushnikova, T.; Heim, C.E.; Thomas, V.C.; Bayles, K.W.; Kielian, T. Transformation of human cathelicidin LL-37 into selective, stable, and potent antimicrobial compounds. ACS Chem. Biol. 2014, 9, 1997–2002. [Google Scholar] [CrossRef]
- Juretić, D.; Simunić, J. Design of α-helical antimicrobial peptides with a high selectivity index. Expert Opin. Drug Discov. 2019, 14, 1053–1063. [Google Scholar] [CrossRef]
- Kim, E.Y.; Rajasekaran, G.; Shin, S.Y. LL-37-derived short antimicrobial peptide KR-12-a5 and its d-amino acid substituted analogs with cell selectivity, anti-biofilm activity, synergistic effect with conventional antibiotics, and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 136, 428–441. [Google Scholar] [CrossRef]
- Souza, B.M.; Mendes, M.A.; Santos, L.D.; Marques, M.R.; César, L.M.; Almeida, R.N.; Pagnocca, F.C.; Konno, K.; Palma, M.S. Structural and functional characterization of two novel peptide toxins isolated from the venom of the social wasp Polybia paulista. Peptides 2005, 26, 2157–2164. [Google Scholar] [CrossRef]
- Kerkis, I.; de Brandão Prieto da Silva, A.R.; Pompeia, C.; Tytgat, J.; de Sá Junior, P.L. Toxin bioportides: Exploring toxin biological activity and multifunctionality. Cell Mol. Life Sci. 2017, 74, 647–661. [Google Scholar] [CrossRef]
- Kyte, J.A. Cancer vaccination with telomerase peptide GV1001. Expert Opin. Investig. Drugs. 2009, 18, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.R.; Zhang, B.Z.; Zhang, W.; Yan, J.X.; Li, J.; Wang, R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MP1. Peptides 2008, 29, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Bitler, B.G.; Schroeder, J.A. Anti-Cancer Therapies that Utilize Cell Penetrating Peptides. Recent Pat. Anticancer Drug Discov. 2010, 5, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-active host defense peptides—Challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 2011, 164, 766–781. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Yang, C.; Zeng, P.; Xu, H.; Pan, F.; Lu, J.R. High Selective Performance of Designed Antibacterial and Anticancer Peptide Amphiphiles. ACS Appl. Mater. Interfaces 2015, 7, 17346–17355. [Google Scholar] [CrossRef]
- Vernen, F.; Craik, D.J.; Lawrence, N.; Henriques, S.T. Cyclic Analogues of Horseshoe Crab Peptide Tachyplesin I with Anticancer and Cell Penetrating Properties. ACS Chem. Biol. 2019, 14, 2895–2908. [Google Scholar] [CrossRef]
- Simmaco, M.; Mignogna, G.; Canofeni, S.; Miele, R.; Mangoni, M.L.; Barra, D. Temporins, antimicrobial peptides from the European red frog Rana temporaria. Eur. J. Biochem. 1996, 242, 788–792. [Google Scholar] [CrossRef]
- Simmaco, M.; De Biase, D.; Severini, C.; Aita, M.; Erspamer, G.F.; Barra, D.; Bossa, F. Purification and characterization of bioactive peptides from skin extracts of Rana esculenta. Biochim. Biophys. Acta 1990, 1033, 318–323. [Google Scholar] [CrossRef]
- Mangoni, M.L. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. 2006, 63, 1060–1069. [Google Scholar] [CrossRef]
- Romero, S.M.; Cardillo, A.B.; Ceron, M.C.M.; Camperi, S.A.; Giudicessi, S.L. Temporins: An Approach of Potential Pharmaceutic Candidates. Surg. Infect. 2020, 21, 309–322. [Google Scholar] [CrossRef]
- Rinaldi, A.C.; Mangoni, M.L.; Rufo, A.; Luzi, C.; Barra, D.; Zhao, H.; Kinnunen, P.K.J.; Bozzi, A.; Di Giulio, A.; Simmaco, M. Temporin L: Antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 2002, 368 Pt 1, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Shai, Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta 2009, 1788, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Oger, P.-C.; Piesse, C.; Ladram, A.; Humblot, V. Engineering of Antimicrobial Surfaces by Using Temporin Analogs to Tune the Biocidal/antiadhesive Effect. Molecules 2019, 24, 814. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The amphibian antimicrobial peptide temporin B inhibits in vitro herpes simplex virus 1 infection. Antimicrob. Agents Chemother. 2018, 62, e02367-17. [Google Scholar] [CrossRef]
- Roy, M.; Lebeau, L.; Chessa, C.; Damour, A.; Ladram, A.; Oury, B.; Boutolleau, D.; Bodet, C.; Lévêque, N. Comparison of Anti-Viral Activity of Frog Skin Anti-Microbial Peptides Temporin-Sha and [K3]SHa to LL-37 and Temporin-Tb against Herpes Simplex Virus Type 1. Viruses 2019, 11, 77. [Google Scholar] [CrossRef]
- Shang, D.; Liang, H.; Wei, S.; Yan, X.; Yang, Q.; Sun, Y. Effects of antimicrobial peptide L-K6, a temporin-1CEb analog on oral pathogen growth, Streptococcus mutans biofilm formation, and anti-inflammatory activity. Appl. Microbiol. Biotechnol. 2014, 98, 8685–8695. [Google Scholar] [CrossRef]
- Russ, W.P.; Engelman, D.M. The GxxxG Motif: A Framework for Transmembrane Helix-Helix Association. J. Mol. Biol. 2000, 296, 911–919. [Google Scholar] [CrossRef]
- Zanetti, M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 2005, 7, 179–196. [Google Scholar] [CrossRef]
- Wang, G.; Watson, K.M.; Buckheit, R.W., Jr. Anti-Human Immunodeficiency Virus Type 1 Activities of Antimicrobial Peptides Derived from Human and Bovine Cathelicidins. Antimicrob. Agents Chemother. 2008, 52, 3438–3440. [Google Scholar] [CrossRef]
- Haines, L.R.; Thomas, J.M.; Jackson, A.M.; Eyford, B.A.; Razavi, M.; Watson, C.N.; Gowen, B.; Hancock, R.E.W.; Pearson, T.W. Killing of Trypanosomatid Parasites by a Modified Bovine Host Defense Peptide, BMAP-18. PLoS Negl. Trop. Dis. 2009, 3, e373. [Google Scholar] [CrossRef]
- Eckert, R.; Qi, F.; Yarbrough, D.K.; He, J.; Anderson, M.H.; Shi, W. Adding selectivity to antimicrobial peptides: Rational design of a multidomain peptide against Pseudomonas spp. Antimicrob. Agents Chemother. 2006, 50, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Santarpia, P.; Lavender, S.; Gittins, E.; Liu, Z.; Anderson, M.H.; He, J.; Shi, W.; Eckert, R. Clinical Efficacy of a Specifically Targeted Antimicrobial Peptide Mouth Rinse: Targeted Elimination of Streptococcus mutans and Prevention of Demineralization. Caries Res. 2011, 45, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Hsiao, F.S.; Ho, Y.H.; Chen, C.S. The proteome targets of intracellular targeting antimicrobial peptides. Proteomics 2016, 16, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- Shah, P.; Chen, C.S. Systematic Screening of Penetratin’s Protein Targets by Yeast Proteome Microarrays. Int. J. Mol. Sci. 2022, 23, 712. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Chen, C.S. Systematic Identification of Protein Targets of Sub5 Using Saccharomyces cerevisiae Proteome Microarrays. Int. J. Mol. Sci. 2021, 22, 760. [Google Scholar] [CrossRef]
- Cerrato, C.P.; Langel, Ü. An update on cell-penetrating peptides with intracellular organelle targeting. Expert Opin. Drug Deliv. 2022, 19, 133–146. [Google Scholar] [CrossRef]
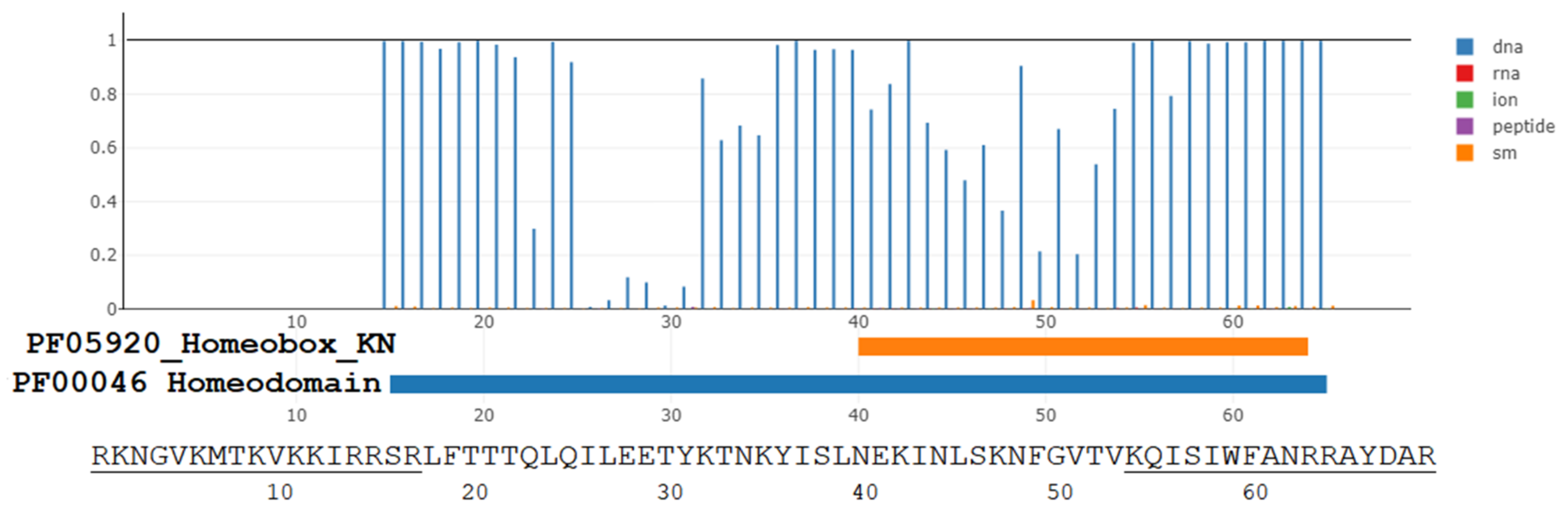
| Organism/ Common Name | Parent Protein or Gene/ GenBank or Uniprot Link | * Penetratin or Penetratin Analog Sequence | #Arg /#Lys/CPP Probability $ |
|---|---|---|---|
| Drosophila melanogaster/fruit fly | pAntp/P02833 | RQIKIWFQNRRMKWKK | 3/4/1.00 |
| Homo sapiens/human | Hox-A5/P20719 PDX-1/P52945 HXD8/P13378 Hox-C12/ P31275 | RQIKIWFQNRRMKWKK RHIKIWFQNRRMKWKK RQVKIWFQNRRMKWKK QQVKIWFQNRRMKKKR | 3/4/1.00 3/4/0.997 3/4/0.97 3/4/0.97 |
| Homo sapiens/human | Pax-6/P26367 Pax-7/P23759 and Pax-3/P23760 | ARIQVWFSNRRAKWRR ARVQVWFSNRRARWRK | 5/1/0.94 5/1/0.94 |
| Homo sapiens/human | PITX2/D6RFI4 | ARVRVWFKNRRAKWRKR | 5/3/0.97 |
| Ciona intestinalis/sea squirt tunicate | Pax3/7-like/NP_001071798.1 | ARVQVWFSNRRAKWRR | 5/1/0.94 |
| Acropora millepora/stony coral | Pax-6/XP_029212196.2 | ARIQVWFSNRRAKWRK | 4/2/0.94 |
| Capitella teleta/annelid worm | Ct-Pax3/7 (Pax6)/A1XC54, ABC68267.1 | ARVQVWFSNRRARWRK | 5/1/0.94 |
| Nematostella vectensis/sea anemone | PaxC homeodomain transcription factor/Q5IGV4 | ARVQVWFSNRRAKWRR | 5/1/0.94 |
| Mnemiopsis leidyi/comb jelly | PRD10a homeobox trancription factor/ ADO22618.1 | ARIQVWFQNRRAKWRK | 4/2/0.93 |
| Amphimedon queenslandica/sponge | Pax-6/XP_003387530.1 | SRVQVWFQNRRAKWRK | 4/2/0.93 |
| Trichoplax adhaerens | PaxB/ACH57172.1 | ARVQVWFSNRRAKWRK | 4/2/0.92 |
| Ceratocystis platani/fungi | Pax-6/KKF93291.1 | AKINNWFQNRRAKAKL | 2/3/0.86 |
| Galerina marginata/Dykaria higher fungi | Homeobox containing protein fragment/A0A067SZU8 | ARIQVWFSNRRAKWRR | 5/1/0.94 |
| Planoprotostelium fungivorum/amoeba | Arf-GAP with homeobox domain/ A0A2P6NXG8 | ARIQVWFSNRRAKWRR | 5/1/0.94 |
| Monosiga brevicollis (Choanoflagellate) | Mb_hbx2 homeobox-domain protein/ A9UP33 | QQINNWFINARRRLLNR | 4/0/0.76 |
| Capsaspora owczarzakiamoebae (Filasterea clade) | CAOG_004648 Homeobox domain-containing protein/ A0A0D2VSA1 | RVIRIWFQNRRAKQRR RRQKARRNQFWIRIVRR§ | 6/1/0.96 7/1/0.96 |
| Candida glabrata/budding yeast | Homeobox containing protein PHO2/ Q6FKZ3 | KNVRIWFQNRRAKVRK KNVRIWFQNRRAKVRKKGKL | 4/3/0.95 4/5/0.95 |
| Hanseniaspora osmophila/ wine-making yeast | Regulatory protein PHO2 with homobox domain 1/A0A1E5RMZ3 | TQVKIWFQNRRMKWKR | 3/3/0.94 |
| Acinetobacter baumannii/Gram- bacteria | Homeobox domain-containing protein (partial)/ WP_139162288.1 | RQVAVWFQNRRARWKT | 4/1/0.87 |
| Klebsiella pneumoniae/Gram- bacteria | Homeobox domain-containing protein WP_185963280.1 | TQIKIWFQNRRAKDHR | 3/2/0.76 |
| Euryarchaeota archaeon | RYE98021.1 | RQVSVWFTNARKRIWL | 3/1/0.77 |
| Acanthamoeba polyphaga mimivirus/ giant virus | Putative homeobox protein/ AKI80488.1 | RQIQIWFQNRRCKDRK | 4/2/0.87 |
| Moumouvirus maliensis/giant virus | Homeodomain containing protein/ QGR53678.1 | KQISIWFANRRAYDARK RKNGVKMTKVKKIRRSR& | 3/2/0.63 4/5/0.94 |
| Megavirus chiliensis/giant virus | Putative homeobox protein/ YP_004894234.1 | RQIQIWFQNRRARDSKKNR | 5/2/0.85 |
| Bandra megavirus/ | Homeobox/ AUV58136.1 | RQIQIWFQNRRARDSKKIR | 5/2/0.85 |
| Unclassified Mimivirus/ giant virus | Homeobox protein/ QZX43434.1 | RQIQIWFQNRRARDSRKNR | 6/1/0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juretić, D. Designed Multifunctional Peptides for Intracellular Targets. Antibiotics 2022, 11, 1196. https://doi.org/10.3390/antibiotics11091196
Juretić D. Designed Multifunctional Peptides for Intracellular Targets. Antibiotics. 2022; 11(9):1196. https://doi.org/10.3390/antibiotics11091196
Chicago/Turabian StyleJuretić, Davor. 2022. "Designed Multifunctional Peptides for Intracellular Targets" Antibiotics 11, no. 9: 1196. https://doi.org/10.3390/antibiotics11091196
APA StyleJuretić, D. (2022). Designed Multifunctional Peptides for Intracellular Targets. Antibiotics, 11(9), 1196. https://doi.org/10.3390/antibiotics11091196







