Abstract
Fever is one of the most common causes of medical evaluation of children, and early discrimination between viral and bacterial infection is essential to reduce inappropriate prescriptions. This study aims to systematically review the effects of point-of-care tests (POCTs) and rapid tests for respiratory tract infections on changing antibiotic prescription rate, length of stay, duration of therapy, and healthcare costs. Embase, MEDLINE, and Cochrane Library databases were systematically searched. All randomized control trials and non-randomized observational studies meeting inclusion criteria were evaluated using the NIH assessment tool. A meta-analysis was performed to assess the effects of rapid influenza diagnostic tests and film-array respiratory panel implementation on selected outcomes. From a total of 6440 studies, 57 were eligible for the review. The analysis was stratified by setting and POCT/rapid test type. The most frequent POCTs or rapid tests implemented were the Rapid Influenza Diagnostic Test and film-array and for those types of test a separate meta-analysis assessed a significant reduction in antibiotic prescription and an improvement in oseltamivir prescription. Implementing POCTs and rapid tests to discriminate between viral and bacterial infections for respiratory pathogens is valuable for improving appropriate antimicrobial prescriptions. However, more studies are needed to assess these findings in pediatric settings.
1. Introduction
Fever in children is one of the most common causes of medical evaluation in the emergency department (ED) or primary care practices and one of the possible complications in children hospitalized for other reasons [1]. Early recognition of severe infections in acutely ill children is crucial for improving their outcomes. Many physicians prescribe antibiotics, especially broad-spectrum antibiotics, for children with fever while waiting for blood tests and microbiological results to avoid the risk of severe infectious complications. However, it has been demonstrated that up to 50% of antibiotic prescriptions are unnecessary or inappropriate [2], and many children receive broad-spectrum antibiotics for viral infections [3]. This unnecessary use of antibiotics leads to increased antibiotic resistance and healthcare costs. For these reasons, discriminating between viral and bacterial infections is essential.
In 2007, the Infectious Disease Society of America (IDSA), alarmed by the increasing antimicrobial resistance rate compared to a reduction in newly available antibiotics, introduced the concept of Antimicrobial Stewardship Programs (ASP), defined as a collection of proactive strategies to improve the antibiotic prescription [2]. An integral part of ASPs is represented by diagnostic stewardship, coordinated guidance, and intervention to improve the appropriate use of microbiological diagnostics, which could help discriminate between viral and bacterial infection and guide therapeutic decisions [4]. Indeed, diagnostic tools are required to be accurate and available in a short period of time to modify decisions effectively and have an impact on the choice of treatment, as well as adequately cost saving.
Rapid tests are designed to give a diagnostic response with a shorter turnaround time than standard analysis (sample culture, serology, etc.). In addition, microbiological point-of-care tests (POCTs) are rapid tests designed to provide a quicker response at the bedside [5]. Indeed, rapid tests and POCTs could reduce inappropriate antibiotic prescriptions and length of stay, improve patient outcomes, and even allow cost savings due to earlier appropriate medical decisions. Many studies have been published on the usability of rapid tests and POCTs for implementing the diagnostic workup in many different settings, especially in adult populations. However, their use is not currently the standard of care for evaluating febrile children.
The primary aim of this review is to summarize the current state of evidence on the impact of rapid tests and POCTs for respiratory tract infections in pediatric settings (ED, inpatient, and outpatient) on changing the antimicrobial prescriptions rate (both antibiotic and oseltamivir), duration of therapy, length of stay, and healthcare costs in high and low–middle income countries.
2. Results
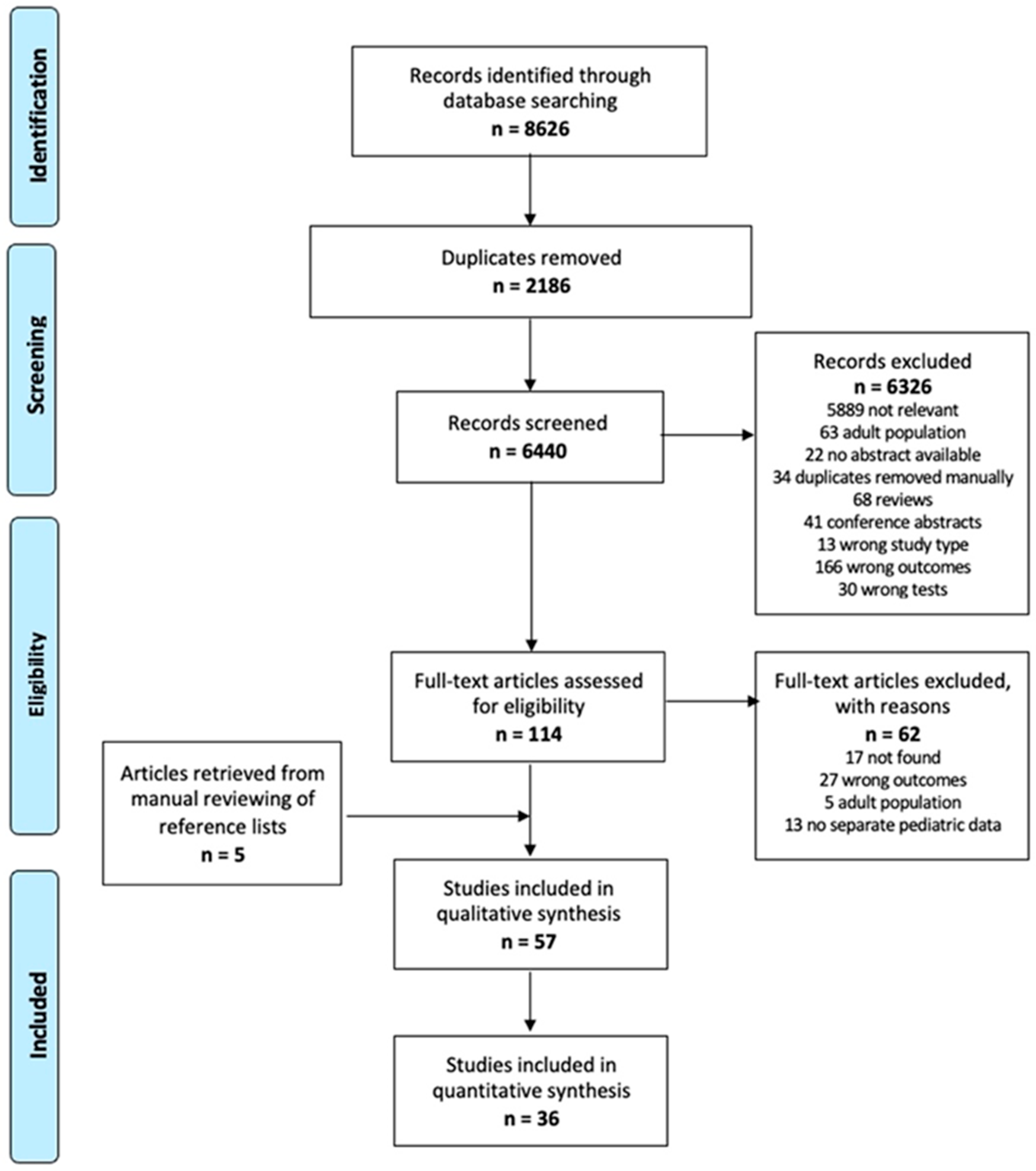
A total of 6440 studies were retrieved from the database search, excluding duplicates. After title and abstract screening, 114 were eligible for full-text reading and 52 were included in this review. In addition, five studies were added from the manual reviewing of reference lists for a total of 57 papers. The selection process is summarized in Figure 1 [6].

Figure 1.
Flowchart of the study selection process (PRISMA).
The title, authors, publication year, study design, country, study period, setting, number of patients included, type of rapid test or POCT, and types of outcomes considered are summarized in Table 1. The results of each study for the different outcomes are reported in Table 2.
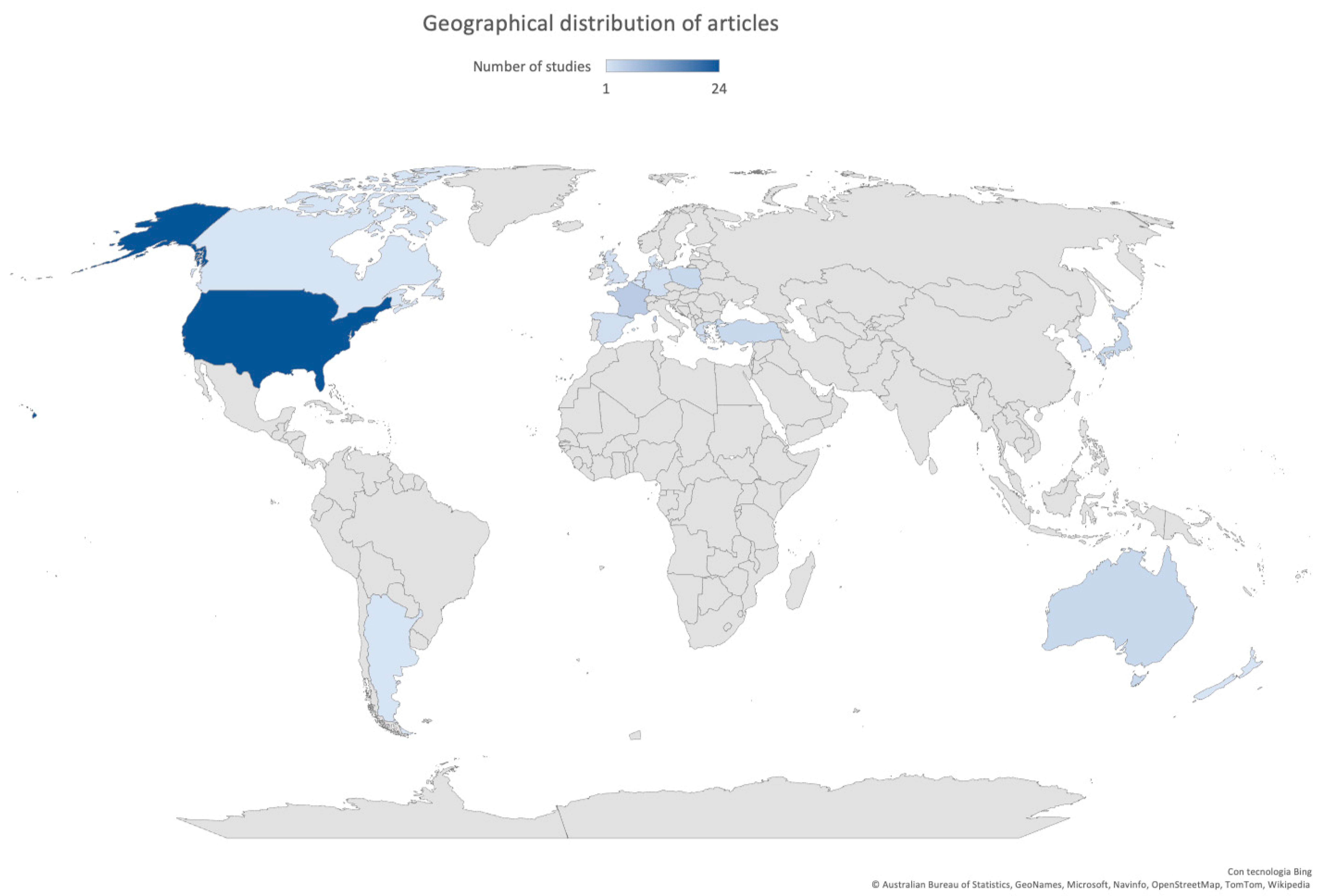
Most of the studies were published after 2007 (47/57, 82.5%), with 18 studies (10/57, 17.5%) between 2020 and 2021. Fifty-three studies (53/57, 93.0%) were conducted in high-income countries [7], and almost 80% of these articles described the implementation of a rapid test or POCT in North America (25/57, 43.9%) or Europe (19/57, 33.3%). Only eight studies (8/57, 14.0%) were conducted in Asia. The geographical distribution of articles is shown in Figure 2.
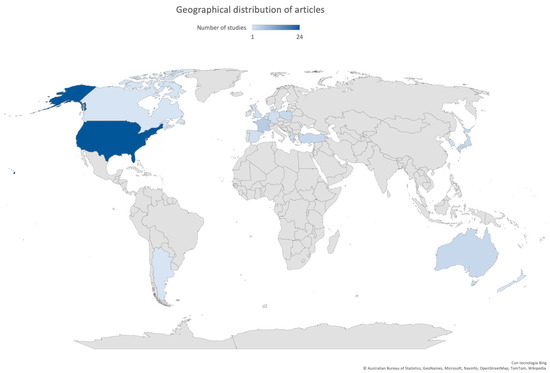
Figure 2.
Geographical distribution of articles included in this review.
Thirteen studies were multicenter: more than half were set in Europe (8/13, 61.5%) and the other 5 in North America (5/13, 38.5%).
The distribution of studies according to their design was: 8 randomized controlled trials (14.0%), 27 observational studies, including retrospective or prospective observational studies and control–case studies (47.4%), 22 quasi-experimental studies including before and after studies and interrupted time series studies (38.6%)
The most frequent rapid tests or POCTs implemented were the Rapid Influenza Diagnostic Test (RIDT) (22/57, 38.6%) and Film Array–Respiratory Panel (FA-RP) (22/57, 38.5%), followed by Strep A rapid tests (7/57, 12.3%) and others testing Respiratory Syncytial Virus (RSV) or the combination of RSV and influenza. Only one study focused on the implementation of a rapid PCR test for Mycoplasma pneumoniae.

Table 1.
Characteristics of studies included in the review.
Table 1.
Characteristics of studies included in the review.
| Authors | Year of Publication | Study Design | Study Location | Study Period | Single or Multi-Center | Age | Care Setting | N° Patients | Type of Test | Outcomes | NIH Tool | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | DOT | LOS | COST | PO | |||||||||||
| Nitsch-Osuch et al. [8] | 2017 | QE | POL | January 2015–March 2015 January 2016–March 2016 | Single | <5 years | Inpatient | 115 | RIDT | X | X | FAIR | |||
| Vecino-Ortiz et al. [9] | 2018 | QE | GBR | 2013–2014 2014–2015 | Single | Children | Inpatient | 574 | RIDT, Rapid VRS test | X | X | X | X | FAIR | |
| Abanses et al. [10] | 2006 | RCT | USA | December 2002–March 2003 | Single | 3–36 months | ED | 1007 | RIDT | X | X | X | FAIR | ||
| Diallo et al. [11] | 2019 | OR | FRA | 2013–2015 | Single | 1 month–18 years | ED | 241 | RIDT | X | FAIR | ||||
| Noyola et al. [12] | 2000 | OR | USA | July 1995– June 1997 | Single | Children | ED | 1530 | RIDT | X | X | X | X | GOOD | |
| Özkaya et al. [13] | 2009 | OP | TUR | November 2006–March 2007 | Single | 3–14 years | ED | 97 | RIDT | X | FAIR | ||||
| Bonner et al. [14] | 2003 | RCT | USA | January 2002–March 2002 | Single | 2 months–21 years | ED | 391 | RIDT | X | X | X | GOOD | ||
| Cantais et al. [15] | 2019 | OP | FRA | January 2016–March 2016 | Single | 0–16 years | ED | 514 | RIDT | X | FAIR | ||||
| Iyer et al. [16] | 2006 | Quasi-RCT | USA | January 2003–March 2003 December 2003–January 2004 | Single | 2–24 months | ED | 700 | RIDT | X | X | X | FAIR | ||
| Jacob et al. [17] | 2020 | OR | AUS | August 2017–September 2017 | Single | <16 years | ED | 1451 | RIDT | X | X | FAIR | |||
| Jun et al. [18] | 2016 | QE | KOR | December 2008–January 2009 February 2013–March 2013 | Single | <16 years | ED | 342 | RIDT | X | X | FAIR | |||
| Li-Kim-Moy et al. [19] | 2016 | OR | AUS | 2009 | Single | <18 years | ED | 364 | RIDT | X | X | X | FAIR | ||
| Sharma et al. [20] | 2002 | OR | USA | November 1998–March 1999 November 1999–March 2000 | Single | 2 months–2 years | ED | 72 | RIDT | X | X | FAIR | |||
| Patel et al. [21] | 2020 | QE | USA | November–March of 2014 to 2017 November–March of 2017 to 2018 | Single | 3 months–18 years | ED | 5307 | RIDT | X | X | X | FAIR | ||
| Pierron et al. [22] | 2008 | OP | FRA | January 2007–March 2007 | Single | 1 month–6 years | ED | 177 | RIDT | X | FAIR | ||||
| Benito-Fernandez et al. [23] | 2006 | OP | ESP | November–December 2003 December 2004–February 2005 | Single | 0–14 years | ED | 206 | RIDT | X | X | FAIR | |||
| Poehling et al. [24] | 2006 | RCT | USA | 2002–2004 | Multi-center | <5 years | ED + outpatient | 468 | RIDT | X | GOOD | ||||
| Jennings et al. [25] | 2009 | OP | DEU | January 2007– April 2007 | Multi-center | 1–12 years | Outpatient | 16907 | RIDT | X | X | FAIR | |||
| Nitsch-Osuch et al. [26] | 2013 | OP | POL | 2009/2010–2010/2011 | Multi-center | <5 years | Outpatient | 256 | RIDT | X | X | FAIR | |||
| Van Esso et al. [27] | 2019 | OP | ESP | 2016–2017 | Multi-center | 0–6 years | Outpatient | 1170 | RIDT | X | POOR | ||||
| Cohen et al. [28] | 2007 | OP | FRA | 2004–2005 | Multi-center | Children | Outpatient | 602 | RIDT | X | X | FAIR | |||
| de La Rocque et al. [29] | 2009 | OP | FRA | December 2006–April 2007 | Multi-center | Children | Outpatient | 695 | RIDT | X | X | GOOD | |||
| Keske et al. [30] | 2018 | QE | TUR | January 2015–December 2016 | Single | <16 years | Inpatient | 258 | FA-RP | X | X | FAIR | |||
| Kitano et al. [31] | 2019 | QE | JPN | March 2018 – April 2019 March 2012–March 2018 | Single | nd | Inpatient | 1281 | FA-RP | X | X | X | FAIR | ||
| Lee et al. [32] | 2020 | QE | USA | December 2009–July 2012 August 2012 – June 2016 | Single | <18 years | Inpatient | 5142 | FA-RP | X | X | X | FAIR | ||
| Reischl et al. [33] | 2020 | QE | DEU | February 2016–February 2017 February 2017–April 2018 | Single | 0–2 years | Inpatient | 786 | FA-RP | X | X | X | X | FAIR | |
| Schulert et al. [34] | 2013 | OR | USA | August 2009–December 2010 | Single | 0–14 years | Inpatient | 790 | FA-RP | X | X | FAIR | |||
| Subramony et al. [35] | 2016 | QE | USA | June 2010 – June 2012 October 2012–May 2014 | Single | 0–18 years | Inpatient | 4779 | FA-RP | X | FAIR | ||||
| Walls et al. [36] | 2016 | OR | NZL | Winter months 2012–2015 | Single | 3 months–5 years | Inpatient | 237 | FA-RP | X | X | FAIR | |||
| Yoshida et al. [37] | 2021 | QE | JPN | December 2017–November 2018 March 2019–February 2020 | Single | 0–18 years | Inpatient | 181 | FA-RP | X | X | X | FAIR | ||
| McCulloh et al. [38] | 2013 | OR | USA | October 2009–April 2010 October 2010–April 2011 | Single | 0–18 years | Inpatient | 1727 | FA-RP | X | X | X | FAIR | ||
| McFall et al. [39] | 2017 | OR | USA | November 2012–August 2015 | Single | 1–3 months | Inpatient | 176 | FA-RP | X | X | FAIR | |||
| Iroh Tam et al. [40] | 2017 | OR | USA | January 2011 – April 2015 | Multi-center | <18 years | ED + inpatient | 1625 | FA-RP | X | X | X | FAIR | ||
| Kim et al. [41] | 2021 | QE | KOR | November 2015–June 2016 November 2016–July 2018 | Single | 1 month–18 years | ED + inpatient | 915 | FA-RP | X | X | X | FAIR | ||
| Rogers et al. [42] | 2015 | QE | USA | November 2011–January 2012 November 2012–January 2013 | Single | 3 months–21 years | ED + inpatient | 1136 | FA-RP | X | X | X | FAIR | ||
| Busson et al. [43] | 2019 | QE | BEL | February 2016–March 2016 | Single | Adults and children, separated data | ED | 142 | FA-RP | X | X | X | FAIR | ||
| Byington et al. [44] | 2002 | OR | USA | December 2000–February 2001 December 2001–January 2002 | Single | <19 years | ED | 338 | FA-RP | X | X | FAIR | |||
| Crook et al. [45] | 2020 | QE | USA | January 2011–December 2014 January 2015– April 2018 May 2018– June 2019 | Single | <90 days | ED | 5317 | FA-RP | X | X | X | FAIR | ||
| Dimopoulou et al. [46] | 2020 | OP | GRC | February 2019 | Single | 0–16 years | ED | 80 | FA-RP | X | X | FAIR | |||
| Rao et al. [47] | 2021 | RCT | USA | December 2018–November 2019 | Single | 1 month–18 | ED | 920 | FA-RP | X | X | X | GOOD | ||
| Echavarría et al. [48] | 2018 | RT | ARG | April–November 2016 April–October 2017 | Single | 2 months–6 years | ED | 156 | FA-RP | X | X | X | GOOD | ||
| May et al. [49] | 2019 | RCT | USA | December 2016–April 2018 | Single | 1–17 years | ED | 71 | FA-RP | X | X | X | GOOD | ||
| Wishaupt et al. [50] | 2011 | RCT | NLD | November 2007–May 2008 October 2008–March 2009 | Single | 0–12 years | ED + outpatient | 583 | FA-RP | X | X | GOOD | |||
| Beal et al. [51] | 2020 | QE | USA | January 2018–January 2019 | Multi-center | <21 years | Outpatient | 430 | FA-RP | X | X | X | FAIR | ||
| Thibeault et al. [52] | 2007 | OR | CAN | Winter 2001–2002 and 2002–2003 | Multi-center | 0–3 years | Inpatient | 448 | Rapid RSV test | X | X | FAIR | |||
| Schnell et al. [53] | 2017 | OR | USA | November–March of 2008–2013 | Single | 2 months–2 years | ED | 713 | Rapid RSV test | X | X | FAIR | |||
| O’ Callaghan et al. [54] | 2019 | QE | AUS | May 2017– August 2017 May 2018–August 2018 | Single | Children | ED | 642 | Rapid PCR for influenza and RSV | X | X | FAIR | |||
| Mitchell et al. [55] | 2018 | QE | USA | November 2014–March 2015 | Single | <18 years | ED | 2171 | Rapid PCR for influenza and RSV | X | FAIR | ||||
| Schneider et al. [56] | 2018 | OP | DNK | February 2018–July 2018 | Multi-center | 0–18 years | ED | 180 | Rapid PCR for influenza and RSV | X | X | FAIR | |||
| Hayashi et al. [57] | 2018 | OP | JPN | May 2016– April 2017 | Single | <18 | Inpatient | 375 | Mycoplasma PCR | X | FAIR | ||||
| Ayanruoh et al. [58] | 2009 | QE | USA | September 2005–September 2007 | Single | 3–18 years | ED | 8280 | RSTs | X | FAIR | ||||
| Bird et al. [59] | 2021 | QE | GBR | October–November 2014 August–November 2015 September–November 2016 | Single | 6 months–16 years | ED | 605 | RSTs | X | FAIR | ||||
| Halverson et al. [60] | 2011 | QE | USA | October 2006–December 2009 | Multi-center | nd | ED | nd | RSTs | X | POOR | ||||
| Kose et al. [61] | 2016 | QE | TUR | February 2012–May 2014 | Single | 3–14 years | ED | 223 | RSTs | X | X | FAIR | |||
| Małecki et al. [62] | 2017 | QE | POL | October 2013– April 2014 | Multi-center | 2–15 years | Outpatient | 1307 | RSTs | X | X | X | X | FAIR | |
| Maltezou et al. [63] | 2008 | OP | GRC | December 2005–June 2006 September 2006–June 2007 | Multi-center | 2–14 years | Outpatient | 820 | RSTs | X | X | X | X | FAIR | |
| Rao et al. [64] | 2019 | OP | USA | Fall-winter 2016–2017 | Single | 3–18 years | Outpatient | 275 | RSTs and PCR Strep A test | X | FAIR | ||||
PR = prescription rate; DOT = days of therapy; LOS = length of stay; PO = prescription of oseltamivir; NIH = National Institutes of Health; OP = observational prospective; RCT= randomized control trial; RT= randomized trial; OR = observational retrospective; QE = quasi-experimental; ED = emergency department; RIDT = Rapid Influenza Diagnostic Test; FA-RP = Film Array–Respiratory Panel; RSV = Respiratory Syncytial Virus; RSTs = Rapid Streptococcal Tests; PCR = polymerase chain reaction; regarding outcomes, X indicates which outcome is considered in each study.

Table 2.
Outcomes of the studies included in the review.
Table 2.
Outcomes of the studies included in the review.
| Authors and Year of Publication | N° Patients | Type of Test | Outcomes | NIH Tool | ||||
|---|---|---|---|---|---|---|---|---|
| PR | DOT | LOS | COST | PO | ||||
| Nitsch-Osuch et al., 2017 [8] | 115 | RIDT | Antibiotic therapy was statistically more frequently administered when RIDT was not available (93% vs. 64%; p < 0.05) | Oseltamivir was statistically more prescribed when RIDT was available (64% of patients with influenza received an antiviral, none of the children received an antiviral without RIDT) | FAIR | |||
| Vecino-Ortiz et al., 2018 [9] | 574 | RIDT, rapid VRS test | No significant differences in the antibiotic prescription rate between periods in those positive for influenza and negative for both influenza and RSV | There was no significant difference between the periods for the total length of stay (median = 2 days for both periods, p = 0.23) | Reductions in the average reimbursement charge for patients with a negative influenza and RSV test. No change in reimbursement for patients with proven influenza or RSV infection | Small but significant increase in the cost of drugs between periods 1 and 2 for admissions in which the patients were positive for influenza and/or RSV | FAIR | |
| Abanses et al., 2006 [10] | 1007 | RIDT | Significant reduction in antibiotic prescription in those testing positive for influenza (215 vs. 102, RR 0.85, CI 95% 0.7–1.02) | Time in ED was significantly less in the intervention group (195 vs. 156 min; 95% CI for the difference, 19–60) | Total medical charges were significantly less in the intervention group (USD 666 vs. USD 393; 95% CI for the difference, 153–392) | FAIR | ||
| Diallo et al., 2019 [11] | 241 | RIDT | The mean length of stay in the PED was significantly lower in the positive RDT group: 4.0 h vs. 7.4 h (p < 10−6) | FAIR | ||||
| Noyola et al., 2000 [12] | 1530 | RIDT | Patients discharged from the ED with a positive influenza test were less likely to receive antibiotics than those with a negative test (20% vs. 53%; p < 0.04) Patients admitted to the hospital with a positive EIA test were as likely to receive antibiotics as those without a rapid diagnosis | Duration of antibiotic administration was significantly shorter in the group with a positive influenza test (3.5 vs. 5.4 days; p = 0.03) | Patients with a positive influenza test were more likely to have a shorter duration of admission than the control group (4.3 mean days versus 7.4; p = 0.02) | Patients with a positive influenza test were more likely to receive antiviral therapy than the control group (25% vs. 0 and 1.8%; p < 0.001) | GOOD | |
| Özkaya et al., 2009 [13] | 97 | RIDT | Patients in group testing prior to prescription were less likely to be prescribed antibiotics when compared to those in the group in which rapid testing was not considered for prescription (32% vs. 100%, respectively, p < 0.0001) | FAIR | ||||
| Bonner et al., 2003 [14] | 391 | RIDT | Reduction in antibiotic prescription in the group in which the results of the rapid test was known in comparison to the group of unknown (7/96 vs. 26/106, p < 0.001); no difference between negative test and negative unaware test (27/97 vs. 27/92, p = 0.0818) | Reduction in the length of stay in the group in which the results of the rapid test were known in comparison to the group of unknown (25 min vs. 49 min, p < 0.001); no difference between the negative test and negative unaware test (45 min vs. 42 min, p = 0.549) | Increase in antiviral prescription in the group in which the results of the rapid test were known in comparison to the group of unknown (18/96 vs. 7/106, p = 0.02); no difference between negative test and negative unaware test (0/97 vs. 2/92, p = 0.236) | GOOD | ||
| Cantais et al., 2019 [15] | 514 | RIDT | Reduction in antibiotic prescriptions, 60 of 245 patients (24.5%) received antibiotics in the DIA negative group, versus 25 of 262 (9.5%) in the positive one (p < 0.001) | FAIR | ||||
| Iyer et al., 2006 [16] | 700 | RIDT | No significant differences were demonstrated between the POCT and standard test groups with respect to antibiotic prescription | No significant differences were demonstrated between the POCT and standard test groups with respect to lengths of stay | No significant differences were demonstrated between the POCT and standard test groups with respect to visit-associated costs | FAIR | ||
| Jacob et al., 2020 [17] | 1451 | RIDT | Antibiotics were used more in patients with ILI with no RIDT (15.2% in the ILI group vs. 2.7% in the laboratory RIDT group and 11.2% in the ED RIDT group; p < 0.0001) | Patients for whom RIDT was performed at the laboratory had a shorter length of stay when compared to patients for whom RIDT was performed bedside in the ED (4.7 and 5.3 h, respectively; p < 0.0001), | FAIR | |||
| Jun et al., 2016 [18] | 342 | RIDT | Reduction in antibiotic prescription in RAT-positive patients, after the 2009 influenza pandemic (none of the pediatric patients received antibiotics) | The duration of ER stay in discharged patients was 268.9 ± 144.2 min in patients with the use of a RAT kit and 210.5 ± 205.3 min in patients with no use of a RAT kit after the 2009 influenza pandemic | FAIR | |||
| Li-Kim-Moy et al., 2016 [19] | 364 | RIDT | Compared with standard testing (n = 65), children diagnosed by positive POCT (n = 236) had a reduction in antibiotic use (odds ratio 0.42, p = 0.003) | Compared with standard testing, children diagnosed by positive POCT had a shorter median hospital LOS by 1 day (p = 0.006). POCT did not decrease LOS in ED | Compared with standard testing, children diagnosed by positive POCT had increased antiviral prescription (odds ratio 3.1, p < 0.001) | FAIR | ||
| Sharma et al., 2002 [20] | 72 | RIDT | Fewer patients in the early diagnosis group received ceftriaxone sodium compared with the late diagnosis group (2% vs. 24%, p = 0.006) | No significant differences were demonstrated between the POCT and standard test groups with respect to lengths of stay | FAIR | |||
| Patel et al., 2020 [21] | 5307 | RIDT | There was no significant difference in rates of antibiotics used | The median LOS decreased from 239 min in the pre-POC period to 232 min in the post-POC period (p < 0.05) | There were increased rates of oseltamivir used in the post-POC period (21.2% vs. 13.3%, p < 0.05 | FAIR | ||
| Pierron et al., 2008 [22] | 177 | RIDT | There was not any significant difference concerning antibiotic prescriptions | FAIR | ||||
| Benito-Fernandez et al., 2006 [23] | 206 | RIDT | There was a significant reduction in the use of antibiotics (38.5% vs. 0%, p < 0.01) | There was a significant reduction in the mean length of stay in the ED (192.9 versus 116.2 min) (p < 0.01) | FAIR | |||
| Poehling et al., 2006 [24] | 468 | RIDT | There was no difference in antibiotic prescribing | GOOD | ||||
| Jennings et al., 2009 [25] | 16907 | RIDT | Antibiotics were less commonly prescribed for children who were influenza positive by rapid test (3.5% (271/7685) versus 17.2% (125/725) for symptom assessment alone) | The antiviral oseltamivir was prescribed for 24.6% (178/725) of children who were influenza positive by symptom assessment alone and 60.1% (4618/7685) of children who were influenza positive by rapid test | FAIR | |||
| Nitsch-Osuch et al., 2013 [26] | 256 | RIDT | Antibiotics were administered more often in the control group compared with the rapid test group (respectively, for 16% vs. 7%). No child with a positive result of RIDT was prescribed an antibiotic | The antiviral treatment (oseltamivir) was prescribed only for four children with positive results of RIDT | FAIR | |||
| Van Esso et al., 2019 [27] | 1170 | RIDT | Influenza-confirmed patients received fewer antibiotics during the 10 days after influenza diagnosis but not statistically significant compared with the groups with a clinical diagnosis of influenza without a microbiologic confirmation | POOR | ||||
| Cohen et al., 2007 [28] | 602 | RIDT | The antibiotic prescription was overall low (9.5% with RIDT vs. 3.9% without RIDT, p = 0,008), and primarily when the result of RIDT was negative (15.7% if RIDT– vs. 4.3% if RIDT+, p = 0.0003) | The pediatricians using RIDT prescribed with positive tests more oseltamivir (68.5 vs. 1.9%, p < 0.0001) | FAIR | |||
| de La Rocque et al., 2009 [29] | 695 | RIDT | The RIDT+ group received antibiotics in 7.6% of cases, RIDT− in 18.5% (p < 0.0001) | The RIDT+ group received an antiviral in 64.7% and the RIDT− group received no antiviral (p < 0.0001) | GOOD | |||
| Keske et al., 2018 [30] | 258 | FA-RP | Significant decrease in antibiotic use (44.5% in 2015 and 28.8% in 2016, p = 0.009) | The duration of antibiotic use after the detection of virus was significantly decreased in children (p < 0.001) | FAIR | |||
| Kitano et al., 2019 [31] | 1281 | FA-RP | The DOT/case was 12.82 vs. 8.56 (p < 0.001), in the rapid antigen test and mPCR groups, respectively | The LOS was 8.18 vs. 6.83 days (p = 0.032) in the rapid antigen test and mPCR groups, respectively | The total costs during admissions were 258,824 (USD 2331.7) and 243,841 (USD 2196.8)/case, in the rapid antigen test and mPCR groups, respectively | FAIR | ||
| Lee et al., 2020 [32] | 5142 | FA-RP | Patients tested with RP were less likely to receive empiric antibiotics (OR: 0.45; p < 0.001; 95% CI: 0.39, 0.52) compared to RVP patients | Patients tested with RP had a shorter duration of empiric broad-spectrum antibiotics (6.4 h vs. 32.9 h; p < 0.001) compared to RVP patients | RP influenza patients had increased oseltamivir use post-test compared to RVP influenza patients (OR: 13.56; p < 0.001; 95% CI: 7.29, 25.20). | FAIR | ||
| Reischl et al., 2020 [33] | 786 | FA-RP | The binary logistic regression analysis shows no significant (p = 0.784) impact of the FA-RP or the multiplex RT-PCR on the antibiotic treatment | The diagnostic method, FA-RP (8.6 days) or multiplex RT-PCR (9.1 days), showed no significant (p = 0.592) impact on the duration of antibiotic treatment in the linear logistic regression analysis | The mean hospital length of stay for both study groups was 4.7 days. The diagnostic method, FA or multiplex RT-PCR, showed no significant impact on the length of hospital stay in the linear regression analysis. | No significant difference in antiviral prescriptions | FAIR | |
| Schulert et al., 2013 [34] | 790 | FA-RP | The median duration of IV antibiotics for patients with a positive RVP was 55 h, compared with 96 h for patients with a negative RVP (p = 0.03) | The median length of stay for patients with a positive RVP was 3 days, compared with 4 days for patients with a negative RVP (p = 0.057) | FAIR | |||
| Subramony et al., 2016 [35] | 4779 | FA-RP | Subjects in the mPCR group received fewer days of antibiotics than subjects in the non-mPCR group (4 vs. 5 median antibiotic days, p < 0.01) | FAIR | ||||
| Walls et al., 2016 [36] | 237 | FA-RP | A significantly larger proportion of children who had an NPS sample taken (42/146, 36%) received no empiric antibiotics compared to children who did not have a sample taken (7/91, 7.7%, p < 0.001) | Of those who did have an NPS sample taken, 17 of 146 (11.6%) had their antibiotics discontinued prior to or at the time of discharge compared with only 3 of 91 (3.3%) of those who did not have an NPS sample (p < 0.025) | FAIR | |||
| Yoshida et al., 2021 [37] | 181 | FA-RP | We did not observe differences in the use of antibiotics between the pre- and post-mPCR periods (p = 0.14) | We did not observe differences in the duration of antibiotic usage between the pre- and post-mPCR periods (p = 0.45) | We did not observe differences in the length of stay between the pre- and post-mPCR periods (p = 0.94) | FAIR | ||
| McCulloh et al., 2013 [38] | 1727 | FA-RP | Children with a positive RVP test result received antibiotics less often (363 of 703 (51.6%) vs. 71 of 106 (67.0%); p = 0.003) | In total, 21 of 348 (6.0%) children who were positive for a viral pathogen by RVP had antibiotics discontinued within 24 h after RVP test results were available, but no children with negative RVP results had antibiotics subsequently stopped | Children with a positive RVP test result received oseltamivir more often (76.9% vs. 18%; p < 0.001) | FAIR | ||
| McFall et al., 2017 [39] | 176 | FA-RP | Duration of antimicrobial consumption was significantly decreased in patients with a positive FA-RP compared to infants with a negative test (mean rank 2.8 vs. mean rank 5.2 days), p < 0.001) | For all infants with a positive FA-RP result, LOS was significantly decreased compared with infants with a negative FA-RP result (5.7 vs. 10.4 days, p = 0.017) | FAIR | |||
| Iroh Tam et al., 2017 [40] | 1625 | FA-RP | No difference in antibiotic prescription for all types of antibiotics | Patients with a positive test from RVPP had shorter LOS (p = 0.0503) | Hospital charges for patients with a positive test from RVPP were lower, but not significantly so | No difference in antiviral prescription (p = 0.76) | FAIR | |
| Kim et al., 2021 [41] | 915 | FA-RP | FA-RP reduced intravenous (IV) antibiotic use (p = 0.002) | FA-RP reduced the duration of intravenous (IV) antibiotic use, for pediatric patients (p < 0.001) | FA-RP reduced the lead time, waiting time, turnaround time, and length of hospital stay (p = 0.004) | FAIR | ||
| Rogers et al., 2015 [42] | 1136 | FA-RP | The number of patients receiving antibiotics and the inpatient LOS did not differ in the 2 groups | Duration of antibiotic use decreased for patients in the post-FA-RP group by 0.4 days (p = 0.003) | The LOS in the ED increased by 26 min in the post-RRP group (p = 0.002) | FAIR | ||
| Busson et al., 2019 [43] | 142 | FA-RP | Results from the FilmArray Respiratory Panel do not appear to impact antibiotic prescription | The mean length of stay was not significantly different between the two groups (3.9 days for the group with a positive FA result vs. 5.2 days for the group with a negative FA result; p = 0.286) | No difference in oseltamivir prescription | FAIR | ||
| Byington et al., 2002 [44] | 338 | FA-RP | Test-positive patients had fewer discharge prescriptions for oral antibiotics (37% vs. 52%, p = 0.02) when compared with test-negative patients. Intravenous antibiotics were initiated less often for test-positive patients during the second winter season than during the first (26% vs. 44%, p = 0.008) | Test-positive patients had fewer days using intravenous antibiotics (2.4 vs. 4, p = 0.04), fewer days using oral antibiotics (0.25 vs. 2.5, p = 0.04), when compared with test-negative patients | FAIR | |||
| Crook et al., 2020 [45] | 5317 | FA-RP | Following introduction of mPCR testing, the percentage of patients who did not receive antimicrobials increased from 32.4% to 43.1% (difference, 10.8%; 95% CI, 6.5–15%) | Median antibiotic duration decreased by 0.47 days (95% CI, 0.16–0.51) | There was a significant reduction in LOS (p < 0.001) | FAIR | ||
| Dimopoulou et al., 2020 [46] | 80 | FA-RP | The implementation of a rapid molecular test had no impact on antibacterial prescription (10% vs. 13.3%). | The implementation of a rapid molecular test had no impact on antiviral prescription | FAIR | |||
| Rao et al., 2021 [47] | 920 | FA-RP | In the intention-to-treat intervention group (result known), children were more likely to receive antibiotics (relative risk (RR), 1.3; 95% CI, 1.0–1.7) compared to the control group (result not known) | No significant differences in length of stay between the two groups | No significant differences in antiviral prescribing between the two groups | GOOD | ||
| Echavarría et al., 2018 [48] | 156 | FA-RP | Diagnosis with FA-RP was associated with significant changes in medical management including withholding antibiotic prescriptions (OR:12.23, 95%CI:1.56–96.09) | The median LOS was lower for the FA-RP group (4 days) than the control group (10 days) although the difference was not statistically significant (p = 0.382) | Oseltamivir usage was very low and no significant changes in treatment with the drug were observed between the two study groups | GOOD | ||
| May et al., 2019 [49] | 71 | FA-RP | In total, 20 (22%) RP patients and 33 (34%) usual-care patients received antibiotics during the ED visit (–12%; 95% confidence interval, –25% to 0.4%; p = 0.06/0.08) | There was no significant difference in length of ED stay, or hospital stay among admitted patients between the 2 groups | No significant difference in antiviral prescription (+3% (–5% to –10%) 0.53/0.61) | GOOD | ||
| Wishaupt et al., 2011 [50] | 583 | FA-RP | Mean durations of antibiotic treatment, if antibiotic treatment was started, did not differ significantly between the groups | There was a trend toward a shorter length of hospital stay in the intervention group, but the difference was not statistically significant | GOOD | |||
| Beal et al., 2020 [51] | 430 | FA-RP | Appropriate treatment occurred for 93.6% of patients when the FA-RP was performed (Clinic A) versus 87.9% of patients who had only antigen tests performed (Clinic B, p = 0.0445) | Utilization of FA-RP testing also significantly reduced appointment duration time (48.0 versus 54.9 min, p = 0.0009) | Patients tested with FA-RP received less oseltamivir compared to children tested with an antigen test (p = 0.0018) | FAIR | ||
| Thibeault et al., 2007 [52] | 448 | Rapid RSV test | There was no significant difference between children with positive and negative RSV RADT results in the percentage receiving IV antibiotics only (10% versus 7%, p = 0.61); PO antibiotics only (38% versus 28%, p = 0.17); or both PO and IV antibiotics (52% versus 65%, p = 0.12) | At 24, 48, or 72 h, stopping or switching of IV antibiotics was not influenced by the RADT result in any of the four strata combining age and presence of pneumonia (<3 months and no pneumonia; <3 months without pneumonia; ≥3 months with pneumonia; ≥3 months with- out pneumonia) | FAIR | |||
| Schnell et al., 2017 [53] | 713 | Rapid RSV test | Antibiotic administration within the ED did not differ between those testing positive for RSV versus those testing negative | The mean time in the department was not statistically significant between the 2 groups at 174.1 (SD, 89.8) minutes for the RSV-negative group and 165.2 (SD, 84.6) minutes for the RSV-positive group | FAIR | |||
| O’ Callaghan et al., 2019 [54] | 642 | Rapid PCR for influenza and RSV | 26.3% of positive influenza A/B RSV patients were treated with antibiotics in 2017, whereas 21.3% were treated with antibiotics in 2018 (p = 0.45) | According to time to discharge in the ED, there were no differences between positive and negative patients (p = 0.85) | FAIR | |||
| Mitchell et al., 2018 [55] | 2171 | Rapid PCR for influenza and RSV | Analysis of the post-implementation period revealed a significantly lower percentage (14.3%, p < 0.001) of negative patients receiving antiviral therapy compared to the pre-implementation period, with no difference in prescription of oseltamivir in those testing positive | FAIR | ||||
| Schneider et al., 2018 [56] | 180 | Rapid PCR for influenza and RSV | A positive POCT result significantly reduced antibiotic prescription (p < 0.0011) | A positive POCT result significantly reduced median hospitalization time by 14.2 h for children | FAIR | |||
| Hayashi et al., 2018 [57] | 375 | Mycoplasma PCR | Antimicrobial agents for atypical pathogens (macrolides, tetracyclines, or quinolones) were prescribed in 97.3% (217/223) at the initial evaluation, and their prescription rates increased to 99.1% (221/223) during management | FAIR | ||||
| Ayanruoh et al., 2009 [58] | 8280 | RSTs | Rapid strep testing was associated with a lower antibiotic prescription rate for children with pharyngitis (41.38% for those treated in the pre-RST phase versus 22.45% for those treated in the post-RST phase; p < 0.001) | FAIR | ||||
| Bird et al., 2021 [59] | 605 | RSTs | The baseline prescribing rate was 79%, whereas rates after intervention were 24% in 2015 and 28% in 2016 | FAIR | ||||
| Halverson et al., 2011 [60] | nd | RSTs | Implementation of POCT was shown to provide a statistically significant drop in LOS of patients who had group A strep testing performed on them, discharging them 25–30 min faster than other patients on average | POOR | ||||
| Kose et al., 2016 [61] | 223 | RSTs | Antibiotic prescription decreased by 42.6% after learning RST results | Antibiotic costs in non-Group A streptococcus pharyngitis, Group A streptococcus pharyngitis, and all subjects’ groups decreased by 80.8%, 48%, and 76.4%, respectively | FAIR | |||
| Małecki et al., 2017 [62] | 1307 | RSTs | Reduction in antibiotic use by 5.1%. | The anticipated cost of treatment decreased by 17% | FAIR | |||
| Maltezou et al., 2008 [63] | 820 | RSTs | Pediatricians without access to laboratory tests were more likely to prescribe antibiotics compared with pediatricians with access to tests (72.2% versus 28.2%, p < 0.001) | FAIR | ||||
| Rao et al., 2019 [64] | 275 | RSTs and PCR Strep A test | The use of POC PCR resulted in the appropriate use of antibiotics in 97.1% of cases compared with 87.5% of cases for the standard of care, RST plus confirmatory bacterial culture (p = 0.0065) | FAIR | ||||
PR = prescription rate; DOT = days of therapy; LOS = length of stay; PO = prescription of oseltamivir; NIH = National Institutes of Health; RIDT = Rapid Influenza Diagnostic Test; FA-RP = Film Array–Respiratory Panel; RSV = Respiratory Syncytial Virus; RSTs = Rapid Streptococcal Tests; PCR = polymerase chain reaction; regarding outcomes, X indicates which outcome is considered in each study; RP = respiratory panel; RVP = respiratory viral panel; NPS = nasopharyngeal swab; RVPP = respiratory virus PCR panel.
2.1. Implementation Setting
More than three-quarters of the studies described the implementation of rapid tests or POCTs in a hospital setting (46/57, 80.7%). More than half were exclusively conducted in the ED (29/46, 63.0%), and 14 studies (14/46, 30.4%) referred only to hospital wards. Three studies (3/46, 6.52%) described the implementation of rapid tests or POCTs both in ED and hospital wards. Nine studies (9/57, 15.8%) were conducted in the outpatient setting and two studies both in the outpatient and ED settings.
The implementation of different rapid tests and POCTs according to different settings is summarized in Table 1.
2.1.1. Hospital Setting: Inpatient—Emergency Department
Most of the studies on the implementation of FA-RP were conducted in a hospital setting (20/22, 90.9%), 7 studies in the ED (7/20, 35.0%) and 10 studies in different hospital wards (10/20, 50.0%); 3 studies were conducted both in the ED and hospital wards (3/20, 15.0%). More than half of the studies on the implementation of RIDT were conducted in a hospital setting (16/22, 72.7%), almost all in the ED (14/16, 87.5%). Rapid tests for RSV, Mycoplasma, or rapid tests combined for RSV and influenza were implemented only in the hospital setting (three studies about the RSV rapid test, three studies for the combined test, one study about Mycoplasma). Almost all the studies on the implementation of rapid tests or POCTs in hospital wards were conducted in high-income countries.
2.1.2. Outpatient—Primary Care
The most implemented POCT in the outpatient setting was RIDT (5/9, 55.6%), followed by Strep A test (3/9, 33.3%). Only one study described the implementation of FA-RP in the outpatient setting.
Almost all the studies about POCTs in outpatient settings were conducted in Europe (7/9, 77.8%), and only two in the USA (2/9, 22.2%).
2.2. Implementation of Rapid Tests or POCTs and Outcomes
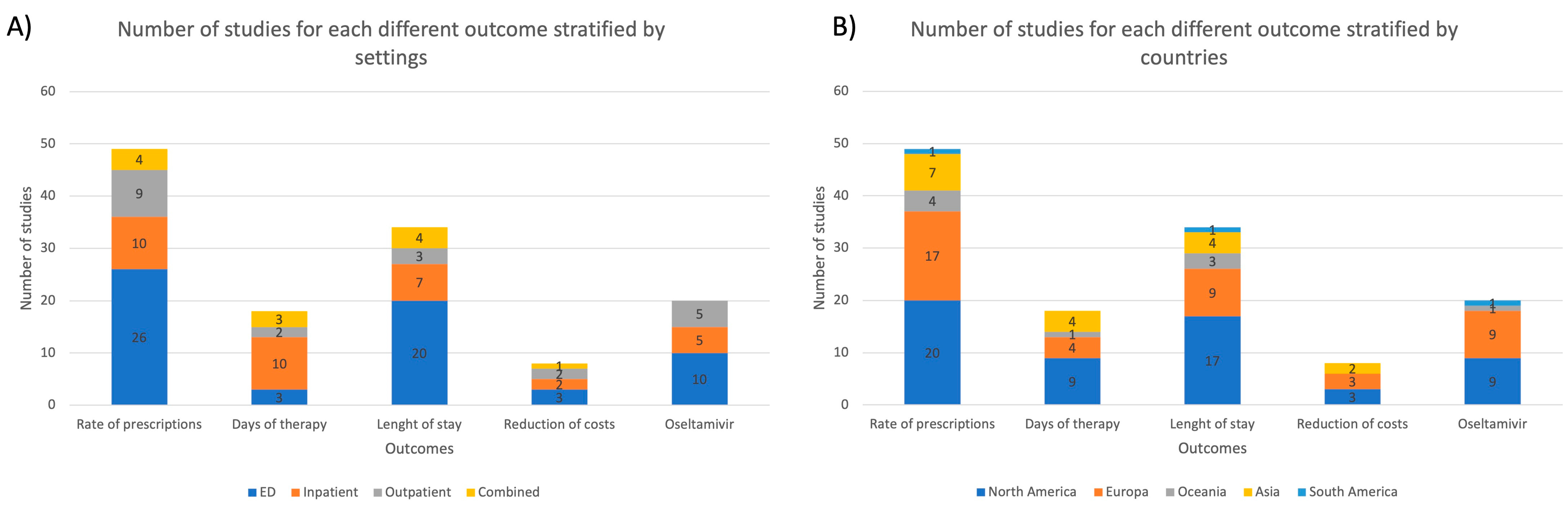
In Figure 3, the distribution of studies for each outcome is reported, stratified for setting and country.

Figure 3.
The number of studies for each different outcome stratified by (A) settings; (B) countries; ED = emergency department.
Considering the studies’ variety, separate meta-analyses for primary and secondary outcomes were conducted for the two types of rapid tests/POCTs with the most consistent data: film-array respiratory panel FA-RP and RIDT, including a total of 15 articles and 21 articles, respectively.
2.2.1. Antibiotics Prescription
More than three-quarters of the studies reported as their main outcome the change in antibiotics prescription after rapid tests or POCT implementation (49/57, 86.0%), with almost two-thirds (32/49, 65.3%) assessing a statistically significant reduction.
All the articles that focused on the change in antibiotic prescription after the introduction of the Strep A test reported a significant reduction in the antibiotic use, both in inpatient and outpatient settings.
On the contrary, the two articles about the implementation of a rapid test for RSV showed no difference in the antibiotic prescriptions between patients testing positive and those testing negative, both in the ED and hospital wards. One of the studies on the rapid test for RSV combined with influenza reported a statistically significant reduction in antibiotic use in children with a positive test, while another showed a slight reduction in antibiotic prescription. The study about the implementation of a rapid PCR for Mycoplasma pneumoniae showed increased appropriateness in prescribing antibiotics for atypical pathogens in those patients testing positive.
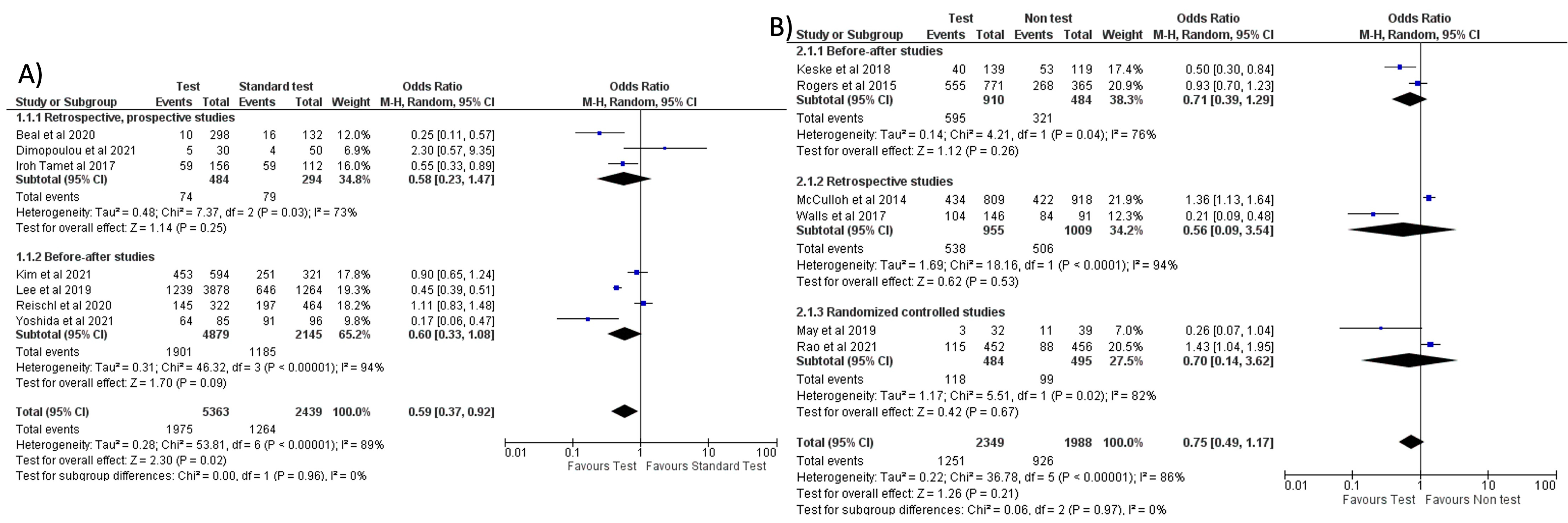
Separate meta-analyses for FA-RP and RIDT are reported in Figure 4 and Figure 5, and subgroup analyses are reported in Supplementary Material. It was not possible to conduct the pre-planned metanalysis by age groups due to the lack of data.
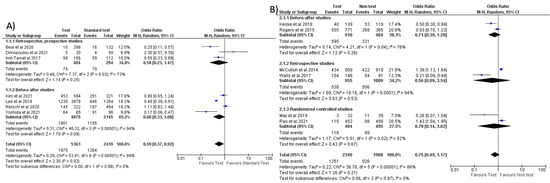
Figure 4.
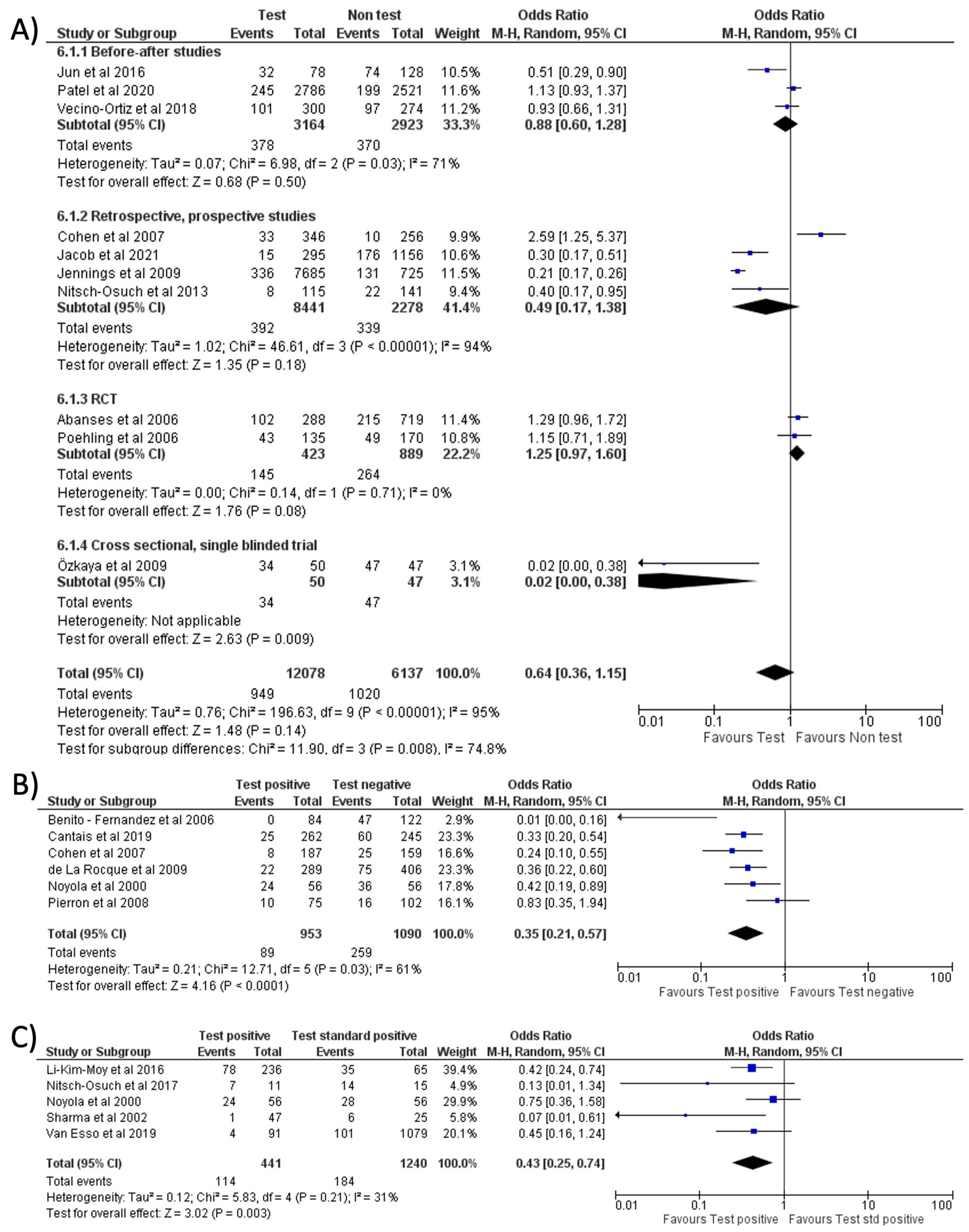
Forest plot of prescription of antibiotics after implementation of FA-RP: (A) FA-RP vs. standard test; (B) FA-RP versus clinical diagnosis.

Figure 5.
Forest plot of prescription of antibiotics after implementation of RIDT: (A) RIDT vs. clinical diagnosis; (B) RIDT positive versus RIDT negative; (C) RIDT positive vs. standard of care positive.
When comparing the use of FA-RP vs. standard test, an overall reduction in antibiotic prescriptions was noted (OR = 0.59, 95% CI 0.37–0.92, p = 0.02) [32,33,37,40,41,46,51], however, with a high heterogeneity (I2) of 89% (Figure 4A). In a subgroup analysis stratified by countries, this reduction was more significant in the studies conducted in the US compared to Europe and East Asia (see Figure S1A). However, when comparing FA-RP vs. clinical diagnosis [30,36,38,42,47,49], prescription rate did not significantly decrease (OR = 0.75, 95% CI 0.49–1.17, p = 0.21; I2 = 86%) (Figure 4B). A subgroup analysis stratified for different types of study is shown in Figure S1B,C.
No significant reduction in antibiotic prescription (OR = 0.64, 95% CI 0.36–1.15, p = 0.14; I2 = 95%) was noted when comparing the use of RIDT vs. clinical diagnosis (Figure 5A) [9,10,13,17,18,21,24,25,26,28]. One of the studies (Ozkaya et al.) seems to have a more evident reduction in antibiotic prescribing compared to the other studies. This study, conducted in Turkey, involved only 97 children and all the patients not tested with the rapid antigen detection test received antibiotic therapy. We evaluated this study as “fair” and it had a low weight in our metanalysis.
However, when stratified by geographical location (continent, nation, or single state based on the number of articles included in the analysis), a trend toward fewer prescriptions emerged in Europe and East Asia with respect to the US (see Figure S2A).
Comparing a positive result of RIDT vs. a negative result, a significant reduction in the prescriptions rate was noted (OR = 0.35, 95% CI 0.21–0.57, p < 0.0001; I2 = 61%) (Figure 5B) [12,15,22,23,28,29]. A positive RIDT was also associated with a significant reduction in antibiotics prescriptions with respect to a positive standard test [8,12,19,20,27] (OR = 0.43, 95% CI 0.25–0.74, p = 0.003; I2 = 31%) (Figure 5C). This evidence seemed stronger in Europe than in the US (see Figure S2B). A subgroup analysis stratified for different types of study is shown in Figure S3.
2.2.2. Oseltamivir Prescription
Twenty studies (20/57, 35.1%) also reported the change in antiviral prescriptions (oseltamivir). A significant increase in oseltamivir prescription with POCT emerged from 60.0% of the studies (12/20), including one study focused on the implementation of the rapid test for RSV combined with influenza.
Separate meta-analyses stratified by FA-RP and RIDT are reported in Figure 6 and Figure 7. Subgroup analyses are reported in Figure S4.

Figure 6.
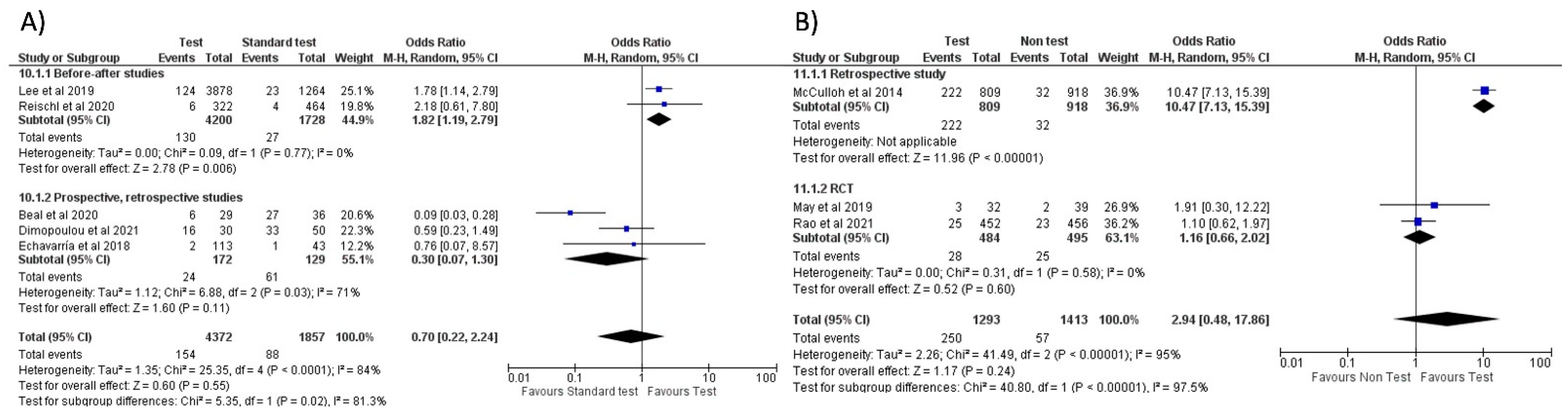
Forest plot of prescription of oseltamivir after implementation of FA-RP: (A) FA-RP vs. standard of care; (B) FA-RP vs. clinical diagnosis.
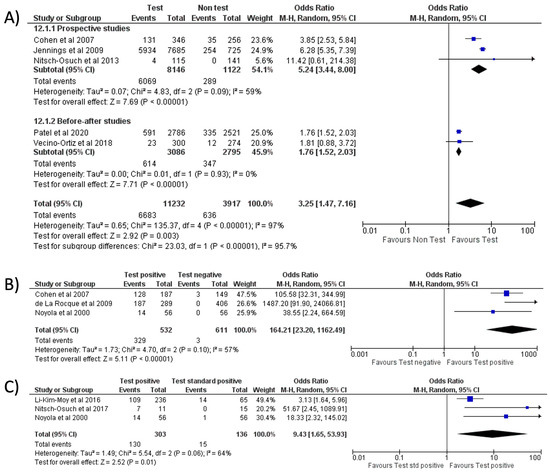
Figure 7.
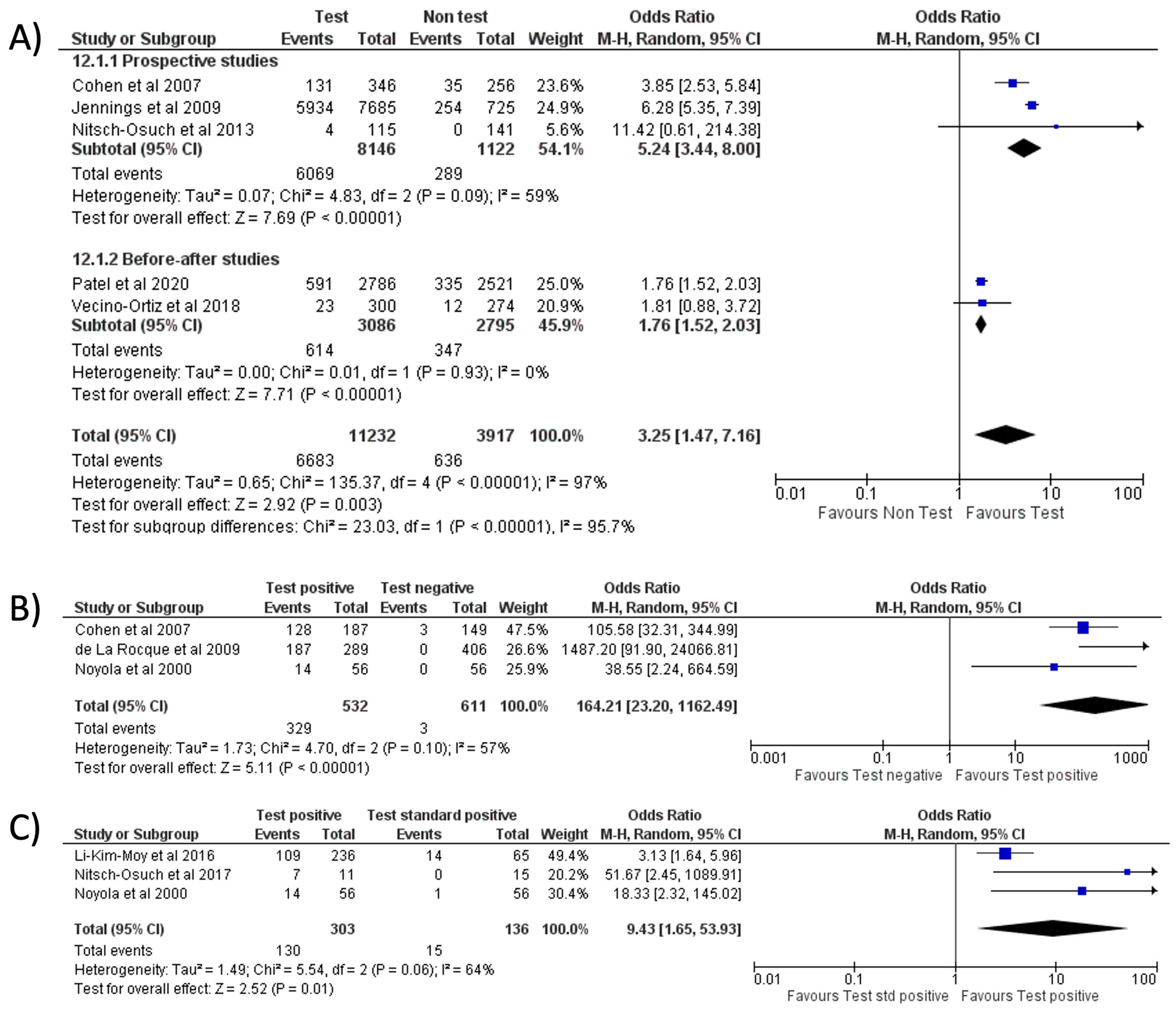
Forest plot of prescription of oseltamivir after implementation of RIDT: (A) RIDT vs. clinical diagnosis; (B) RIDT positive vs. RIDT negative; (C) RIDT positive vs. standard of care positive.
No significant difference in oseltamivir prescription was noted when comparing FA-RP to the standard test (OR = 0.70, 95% CI 0.22–2.24, p = 0.55; I2 = 84%) (Figure 6A) [32,33,46,48,51], which is confirmed in the sub-group analysis stratified by country (Europe and the US, see Figure S4A). When comparing the use of FA-RP vs. clinical diagnosis of influenza [38,47,49], there was an increased trend in prescriptions but without a significant difference (OR = 2.94, 95% CI 0.48–17.86, p = 0.24; I2 = 95%) (Figure 6B).
With respect to RIDT, all comparisons from the meta-analysis highlighted a significant increase in oseltamivir prescriptions: the use of RIDT vs. clinical diagnosis [9,21,25,26,28] tripled the odds of having prescribed oseltamivir (OR = 3.25, 95% CI 1.47–7.16, p = 0.003; I2 = 97%) (Figure 7A), while a more conclusive association emerged when comparing a positive RIDT result vs. negative (OR = 164.21, 95% CI 23.20–1162.49, p < 0.001; I2 = 57%) (Figure 7B) [12,28,29] and when evaluating a positive RIDT vs. a positive standard test (OR = 9.43, 95% CI 1.65–53.93, p = 0.01; I2 = 64%) (Figure 7C) [8,12,19].
2.2.3. Length of Stay
Thirty-four studies (34/57, 59.6%) evaluated the length of stay in relation to rapid tests or POCTs, with most of the studies conducted in the hospital setting (20/34 and 7/34 in ED other hospital wards, respectively). Almost half of the studies (16/34, 47.1%) reported a significant reduction in the length of stay with POCTs.
Data were not available from RIDT and FA-RP studies to conduct a meta-analysis.
2.2.4. Days of Therapy
Eighteen different studies (18/57, 31.6%), mostly conducted in the hospital wards (15/18, 83.3%), evaluated a potential change in days of therapy (DOT) related to rapid tests or POCT implementation.
In 61.1% of the studies a reduction in DOT was noted (11/18, 61%).
A meta-analysis was possible only for three observational studies [31,33,41], which compared the use of FA-RP vs. standard tests for the duration of therapy in inpatients. No significant difference was found (see Figure S4B). Data from RIDT studies were not available to conduct a meta-analysis for this outcome.
2.2.5. Healthcare Cost
The reduction of healthcare costs was less frequent analyzed; only eight studies, mostly conducted in the ED and hospital wards (6/8, 75.0%), reported data about costs (8/57, 14.0%). In only three studies (3/8, 37.5%) a significant reduction in costs was noted. No meta-analysis was conducted for this outcome.
2.3. Quality Assessment of Studies
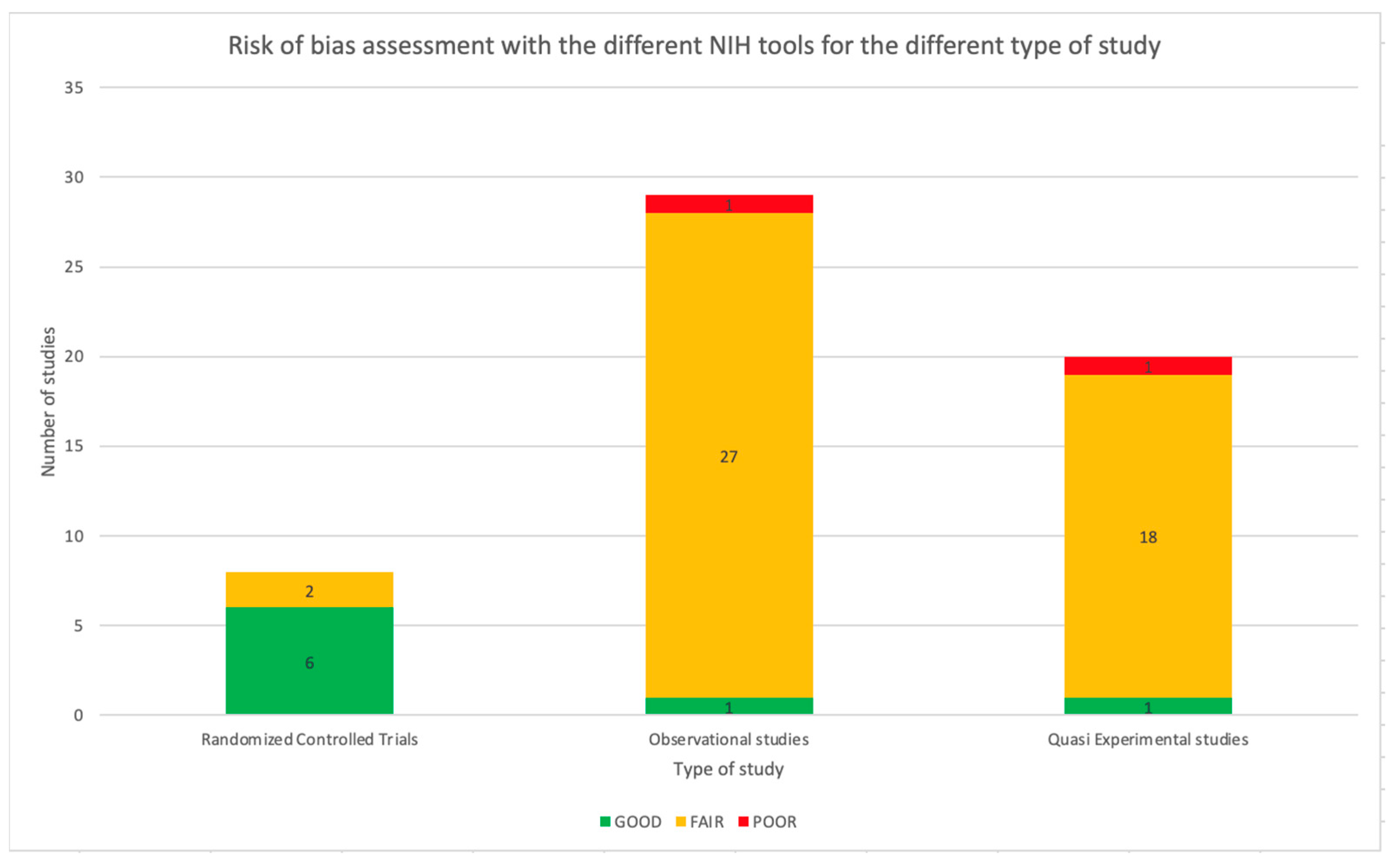
The NIH Quality Assessment Tool was applied to all 57 studies. As reported in Table 1, 47 studies (82.5%) were assessed as fair, two as poor, and eight as good using a specific tool based on the type of study. Referring to RCT, 75.0% of the studies were considered good (6/8). The remaining two studies were assessed as fair. Considering the observational studies, meant as cohort studies, prospective and retrospective studies, most of the papers were considered as fair (27/29, 93.1%) and one as poor (1/29, 3.4%). Only one study was assessed as good (1/29, 3.4%). Most of the before and after studies were considered fair (18/19, 94.7%), while the other study was assessed as poor (1/19, 5.3%). Figure 8 shows the distribution of the quality assessment for the different types of studies. The complete quality assessment of each study is reported in Tables S1–S4 in the Supplementary Material.
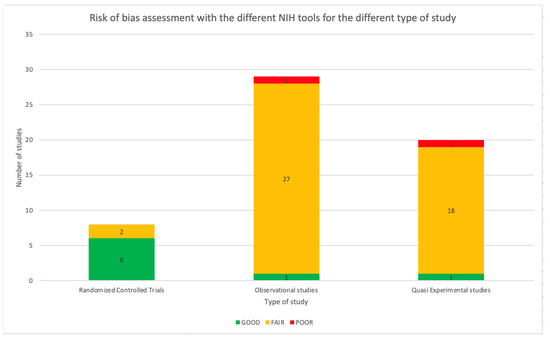
Figure 8.
Risk of bias assessment with the different NIH tools for the different types of studies.
3. Discussion
Rapid tests and POCTs are important tools to help clinicians discriminate the risk of infection and differentiate between viral and bacterial pathogens. Many rapid tests and POCTs are available nowadays, ranging from quick lab tests to microbiological arrays to even ultrasound bedside imaging protocols.
Advances in technology and recent innovations have allowed diagnostics to enlarge their scope, increase accuracy, minimize the time required for results and, above all, reduce their costs. This leads to the actual usage and applicability of these tests in routine diagnostic evaluation in adults [65]. When efficiently implemented into the diagnostic process, the most valuable characteristic of rapid tests is the shorter time to results, leading to more rapid clinical decisions than the standard test.
Respiratory, gastrointestinal, blood, and cerebrospinal fluid (CSF) arrays have been recently developed and evaluated for the diagnostic workup of outpatient, inpatient, and ED settings also in children, with no univocal interpretation of their utility. Despite these doubts, the potential of the new technologies and new diagnostics is still considered of great value [66].
Indeed, the chance to discriminate the presence of infection with more rapid tests is essential for developing institutional programs to enhance antimicrobial stewardship. As predictable, almost 80% of studies on rapid test implementation were performed after 2007. A further increase occurred during the last two years of the COVID-19 pandemic, with a quarter of studies conducted from 2019 to 2021 since differentiating between viral and bacterial infections has become more crucial [67].
Although rapid tests and POCTs have already been proven valuable and feasible in the adult population, both in outpatient and hospital settings [68,69], studies in children are still lacking.
To our knowledge, this is the first systematic review evaluating rapid tests and POCTs in pediatric settings worldwide and their impact on antimicrobial prescription, healthcare costs, and patient outcomes.
A wide heterogeneity in the use of rapid tests for febrile children with respiratory tract infections emerged. More than 75% of studies reported the implementation of POCTs in hospital settings. In outpatient and primary care practice, Strep A rapid test and influenza tests were the most commonly used POCTs. This is not surprising considering the need for a nearby laboratory to perform other tests such as FA-RP that require different procedures for the analysis of the sample (immunofluorescence, array, molecular probe, etc.). In contrast, the rapid antigen tests represent a quicker (15–30 min) tool that can be undertaken at the bedside. Nevertheless, their use still seems not as widespread compared to the in-hospital setting, and this could also be related to the costs of the POCT and the lack of diagnostic stewardship programs that involve general practitioners and pediatricians working in primary care practices.
Almost 80% of studies on rapid test or POCT usage have been performed in Europe and North America, with only a few in East Asia and none in Africa. Due to their simplicity and quick response, there is wide potential in the implementation of POCTs in low- and middle-income countries and in remote or resource-poor settings with no laboratory infrastructure. However, the simple availability of rapid or easy tests does not ensure their implementation [70,71]. As reported by Pai et al. [72], there are considerable barriers to the widespread use of POCTs at primary levels of the healthcare system, which could be related to the lack of training of physicians or of economic resources to buy them. Nevertheless, it is important to highlight that we did not consider for our review many POCTs that could be more useful in low- and middle-income countries than in high-income countries, such as HIV, HCV, malaria, and tuberculosis POCTs.
Rapid differentiation of respiratory infection in non-critically ill children is essential to positively affect the rate of antibiotic prescriptions. This outcome was assessed for the out-patient setting in 86% of all studies worldwide (80%, 89.5%, and 87.5% of studies in North America, Europe, and Asia, respectively). Regardless of setting, country, and type of test, prescriptions rate seems to be positively affected in 65.3% of cases by rapid tests or POCTs implementation.
More evidence on rapid tests and POCTs for respiratory pathogens in improving both antibacterial and antiviral prescriptions results from the meta-analysis of RIDT and FA-RP.
Different results are reported comparing a positive RIDT result with a negative one or comparing the use of RIDT, regardless of the results, to the clinical diagnosis. In the first case, assessing influenza virus as causative of the clinical presentation of the patients, leads to a reduction in antibiotic prescription. Instead, in the second case, the reduction in antibiotic prescription seems to be less significant. This could be related to different reasons, probably depending on physicians’ prescribing attitudes and intrinsic study limitations.
Even if no definite statement can be made, it is interesting to notice that the trend towards fewer prescriptions is stronger in Europe than in the USA. IDSA guidelines for influenza strengthen the efficacy of an empirical antibacterial therapy to be started in case of deterioration or failure to improve in patients with this diagnosis, especially those with comorbidities [73,74]. Moreover, in the US, antimicrobial stewardship programs have been widespread for longer, so the impact of rapid tests and POCTs in further reducing antibiotics may appear less significant.
Similar results can be extracted from studies evaluating FA-RP, confirming an overall reduction in antibiotic prescriptions. However, as displayed for RIDT, when comparing FA-RP to clinical diagnosis, the evidence for this outcome becomes less strong, despite results from two RCTs assessed as good through the NIH quality assessment rate. Unlike RIDT, this trend appears more significant in the US than in Europe, maybe because the prescription of antibiotics in Europe remains higher for respiratory non-influenza viruses for fear of possible bacterial co-infections [75,76,77]. For example, British guidelines for community-acquired pneumonia (CAP) suggest that all children with a clear clinical diagnosis of CAP should receive antibiotics as bacterial and viral pathogens cannot reliably be distinguished from each other, especially with persistent symptoms [78].
Interestingly, the two studies on the implementation of the rapid test for RSV showed no significant difference in the antibiotic prescription between those testing positive and those negative, suggesting that factors others than the specific etiology determined the decision about prescription. In the study of Thibeault [52], factors associated independently with the cessation of intravenous antibiotics in the case of a positive test for RSV were the age > 3 months and the absence of pneumonia. This is probable due to the fact that young infants are more likely to have severe outcomes than older ones and that the risk of bacterial coinfection could be perceived greater by physicians in case of presence of pneumonia. This is in line with what was found by a Spanish study based on a mail questionnaire, showing that the fear of complications from infections is one of the factors related to the prescribing of antibiotics by general practitioners even if not necessary. Indeed, many physicians agreed that when in doubt as to whether a patient had a bacterial infection, it was better to prescribe a broad-spectrum antibiotic [79,80].
It is important to highlight this point, because it is known that the implementation of these tests alone is often not enough to change antimicrobial prescription patterns [81].
Unfortunately, only six studies included in this review specified whether an antimicrobial stewardship program (ASP) was implemented at the same time of the introduction of the point of care test and in only one the introduction of a rapid test was concomitant with the implementation of an ASP. The implementation of educational interventions combined with the introduction of rapid tests could further improve the antibiotic prescription, as it is also emphasized by a review published in 2018 to ensure rational use of antibiotics [82].
The meta-analysis highlighted as the strongest evidence the improvement of oseltamivir prescription from overall use of RIDT. According to the IDSA guideline on managing seasonal influenza, oseltamivir should be prescribed to patients at high risk of complications from influenza. Regarding children, it is recommended for children aged < 5 years and especially aged < 2 years, children with hematological disease (including sickle cell disease), metabolic disease (diabetes, obesity), immunosuppression, and children and adolescents through 18 years who are receiving aspirin and who might be at risk of Reye Syndrome [73].
Analysis for FA-RP shows a trend toward a more appropriate prescription of antivirals, despite a significant difference. It could be related to the usefulness of this test for detecting a broad spectrum of pathogens rather than influenza only; therefore, this outcome can be limited by less a pre-test probability for clinical, seasonal, or epidemiological reasons.
Unfortunately, no significant data could be extrapolated from RIDT and FA-RP studies for meta-analysis regarding secondary outcomes considered in this review (DOTs, LOS, and healthcare costs). A trend in reduction of DOTs was only evaluated from three studies of FA-RP without a significant difference.
Almost half of the studies in our review reported an overall significant reduction of LOS using the rapid tests, particularly in ED, due to faster clinical decisions. Nevertheless, it is difficult to assess this outcome in hospitalized children because of other possible confounders (e.g., severity of symptoms, comorbidities, complications). For such patients, a decline in the duration of treatment seems to be more relevant. Interestingly, three-quarters of the studies reporting DOTs and LOS as outcomes were conducted in Europe (4/18, 22.2% and 9/34, 26.5%, respectively) and North America (9/18, 50.0% and 17/34, 50.0%, respectively).
The effects of rapid tests and POCTs on healthcare costs have been assessed in only one-third of studies, almost all conducted in hospital settings, with a positive correlation in terms of cost-saving. Whereas other outcomes (rate of prescriptions, LOS, or DOTs) can be easily measured, healthcare cost evaluation involves a more complex analysis to be performed by the authors. Thus, although rapid tests and POCTs should theoretically be cost-saving tools, less evidence emerged from this review that cannot be transferred to outpatient settings.
Finally, considerations can be made from the quality assessment of studies. Most RCTs have been assessed as good using the standardized NIH tool. Only two studies were considered fair, mainly due to an overall drop-out rate higher than 20% and an included population that had not reached the pre-planned sample size. In none of the studies, participants, providers, or people assessing the outcomes were blinded to the intervention. The majority of the observational and before and after studies have been judged as fair. Most of the studies did not have a pre-planned sample size calculation; therefore, it was not possible to assess the statistical power of the studies. Indeed, no studies reported the blinding of the outcome assessors and not all the potentially confounding variables were always measured and adjusted statistically.
It would be desirable that before and after studies, with preplanned sample size calculation or blinded, randomized, clinical trials would be proposed and conducted both in high and low–middle income countries.
Limitations
The limitations of this study are primarily related to the vast heterogeneity of studies focused on the use of rapid tests and POCTs in pediatric settings. Beyond the type of rapid tests evaluated, another source of variability was due to different brand names used for testing, which involves non-uniform reliability of tests in terms of sensitivity, specificity, and positive or negative predictive value. The authors also did not consider different pre-test probability related to the type of infection for most cases. Moreover, the majority of studies analyzed for this review were set in high-income countries, thus, results and conclusions cannot be broadened to low-resources countries.
All of this was also exacerbated by different modalities for outcome assessment which complicated the comparison between studies.
4. Materials and Methods
4.1. Study Design and Search Strategy
This systematic review is based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [83]. Embase, MEDLINE, and Cochrane Library databases were systematically searched, combining terms for “children”, “rapid test”, “Point of care test”, and “Outcome Assessment”, with restrictions on dates from 1 January 2000, to 11 September 2021. The full strategy is provided in Supplementary Material File S1.
The review protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews: Registration Number CRD 42022345124.
4.2. Inclusion Criteria and Outcomes
Studies evaluating the effect of the implementation of rapid tests and POCTs for respiratory tract infections that included patients younger than 21 years, both in outpatient or in-hospital settings, were considered for full-text review. Even if the child age is usually considered until 14 years old, we decided to include children until 21 years old because some centers accept these younger adults in the Pediatric Emergency Department.
POCTs were considered those tests for which the sample was analyzed near the patient itself, for example, the Rapid Influenza Diagnostic Test (RIDT) and Strep A test. Instead, rapid tests were those tests for which the sample was collected and then sent to a laboratory that analyzed it and gave results in few hours, for example, the film-array test or PCR for respiratory virus.
Randomized controlled trials, controlled and non-controlled before and after studies, controlled and non-controlled interrupted time series, and cohort studies conducted in all income level countries were included. The primary outcome considered for the final review was the change in the rate of antimicrobial prescriptions (antibacterial and/or antiviral); secondary outcomes included days of therapy (DOTs), length of stay (LOS), and healthcare costs.
4.3. Exclusion Criteria
Review, case series, notes and letters, conference abstracts, and opinion articles were excluded. Papers on both adults and children were also excluded if extraction of pediatric data was not possible. Papers on rapid tests and POCTs for HIV, malaria, tuberculosis, hepatitis B or C virus, parasites, or sexually transmitted infections were also excluded. Studies validating rapid tests’ or POCTs’ diagnostic accuracy or only with epidemiological analysis were excluded. Papers on rapid tests on blood, stool, or cerebrospinal fluid were excluded.
4.4. Study Selection
In line with the PRISMA guidelines for systemic reviews, titles and abstracts identified through an electronic database were independently screened by three reviewers (GB, AG, MM), and any references which did not meet the inclusion criteria were excluded. For all remaining references, three reviewers obtained full-text copies and independently examined them in detail to determine whether they met all the inclusion criteria for the review. Discussion with a fourth reviewer (DD) resolved any disagreement regarding study selection.
4.5. Data Collection
Data were extracted using a standardized data collection form. The following data from each included paper were extracted, where reported: study characteristics (authors, year of publication, study design, study location, multicenter involvement, country); patient characteristics (age, care setting, inclusion and exclusion criteria); type of rapid test or POCT; main results with accuracy measures; outcomes (e.g., rate of antibiotic and antiviral prescription, days of therapy); patient care (e.g., length of stay); economic outcomes.
The income level of each country was defined according to the World Bank List of economies published in July 2021 [7].
4.6. Risk of Bias in Individual Studies
Three reviewers independently rated the quality of the included studies using the National Institutes of Health (NIH) Quality Assessment Tool [84]. Based on the type of study, a different NIH tool was used. The overall assessment was good, fair, or poor. The tools with 14 questions were classified as poor if the score was between 0 and 5, fair if between 6 and 10, and good if between 11 and 14. The tools with 12 questions were classified as poor if the score was between 0 and 4, fair if between 5 and 8, and good if between 9 and 12.
4.7. Statistical Analysis
When sufficient outcome data were available, we performed meta-analyses. The effect of the intervention was evaluated using the dichotomized measure of odds ratio (OR) and a mean difference (MD) value when different outcome measurements were present for continuous outcomes, along with 95% confidence intervals (CI). Heterogeneity between study-specific estimates was tested using chi-squared statistics and measured with the I2 index (a measure of the percentage variation across the studies caused by heterogeneity) and the Cochran’s Q test [85,86]. A pooled estimate was obtained by fitting the DerSimonian and Laird random-effects model [87], which takes into account both the sampling variance within the studies and the variation in the underlying effect across studies. Separated analyses were performed depending on the study design, observational or randomized controlled studies. Furthermore, subgroup analyses were planned (1) by age group (e.g., less than 5 years or 18 years) and (2) geographical locations (e.g., USA or European countries). All tests were considered significant statistically, for p < 0.05. The analyses were performed using Review Manager Version 5.4 (Cochrane Collaboration, London, UK).
5. Conclusions
The findings of this systematic review seem to support the implementation of rapid tests and POCTs as a valuable tool to improve antimicrobial prescribing, both reducing unnecessary antibiotics administration and the duration of therapy and increasing appropriate oseltamivir usage. Furthermore, even if it was not possible to perform a metanalysis, the use of rapid tests also seems to be useful in reducing turnaround time and length of stay, particularly in hospital settings.
However, more well-designed studies are still needed to reliably assess the implementation of rapid tests and POCTs in pediatric settings, both in high and low-middle income countries, in order to improve patients’ outcomes and reduce unnecessary prescriptions and healthcare costs.
It is also advocated that implementation of rapid tests should be constantly combined within well-structured antimicrobial stewardship programs, as recommended by international societies for infection and antibiotic resistance control, to improve antimicrobial prescription further.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11091192/s1, Supplementary Material File S1: Search strategy; Figure S1: Forest plot of prescription of antibiotics after implementation of FA-RP; Figure S2: Forest plot of prescription of antibiotics after implementation of RIDT; Figure S3: Forest plot of prescription of antibiotics after implementation of RIDT; Figure S4: Forest plot (A) of prescription of oseltamivir after implementation of FA-RP versus standard test stratified for different countries; (B) of days of therapy after implementation of FA-RP versus standard test; Table Supplementary: NIH assessment tools.
Author Contributions
D.D., G.B. and A.G. conceived the idea. G.B., A.G. and M.M. conducted the assessments of the titles, abstracts, and full texts independently. D.D., E.B., G.B., A.G., M.M. and S.R. discussed study selection and study details and decided which studies were to be included in the review. G.B., A.G. and M.M. performed the numerical calculations and the assessment of bias. A.B., A.C. performed statistical analysis. G.B., A.G. and M.M. wrote the manuscript with input from all authors. D.D. and E.B. supervised and revised the manuscript. C.G. and L.D.D. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Italian Ministry of the Education, University and Research (‘PRIN’ 2017, project 2017728JPK. Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the MOTIVE Project).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sands, R.; Shanmugavadivel, D.; Stephenson, T.; Wood, D. Medical problems presenting to paediatric emergency departments: 10 Years on. Emerg. Med. J. 2012, 29, 379–382. [Google Scholar] [CrossRef]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar]
- Hecker, M.T.; Aron, D.C.; Patel, N.P.; Lehmann, M.K.; Donskey, C.J. Unnecessary Use of Antimicrobials in Hospitalized Patients. Arch. Intern. Med. 2003, 163, 972–978. [Google Scholar] [CrossRef]
- Messacar, K.; Gaensbauer, J.T.; Birkholz, M.; Palmer, C.; Todd, J.K.; Tyler, K.L.; Dominguez, S.R. Impact of FilmArray meningitis encephalitis panel on HSV testing and empiric acyclovir use in children beyond the neonatal period. Diagn. Microbiol. Infect. Dis. 2020, 97, 115085. [Google Scholar] [CrossRef]
- Samuel, L. Point-of-Care Testing in Microbiology. Clin. Lab. Med. 2020, 40, 483–494. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- New World Bank Country Classifications by Income Level: 2021–2022. Available online: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2021-2022 (accessed on 22 February 2022).
- Nitsch-Osuch, A.; Kuchar, E.; Gołębiak, I.; Kanecki, K.; Tarka, P.; Brydak, L.B. Rapid influenza diagnostic tests improve suitability of antiviral treatment in hospitalized children. Adv. Exp. Med. Biol. 2017, 968, 1–6. [Google Scholar]
- Vecino-Ortiz, A.I.; Goldenberg, S.D.; Douthwaite, S.T.; Cheng, C.-Y.; Glover, R.E.; Mak, C.; Adams, E.J. Impact of a multiplex PCR point-of-care test for influenza A/B and respiratory syncytial virus on an acute pediatric hospital ward. Diagn. Microbiol. Infect. Dis. 2018, 91, 331–335. [Google Scholar] [CrossRef]
- Abanses, J.C.; Dowd, M.D.; Simon, S.D.; Sharma, V. Impact of rapid influenza testing at triage on management of febrile infants and young children. Pediatr. Emerg. Care 2006, 22, 145–149. [Google Scholar] [CrossRef]
- Diallo, D.; Hochart, A.; Lagree, M.; Dervaux, B.; Martinot, A.; Dubos, F. Impact of the Sofia® Influenza A+B FIA rapid diagnostic test in a pediatric emergency department. Arch. Pediatr. 2019, 26, 6–11. [Google Scholar]
- Noyola, D.E.; Demmler, G.J. Effect of rapid diagnosis on management of influenza A infections. Pediatr. Infect. Dis. J. 2000, 19, 303–307. [Google Scholar] [CrossRef]
- Özkaya, E.; Cambaz, N.; Çoşkun, Y.; Mete, F.; Geyik, M.; Samanci, N. The effect of rapid diagnostic testing for influenza on the reduction of antibiotic use in paediatric emergency department. Acta Paediatr. Int. J. Paediatr. 2009, 98, 1589–1592. [Google Scholar] [CrossRef]
- Bonner, A.B.; Monroe, K.W.; Talley, L.I.; Klasner, A.E.; Kimberlin, D.W. Impact of the Rapid Diagnosis of Influenza on Physician Decision-Making and Patient Management in the Pediatric Emergency Department: Results of a Randomized, Prospective, Controlled Trial. Pediatrics 2003, 112, 363–367. [Google Scholar] [CrossRef]
- Cantais, A.; Mory, O.; Plat, A.; Bourmaud, A.; Giraud, A.; Costille, M.; Pozzetto, B.; Pillet, S. Impact of bedside diagnosis of influenza in the paediatric emergency ward. Clin. Microbiol. Infect. 2018, 25, 898–903. [Google Scholar]
- Iyer, S.B.; Gerber, M.A.; Pomerantz, W.J.; Mortensen, J.E.; Ruddy, R.M. Effect of Point-of-care Influenza Testing on Management of Febrile Children. Acad. Emerg. Med. 2006, 13, 1259–1268. [Google Scholar]
- Jacob, R.; White, B.; McCaskill, M.E. Does location of rapid influenza diagnostic testing influence treatment time and ancillary testing in a paediatric emergency department? EMA-Emerg. Med. Australas. 2021, 33, 88–93. [Google Scholar] [CrossRef]
- Jun, S.H.; Kim, J.Y.; Yoon, Y.H.; Lim, C.S.; Ch, H.J.; Choi, S.H. The effect of the rapid antigen test for influenza on clinical practice in the emergency department: A comparison of periods before and after the 2009 H1N1 influenza pandemic. Signa Vitae 2016, 11, 74–89. [Google Scholar]
- Li-Kim-Moy, J.; Dastouri, F.; Rashid, H.; Khandaker, G.; Kesson, A.; McCaskill, M.; Wood, N.; Jones, C.; Zurynski, Y.; Macartney, K.; et al. Utility of early influenza diagnosis through point-of-care testing in children presenting to an emergency department. J. Paediatr. Child Health 2016, 52, 422–429. [Google Scholar]
- Sharma, V.; Dowd, M.D.; Slaughter, A.J.; Simon, S.D. Effect of rapid diagnosis of influenza virus type A on the emergency department management of febrile infants and toddlers. Arch. Pediatr. Adolesc. Med. 2002, 156, 41–43. [Google Scholar] [CrossRef][Green Version]
- Patel, P.; Laurich, V.M.; Smith, S.; Sturm, J. Point-of-care influenza testing in the pediatric emergency department. Pediatr. Emerg. Care 2020, 36, 515–518. [Google Scholar] [CrossRef]
- Pierron, S.; Haas, H.; Berlioz, M.; Ollier, L.; Albertini, M. Impact of rapid influenza test during influenza epidemic in all febrile children less than 6 years old in a pediatric emergency departmentl. Arch. Pediatr. Organe Off. Soc. Fr. Pediatr. 2008, 15, 1283–1288. [Google Scholar] [CrossRef]
- Benito-Fernández, J.; Vázquez-Ronco, M.A.; Morteruel-Aizkuren, E.; Mintegui-Raso, S.; Sánchez-Etxaniz, J.; Fernández-Landaluce, A. Impact of rapid viral testing for influenza A and B viruses on management of febrile infants without signs of focal infection. Pediatr. Infect. Dis. J. 2006, 25, 1153–1157. [Google Scholar] [CrossRef]
- Poehling, K.A.; Zhu, Y.; Tang, Y.W.; Edwards, K. Accuracy and impact of a point-of-care rapid influenza test in young children with respiratory illnesses. Arch. Pediatr. Adolesc. Med. 2006, 160, 713–718. [Google Scholar] [CrossRef]
- Jennings, L.C.; Skopnik, H.; Burckhardt, I.; Hribar, I.; del Piero, L.; Deichmann, K.A. Effect of rapid influenza testing on the clinical management of paediatric influenza. Influenza Other Respi. Viruses 2009, 3, 91–98. [Google Scholar] [CrossRef]
- Nitsch-Osuch, A.; Stefańska, I.; Kuchar, E.; Brydak, L.B.; Pirogowicz, I.; Życińska, K.; Wardyn, K. Influence of rapid influenza test on clinical management of children younger than five with febrile respiratory tract infections. Adv. Exp. Med. Biol. 2013, 755, 237–241. [Google Scholar] [PubMed]
- van Esso, D.L.; Valente, A.M.; Vilà, M.; Casanovas, J.M.; de Quixano, M.; Rodrigo, C.; Anton, A.; Pumarola, T. Rapid Influenza Testing in Infants and Children Younger than 6 Years in Primary Care: Impact on Antibiotic Treatment and Use of Health Services. Pediatr. Infect. Dis. J. 2019, 38, E187–E189. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Thollot, F.; Lécuyer, A.; Koskas, M.; Touitou, R.; Boucherat, M.; D’Athis, P.; Corrard, F.; Pecking, M.; de La Rocque, F. Impact des tests de diagnostic rapide en ville dans la prise en charge des enfants en période de grippe. Arch. Pediatr. 2007, 14, 926–931. [Google Scholar] [CrossRef]
- de La Rocque, F.; Lecuyer, A.; Wollner, C.; d’Athis, P.; Pecking, M.; Thollot, F.; Cohen, R. Impact des tests de diagnostic rapide de la grippe dans la prise en charge des enfants en période d’épidémie en pédiatrie de ville. Arch. Pediatr. 2009, 16, 288–293. [Google Scholar] [CrossRef]
- Keske, Ş.; Ergönül, Ö.; Tutucu, F.; Karaaslan, D.; Palaoğlu, E.; Can, F. The rapid diagnosis of viral respiratory tract infections and its impact on antimicrobial stewardship programs. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 779–783. [Google Scholar] [CrossRef]
- Kitano, T.; Nishikawa, H.; Suzuki, R.; Onaka, M.; Nishiyama, A.; Kitagawa, D.; Oka, M.; Masuo, K.; Yoshida, S. The impact analysis of a multiplex PCR respiratory panel for hospitalized pediatric respiratory infections in Japan. J. Infect. Chemother. 2019, 26, 82–85. [Google Scholar] [CrossRef]
- Lee, B.R.; Hassan, F.; Anne, M.; Selvarangan, R. Impact of multiplex molecular assay turn-around-time on antibiotic utilization and clinical management of hospitalized children with acute respiratory tract infections. J. Clin. Virol. 2018, 110, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Reischl, A.T.; Schreiner, D.; Poplawska, K.; Kidszun, A.; Zepp, F.; Gröndahl, B.; Gehring, S. The clinical impact of PCR-based point-of-care diagnostic in respiratory tract infections in children. J. Clin. Lab. Anal. 2020, 34, e23203. [Google Scholar] [CrossRef] [PubMed]
- Schulert, G.S.; Lu, Z.; Wingo, T.; Tang, Y.W.; Saville, B.R.; Hain, P.D. Role of a respiratory viral panel in the clinical management of pediatric inpatients. Pediatr. Infect. Dis. J. 2013, 32, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Subramony, A.; Zachariah, P.; Krones, A.; Whittier, S.; Saiman, L. Impact of multiplex polymerase chain reaction testing for respiratory pathogens on healthcare resource utilization for pediatric inpatients. J. Pediatr. 2016, 173, 196–201.e2. [Google Scholar] [CrossRef] [PubMed]
- Walls, T.; Stark, E.; Pattemore, P.; Jennings, L. Missed opportunities for antimicrobial stewardship in pre-school children admitted to hospital with lower respiratory tract infection. J. Paediatr. Child Health 2017, 53, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hatachi, T.; Okamoto, Y.; Aoki, Y.; Kyogoku, M.; Miyashita, K.M.; Inata, Y.; Shimizu, Y.; Fujiwara, F.; Takeuchi, M. Application of Multiplex Polymerase Chain Reaction for Pathogen Identification and Antibiotic Use in Children With Respiratory Infections in a PICU. Pediatr. Crit. Care Med. 2021, 22, e644–e648. [Google Scholar] [CrossRef]
- McCulloh, R.J.; Andrea, S.; Reinert, S.; Chapin, K. Potential utility of multiplex amplification respiratory viral panel testing in the management of acute respiratory infection in children: A retrospective analysis. J. Pediatr. Infect. Dis. Soc. 2014, 3, 146–153. [Google Scholar] [CrossRef]
- McFall, C.; Salimnia, H.; Lephart, P.; Thomas, R.; McGrath, E. Impact of Early Multiplex FilmArray Respiratory Pathogen Panel (RPP) Assay on Hospital Length of Stay in Pediatric Patients Younger Than 3 Months Admitted for Fever or Sepsis Workup. Clin. Pediatr. 2018, 57, 1224–1226. [Google Scholar] [CrossRef]
- Tam, P.Y.I.; Zhang, L.; Cohen, Z. Impact of a transition from respiratory virus shell vial to multiplex PCR on clinical outcomes and cost in hospitalized children. Children 2017, 4, 3. [Google Scholar]
- Kim, Y.K.; Lee, J.H.; Kim, S.Y.; Ahn, J.Y.; Choi, K.H.; Lee, Y.H.; Jang, K.M.; Hau, Y.S.; Lee, J.M. Rapid Molecular Tests for Detecting Respiratory Pathogens Reduced the Use of Antibiotics in Children. Antibiotics 2021, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.B.; Shankar, P.; Jerris, R.C.; Kotzbauer, D.; Anderson, E.J.; Watson, J.R.; O’Brien, L.A.; Uwindatwa, F.; McNamara, K.; Bost, J.E. Impact of a Rapid Respiratory Panel Test on Patient Outcomes. Arch. Pathol. Lab. Med. 2014, 139, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Busson, L.; Bartiaux, M.; Brahim, S.; Konopnicki, D.; Dauby, N.; Gérard, M. Contribution of the FilmArray Respiratory Panel in the management of adult and pediatric patients attending the emergency room during 2015–2016 influenza epidemics: An interventional study. Int. J. Infect. Dis. 2019, 83, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Byington, C.L.; Castillo, H.; Gerber, K.; Daly, J.A.; Brimley, L.A.; Adams, S.; Christenson, J.C.; Pavia, A.T. The Effect of Rapid Respiratory Viral Diagnostic Testing on Antibiotic Use in a Children’s Hospital. Arch. Pediatr. Adolesc. Med. 2002, 156, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.; Xu, M.; Slaughter, J.C.; Willis, J.; Browning, W.; Estrada, C.; Gay, J.; Thomas, G.; Benton, A.; Quinn, C.; et al. Impact of clinical guidance and rapid molecular pathogen detection on evaluation and outcomes of febrile or hypothermic infants. Infect. Control Hosp. Epidemiol. 2020, 41, 1285–1291. [Google Scholar] [CrossRef]
- Dimopoulou, D.; Vourli, S.; Douros, K.; Pournaras, S.; Papaevangelou, V. Use of point-of-care molecular tests reduces hospitalization and oseltamivir administration in children presenting with influenza-like illness. J. Med. Virol. 2021, 93, 3944–3948. [Google Scholar] [CrossRef]
- Rao, S.; Lamb, M.M.; Moss, A.; Mistry, R.D.; Grice, K.; Ahmed, W.; Santos-Cantu, D.; Kitchen, E.; Patel, C.; Ferrari, I. Effect of Rapid Respiratory Virus Testing on Antibiotic Prescribing among Children Presenting to the Emergency Department with Acute Respiratory Illness: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2111836. [Google Scholar] [CrossRef]
- Echavarría, M.; Marcone, D.; Querci, M.; Seoane, A.; Ypas, M.; Videla, C.; O’Farrell, C.; Vidaurreta, S.; Ekstrom, J.; Carballal, G. Clinical impact of rapid molecular detection of respiratory pathogens in patients with acute respiratory infection. J. Clin. Virol. 2018, 108, 90–95. [Google Scholar] [CrossRef]
- May, L.; Tatro, G.; Poltavskiy, E.; Mooso, B.; Hon, S.; Bang, H.; Polage, C. Rapid Multiplex Testing for Upper Respiratory Pathogens in the Emergency Department: A Randomized Controlled Trial. Open Forum Infect. Dis. 2019, 6, ofz481. [Google Scholar] [CrossRef]
- Wishaupt, J.O.; Russcher, A.; Smeets, L.C.; Versteegh, F.G.A.; Hartwig, N.G. Clinical Impact of RT-PCR for Pediatric Acute Respiratory Infections: A Controlled Clinical Trial. Pediatrics 2011, 128, e1113–e1120. [Google Scholar] [CrossRef]
- Beal, S.G.; Posa, M.; Gaffar, M.; Reppucci, J.; Mack, J.A.; Gurka, M.J.; Rand, K.; Houck, H.; Kelly, M.N. Performance and Impact of a CLIA-waived, Point-of-care Respiratory PCR Panel in a Pediatric Clinic. Pediatr. Infect. Dis. J. 2020, 39, 188–191. [Google Scholar] [CrossRef]
- Thibeault, R.; Gilca, R.; Côté, S.; de Serres, G.; Boivin, G.; Déry, E.P. Antibiotic use in children is not influenced by the result of rapid antigen detection test for the respiratory syncytial virus. J. Clin. Virol. 2007, 39, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.; Schroeder, L.; Sinclair, K.; Patel, L.; Dowd, D. The Effect of Early Knowledge of Respiratory Syncytial Virus Positivity on Medical Decision Making and Throughput Time within the Pediatric Emergency Department. Pediatr. Emerg. Care 2020, 36, 134–137. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K.; Jones, K. Rapid testing for respiratory viruses: Impact on antibiotic use and time to patient discharge. Infect. Dis. Health 2019, 24, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Chang, Y.C.; Feemster, K.; Cárdenas, A.M. Implementation of a rapid influenza A/B and RSV direct molecular assay improves emergency department oseltamivir use in paediatric patients. J. Med. Microbiol. 2018, 67, 358–363. [Google Scholar] [CrossRef]
- Schneider, U.V.; Holm MK, A.; Bang, D.; Petersen, R.F.; Mortensen, S.; Trebbien, R.; Lisby, J.G. Point-of-care tests for influenza A and B viruses and RSV in emergency departments-indications, impact on patient management and possible gains by syndromic respiratory testing, Capital Region, Denmark, 2018. Eurosurveillance 2020, 25, 1900430. [Google Scholar]
- Hayashi, D.; Akashi, Y.; Suzuki, H.; Shiigai, M.; Kanemoto, K.; Notake, S.; Ishiodori, T.; Ishikawa, H.; Imai, H. Implementation of Point-of-Care Molecular Diagnostics for Mycoplasma pneumoniae Ensures the Correct Antimicrobial Prescription for Pediatric Pneumonia Patients. Tohoku J. Exp. Med. 2018, 246, 225–231. [Google Scholar] [CrossRef]
- Ayanruoh, S.; Waseem, M.; Quee, F.; Humphrey, A.; Reynolds, T. Impact of rapid streptococcal test on antibiotic use in a pediatric emergency department. Pediatr. Emerg. Care 2009, 25, 748–750. [Google Scholar] [CrossRef]
- Bird, C.; Winzor, G.; Lemon, K.; Moffat, A.; Newton, T.; Gray, J. A Pragmatic Study to Evaluate the Use of a Rapid Diagnostic Test to Detect Group A Streptococcal Pharyngitis in Children with the Aim of Reducing Antibiotic Use in a UK Emergency Department. Pediatr. Emerg. Care 2021, 37, E249–E251. [Google Scholar] [CrossRef]
- Halverson, K.A.; Milner, D. Implementation of Point-of-Care Testing in the Emergency Department. Point Care J. Near-Patient Test. Technol. 2011, 10, 116–119. [Google Scholar] [CrossRef]
- Kose, E.; Kose, S.S.; Akca, D.; Yildiz, K.; Elmas, C.; Baris, M.; Anil, M. The Effect of Rapid Antigen Detection Test on Antibiotic Prescription Decision of Clinicians and Reducing Antibiotic Costs in Children with Acute Pharyngitis. J. Trop. Pediatr. 2016, 62, 308–315. [Google Scholar] [CrossRef][Green Version]
- Małecki, M.; Mazur, A.; Sobolewski, M.; Binkowska-Bury, M.; Marć, M.; Januszewicz, P. Rapid strip tests as a decision-making tool about antibiotic treatment in children–A prospective study. Pediatr. Polska 2017, 92, 149–155. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Tsagris, V.; Antoniadou, A.; Galani, L.; Douros, C.; Katsarolis, I.; Maragos, A.; Raftopoulos, V.; Biskini, P.; Kanellakopoulou, K.; et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J. Antimicrob. Chemother. 2008, 62, 1407–1412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rao, A.; Berg, B.; Quezada, T.; Fader, R.; Walker, K.; Tang, S.; Cowen, U.; Duncan, D.; Sickler, J. Diagnosis and antibiotic treatment of group a streptococcal pharyngitis in children in a primary care setting: Impact of point-of-care polymerase chain reaction. BMC Pediatr. 2019, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.V. Advances in Diagnostic Testing that Impact Infection Prevention and Antimicrobial Stewardship Programs. Curr. Infect. Dis. Rep. 2019, 21, 6. [Google Scholar] [CrossRef]
- Martínez-González, N.A.; Keizer, E.; Plate, A.; Coenen, S.; Valeri, F.; Verbakel, J.Y.J.; Rosemann, T.; Neuner-Jehle, S.; Senn, O. Point-of-care c-reactive protein testing to reduce antibiotic prescribing for respiratory tract infections in primary care: Systematic review and meta-analysis of randomised controlled trials. Antibiotics 2020, 9, 610. [Google Scholar] [CrossRef]
- Lippi, G.; Nocini, R.; Mattiuzzi, C.; Henry, B.M. FebriDx for rapid screening of patients with suspected COVID-19 upon hospital admission: Systematic literature review and meta-analysis. J. Hosp. Infect. 2022, 123, 61–66. [Google Scholar] [CrossRef]
- Egilmezer, E.; Walker, G.J.; Bakthavathsalam, P.; Peterson, J.R.; Gooding, J.J.; Rawlinson, W.; Stelzer-Braid, S. Systematic review of the impact of point-of-care testing for influenza on the outcomes of patients with acute respiratory tract infection. Rev. Med Virol. 2018, 28, e1995. [Google Scholar] [CrossRef]
- Marrie, T.J.; Lau, C.Y.; Wheeler, S.L.; Wong, C.J.; Vandervoort, M.K.; Feagan, B.G. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA 2000, 283, 749–755. [Google Scholar] [CrossRef]
- Asiimwe, C.; Kyabayinze, D.; Kyalisiima, Z.; Nabakooza, J.; Bajabaite, M.; Counihan, H.; Tibenderana, J.K. Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators. Implement. Sci. 2012, 7, 5. [Google Scholar] [CrossRef]
- Schito, M.; Peter, T.F.; Cavanaugh, S.; Piatek, A.S.; Young, G.J.; Alexander, H.; Coggin, W.; Domingo, G.; Ellenberger, D.; Ermantraut, E.; et al. Opportunities and Challenges for Cost-Efficient Implementation of New Point-of-Care Diagnostics for HIV and Tuberculosis. J. Infect. Dis. 2012, 205, S169–S180. [Google Scholar] [CrossRef]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- and Middle-Income Countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M., Jr.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin. Infect. Dis. IDSA Guidel. 2019, 30, 97–98. [Google Scholar]
- Tse, J.; Near, A.M.; Cheng, M.; Karichu, J.; Lee, B.; Chang, S.N. Outpatient Antibiotic and Antiviral Utilization Patterns in Patients Tested for Respiratory Pathogens in the United States: A Real-World Database Study. Antibiotics 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Cheysson, F.; Brun-Buisson, C.; Opatowski, L.; Le Fouler, L.; Caserio-Schönemann, C.; Pontais, I.; Guillemot, D.; Watier, L. Outpatient antibiotic use attributable to viral acute lower respiratory tract infections during the cold season in France, 2010–2017. Int. J. Antimicrob. Agents 2021, 57, 106339. [Google Scholar] [CrossRef]
- Thompson, P.L.; Spyridis, N.; Sharland, M.; Gilbert, R.; Saxena, S.; Long, P.; Johnson, A.P.; Wong, I.C.K. Changes in clinical indications for community antibiotic prescribing for children in the UK from 1996 to 2006: Will the new NICE prescribing guidance on upper respiratory tract infections just be ignored? Arch. Dis. Child. 2009, 94, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.; Malcolm, W.; McMenamin, J.; Reynolds, A.; Guttmann, A.; Hardelid, P. Community-Based Antibiotic Prescribing Attributable to Respiratory Syncytial Virus and Other Common Respiratory Viruses in Young Children: A Population-Based Time-series Study of Scottish Children. Clin. Infect. Dis. 2021, 72, 2144–2153. [Google Scholar] [PubMed]
- Harris, M.; Clark, J.; Coote, N.; Fletcher, P.; Harnden, A.; Mckean, M.; Thomson, A.; on behalf of British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax 2011, 66 (Suppl. S2), ii1–ii23. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, C.; López-Vázquez, P.M.; Vazquez-Lago, J.; Piñeiro-Lamas, M.; Herdeiro, M.T.; Arzamendi, P.C.; Figueiras, A.; GREPHEPI Group. Effect of Physicians’ Attitudes and Knowledge on the Quality of Antibiotic Prescription: A Cohort Study. PLoS ONE 2015, 10, e0141820. [Google Scholar]
- Borg, M.A.; Camilleri, L. Broad-spectrum antibiotic use in Europe: More evidence of cultural influences on prescribing behaviour. J. Antimicrob. Chemother. 2019, 74, 3379–3383. [Google Scholar] [CrossRef]
- Zeitler, K.; Narayanan, N. The Present and Future State of Antimicrobial Stewardship and Rapid Diagnostic Testing: Can One Ideally Succeed Without the Other? Curr. Treat. Options Infect. Dis. 2019, 11, 177–187. [Google Scholar] [CrossRef]
- Machowska, A.; Lundborg, C.S. Drivers of irrational use of antibiotics in Europe. Int. J. Environ. Res. Public Health 2019, 16, 27. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- NIH Tool for Bias Assessment. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 3 February 2022).
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Der Simonian, N.; Lair, R. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).