Systemic Uptake of Oxytetracycline and Streptomycin in Huanglongbing-Affected Citrus Groves after Foliar Application and Trunk Injection
Abstract
:1. Introduction
2. Results
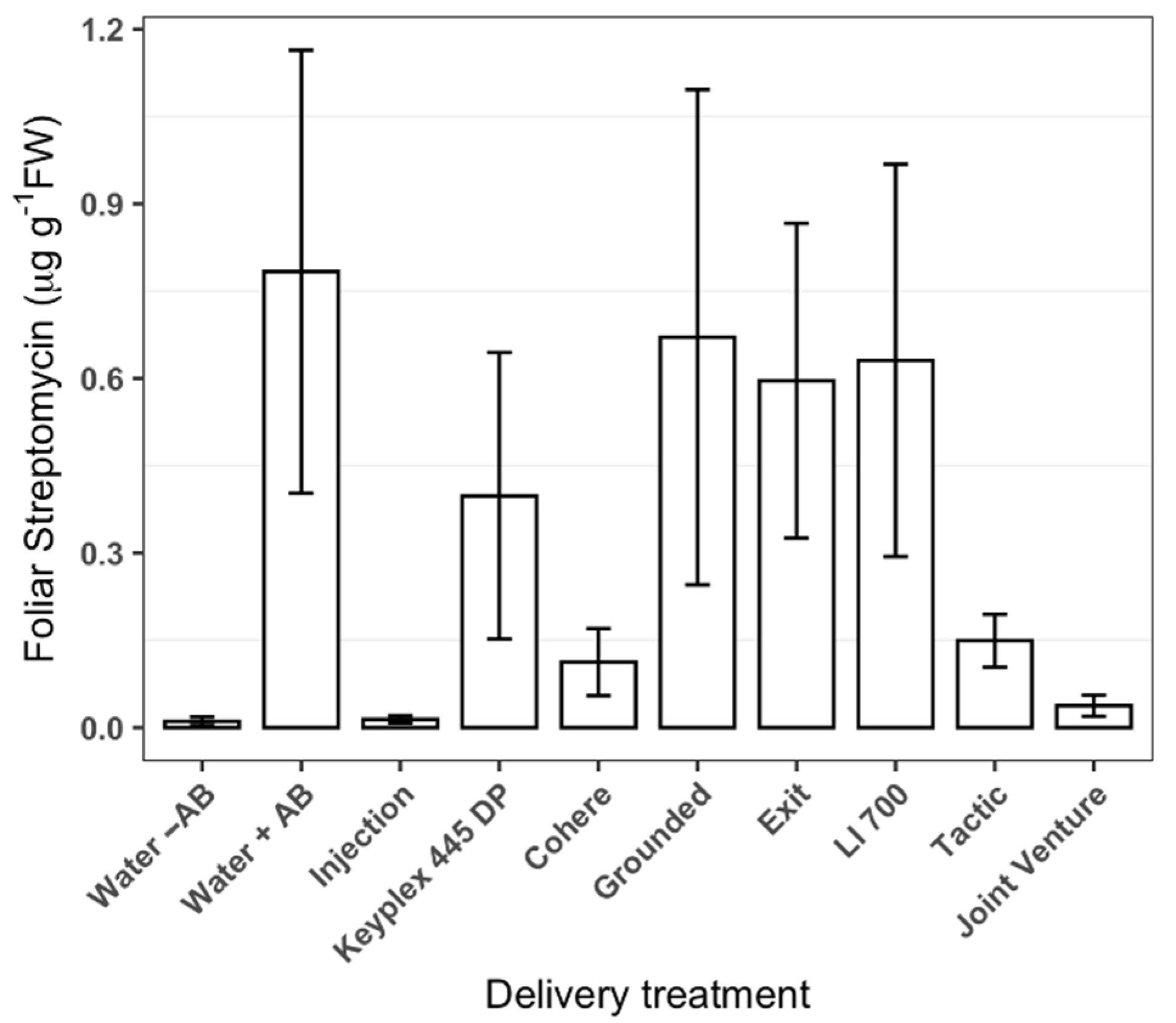
2.1. Effect of Adjuvants on the Uptake of Streptomycin (Study 1)
2.2. Comparison of Oxytetracycline and Streptomycin Delivery (Study 2)
3. Discussion
4. Material and Methods
4.1. Study 1: Effect of Adjuvants on Delivery of Streptomycin
4.1.1. Plant Material
4.1.2. Experimental Design
4.1.3. Treatments
4.2. Study 2: Comparison of Streptomycin and Oxytetracycline Delivery
4.2.1. Plant Material
4.2.2. Experimental Design
4.2.3. Extraction and Analysis of Oxytetracycline and Streptomycin
4.2.4. ‘Ca. L. asiaticus’ Detection
4.2.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bové, J.M. Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Singerman, A.; Burani-Arouca, M.; Futch, S.H. The Profitability of New Citrus Plantings in Florida in the Era of Huanglongbing. HortScience 2018, 53, 1655–1663. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Vincent, C.I.; Urbaneja, A.; Machado, M.A. Editorial: Unravelling Citrus Huanglongbing Disease. Front. Plant Sci. 2020, 11, 609655. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, C.; Brlansky, R.H.; Gmitter, F.G., Jr.; Li, Z.-G. Changes in carbohydrate metabolism in Citrus sinensis infected with ‘Candidatus Liberibacter asiaticus’. Plant Pathol. 2010, 59, 1037–1043. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of “Candidatus Liberibacter asiaticus” root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Wu, J.; Johnson, E.G.; Gerberich, K.M.; Bright, D.B.; Graham, J.H. Contrasting canopy and fibrous root damage on Swingle citrumelo caused by ‘Candidatus Liberibacter asiaticus’ and Phytophthora nicotianae. Plant Pathol. 2018, 67, 202–209. [Google Scholar] [CrossRef]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Deng, H.; Achor, D.; Exteberria, E.; Yu, Q.; Du, D.; Stanton, D.; Liang, G.; Gmitter, F.G. Phloem regeneration is a mechanism for huanglongbing-tolerance of “bearss” lemon and “LB8-9” sugar belle® mandarin. Front. Plant Sci. 2019, 10, 277. [Google Scholar] [CrossRef]
- Yang, C.; Powell, C.A.; Duan, Y.; Shatters, R.G.; Lin, Y.; Zhang, M. Mitigating citrus huanglongbing via effective application of antimicrobial compounds and thermotherapy. Crop Prot. 2016, 84, 150–158. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. OIE Rev. Sci. Tech. 2012, 31, 199–210. [Google Scholar] [CrossRef]
- Blaustein, R.A.; Lorca, G.L.; Teplitski, M. Challenges for Managing Candidatus liberibacter spp. (Huanglongbing Disease Pathogen): Current Control Measures and Future Directions. Phytopathology 2017, 108, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.E.; van Vuuren, S.P. Decreases in fruit greening of sweet orange by trunk injections with tetracyclines. Plant Dis. Reptr. 1970, 55, 747–750. [Google Scholar] [CrossRef]
- Zhao, X.Y. Citrus yellow shoot disease (Huanglongbing) in China—A review. In Proceedings of the International Society of Citriculture, International Citrus Congress, Tokyo, Japan, 9–12 November 1981; Matsumoto, K., Ed.; International Society for Horticultural Science: Leuven, Belgium, 1982; Volume 1, pp. 466–469. [Google Scholar]
- Aubert, B.; Bove, J.M. Effect of Penicillin or Tetracycline injections of citrus trees affected by greening disease under field conditions in Reunion Island. Proc. Eighth Conf. Int. Organ. Citrus Virol. 1980, 8, 103–108. [Google Scholar] [CrossRef]
- Martinez, A.L.; Nora, D.M.; Armedilla, A.L. Suppression of symptoms of citrus greening disease in the Philippines by treatment with tetracycline antibiotics. Plant Dis. Report. 1970, 54, 1007–1009. [Google Scholar]
- Capoor, S.P.; Thirumal, M. J Cure of Greening Affected Citrus Plants by Chemotherapeutic Agents. Plant Dis. Report. 1973, 57, 160–163. [Google Scholar]
- Li, J.; Pang, Z.; Duan, S.; Lee, D.; Kolbasov, V.G.; Wang, N. The in Planta Effective Concentration of Oxytetracycline against ‘Candidatus Liberibacter asiaticus’ for Suppression of Citrus Huanglongbing. Phytopathology 2019, 109, 2046–2054. [Google Scholar] [CrossRef]
- Vincent, C.; Pierre, M.; Li, J.; Wang, N. Implications of Heat Treatment and Systemic Delivery of Foliar-Applied Oxytetracycline on Citrus Physiological Management and Therapy Delivery. Front. Plant Sci. 2019, 10, 41. [Google Scholar] [CrossRef]
- Daniels, M.J. Editorial: Possible Adverse Effects of Antibiotic Therapy in Plants. Clin. Infect. Dis. 1982, 4, S167–S170. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Hijaz, F.; Nehela, Y.; Batuman, O.; Killiny, N. Uptake, Translocation, and Stability of Oxytetracycline and Streptomycin in Citrus Plants. Antibiotics 2019, 8, 196. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Al-Rimawi, F.; Vincent, C.I.; Killiny, N. The role of the xylem in oxytetracycline translocation within citrus trees. Antibiotics 2020, 9, 691. [Google Scholar] [CrossRef]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Killiny, N.; Hijaz, F.; Gonzalez-Blanco, P.; Jones, S.E.; Pierre, M.O.; Vincent, C.I. Effect of adjuvants on oxytetracycline uptake upon foliar application in citrus. Antibiotics 2020, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.C.; Kleier, D.A. Phloem mobility of xenobiotics VIII. A short review. J. Exp. Bot. 1996, 47, 1265–1271. [Google Scholar] [CrossRef]
- Baur, P. Mechanistic aspects of foliar penetration of agrochemicals and the effect of adjuvants. Recent Res. Devel. Agric. Food Chem. 1998, 2, 809–837. [Google Scholar]
- DeBoer, G.J.; Satchivi, N. Comparison of translocation properties of insecticides versus herbicides that leads to efficacious Control of pests as specifically illustrated by IsoclastTM active, a new insecticide, and ArylexTM active, a new herbicide. In Proceedings of the ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1171, pp. 75–93. [Google Scholar]
- Kleier, D.A. Phloem Mobility of Xenobiotics. Plant Physiol. 1988, 86, 803–810. [Google Scholar] [CrossRef]
- Brudenell, A.J.P.; Baker, D.A.; Grayson, B.T. Phloem mobility of xenobiotics: Tabular review of physicochemical properties governing the output of the Kleier model. Plant Growth Regul. 1995, 16, 215–231. [Google Scholar] [CrossRef]
- Li, J.; Kolbasov, V.G.; Lee, D.; Pang, Z.; Huang, Y.; Collins, N.; Wang, N. Residue dynamics of streptomycin in citrus delivered by foliar spray and trunk injection and effect on “Candidatus liberibacter asiaticus” titer. Phytopathology 2021, 111, 1095–1103. [Google Scholar] [CrossRef]
- Tu, M.; Randall, J.M. Adjuvants. In Weed Control Methods Handbook, The Nature Conservancy; Tu, M., Hurd, C., Randall, J.M., Eds.; University of California-Davis: Davis, CA, USA, 2001; pp. 8.1–8.25. [Google Scholar]
- Orbović, V.; Achor, D.; Syvertsen, J.P. Adjuvants affect penetration of copper through isolated cuticles of Citrus leaves and fruit. HortScience 2007, 42, 1405–1408. [Google Scholar] [CrossRef]
- Baker, E.A.; Procopiou, J.; Hunt, G.M. The cuticles of Citrus species. Composition of leaf and fruit waxes. J. Sci. Food Agric. 1975, 26, 1093–1101. [Google Scholar] [CrossRef]
- Baker, E.A.; Procopiou, J. The cuticles of citrus species. Composition of the intracuticular lipids of leaves and fruits. J. Sci. Food Agric. 1975, 26, 1347–1352. [Google Scholar] [CrossRef]
- Bondada, B.; Petracek, P.; HortScience, J.S. Undefined Finite Dose Diffusion of Urea through Isolated Citrus Leaf Cuticles. HortScience 1997, 32, 541–542. [Google Scholar] [CrossRef]
- Etxeberria, E.; Gonzalez, P.; Borges, A.F.; Brodersen, C. The Use of Laser Light to Enhance the Uptake of Foliar-Applied Substances into Citrus (Citrus sinensis) Leaves. Appl. Plant Sci. 2016, 4, 1500106. [Google Scholar] [CrossRef] [PubMed]
- Geßler, A.; Weber, P.; Schneider, S.; Rennenberg, H. Bidirectional exchange of amino compounds between phloem and xylem during long-distance transport in Norway spruce trees (Picea abies [L.] Karst). J. Exp. Bot. 2003, 54, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Dye, M.H. Studies on the uptake and translocation of streptomycin by peach seedlings. Ann. Appl. Biol. 1956, 44, 567–575. [Google Scholar] [CrossRef]
- Crowdy, S.H. The uptake and translocation of griseofulvin, streptomycin and chloramphenicol in plants. Ann. Appl. Biol. 1957, 45, 208–215. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package version 1.3-5. 2021. Available online: https://rdrr.io/cran/agricolae/man/agricolae-package.html (accessed on 26 July 2022).
- Core R Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Boston, MA, USA, 2022. [Google Scholar]


| Study | Compound | Rate per Tree | Antimicrobial Concentration (µg g−1 FW) | |
|---|---|---|---|---|
| Covered Leaves | Sprayed Leaves | |||
| Streptomycin—9 adjuvants | Streptomycin | 0.78 g | 0.33 ± 0.07 b | 8.1 ± 0.61 a |
| Combined—4 adjuvants | Streptomycin | 0.78 g | 0.78 ± 0.27 b | 10.7 ± 1.6 a |
| Combined—4 adjuvants | Oxytetracycline | 0.72 g | 0.95 ± 0.34 a | 1.3 ± 0.42 a |
| Study | Anti-Microbial Compound | Treatment | Pre-Treatment | Post-Treatment | P(T) | ||
|---|---|---|---|---|---|---|---|
| Mean | ±SE | Mean | ±SE | ||||
| Streptomycin only | Streptomycin | Cohere | 31.3 | 0.72 | 31.9 | 0.67 | 0.41 |
| Exit | 31.2 | 0.92 | 31.0 | 0.62 | 0.86 | ||
| Grounded | 29.7 | 0.67 | 30.1 | 0.54 | 0.59 | ||
| Joint Venture | 29.9 | 0.66 | 31.8 | 0.67 | 0.14 | ||
| Keyplex 445 DP | 32.3 | 0.76 | 31.2 | 0.51 | 0.27 | ||
| LI 700 | 30.7 | 0.95 | 31.0 | 0.59 | 0.82 | ||
| Tactic | 30.5 | 0.72 | 30.9 | 0.48 | 0.73 | ||
| Injection | 30.5 | 0.86 | 31.2 | 0.77 | 0.55 | ||
| Water − AB | 30.3 | 0.83 | 30.3 | 0.77 | 0.92 | ||
| Water + AB | 30.5 | 0.74 | 32.0 | 0.46 | 0.12 | ||
| None | Water − AB | 30.3 | 0.83 | 30.3 | 0.77 | 0.92 | |
| Oxytetracycline and Streptomycin | Streptomycin | Injection | 29.8 | 1.25 | 30.8 | 0.83 | 0.87 |
| Joint Venture | 30.4 | 0.60 | 30.8 | 0.68 | 0.75 | ||
| Nutrisync Micro Pak | 31.3 | 0.59 | 30.4 | 0.68 | 0.37 | ||
| Water | 29.7 | 0.40 | 30.3 | 0.72 | 0.41 | ||
| Oxytetracycline | Injection | 30.7 | 0.61 | 29.8 | 0.98 | 0.002 | |
| Joint Venture | 30.4 | 0.92 | 31.0 | 0.52 | 0.47 | ||
| LI 700 | 29.8 | 0.64 | 30.8 | 0.68 | 0.19 | ||
| Water | 29.3 | 0.86 | 30.7 | 0.66 | 0.17 | ||
| None | Water | 29.8 | 0.88 | 29.7 | 0.71 | 0.82 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincent, C.I.; Hijaz, F.; Pierre, M.; Killiny, N. Systemic Uptake of Oxytetracycline and Streptomycin in Huanglongbing-Affected Citrus Groves after Foliar Application and Trunk Injection. Antibiotics 2022, 11, 1092. https://doi.org/10.3390/antibiotics11081092
Vincent CI, Hijaz F, Pierre M, Killiny N. Systemic Uptake of Oxytetracycline and Streptomycin in Huanglongbing-Affected Citrus Groves after Foliar Application and Trunk Injection. Antibiotics. 2022; 11(8):1092. https://doi.org/10.3390/antibiotics11081092
Chicago/Turabian StyleVincent, Christopher I., Faraj Hijaz, Myrtho Pierre, and Nabil Killiny. 2022. "Systemic Uptake of Oxytetracycline and Streptomycin in Huanglongbing-Affected Citrus Groves after Foliar Application and Trunk Injection" Antibiotics 11, no. 8: 1092. https://doi.org/10.3390/antibiotics11081092
APA StyleVincent, C. I., Hijaz, F., Pierre, M., & Killiny, N. (2022). Systemic Uptake of Oxytetracycline and Streptomycin in Huanglongbing-Affected Citrus Groves after Foliar Application and Trunk Injection. Antibiotics, 11(8), 1092. https://doi.org/10.3390/antibiotics11081092







