Control of Healthcare-Associated Carbapenem-Resistant Acinetobacter baumannii by Enhancement of Infection Control Measures
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Promotion of Hand Hygiene and Directly Observed Hand hygiene
2.3. Infection Control Measures for Antimicrobial-Resistant Organisms
2.4. Data Source
2.5. Trends of Antimicrobial-Resistant Organisms
2.6. Hospital-onset Events before and after the Enhancement of Infection Control Measures
2.7. Antimicrobial Consumption before and after the Enhancement of Infection Control Measures
2.8. Statistical Analysis
3. Results
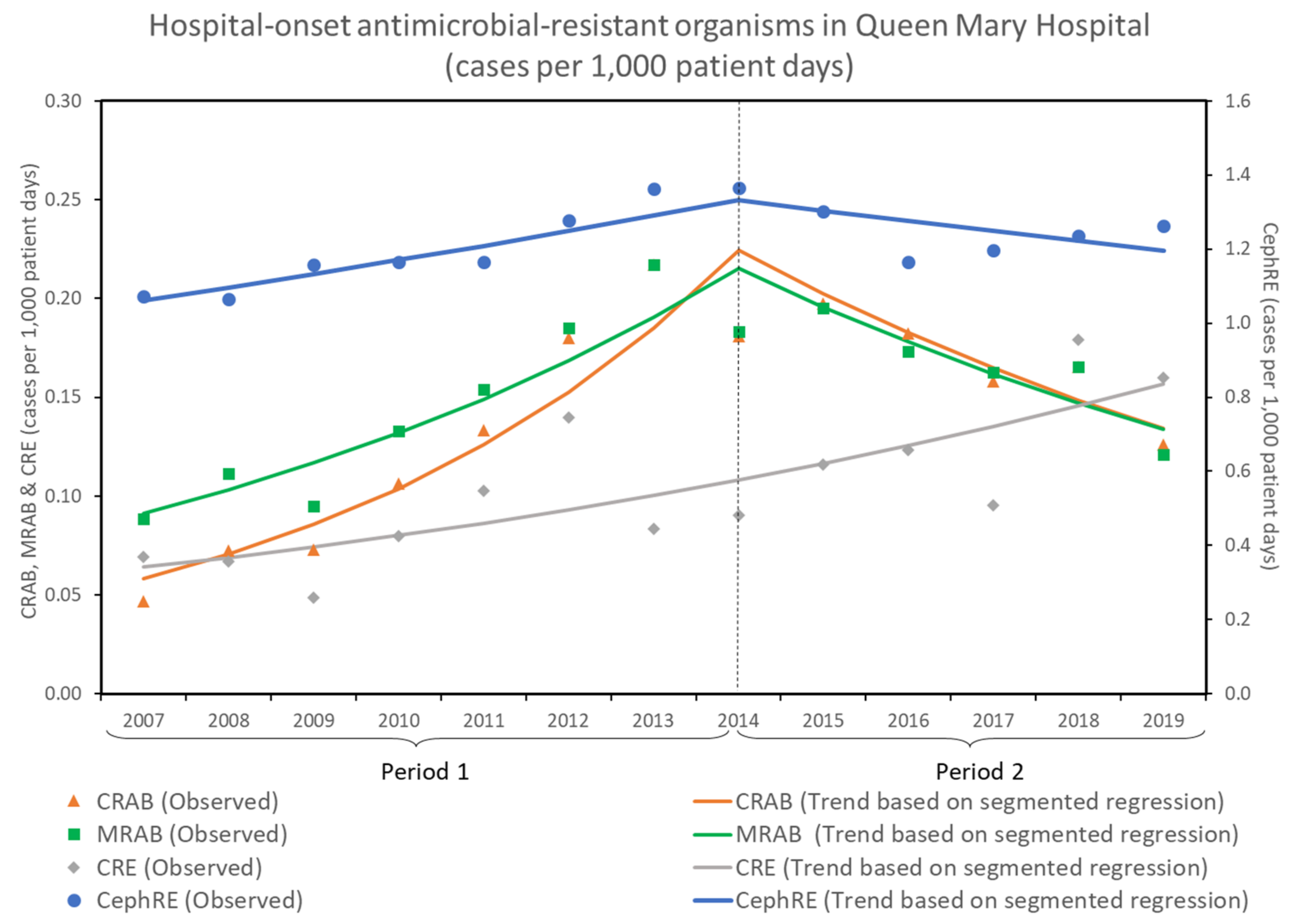
3.1. Trends of Antimicrobial-Resistant Organisms
3.2. Compliance with Hand Hygiene and Directly Observed Hand Hygiene
3.3. Hospital-onset Events before and after the Enhancement of Infection Control Measures
3.4. Antimicrobial Consumption before and after the Enhancement of Infection Control Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pogue, J.M.; Mann, T.; Barber, K.E.; Kaye, K.S. Carbapenem-resistant Acinetobacter baumannii: Epidemiology, surveillance and management. Expert. Rev. Anti. Infect. Ther. 2013, 11, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Joshi, S.G. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: A scientific review. J. Appl. Microbiol. 2021, 131, 2715–2738. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.J.; Chopra, T.; Abdel-Haq, N.; Preney, K.; Koo, W.; Asmar, B.I.; Kaye, K.S. An outbreak of carbapenem-resistant Acinetobacter baumannii infection in a neonatal intensive care unit: Investigation and control. Infect. Control Hosp. Epidemiol. 2011, 32, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Tsiatsiou, O.; Iosifidis, Ε.; Katragkou, A.; Dimou, V.; Sarafidis, K.; Karampatakis, T.; Antachopoulos, C.; Orfanou, A.; Tsakris, A.; Drossou-Agakidou, V.; et al. Successful management of an outbreak due to carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. Eur. J. Pediatr. 2015, 174, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Nhu, N.T.-K.; Lan, N.P.-H.; Campbell, J.I.; Parry, C.M.; Thompson, C.; Tuyen, H.T.; Hoang, N.V.-M.; Tam, P.T.-T.; Le, V.M.; Nga, T.V.-T.; et al. Emergence of carbapenem-resistant Acinetobacter baumannii as the major cause of ventilator-associated pneumonia in intensive care unit patients at an infectious disease hospital in southern Vietnam. J. Med. Microbiol. 2014, 63, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.H.-L.; Marimuthu, K.; Lee, J.J.; Khong, W.X.; Ng, O.T.; Zhang, W.; Poh, B.F.; Rao, P.; Raj, M.D.-R.; Ang, B.; et al. Environmental colonization and onward clonal transmission of carbapenem-resistant Acinetobacter baumannii (CRAB) in a medical intensive care unit: The case for environmental hygiene. Antimicrob. Resist. Infect. Control 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, O.; Willrich, N.; Harder, T.; Okeke, I.N.; Eckmanns, T.; Markwart, R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A systematic review and meta-analysis. Emerg. Microbes. Infect. 2019, 8, 1747–1759. [Google Scholar] [CrossRef]
- Bitterman, R.; Hussein, K.; Leibovici, L.; Carmeli, Y.; Paul, M. Systematic review of antibiotic consumption in acute care hospitals. Clin. Microbiol. Infect. 2016, 22, 561.e7–561.e19. [Google Scholar] [CrossRef]
- Tan, C.K.; Tang, H.J.; Lai, C.C.; Chen, Y.Y.; Chang, P.C.; Liu, W.L. Correlation between antibiotic consumption and carbapenem-resistant Acinetobacter baumannii causing health care-associated infections at a hospital from 2005 to 2010. J. Microbiol. Immunol. Infect. 2015, 48, 540–544. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Y.; Jiang, S.; Shen, P.; Lu, X.; Xiao, Y. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob. Resist. Infect. Control 2018, 7, 137. [Google Scholar] [CrossRef]
- Paiboonvong, T.; Tedtaisong, P.; Montakantikul, P.; Gorsanan, S.; Tantisiriwat, W. Correlation between Carbapenem Consumption and Carbapenems Susceptibility Profiles of Acinetobacter baumannii and Pseudomonas aeruginosa in an Academic Medical Center in Thailand. Antibiotics 2022, 11, 143. [Google Scholar] [CrossRef]
- Al-Hashimy, Z.S.; Conway, B.R.; Al-Yaqoobi, M.; Khamis, F.; Al Mawali, G.Z.; Al Maashani, A.M.; Al Hadhrami, Y.S.; Al Alawi, S.S.; Al Mamari, M.S.; Lattyak, W.J.; et al. Identifying Targets for Antibiotic Use for the Management of Carbapenem-Resistant Acinetobacter baumannii (CRAb) in Hospitals—A Multi-Centre Nonlinear Time-Series Study. Antibiotics 2022, 11, 775. [Google Scholar] [CrossRef]
- Yusef, D.; Hayajneh, W.A.; Bani Issa, A.; Haddad, R.; Al-Azzam, S.; Lattyak, E.A.; Lattyak, W.J.; Gould, I.; Conway, B.R.; Bond, S.; et al. Impact of an antimicrobial stewardship programme on reducing broad-spectrum antibiotic use and its effect on carbapenem-resistant Acinetobacter baumannii (CRAb) in hospitals in Jordan. J. Antimicrob. Chemother. 2021, 76, 516–523. [Google Scholar] [CrossRef]
- Risser, C.; Pottecher, J.; Launoy, A.; Ursenbach, A.; Belotti, L.; Boyer, P.; Willemain, R.; Lavigne, T.; Deboscker, S. Management of a Major Carbapenem-Resistant Acinetobacter baumannii Outbreak in a French Intensive Care Unit While Maintaining Its Capacity Unaltered. Microorganisms 2022, 10, 720. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, S.H.; Jeon, E.G.; Son, M.H.; Yoon, Y.K.; Kim, J.Y.; Kim, M.J.; Sohn, J.W.; Kim, M.J.; Park, D.W. Nosocomial outbreak of carbapenem-resistant Acinetobacter baumannii in intensive care units and successful outbreak control program. J. Korean Med. Sci. 2010, 25, 999–1004. [Google Scholar] [CrossRef][Green Version]
- Doidge, M.; Allworth, A.M.; Woods, M.; Marshall, P.; Terry, M.; O’Brien, K.; Goh, H.M.; George, N.; Nimmo, G.R.; Schembri, M.A.; et al. Control of an outbreak of carbapenem-resistant Acinetobacter baumannii in Australia after introduction of environmental cleaning with a commercial oxidizing disinfectant. Infect. Control Hosp. Epidemiol. 2010, 31, 418–420. [Google Scholar] [CrossRef]
- Ben-Chetrit, E.; Wiener-Well, Y.; Lesho, E.; Kopuit, P.; Broyer, C.; Bier, L.; Assous, M.V.; Benenson, S.; Cohen, M.J.; McGann, P.T.; et al. An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: Long-term impact for the ICU and hospital. Crit. Care 2018, 22, 319. [Google Scholar] [CrossRef]
- Meschiari, M.; Lòpez-Lozano, J.M.; Di Pilato, V.; Gimenez-Esparza, C.; Vecchi, E.; Bacca, E.; Orlando, G.; Franceschini, E.; Sarti, M.; Pecorari, M.; et al. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob. Resist. Infect. Control 2021, 10, 123. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Carling, P.; Cleary, T.; Fajardo-Aquino, Y.; DePascale, D.; Jimenez, A.; Hughes, M.; Namias, N.; Pizano, L.; Kett, D.H.; et al. Control of a two-decade endemic situation with carbapenem-resistant Acinetobacter baumannii: Electronic dissemination of a bundle of interventions. Am. J. Infect. Control 2014, 42, 466–471. [Google Scholar] [CrossRef]
- Cho, O.H.; Bak, M.H.; Baek, E.H.; Park, K.H.; Kim, S.; Bae, I.G. Successful control of carbapenem-resistant Acinetobacter baumannii in a Korean university hospital: A 6-year perspective. Am. J. Infect. Control 2014, 42, 976–979. [Google Scholar] [CrossRef]
- Chung, Y.K.; Kim, J.S.; Lee, S.S.; Lee, J.A.; Kim, H.S.; Shin, K.S.; Park, E.Y.; Kang, B.S.; Lee, H.J.; Kang, H.J. Effect of daily chlorhexidine bathing on acquisition of carbapenem-resistant Acinetobacter baumannii (CRAB) in the medical intensive care unit with CRAB endemicity. Am. J. Infect. Control 2015, 43, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Jang, O.J.; Bak, M.H.; Baek, E.H.; Park, K.H.; Hong, S.I.; Cho, O.H.; Bae, I.G. Management of carbapenem-resistant Acinetobacter baumannii epidemic in an intensive care unit using multifaceted intervention strategy. Korean J. Intern. Med. 2018, 33, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Hamaguchi, S.; Akeda, Y.; Santanirand, P.; Chaihongsa, N.; Sirichot, S.; Chiaranaicharoen, S.; Hagiya, H.; Yamamoto, K.; Kerdsin, A.; et al. Rapid screening and early precautions for carbapenem-resistant Acinetobacter baumannii carriers decreased nosocomial transmission in hospital settings: A quasi-experimental study. Antimicrob. Resist. Infect. Control 2019, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Thatrimontrichai, A.; Pannaraj, P.S.; Janjindamai, W.; Dissaneevate, S.; Maneenil, G.; Apisarnthanarak, A. Intervention to reduce carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 2020, 41, 710–715. [Google Scholar] [CrossRef]
- Alon, D.; Mudrik, H.; Chowers, M.; Shitrit, P. Control of a hospital-wide outbreak of carbapenem-resistant Acinetobacter baumannii (CRAB) using the Israeli national carbapenem-resistant Enterobacteriaceae (CRE) guidelines as a model. Infect. Control Hosp. Epidemiol. 2020, 41, 926–930. [Google Scholar] [CrossRef]
- Dickstein, Y.; Eluk, O.; Warman, S.; Aboalheja, W.; Alon, T.; Firan, I.; Putler, R.K.B.; Hussein, K. Wall painting following terminal cleaning with a chlorine solution as part of an intervention to control an outbreak of carbapenem-resistant Acinetobacter baumannii in a neurosurgical intensive care unit in Israel. J. Infect. Chemother. 2021, 27, 1423–1428. [Google Scholar] [CrossRef]
- Chhatwal, P.; Ebadi, E.; Schwab, F.; Ziesing, S.; Vonberg, R.P.; Simon, N.; Gerbel, S.; Schlüter, D.; Bange, F.C.; Baier, C. Epidemiology and infection control of carbapenem resistant Acinetobacter baumannii and Klebsiella pneumoniae at a German university hospital: A retrospective study of 5 years (2015–2019). BMC Infect. Dis. 2021, 21, 1196. [Google Scholar] [CrossRef]
- Jung, J.; Choe, P.G.; Choi, S.; Kim, E.; Lee, H.Y.; Kang, C.K.; Lee, J.; Park, W.B.; Lee, S.; Kim, N.J.; et al. Reduction in the acquisition rate of carbapenem-resistant Acinetobacter baumannii (CRAB) after room privatization in an intensive care unit. J. Hosp. Infect. 2022, 121, 14–21. [Google Scholar] [CrossRef]
- Cheng, V.C.-C.; Wong, S.-C.; Wong, S.C.-Y.; Yuen, K.-Y. Directly observed hand hygiene—from healthcare workers to patients. J. Hosp. Infect. 2019, 101, 380–382. [Google Scholar] [CrossRef]
- Cheng, V.C.; Tai, J.W.; Li, W.-S.; Chau, P.-H.; So, S.Y.; Wong, L.M.; Ching, R.H.; Ng, M.M.; Ho, S.K.; Lee, D.W.; et al. Implementation of directly observed patient hand hygiene for hospitalized patients by hand hygiene ambassadors in Hong Kong. Am. J. Infect. Control 2016, 44, 621–624. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wu, A.K.; Cheung, C.-H.; Lau, S.K.; Woo, P.C.; Chan, K.-H.; Li, K.-S.; Ip, I.-K.; Dunn, E.L.; Lee, R.A.; et al. Outbreak of human metapneumovirus infection in psychiatric inpatients: Implications for directly observed use of alcohol hand rub in prevention of nosocomial outbreaks. J. Hosp. Infect. 2007, 67, 336–343. [Google Scholar] [CrossRef]
- Cheng, V.C.; Tai, J.W.; Wong, L.M.; Chan, J.F.; Li, I.W.; To, K.K.; Hung, I.F.; Chan, K.-H.; Ho, P.-L.; Yuen, K.-Y. Prevention of nosocomial transmission of swine-origin pandemic influenza virus A/H1N1 by infection control bundle. J. Hosp. Infect. 2010, 74, 271–277. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wong, L.M.; Tai, J.W.; Chan, J.F.; To, K.K.; Li, I.W.; Hung, I.F.; Chan, K.-H.; Ho, P.-L.; Yuen, K.-Y. Prevention of nosocomial transmission of norovirus by strategic infection control measures. Infect. Control Hosp. Epidemiol. 2011, 32, 229–237. [Google Scholar] [CrossRef]
- Cheng, V.C.; Tai, J.W.; Chen, J.H.; So, S.Y.; Ng, W.-C.; Hung, I.F.; Leung, S.S.; Wong, S.C.; Chan, T.-C.; Chan, F.H.; et al. Proactive infection control measures to prevent nosocomial transmission of vancomycin-resistant enterococci in Hong Kong. J. Formos. Med. Assoc. 2014, 113, 734–741. [Google Scholar] [CrossRef]
- Cheng, V.C.; Chen, J.H.; Poon, R.W.; Lee, W.-M.; So, S.Y.; Wong, S.C.; Chau, P.-H.; Yip, C.C.; Wong, S.S.; Chan, J.F.; et al. Control of hospital endemicity of multiple-drug-resistant Acinetobacter baumannii ST457 with directly observed hand hygiene. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 713–718. [Google Scholar] [CrossRef]
- Cheng, V.C.; Tai, J.W.; Chau, P.-H.; Lai, C.-K.; Chuang, V.W.; So, S.Y.; Wong, S.C.; Chen, J.H.; Ho, P.-L.; Tsang, D.N.; et al. Successful control of emerging vancomycin-resistant enterococci by territory-wide implementation of directly observed hand hygiene in patients in Hong Kong. Am. J. Infect. Control 2016, 44, 1168–1171. [Google Scholar] [CrossRef]
- Cheng, V.C.-C.; Wong, S.-C.; Wong, I.W.-Y.; Chau, P.-H.; So, S.Y.-C.; Wong, S.C.-Y.; Chen, J.H.-K.; Lee, W.-M.; Tai, J.W.-M.; Chau, C.-H.; et al. The challenge of patient empowerment in hand hygiene promotion in health care facilities in Hong Kong. Am. J. Infect. Control 2017, 45, 562–565. [Google Scholar] [CrossRef]
- Hand Hygiene Observation form World Health Organization. Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fcdn.who.int%2Fmedia%2Fdocs%2Fdefault-source%2Fintegrat-ed-health-services-(ihs)%2Fhand-hygiene%2Fmonitoring%2Fsurveyform%2Fobservation-form.doc%3Fsfvrsn%3D39b780c9_6&wdOrigin=BROWSELINK (accessed on 19 June 2022).
- Cheng, V.C.-C.; Wong, S.-C.; So, S.Y.-C.; Chen, J.H.-K.; Chau, P.-H.; Au, A.K.-W.; Chiu, K.H.-Y.; Li, X.; Ip, P.; Chuang, V.W.-M.; et al. Decreased Antibiotic Consumption Coincided with Reduction in Bacteremia Caused by Bacterial Species with Respiratory Transmission Potential during the COVID-19 Pandemic. Antibiotics 2022, 11, 746. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Available online: https://clsi.org/ (accessed on 19 June 2022).
- Multidrug-Resistant Organism & Clostridioides Difficile Infection (MDRO/CDI) Module. National Healthcare Safety Network. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/12pscmdro_cdadcurrent.pdf (accessed on 19 June 2022).
- Acinetobacter in Healthcare Settings. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/hai/organisms/acinetobacter.html (accessed on 19 June 2022).
- Carbapenem-Resistant Enterobacterales (CRE). Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/hai/organisms/cre/index.html (accessed on 19 June 2022).
- Ho, P.-L.; Ho, A.-Y.; Chow, K.-H.; Lai, E.-L.; Ching, P.; Seto, W.-H. Epidemiology and clonality of multidrug-resistant Acinetobacter baumannii from a healthcare region in Hong Kong. J. Hosp. Infect. 2010, 74, 358–364. [Google Scholar] [CrossRef]
- Ho, P.-L.; Ho, A.-Y.; Chow, K.-H.; Cheng, V.C. Surveillance for multidrug-resistant Acinetobacter baumannii: A lesson on definitions. Int. J. Antimicrob. Agents. 2010, 36, 469–471. [Google Scholar] [CrossRef]
- Cheng, V.C.-C.; Chen, J.H.-K.; Ng, W.-C.; Wong, J.Y.-H.; Chow, D.M.-K.; Law, T.-C.; So, S.Y.-C.; Wong, S.C.-Y.; Chan, T.-C.; Chan, F.H.-W.; et al. Emergence of Carbapenem-Resistant Acinetobacter baumannii in Nursing Homes with High Background Rates of MRSA Colonization. Infect. Control Hosp. Epidemiol. 2016, 37, 983–986. [Google Scholar] [CrossRef]
- Li, P.H.; Cheng, V.C.; Yip, T.; Yap, D.Y.; Lui, S.-L.; Lo, W.-K. Epidemiology and Clinical Characteristics of Acinetobacter Peritoneal Dialysis-Related Peritonitis in Hong Kong-With a Perspective on Multi-Drug and Carbapenem Resistance. Perit. Dial. Int. 2017, 37, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.-C.; Wong, S.-C.; Chen, J.H.-K.; So, S.Y.-C.; Wong, S.C.-Y.; Ho, P.-L.; Yuen, K.-Y. Control of multidrug-resistant Acinetobacter baumannii in Hong Kong: Role of environmental surveillance in communal areas after a hospital outbreak. Am. J. Infect. Control 2018, 46, 60–66. [Google Scholar] [CrossRef]
- Wong, S.-C.; Lam, G.K.; Chen, J.H.; Li, X.; Ip, F.T.; Yuen, L.L.; Chan, V.W.; AuYeung, C.H.; So, S.Y.; Ho, P.-L.; et al. Air dispersal of multidrug-resistant Acinetobacter baumannii: Implications for nosocomial transmission during the COVID-19 pandemic. J. Hosp. Infect. 2021, 116, 78–86. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug. Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Hijazi, K.; Joshi, C.; Gould, I.M. Challenges and opportunities for antimicrobial stewardship in resource-rich and resource-limited countries. Expert. Rev. Anti. Infect. Ther. 2019, 17, 621–634. [Google Scholar] [CrossRef]
- Kumana, C.R.; Ching, T.-Y.; Kong, Y.; Ma, E.C.; Kou, M.; Lee, R.A.; Cheng, V.C.; Chiu, S.S.; Seto, W.-H. Curtailing unnecessary vancomycin usage in a hospital with high rates of methicillin resistant Staphylococcus aureus infections. Br. J. Clin. Pharmacol. 2001, 52, 427–432. [Google Scholar] [CrossRef]
- Cheng, V.C.; To, K.K.; Li, I.W.; Tang, B.S.; Chan, J.F.; Kwan, S.; Mak, R.; Tai, J.; Ching, P.; Ho, P.-L.; et al. Antimicrobial stewardship program directed at broad-spectrum intravenous antibiotics prescription in a tertiary hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D. Improving adherence to hand hygiene practice: A multidisciplinary approach. Emerg. Infect. Dis. 2001, 7, 234–240. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines on Hand Hygiene in Health Care. World Health Organization. Available online: https://www.who.int/publications/i/item/9789241597906 (accessed on 26 June 2022).
- D’Angelo, I.; Provenzano, R.; Florio, E.; Pagliuca, C.; Mantova, G.; Scaglione, E.; Vitiello, M.; Colicchio, R.; Salvatore, P.; Ungaro, F.; et al. Alcohol-Based Hand Sanitizers: Does Gelling Agent Really Matter? Gels 2022, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Booq, R.Y.; Alshehri, A.A.; Almughem, F.A.; Zaidan, N.M.; Aburayan, W.S.; Bakr, A.A.; Kabli, S.H.; Alshaya, H.A.; Alsuabeyl, M.S.; Alyamani, E.J.; et al. Formulation and Evaluation of Alcohol-Free Hand Sanitizer Gels to Prevent the Spread of Infections during Pandemics. Int. J. Environ. Res. Public Health 2021, 18, 6252. [Google Scholar] [CrossRef]
- Wong, S.-C.; AuYeung, C.H.; Lam, G.K.; Leung, E.Y.; Chan, V.W.; Yuen, K.Y.; Cheng, V.C. Is it possible to achieve 100 percent hand hygiene compliance during the coronavirus disease 2019 (COVID-19) pandemic? J. Hosp. Infect. 2020, 105, 779–781. [Google Scholar] [CrossRef]
- Cheng, V.C.; Tai, J.W.; Ho, Y.Y.; Chan, J.F. Successful control of norovirus outbreak in an infirmary with the use of alcohol-based hand rub. J. Hosp. Infect. 2009, 72, 370–371. [Google Scholar] [CrossRef]
- McGuckin, M.; Storr, J.; Longtin, Y.; Allegranzi, B.; Pittet, D. Patient empowerment and multimodal hand hygiene promotion: A win-win strategy. Am. J. Med. Qual. 2011, 26, 10–17. [Google Scholar] [CrossRef][Green Version]
- McGuckin, M.; Govednik, J. Patient empowerment and hand hygiene, 1997–2012. J. Hosp. Infect. 2013, 84, 191–199. [Google Scholar] [CrossRef]
- Longtin, Y.; Sax, H.; Allegranzi, B.; Hugonnet, S.; Pittet, D. Patients’ beliefs and perceptions of their participation to increase healthcare worker compliance with hand hygiene. Infect. Control Hosp. Epidemiol. 2009, 30, 830–839. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wong, S.C.; Ho, P.-L.; Yuen, K.-Y. Strategic measures for the control of surging antimicrobial resistance in Hong Kong and mainland of China. Emerg. Microbes Infect. 2015, 4, e8. [Google Scholar] [CrossRef]
- Cheng, V.C.; Chen, J.H.; So, S.Y.; Wong, S.C.; Yan, M.-K.; Chau, P.-H.; Lee, W.-M.; To, K.K.; Chan, J.F.; Hung, I.F.; et al. Use of fluoroquinolones is the single most important risk factor for the high bacterial load in patients with nasal and gastrointestinal colonization by multidrug-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2359–2366. [Google Scholar] [CrossRef]
- Shen, Z.; Hu, Y.; Sun, Q.; Hu, F.; Zhou, H.; Shu, L.; Ma, T.; Shen, Y.; Wang, Y.; Li, J.; et al. Emerging Carriage of NDM-5 and MCR-1 in Escherichia coli From Healthy People in Multiple Regions in China: A Cross Sectional Observational Study. EclinicalMedicine 2018, 6, 11–20. [Google Scholar] [CrossRef]
- Ho, P.-L.; Wang, Y.; Liu, M.-C.; Lai, E.-L.; Law, P.-Y.; Cao, H.; Chow, K.-H. IncX3 Epidemic Plasmid Carrying blaNDM-5 in Escherichia coli from Swine in Multiple Geographic Areas in China. Antimicrob. Agents Chemother. 2018, 62, e02295-17. [Google Scholar] [CrossRef]
- Peng, Z.; Li, X.; Hu, Z.; Li, Z.; Lv, Y.; Lei, M.; Wu, B.; Chen, H.; Wang, X. Characteristics of Carbapenem-Resistant and Colistin-Resistant Escherichia coli Co-Producing NDM-1 and MCR-1 from Pig Farms in China. Microorganisms 2019, 7, 482. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.T.; Zhang, X.Y.; Wan, S.W.; Hao, J.J.; Jiang, R.D.; Song, F.J. Characteristics of Carbapenem-Resistant Enterobacteriaceae in Ready-to-Eat Vegetables in China. Front. Microbiol. 2018, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Chan, J.F.; Lau, E.H.; Yam, W.-C.; Ho, S.K.; Yau, M.C.; Tse, E.Y.; Wong, A.C.; Tai, J.W.; Fan, S.-T.; et al. Studying the transmission dynamics of meticillin-resistant Staphylococcus aureus in Hong Kong using spa typing. J. Hosp. Infect. 2011, 79, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Tai, J.W.; Wong, Z.S.; Chen, J.H.; Pan, K.B.; Hai, Y.; Ng, W.-C.; Chow, D.M.; Yau, M.C.; Chan, J.F.; et al. Transmission of methicillin-resistant Staphylococcus aureus in the long term care facilities in Hong Kong. BMC Infect. Dis. 2013, 13, 205. [Google Scholar] [CrossRef]
- Wong, S.-C.; Chan, V.W.-M.; Lam, G.K.; AuYeung, C.H.; Leung, E.Y.; So, S.Y.; Chen, J.H.; Sridhar, S.; Tam, A.R.; Hung, I.F.; et al. The use of multi-pronged screening strategy to understand the epidemiology of carbapenemase-producing Enterobacteriaceae in Hong Kong: Transition from epidemic to endemic setting. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2017–2022. [Google Scholar] [CrossRef]
- Cheng, V.C.; Chen, J.H.; So, S.Y.; Wong, S.C.; Chau, P.-H.; Wong, L.M.; Ching, R.H.; Ng, M.M.; Lee, W.-M.; Hung, I.F.; et al. A Novel Risk Factor Associated with Colonization by Carbapenemase-Producing Enterobacteriaceae: Use of Proton Pump Inhibitors in Addition to Antimicrobial Treatment. Infect. Control Hosp. Epidemiol. 2016, 37, 1418–1425. [Google Scholar] [CrossRef]
- Wong, S.-C.; Chen, J.H.; So, S.Y.; Ho, P.-L.; Yuen, K.-Y.; Cheng, V.C. Gastrointestinal colonization of meticillin-resistant Staphylococcus aureus: An unrecognized burden upon hospital infection control. J. Hosp. Infect. 2022, 121, 65–74. [Google Scholar] [CrossRef]
- Cheng, V.C.-C.; Wong, S.-C.; Chen, J.H.-K.; Yip, C.C.-Y.; Chuang, V.W.-M.; Tsang, O.T.-Y.; Sridhar, S.; Chan, J.F.-W.; Ho, P.-L.; Yuen, K.-Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020, 41, 493–498. [Google Scholar] [CrossRef]
- Wong, S.-C.; Lam, G.K.; AuYeung, C.H.; Chan, V.W.; Wong, N.L.; So, S.Y.; Chen, J.H.; Hung, I.F.; Chan, J.F.; Yuen, K.-Y.; et al. Absence of nosocomial influenza and respiratory syncytial virus infection in the coronavirus disease 2019 (COVID-19) era: Implication of universal masking in hospitals. Infect. Control Hosp. Epidemiol. 2021, 42, 218–221. [Google Scholar] [CrossRef]
- Wong, S.-C.; Leung, M.; Tong, D.W.; Lee, L.L.; Leung, W.L.; Chan, F.W.; Chen, J.H.; Hung, I.F.; Yuen, K.-Y.; Yeung, D.T.; et al. Infection control challenges in setting up community isolation and treatment facilities for patients with coronavirus disease 2019 (COVID-19): Implementation of directly observed environmental disinfection. Infect. Control Hosp. Epidemiol. 2021, 42, 1037–1045. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wong, S.-C.; Tong, D.W.; Chuang, V.W.; Chen, J.H.; Lee, L.L.; To, K.K.; Hung, I.F.; Ho, P.-L.; Yeung, D.T.; et al. Multipronged infection control strategy to achieve zero nosocomial coronavirus disease 2019 (COVID-19) cases among Hong Kong healthcare workers in the first 300 days of the pandemic. Infect. Control Hosp. Epidemiol. 2022, 43, 334–343. [Google Scholar] [CrossRef]



| Episodes of Audit | Episodes with Compliance | Percentage of Compliance | 95% CI of the Percentage of Compliance | |
|---|---|---|---|---|
| 2018 3Q | 636 | 508 | 79.9 | (76.8–83.0) |
| 2018 4Q | 542 | 456 | 84.1 | (81.1–87.2) |
| 2019 1Q | 474 | 391 | 82.5 | (79.1–85.9) |
| 2019 2Q | 453 | 373 | 82.3 | (78.8–85.9) |
| 2019 3Q | 479 | 391 | 81.6 | (78.2–85.1) |
| 2019 4Q | 542 | 423 | 78.0 | (74.6–81.5) |
| No. | Year (Country) of Publication | Nature of Study (Setting) | Infection Control Measures | Ref |
|---|---|---|---|---|
| 1 | 2010 (Korea) | Observational study during outbreak (ICU) | Enforcing contact precautions, environmental cleaning, and use of a closed-suctioning system | [15] |
| 2 | 2010 (Australia) | Observational study during outbreak (ICU) | Single room isolation with contact precautions; using commercial oxidizing disinfectant with ICU closure for 3 days | [16] |
| 3 | 2011 (USA) | Observational study during outbreak (NICU) | Active surveillance cultures of all infants, cohorting of affected infants and their nursing staff, contact isolation, environmental cleaning, and use of educational modules | [3] |
| 4 | 2015 (Greece) | Observational study during outbreak (NICU) | Active surveillance (weekly stool samples), staff education, daily infection control audits and discontinuation of new admissions for 12 days | [4] |
| 5 | 2018 (Israel) | Observational study during outbreak (ICU) | Unit closure for 3 days, environmental cleaning, hand hygiene interventions, and environmental culture | [17] |
| 6 | 2021 (Italy) | Observational study during outbreak (ICU) | Enforcing hand hygiene, contact precautions to all patients, enhanced environmental sampling, and one-time application of a cycling radical environmental cleaning and disinfection | [18] |
| 7 | 2014 (USA) | Quasi-experimental study (public hospital) | Weekly and systematic dissemination of the findings of infection control interventions | [19] |
| 8 | 2014 (Korea) | Intervention study (tertiary hospital) | Onsite education and hand hygiene campaign in addition to cohorting, active surveillance, and environmental cleaning | [20] |
| 9 | 2015 (Korea) | Intervention study (MICU) a | Daily chlorhexidine bathing | [21] |
| 10 | 2017 (Korea) | Intervention study (MICU) | Universal glove and gown use with daily chlorhexidine bathing for all patients in addition to surveillance cultures, contact precautions, and environmental cleaning | [22] |
| 11 | 2019 (Japan) | Quasi-experimental study (ICU) | Active surveillance upon admission, weekly thereafter, and upon discharge | [23] |
| 12 | 2020 (Thailand) | Intervention study (NICU) a | Use of heat and moisture exchangers and sodium hypochlorite cleaning (5000 ppm in the NICU and 500 ppm in the environment) | [24] |
| 13 | 2020 (Israel) | Intervention study (secondary-care hospital) | Maintaining a case registry of all CRAB patients, cohorting patients under strict contact isolation, using dedicated nursing staff and equipment, rigorous cleaning, education and close monitoring of hospital staff, and involvement of hospital management | [25] |
| 14 | 2021 (Israel) | Intervention study (NSICU) a | Wall painting using a water based acrylic paint following patient discharge and terminal cleaning with Sodium dichloroisocyanurate (sodium troclosene) | [26] |
| 15 | 2021 (German) | Retrospective study (university hospital) | Single-room isolation and mandatory personal protective equipment (gloves, gowns, and surgical mask) for staff when caring for CRAB patients, and using disposable medical items | [27] |
| 16 | 2022 (Korea) | Intervention study (MICU) | Renovated from a multi-bed bay room to single rooms for isolation of CRAB patients | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.-C.; Chau, P.-H.; So, S.Y.-C.; Lam, G.K.-M.; Chan, V.W.-M.; Yuen, L.L.-H.; Au Yeung, C.H.-Y.; Chen, J.H.-K.; Ho, P.-L.; Yuen, K.-Y.; et al. Control of Healthcare-Associated Carbapenem-Resistant Acinetobacter baumannii by Enhancement of Infection Control Measures. Antibiotics 2022, 11, 1076. https://doi.org/10.3390/antibiotics11081076
Wong S-C, Chau P-H, So SY-C, Lam GK-M, Chan VW-M, Yuen LL-H, Au Yeung CH-Y, Chen JH-K, Ho P-L, Yuen K-Y, et al. Control of Healthcare-Associated Carbapenem-Resistant Acinetobacter baumannii by Enhancement of Infection Control Measures. Antibiotics. 2022; 11(8):1076. https://doi.org/10.3390/antibiotics11081076
Chicago/Turabian StyleWong, Shuk-Ching, Pui-Hing Chau, Simon Yung-Chun So, Germaine Kit-Ming Lam, Veronica Wing-Man Chan, Lithia Lai-Ha Yuen, Christine Ho-Yan Au Yeung, Jonathan Hon-Kwan Chen, Pak-Leung Ho, Kwok-Yung Yuen, and et al. 2022. "Control of Healthcare-Associated Carbapenem-Resistant Acinetobacter baumannii by Enhancement of Infection Control Measures" Antibiotics 11, no. 8: 1076. https://doi.org/10.3390/antibiotics11081076
APA StyleWong, S.-C., Chau, P.-H., So, S. Y.-C., Lam, G. K.-M., Chan, V. W.-M., Yuen, L. L.-H., Au Yeung, C. H.-Y., Chen, J. H.-K., Ho, P.-L., Yuen, K.-Y., & Cheng, V. C.-C. (2022). Control of Healthcare-Associated Carbapenem-Resistant Acinetobacter baumannii by Enhancement of Infection Control Measures. Antibiotics, 11(8), 1076. https://doi.org/10.3390/antibiotics11081076








