Decreasing Incidence of Gastric Cancer with Increasing Time after Helicobacter pylori Treatment: A Nationwide Population-Based Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Study Design
2.3. Study Populations and Cohort Definitions
2.4. Outcomes
2.5. Statistical Analysis
2.6. Sensitivity and Negative Control Analyses
3. Results
3.1. Study Flow and Baseline Characteristics
3.2. Effect of HP Treatment on GC Risk in the General Population
3.3. Effect of HP Treatment on GC Risk in the High-Risk Groups (Age ≥ 65 Years and Male Sex)
3.4. Incidence of GC According to the Period after HP Treatment by Age Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Behzadi, P.; Farshad, S. Advances in diagnosis and treatment of Helicobacter pylori infection. Acta Microbiol. Immunol. Hung. 2017, 64, 273–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.R.; Lee, D.H.; Shim, H.I.; Kim, J.W.; Park, S.; Shin, C.M.; Han, K.; Youn, S.W. Risk of Psoriasis in Postgastrectomy Gastric Cancer Survivors: A Nationwide Population-Based Cohort Study. Ann. Dermatol. 2022, 34, 191–199. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 83, 1–1438. [Google Scholar]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.Y. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015, 148, 719–731.e713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.M.; Kim, S.G.; Choi, J.; Park, J.Y.; Oh, S.; Yang, H.J.; Lim, J.H.; Im, J.P.; Kim, J.S.; Jung, H.C. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: A randomized controlled trial. Gastrointest. Endosc. 2018, 88, 475–485.e472. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Kook, M.C.; Kim, Y.I.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.H. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.C.; Zhang, L.; Ma, J.L.; Pan, K.F.; Li, J.Y.; Shen, L.; Liu, W.D.; Feng, G.S.; Zhang, X.D.; Li, J.; et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut 2012, 61, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.L.; Zhang, L.; Brown, L.M.; Li, J.Y.; Shen, L.; Pan, K.F.; Liu, W.D.; Hu, Y.; Han, Z.X.; Crystal-Mansour, S.; et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J. Natl. Cancer Inst. 2012, 104, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Zhang, J.Y.; Ma, J.L.; Li, Z.X.; Zhang, L.; Zhang, Y.; Guo, Y.; Zhou, T.; Li, J.Y.; Shen, L.; et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: Follow-up of a randomized intervention trial. BMJ 2019, 366, l5016. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.I.; Kook, M.C.; Park, B.; Joo, J. Family History of Gastric Cancer and Helicobacter pylori Treatment. N. Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014, 348, g3174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugano, K. Effect of Helicobacter pylori eradication on the incidence of gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2019, 22, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Leung, W.K.; Wong, I.O.L.; Cheung, K.S.; Yeung, K.F.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Graham, D.Y. Effects of Helicobacter pylori Treatment on Incidence of Gastric Cancer in Older Individuals. Gastroenterology 2018, 155, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk Factors and Incidence of Gastric Cancer after Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020, 158, 527–536.e527. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Eom, B.W.; Jung, K.W.; Won, Y.J.; Yang, H.; Kim, Y.W. Trends in Gastric Cancer Incidence According to the Clinicopathological Characteristics in Korea, 1999–2014. Cancer Res. Treat. 2018, 50, 1343–1350. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- You, S.C.; Lee, S.; Cho, S.Y.; Park, H.; Jung, S.; Cho, J.; Yoon, D.; Park, R.W. Conversion of National Health Insurance Service-National Sample Cohort (NHIS-NSC) Database into Observational Medical Outcomes Partnership-Common Data Model (OMOP-CDM). Stud. Health Technol. Inform. 2017, 245, 467–470. [Google Scholar] [PubMed]
- Suchard, M.A.; Schuemie, M.J.; Krumholz, H.M.; You, S.C.; Chen, R.; Pratt, N.; Reich, C.G.; Duke, J.; Madigan, D.; Hripcsak, G.; et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: A systematic, multinational, large-scale analysis. Lancet 2019, 394, 1816–1826. [Google Scholar] [CrossRef] [Green Version]
- You, S.C.; Jung, S.; Swerdel, J.N.; Ryan, P.B.; Schuemie, M.J.; Suchard, M.A.; Lee, S.; Cho, J.; Hripcsak, G.; Park, R.W.; et al. Comparison of First-Line Dual Combination Treatments in Hypertension: Real-World Evidence from Multinational Heterogeneous Cohorts. Korean Circ. J. 2020, 50, 52–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.I.; Park, C.H.; You, S.C.; Kim, J.Y.; Lee, K.J.; Kim, J.; Kim, Y.; Yoo, J.J.; Seo, W.W.; Lee, H.S.; et al. Association between proton pump inhibitor use and gastric cancer: A population-based cohort study using two different types of nationwide databases in Korea. Gut 2021, 70, 2066–2075. [Google Scholar] [CrossRef]
- Hripcsak, G.; Duke, J.D.; Shah, N.H.; Reich, C.G.; Huser, V.; Schuemie, M.J.; Suchard, M.A.; Park, R.W.; Wong, I.C.; Rijnbeek, P.R.; et al. Observational Health Data Sciences and Informatics (OHDSI): Opportunities for Observational Researchers. Stud. Health Technol. Inform. 2015, 216, 574–578. [Google Scholar]
- Korean, H. pylori Study Group. Diagnosis and Treatment of Helicobacter pylori infection in Korea. Korean J. Gastroenterol. 1998, 32, 275–289. [Google Scholar]
- Kim, N.; Kim, J.J.; Choe, Y.H.; Kim, H.S.; Kim, J.I.; Chung, I.-S.; Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Review: Diagnosis and Treatment Guidelines for Helicobacter pylori Infection in Korea. Korean J. Gastroenterol. 2009, 54, 269–278. [Google Scholar] [CrossRef]
- Tian, Y.; Schuemie, M.J.; Suchard, M.A. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int. J. Epidemiol. 2018, 47, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Tchetgen Tchetgen, E.; Cohen, T. Negative controls: A tool for detecting confounding and bias in observational studies. Epidemiology 2010, 21, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef]
- Zhou, M.J.; Huang, R.J. Catching Up with the World: Pepsinogen Screening for Gastric Cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1257–1258. [Google Scholar] [CrossRef]
- Luu, X.Q.; Lee, K.; Jun, J.K.; Suh, M.; Jung, K.W.; Choi, K.S. Effect of gastric cancer screening on long-term survival of gastric cancer patients: Results of Korean national cancer screening program. J. Gastroenterol. 2022, 57, 464–475. [Google Scholar] [CrossRef]
- Seo, S.I.; Park, C.H.; Kim, T.J.; Bang, C.S.; Kim, J.Y.; Lee, K.J.; Kim, J.; Kim, H.H.; You, S.C.; Shin, W.G. Aspirin, metformin, and statin use on the risk of gastric cancer: A nationwide population-based cohort study in Korea with systematic review and meta-analysis. Cancer Med. 2022, 11, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Shin, C.M.; Han, K.-D.; Lee, S.W.; Jin, E.H.; Choi, Y.J.; Yoon, H.; Park, Y.S.; Kim, N.; Lee, D.H. Association between the Persistence of Obesity and the Risk of Gastric Cancer: A Nationwide Population-Based Study. Cancer Res. Treat. 2022, 54, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Bair, M.J.; Chen, P.Y.; Lee, J.Y.; Yang, T.H.; Fang, Y.J.; Chen, C.C.; Chang, A.T.; Hsiao, W.D.; Yu, J.J.; et al. Declining trends of prevalence of Helicobacter pylori infection and incidence of gastric cancer in Taiwan: An updated cross-sectional survey and meta-analysis. Helicobacter 2022, e12914. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Kim, Y.A.; Lee, J.W.; Kim, H.J.; Kim, S.H.; Kim, S.G.; Kim, J.I.; Kim, J.J.; Choi, I.J. Effect of Helicobacter pylori Treatment on Long-term Mortality in Patients with Hypertension. Gut Liver 2020, 14, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.I.; Kim, Y.A.; Kim, H.J.; Kim, S.H.; Hwangbo, Y.; Kim, J.G.; Kim, J.J.; Choi, I.J. Effect of Helicobacter pylori treatment on the long-term mortality in patients with type 2 diabetes. Korean J. Intern. Med. 2021, 36, 584–595. [Google Scholar] [CrossRef]
- Jung, H.K.; Kang, S.J.; Lee, Y.C.; Yang, H.J.; Park, S.Y.; Shin, C.M.; Kim, S.E.; Lim, H.C.; Kim, J.H.; Nam, S.Y.; et al. Evidence-Based Guidelines for the Treatment of Helicobacter pylori Infection in Korea 2020. Gut Liver 2021, 15, 168–195. [Google Scholar] [CrossRef] [PubMed]
- Sverdén, E.; Brusselaers, N.; Wahlin, K.; Lagergren, J. Time latencies of Helicobacter pylori eradication after peptic ulcer and risk of recurrent ulcer, ulcer adverse events, and gastric cancer: A population-based cohort study. Gastrointest. Endosc. 2018, 88, 242–250.e241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Kim, Y.J.; Seo, S.I.; Shin, W.G.; Park, C.H. Impact of the timing of Helicobacter pylori eradication on the risk of development of metachronous lesions after treatment of early gastric cancer: A population-based cohort study. Gastrointest. Endosc. 2020, 92, 613–622.e611. [Google Scholar] [CrossRef] [PubMed]


| Characteristic, % | Before PS Adjustment | After PS Adjustment | ||||
|---|---|---|---|---|---|---|
| HP Treatment (n = 21,801) | Non-Treatment (n = 31,642) | SMD | HP Treatment (n = 2735) | Non-Treatment (n = 5328) | SMD | |
| Age group (years) | ||||||
| 40–44 | 17.9 | 40.6 | 0.52 | 39.2 | 40.2 | 0.02 |
| 45–49 | 19.8 | 13.4 | 0.17 | 17.6 | 17.7 | 0 |
| 50–54 | 18.4 | 10.7 | 0.22 | 13.4 | 13.6 | 0.01 |
| 55–59 | 14.6 | 9.8 | 0.15 | 10.7 | 10.3 | 0.01 |
| 60–64 | 11.4 | 10.1 | 0.04 | 8.0 | 8.2 | 0 |
| 65–69 | 9.2 | 7.7 | 0.05 | 6.3 | 6.0 | 0.01 |
| 70–74 | 5.3 | 4.5 | 0.04 | 2.8 | 2.5 | 0.02 |
| 75–79 | 2.5 | 2.0 | 0.03 | 1.1 | 1.0 | 0.02 |
| 80–84 | 0.8 | 0.8 | 0 | 0.6 | 0.4 | 0.03 |
| 85–89 | 0.2 | 0.2 | 0.01 | <0.2 | 0.1 | 0 |
| Sex: Female | 44.0 | 57.0 | 0.26 | 42.6 | 42.4 | 0 |
| Smoking | 8.4 | 5.3 | 0.12 | 7.6 | 7.6 | 0 |
| Alcohol consumption | 32.3 | 27.6 | 0.10 | 27.6 | 27.1 | 0.01 |
| Medical history | ||||||
| Acute respiratory disease | 60.6 | 62.1 | 0.03 | 47.5 | 47.6 | 0 |
| Chronic liver disease | 11.9 | 15.2 | 0.1 | 13.8 | 14.3 | 0.01 |
| Depressive disorder | 7.7 | 7.9 | 0.01 | 5.5 | 5.4 | 0 |
| Diabetes mellitus | 16.7 | 13.1 | 0.1 | 10.0 | 10.4 | 0.01 |
| Gastroesophageal reflux disease | 19.1 | 16.1 | 0.08 | 14.7 | 15.4 | 0.02 |
| Gastrointestinal hemorrhage | 13.6 | 8.0 | 0.18 | 10.7 | 10.6 | 0 |
| Hyperlipidemia | 30.2 | 17.9 | 0.29 | 18.1 | 19.2 | 0.03 |
| Hypertensive disorder | 29.0 | 19.6 | 0.22 | 14.3 | 14.5 | 0 |
| Osteoarthritis | 12.6 | 10.0 | 0.08 | 6.0 | 6.3 | 0.01 |
| Visual system disorder | 34.1 | 29.8 | 0.09 | 23.2 | 22.4 | 0.02 |
| Heart disease | 17.3 | 16.2 | 0.03 | 12.2 | 13.2 | 0.03 |
| Ischemic heart disease | 9.7 | 7.9 | 0.06 | 5.9 | 6.4 | 0.02 |
| Peripheral vascular disease | 10.4 | 5.1 | 0.2 | 4.1 | 4.0 | 0 |
| Malignant neoplastic disease | 5.6 | 5.6 | 0 | 3.6 | 3.8 | 0.01 |
| Medication use | ||||||
| Agents acting on the renin-angiotensin system | 14.4 | 7.4 | 0.23 | 5.2 | 5.6 | 0.02 |
| Antibacterials for systemic use | 63.2 | 65.5 | 0.05 | 51.9 | 50.5 | 0.03 |
| Antidepressants | 10.8 | 10.9 | 0 | 6.7 | 6.5 | 0.01 |
| Antiepileptics | 5.2 | 3.3 | 0.1 | 2.3 | 2.4 | 0 |
| Anti-inflammatory and antirheumatic products | 58.5 | 56.4 | 0.04 | 43.6 | 43.3 | 0.01 |
| Antithrombotic agents | 49.7 | 40.1 | 0.19 | 32.1 | 31.6 | 0.01 |
| Aspirin | 14.7 | 8.2 | 0.21 | 6.1 | 6.3 | −0.01 |
| Beta blocking agents | 12.2 | 10.4 | 0.06 | 6.9 | 7.2 | 0.01 |
| Calcium channel blockers | 18.1 | 11.3 | 0.19 | 7.6 | 9.0 | 0.05 |
| Diuretics | 16.3 | 10.6 | 0.17 | 6.9 | 7.3 | 0.02 |
| Drugs for acid-related disorders | 78.7 | 83.3 | 0.12 | 75.5 | 75.0 | 0.01 |
| Drugs for obstructive airway diseases | 38.6 | 37.2 | 0.03 | 27.1 | 26.9 | 0 |
| Drugs used in diabetes | 9.3 | 5.9 | 0.13 | 4.8 | 4.6 | 0.01 |
| Metformin | 6.5 | 3.2 | 0.15 | 2.7 | 2.5 | 0.02 |
| Lipid modifying agent | 15.2 | 6.2 | 0.3 | 5.3 | 6.1 | 0.03 |
| Simvastatin | 5.1 | 1.4 | 0.21 | 1.6 | 1.7 | −0.01 |
| Rosuvastatin | 1.1 | 0.1 | 0.13 | 0.3 | 0.2 | 0.01 |
| Pravastatin | 0.9 | 0.7 | 0.03 | 0.4 | 0.8 | −0.05 |
| Pitavastatin | 0.7 | 0.1 | 0.10 | 0.1 | 0.2 | −0.04 |
| Lovastatin | 0.9 | 1.8 | −0.08 | 1.1 | 1.0 | 0.01 |
| Fluvastatin | 0.5 | 0.2 | 0.05 | 0.3 | 0.1 | 0.04 |
| Atorvastatin | 6.5 | 1.6 | 0.25 | 1.5 | 1.7 | −0.01 |
| Opioids | 47.5 | 41.8 | 0.11 | 31.4 | 31.9 | 0.01 |
| Psycholeptics | 69.6 | 72.1 | 0.06 | 62.5 | 62.4 | 0 |
| Charlson index—Romano adaptation | 2.3 | 2.0 | 0.03 | 1.3 | 1.1 | 0.02 |
| Cohorts | Number of Subjects | Observation, Person-Years | Incidence Rate of Gastric Cancer a | HR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Helicobacter pylori treatment in the general population | |||||
| Non-HP treatment | 5328 | 35,654 | 2.92 | Ref | |
| HP treatment | 2735 | 17,938 | 2.34 | 0.76 (0.50–1.13) | 0.19 |
| Helicobacter pylori treatment in individuals aged ≥65 years | |||||
| Non-HP treatment | 1160 | 6237 | 7.37 | Ref | |
| HP treatment | 559 | 2940 | 5.44 | 0.87 (0.44–1.68) | 0.69 |
| Helicobacter pylori treatment in men | |||||
| Non-HP treatment | 3002 | 19,814 | 3.84 | Ref | |
| HP treatment | 1652 | 10,713 | 3.45 | 0.82 (0.51–1.27) | 0.38 |
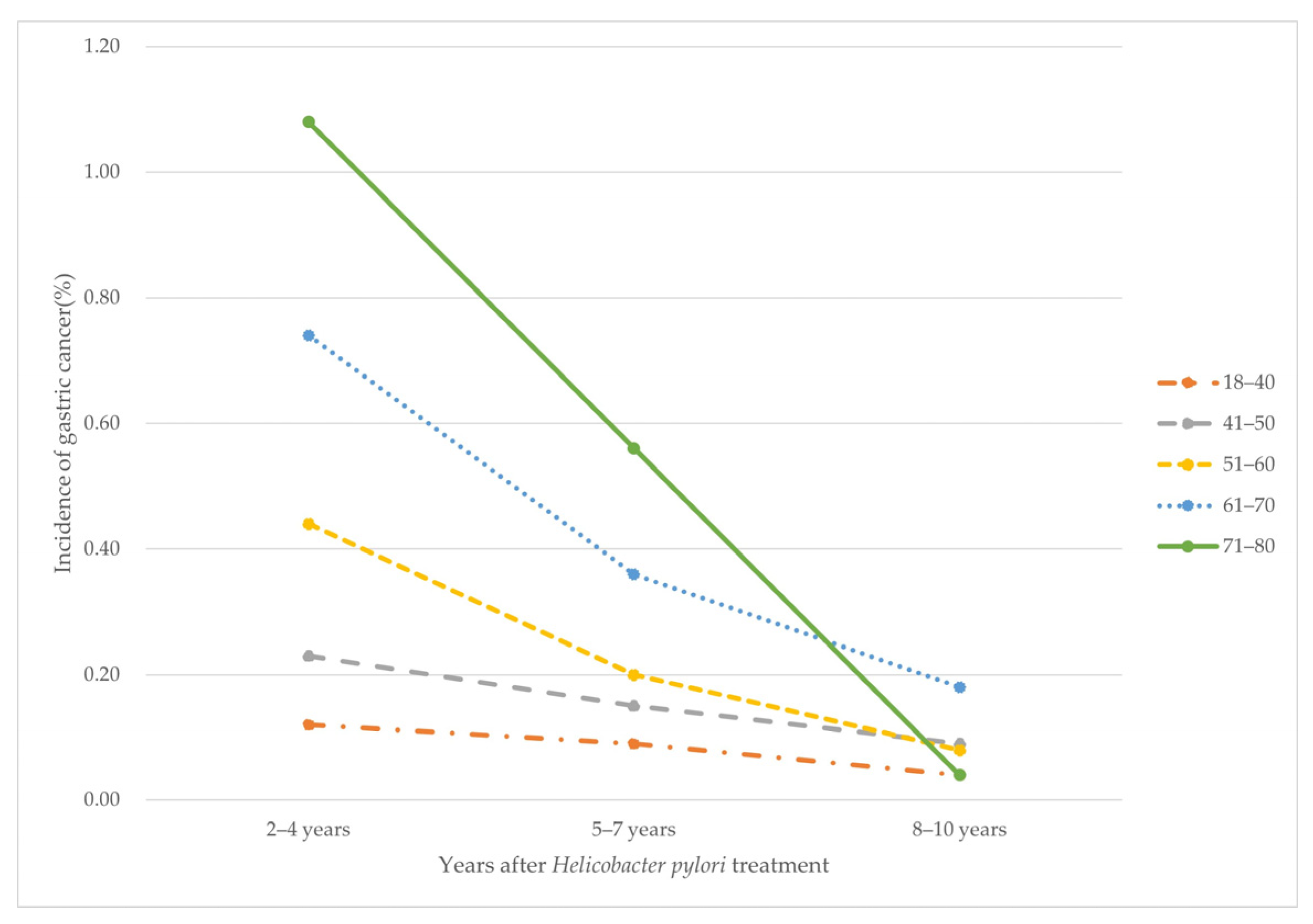
| Years after HP Treatment | 2–4 Years | 5–7 Years | 8–10 Years | p for Trend | |
|---|---|---|---|---|---|
| Age Group (Years) | |||||
| 18–40 | 0.12% (17/14,775) | 0.09% (13/14,758) | 0.04% (6/14,745) | 0.025 | |
| 41–50 | 0.23% (41/17,573) | 0.15% (27/17,532) | 0.09% (15/17,505) | <0.001 | |
| 51–60 | 0.44% (68/15,441) | 0.23% (36/15,373) | 0.08% (13/15,337) | <0.001 | |
| 61–70 | 0.74% (62/8385) | 0.36% (30/8323) | 0.18% (15/8293) | <0.001 | |
| 71–80 | 1.08% (27/2510) | 0.56% (14/2483) | 0.04% (1/2469) | <0.001 | |
| p for trend | <0.001 | <0.001 | 0.018 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Seo, S.I.; Lee, K.J.; Park, C.H.; Kim, T.J.; Kim, J.; Shin, W.G. Decreasing Incidence of Gastric Cancer with Increasing Time after Helicobacter pylori Treatment: A Nationwide Population-Based Cohort Study. Antibiotics 2022, 11, 1052. https://doi.org/10.3390/antibiotics11081052
Kim T, Seo SI, Lee KJ, Park CH, Kim TJ, Kim J, Shin WG. Decreasing Incidence of Gastric Cancer with Increasing Time after Helicobacter pylori Treatment: A Nationwide Population-Based Cohort Study. Antibiotics. 2022; 11(8):1052. https://doi.org/10.3390/antibiotics11081052
Chicago/Turabian StyleKim, Taewan, Seung In Seo, Kyung Joo Lee, Chan Hyuk Park, Tae Jun Kim, Jinseob Kim, and Woon Geon Shin. 2022. "Decreasing Incidence of Gastric Cancer with Increasing Time after Helicobacter pylori Treatment: A Nationwide Population-Based Cohort Study" Antibiotics 11, no. 8: 1052. https://doi.org/10.3390/antibiotics11081052
APA StyleKim, T., Seo, S. I., Lee, K. J., Park, C. H., Kim, T. J., Kim, J., & Shin, W. G. (2022). Decreasing Incidence of Gastric Cancer with Increasing Time after Helicobacter pylori Treatment: A Nationwide Population-Based Cohort Study. Antibiotics, 11(8), 1052. https://doi.org/10.3390/antibiotics11081052






