Antibiotic Resistance Risk with Oral Tetracycline Treatment of Acne Vulgaris
Abstract
:1. Acne Vulgaris
2. Oral Antibiotic Treatment: Tetracyclines
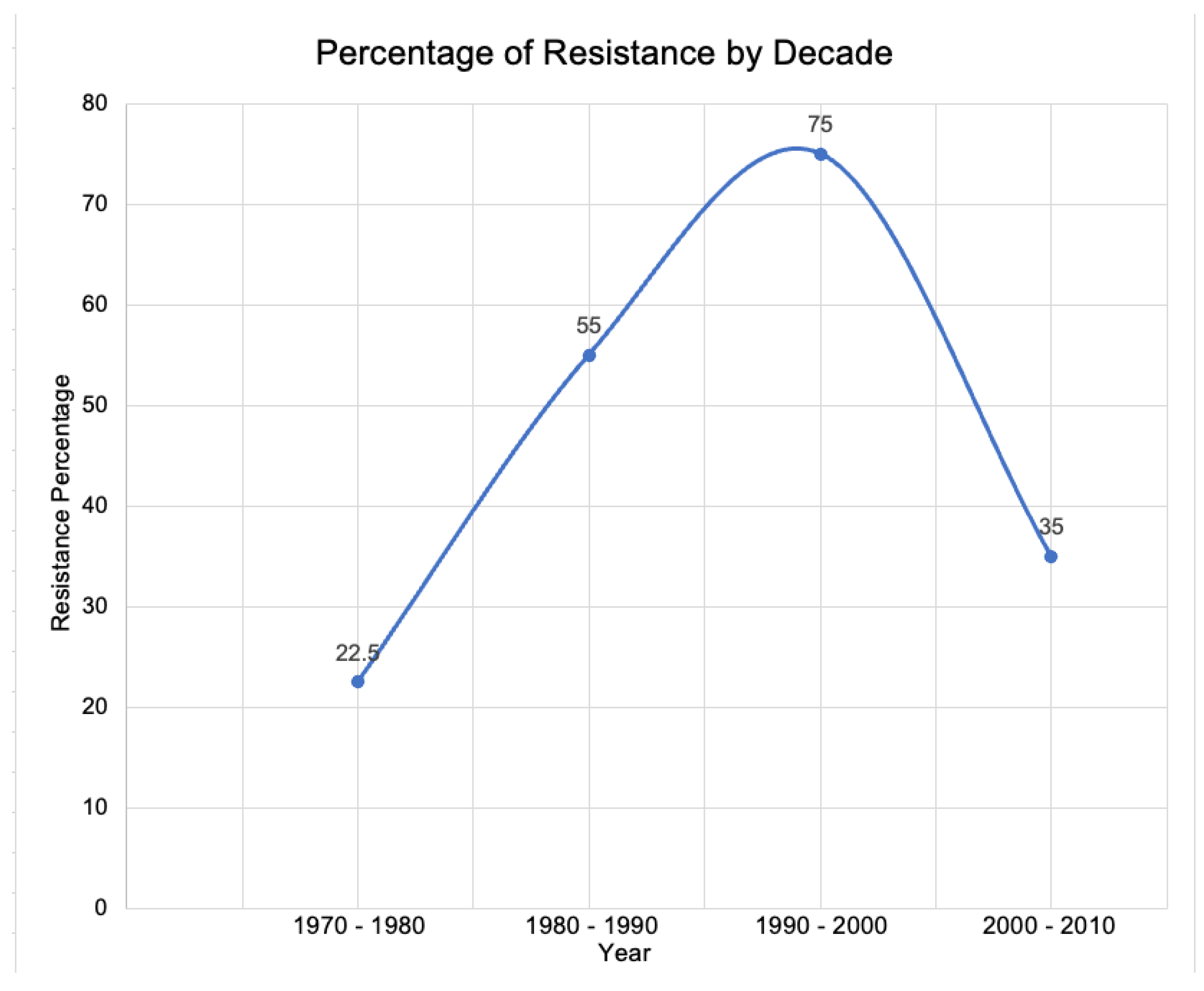
3. The Risk of Antibiotic Resistance
4. Other Negative Effects of Traditional Tetracycline Usage
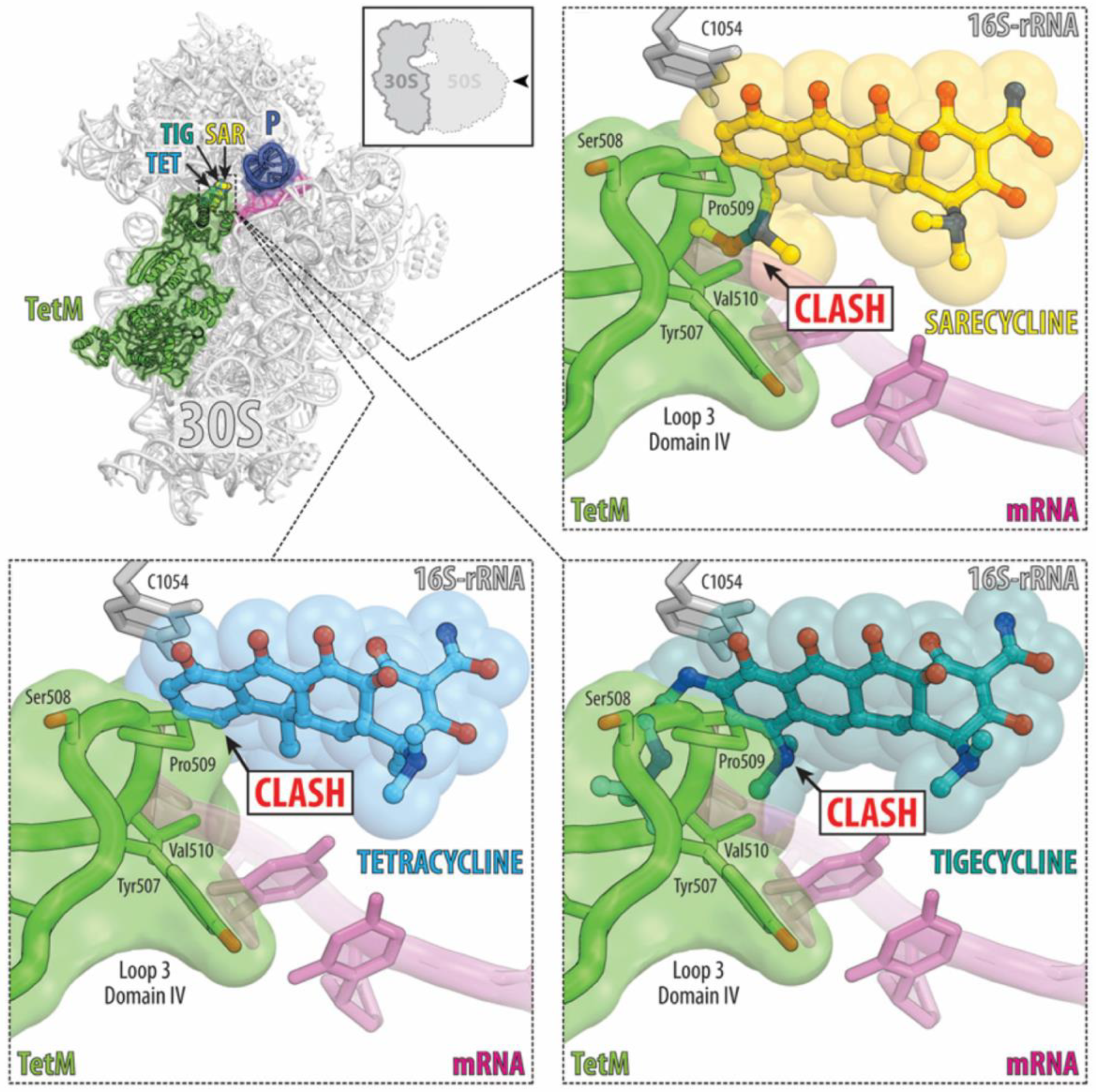
5. Benefits of Sarecycline: Reduced Antibiotic Resistance
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tan, J.K.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172 (Suppl. S1), 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.D.; Umari, T.; Dunnick, C.A.; Dellavalle, R.P. The epidemiology of acne vulgaris in late adolescence. Adolesc. Health Med. Ther. 2016, 7, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Zaenglein, A.L. Acne vulgaris. N. Engl. J. Med. 2018, 379, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.N.; Harper, J.C.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The prevalence of acne in adults 20 years and older. J. Am. Acad. Dermatol. 2008, 58, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Dermatology: How to manage acne vulgaris. Drugs Context 2020, 10, 2021-8-6. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Silverberg, N.B. Epidemiology and extracutaneous comorbidities of severe acne in adolescence: A U.S. population-based study. Br. J. Dermatol. 2014, 170, 1136–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smithard, A.; Glazebrook, C.; Williams, H.C. Acne prevalence, knowledge about acne and psychological morbidity in mid-adolescence: A community-based study. Br. J. Dermatol. 2001, 145, 274–279. [Google Scholar] [CrossRef]
- Spencer, E.H.; Ferdowsian, H.R.; Barnard, N.D. Diet and acne: A review of the evidence. Int. J. Dermatol. 2009, 48, 339–347. [Google Scholar] [CrossRef]
- Halvorsen, J.A.; Stern, R.S.; Dalgard, F.; Thoresen, M.; Bjertness, E.; Lien, L. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: A population-based study. J. Investig. Dermatol. 2011, 131, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [Green Version]
- Bienenfeld, A.; Nagler, A.R.; Orlow, S.J. Oral Antibacterial Therapy for Acne Vulgaris: An Evidence-Based Review. Am. J. Clin. Dermatol. 2017, 18, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.J.; Bhatia, N. Oral Antibiotics for Acne. Am. J. Clin. Dermatol. 2021, 22, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Ling, M.; Bucko, A.; Manna, V.; Rueda, M.J. Efficacy and Safety of Subantimicrobial Dose, Modified-Release Doxycycline 40 mg Versus Doxycycline 100 mg Versus Placebo for the treatment of Inflammatory Lesions in Moderate and Severe Acne: A Randomized, Double-Blinded, Controlled Study. J. Drugs Dermatol. 2015, 14, 581–586. [Google Scholar] [PubMed]
- Fleischer, A.B., Jr.; Dinehart, S.; Stough, D.; Plott, R.T.; Solodyn Phase 2 Study Group; Solodyn Phase 3 Study Group. Safety and efficacy of a new extended-release formulation of minocycline. Cutis 2006, 78, 21–31. [Google Scholar] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, C. Antibiotics at the crossroads. Nature 2004, 431, 899–902. [Google Scholar] [CrossRef]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- World Economic Forum. Global Risks. 2013. Available online: https://reports.weforum.org/global-risks-2013/?doing_wp_cron=1659126758.5001749992370605468750 (accessed on 27 July 2022).
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 27 July 2022).
- Adler, B.L.; Kornmehl, H.; Armstrong, A.W. Antibiotic Resistance in Acne Treatment. JAMA Dermatol. 2017, 153, 810–811. [Google Scholar] [CrossRef]
- Walsh, T.R.; Efthimiou, J.; Dreno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect. Dis. 2016, 16, e23–e33. [Google Scholar] [CrossRef] [Green Version]
- Leyden, J.J. Antibiotic resistant acne. Cutis 1976, 17, 593–596. [Google Scholar]
- Karadag, A.S.; Kayiran, M.A.; Wu, C.Y.; Chen, W.; Parish, L.C. Antibiotic resistance in acne: Changes, consequences and concerns. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 73–78. [Google Scholar] [CrossRef]
- Brodersen, D.E.; Clemons, W.M., Jr.; Carter, A.P.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishnan, V. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 2000, 103, 1143–1154. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, M.H.; Lupu, A.N.; Franklin, S.S. Clinical and physiological factors determining diagnosis and choice of treatment of renovascular hypertension. Circ. Res. 1967, 21 (Suppl. S2), 201. [Google Scholar]
- Pioletti, M.; Schlunzen, F.; Harms, J.; Zarivach, R.; Gluhmann, M.; Avila, H.; Bashan, A.; Bartels, H.; Auerbach, T.; Jacobi, C.; et al. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 2001, 20, 1829–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, N.M.; Hui, M.; Lee, H.C.; Fu, L.H.; Liu, Z.H.; Lam, L.Y.; Eastel, M.; Chan, Y.-K.; Tang, L.-S.; Cheng, T.-S.; et al. Antibiotic-resistant Propionibacterium acnes among acne patients in a regional skin centre in Hong Kong. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 31–36. [Google Scholar] [CrossRef]
- Alkhawaja, E.; Hammadi, S.; Abdelmalek, M.; Mahasneh, N.; Alkhawaja, B.; Abdelmalek, S.M. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: A cross sectional study. BMC Dermatol. 2020, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Sheffer-Levi, S.; Rimon, A.; Lerer, V.; Shlomov, T.; Coppenhagen-Glazer, S.; Rakov, C.; Zeiter, T.; Nir-Paz, R.; Hazan, R.; Molcho-Pessach, V. Antibiotic Susceptibility of Cutibacterium acnes Strains Isolated from Israeli Acne Patients. Acta Derm. Venereol. 2020, 100, adv00295. [Google Scholar] [CrossRef] [PubMed]
- Eady, E.A.; Cove, J.H.; Holland, K.T.; Cunliffe, W.J. Erythromycin resistant propionibacteria in antibiotic treated acne patients: Association with therapeutic failure. Br. J. Dermatol. 1989, 121, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Lofmark, S.; Edlund, C.; Jansson, J.K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiol. (Read.) 2010, 156 Pt 11, 3216–3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, D.J.; Bowe, W.P.; Hoffstad, O.; Berlin, J.A. Antibiotic treatment of acne may be associated with upper respiratory tract infections. Arch. Dermatol. 2005, 141, 1132–1136. [Google Scholar] [CrossRef] [Green Version]
- Margolis, D.J.; Fanelli, M.; Kupperman, E.; Papadopoulos, M.; Metlay, J.P.; Xie, S.X.; DiRienzo, J.; Edelstein, P. Association of pharyngitis with oral antibiotic use for the treatment of acne: A cross-sectional and prospective cohort study. Arch. Dermatol. 2012, 148, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.Y.; Charles, J.E.M.; Moore, S. Sarecycline: A narrow spectrum tetracycline for the treatment of moderate-to-severe acne vulgaris. Future Microbiol. 2019, 14, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, D.J.; Fanelli, M.; Hoffstad, O.; Lewis, J.D. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q.; Gold, L.S.; Baldwin, H.; Harper, J.C.; Zeichner, J.; Obagi, S.; Graber, E.; Jimenez, X.; Vicente, F.H.; Grada, A. Management of Truncal Acne With Oral Sarecycline: Pooled Results from Two Phase-3 Clinical Trials. J. Drugs Dermatol. 2021, 20, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Gao, P.; Song, Y.X.; Xu, Y.; Sun, J.X.; Chen, X.W.; Zhao, J.H.; Wang, Z.N. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: A pooled analysis of 2740 cancer patients. Oncoimmunology 2019, 8, e1665973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. 2019, 5, 1774–1778. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Moore, A.Y.; Del Rosso, J.; Johnson, J.L.; Grada, A. Sarecycline: A review of preclinical and clinical evidence. Clin. Cosmet. Investig. Dermatol. 2020, 13, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Brahe, C.; Peters, K. Fighting Acne for the Fighting Forces. Cutis 2020, 106, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Ghannoum, M.A.; Bunick, C.G. Sarecycline Demonstrates Clinical Effectiveness against Staphylococcal Infections and Inflammatory Dermatoses: Evidence for Improving Antibiotic Stewardship in Dermatology. Antibiotics 2022, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration Approved Prescribing Information. SEYSARA® (Sarecycline) Tablets for Oral Use (Package Insert). Almirall LLC.: Barcelona, Spain, 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/209521Orig1s000TOC.cfm (accessed on 10 April 2022).
- Bunick, C.G.; Keri, J.; Tanaka, S.K.; Furey, N.; Damiani, G.; Johnson, J.L.; Grada, A. Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation. Antibiotics 2021, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Long, L.; Bunick, C.G.; Del Rosso, J.Q.; Gamal, A.; Tyring, S.K.; McCormick, T.S.; Grada, A. Sarecycline Demonstrated Reduced Activity Compared to Minocycline against Microbial Species Representing Human Gastrointestinal Microbiota. Antibiotics 2022, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.; Critchley, I.; Lin, L.Y.; Alvandi, N. Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris. Antimicrob. Agents Chemother. 2019, 63, e01297-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marson, J.W.; Baldwin, H.E. New Concepts, Concerns, and Creations in Acne. Dermatol. Clin. 2019, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Del Rosso, J.Q.; Graber, E.; Bunick, C.G.; Gold, L.S.; Moore, A.Y.; Baldwin, H.; Obagi, Z.; Damiani, G.; Carrothers, T.; et al. Sarecycline treatment for acne vulgaris: Rationale for weight-based dosing and limited impact of food intake on clinical efficacy. Dermatol. Ther. 2022, 35, e15275. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; Sniukiene, V.; Berk, D.R.; Kaoukhov, A. Efficacy and Safety of Sarecycline, a Novel, Once-Daily, Narrow Spectrum Antibiotic for the Treatment of Moderate to Severe Facial Acne Vulgaris: Results of a Phase 2, Dose-Ranging Study. J. Drugs Dermatol. 2018, 17, 333–338. [Google Scholar] [PubMed]
- Batool, Z.; Lomakin, I.B.; Polikanov, Y.S.; Bunick, C.G. Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome. Proc. Natl. Acad. Sci. USA 2020, 117, 20530–20537. [Google Scholar] [CrossRef]
- Arenz, S.; Nguyen, F.; Beckmann, R.; Wilson, D.N. Cryo-EM structure of the tetracycline resistance protein TetM in complex with a translating ribosome at 3.9-A resolution. Proc. Natl. Acad. Sci. USA 2015, 112, 5401–5406. [Google Scholar] [CrossRef] [Green Version]
- Jenner, L.; Starosta, A.L.; Terry, D.S.; Mikolajka, A.; Filonava, L.; Yusupov, M.; Blanchard, S.C.; Wilson, D.N.; Yusupova, G. Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis. Proc. Natl. Acad. Sci. USA 2013, 110, 3812–3816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elston, M.J.; Dupaix, J.P.; Opanova, M.I.; Atkinson, R.E. Cutibacterium acnes (formerly Proprionibacterium acnes) and Shoulder Surgery. Hawaii J. Health Soc. Welf. 2019, 78 (Suppl. S2), 3–5. [Google Scholar] [PubMed]
- Silverman, E. McDonald’s Accused of Dragging Its Feet on Goal of Reducing Antibiotic Use in Beef Supplies. STAT News. 2021. Available online: https://www.statnews.com/pharmalot/2021/11/29/mcdonalds-antibiotics-superbugs-livestock-beef/ (accessed on 27 July 2022).
- WHO Antibacterial Pipeline Team. Lack of Innovation Set to Undermine Antibiotic Performance and Health Gains. June 2022. Available online: https://www.who.int/news/item/22-06-2022-22-06-2022-lack-of-innovation-set-to-undermine-antibiotic-performance-and-health-gains (accessed on 27 July 2022).
- The AMR Innovation Challenge. The AMR Action Fund. 2022. Available online: https://www.amractionfund.com/amr-innovation-challenge#page-section-3 (accessed on 27 July 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swallow, M.A.; Fan, R.; Cohen, J.M.; Bunick, C.G. Antibiotic Resistance Risk with Oral Tetracycline Treatment of Acne Vulgaris. Antibiotics 2022, 11, 1032. https://doi.org/10.3390/antibiotics11081032
Swallow MA, Fan R, Cohen JM, Bunick CG. Antibiotic Resistance Risk with Oral Tetracycline Treatment of Acne Vulgaris. Antibiotics. 2022; 11(8):1032. https://doi.org/10.3390/antibiotics11081032
Chicago/Turabian StyleSwallow, Madisen A., Ryan Fan, Jeffrey M. Cohen, and Christopher G. Bunick. 2022. "Antibiotic Resistance Risk with Oral Tetracycline Treatment of Acne Vulgaris" Antibiotics 11, no. 8: 1032. https://doi.org/10.3390/antibiotics11081032
APA StyleSwallow, M. A., Fan, R., Cohen, J. M., & Bunick, C. G. (2022). Antibiotic Resistance Risk with Oral Tetracycline Treatment of Acne Vulgaris. Antibiotics, 11(8), 1032. https://doi.org/10.3390/antibiotics11081032






