Evaluation of Recombinant Bovine Interleukin-8 (rbIL-8) as a Treatment for Chronic Intramammary Infection in Dairy Cows
Abstract
:1. Introduction
2. Results
2.1. White Blood Cell Count and Somatic Cell Count
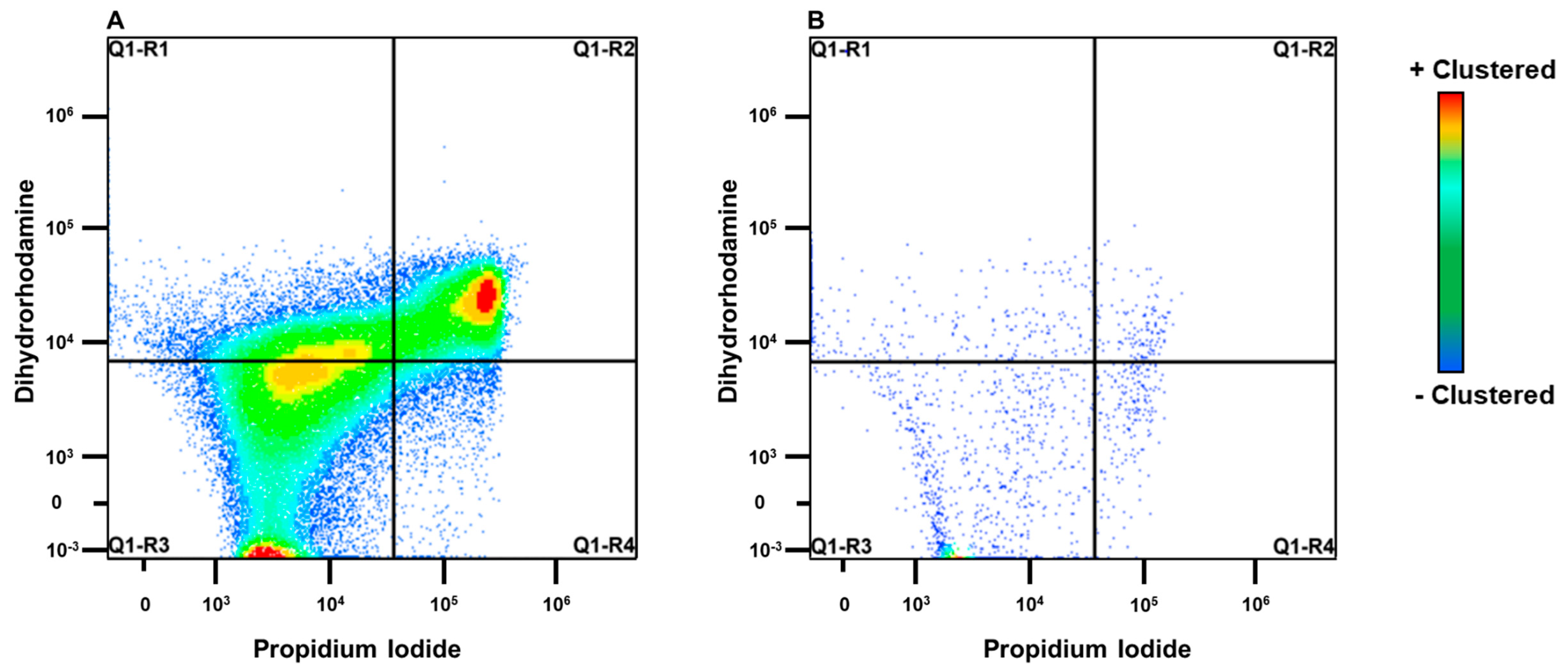
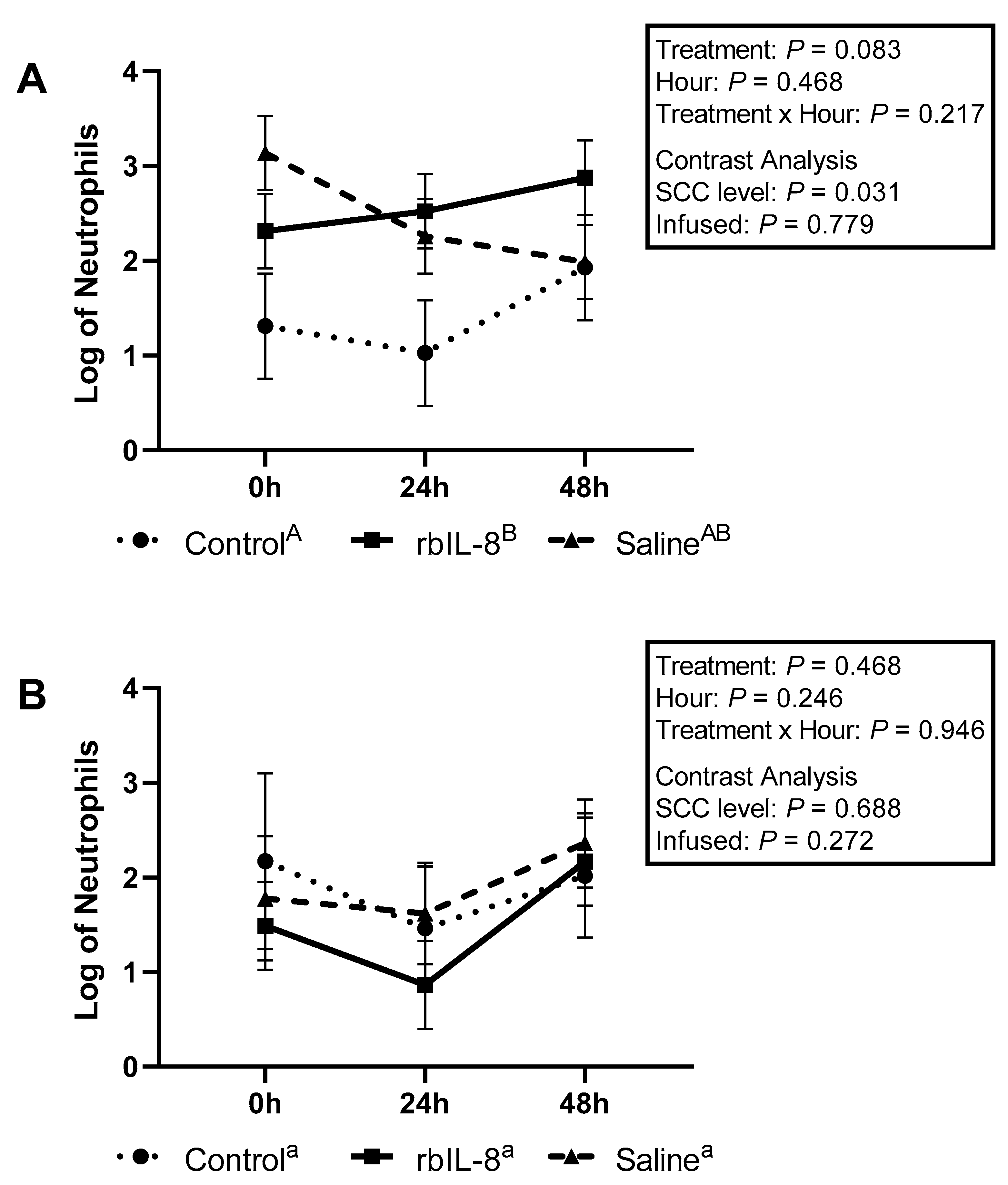
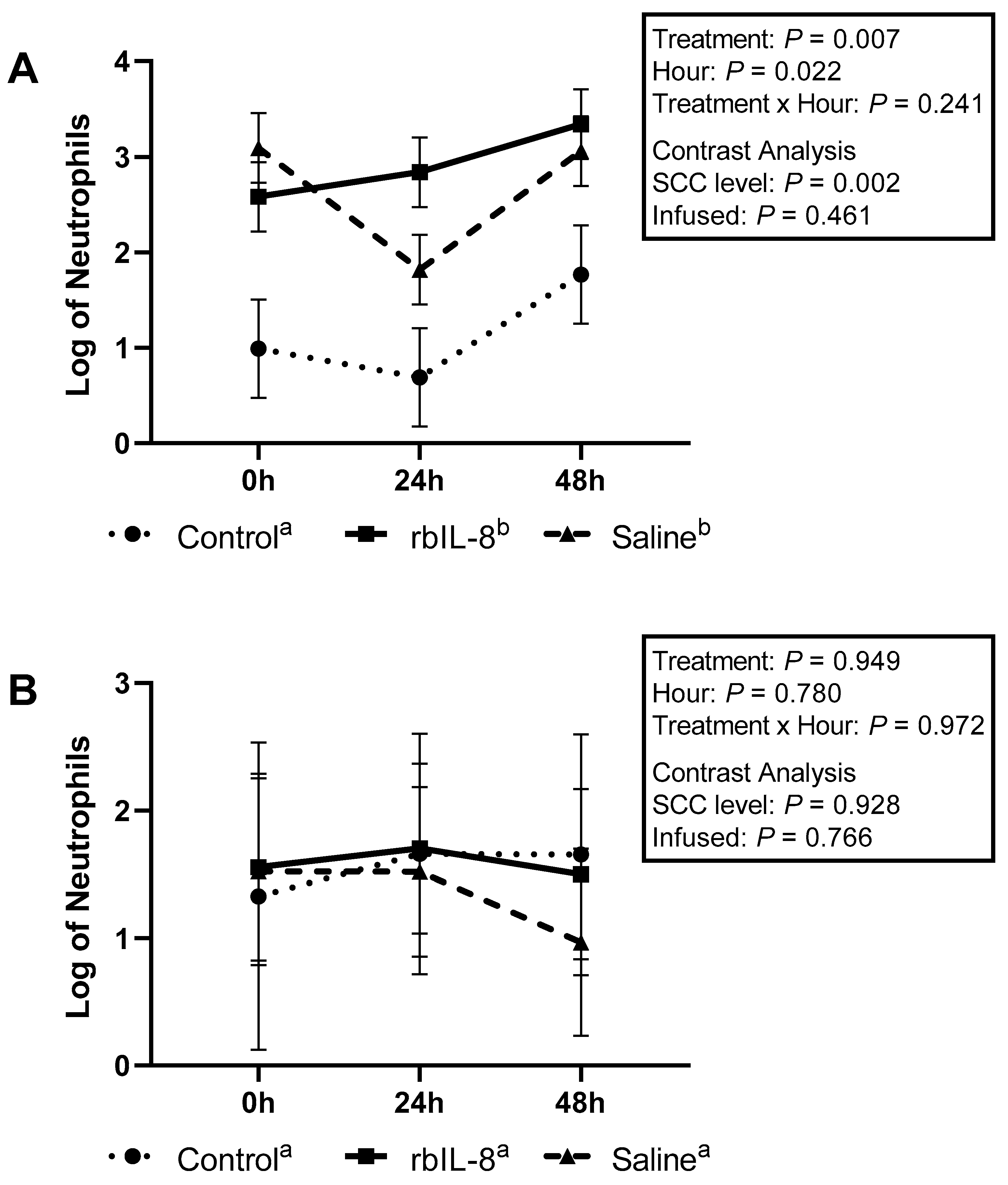
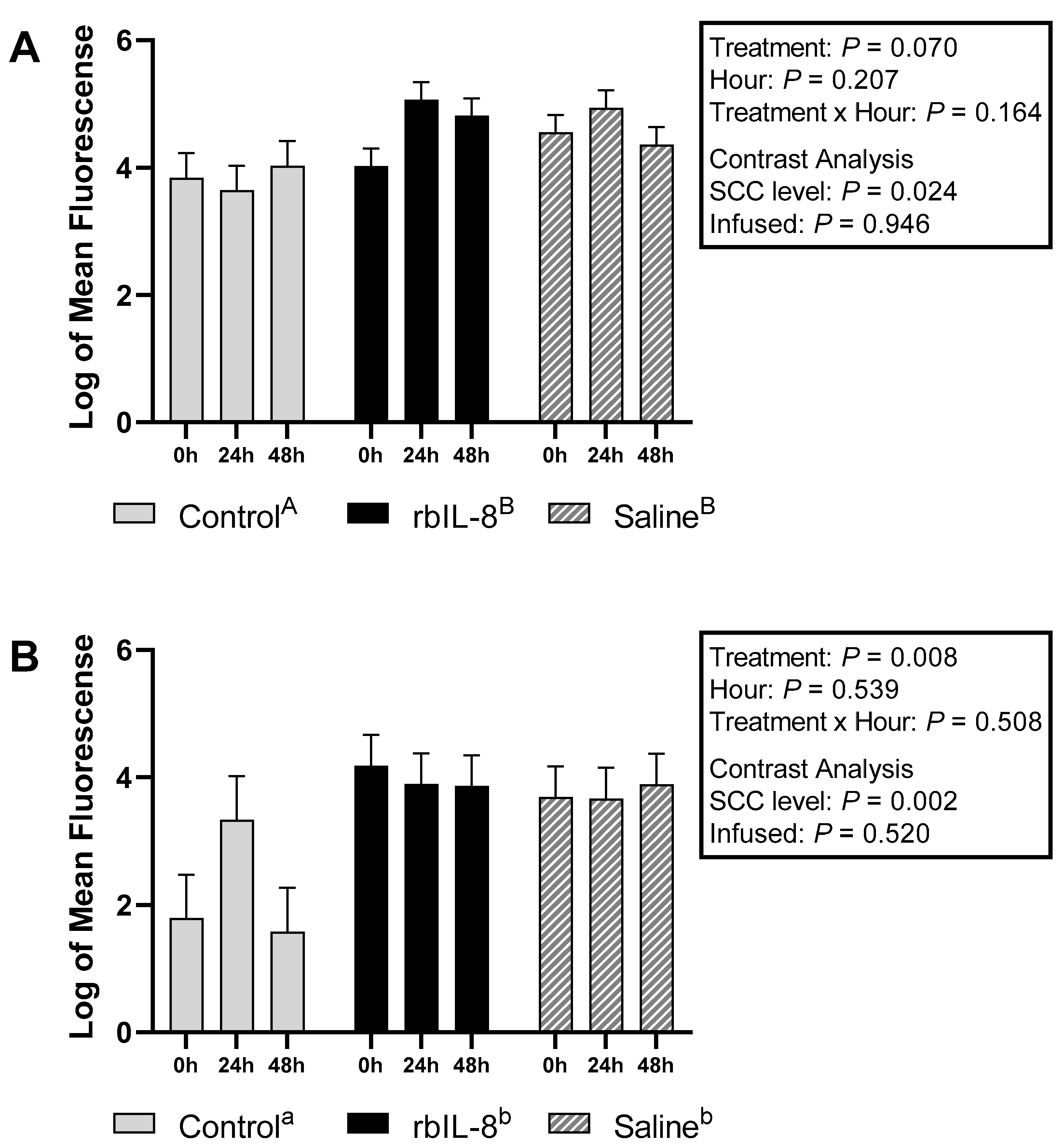
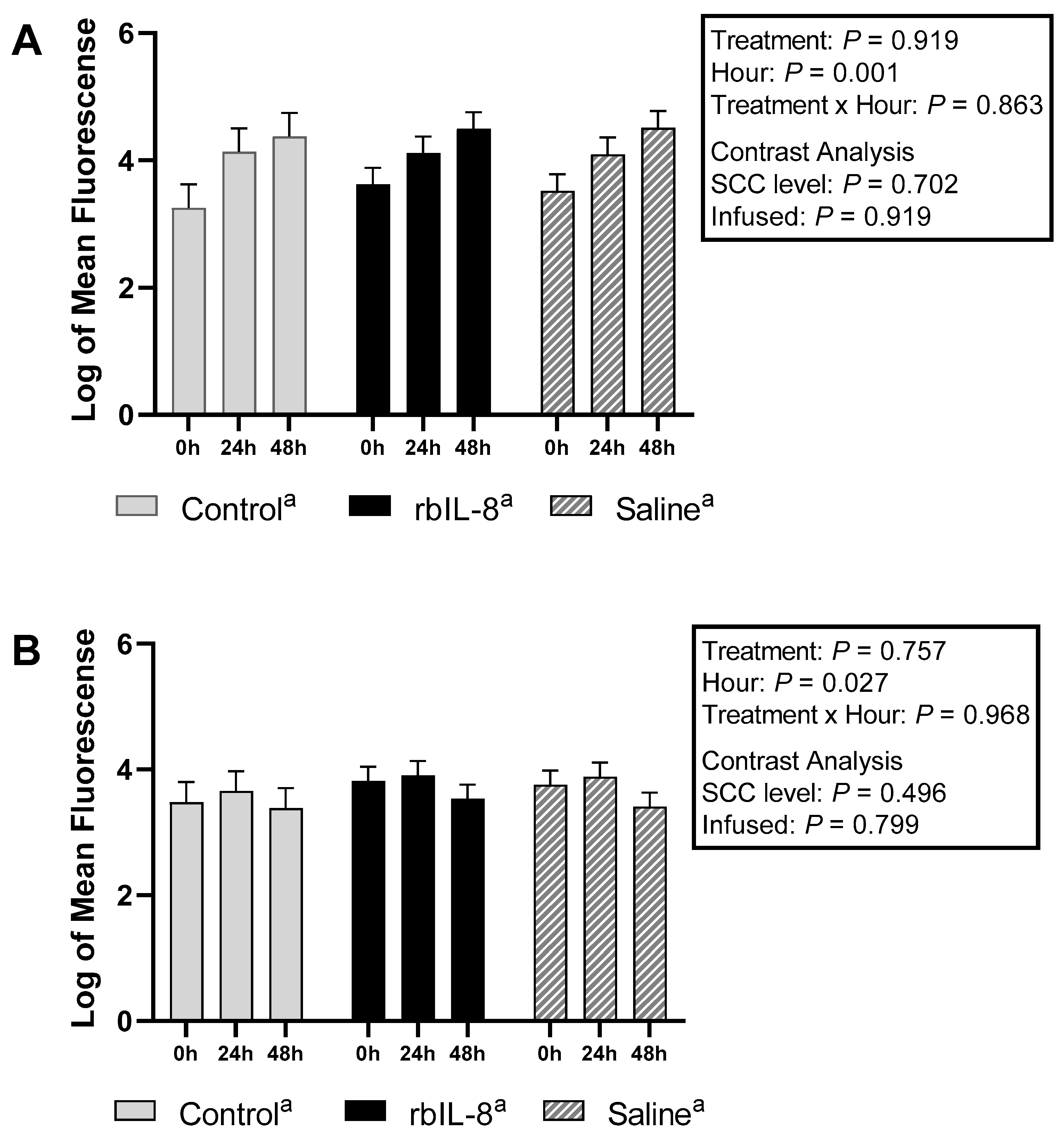
2.2. Evaluation of Milk and Blood Neutrophils Phagocytosis
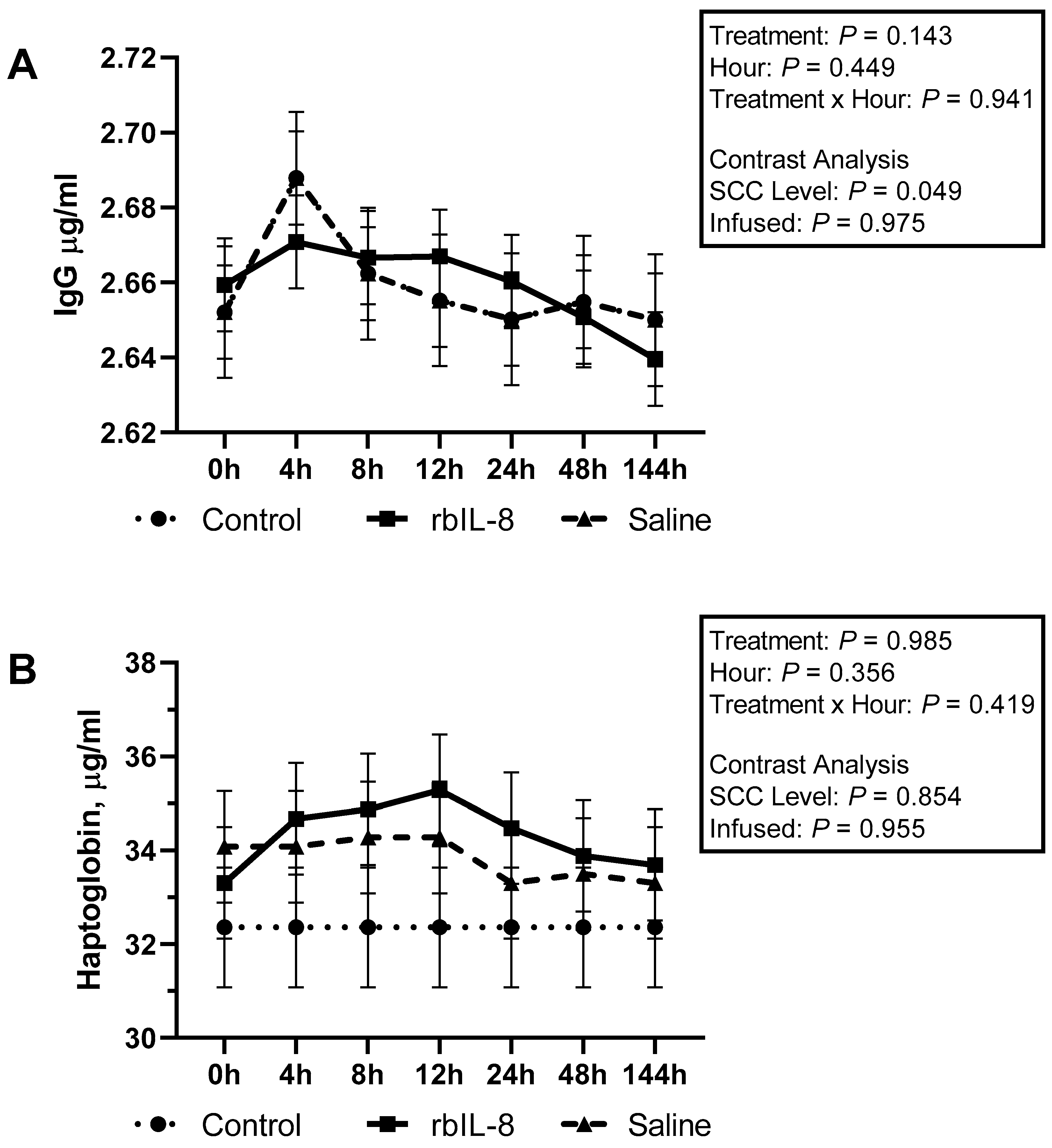
2.3. Evaluation of Blood Biochemical Profile, Milk Haptoglobin, and IgG
3. Discussion
4. Conclusions
5. Materials and Methods
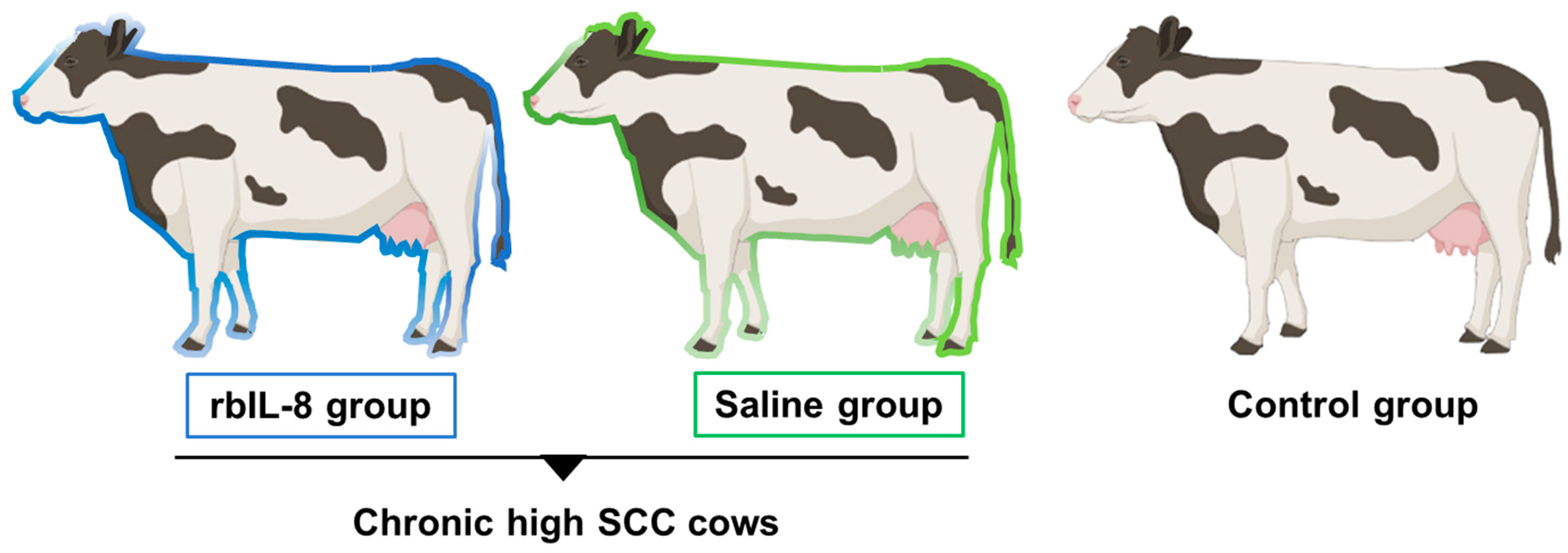
5.1. Animal Selection, Housing, and Treatments
5.2. Somatic Cell Count, PMN Isolation from Milk and Blood Samples
5.3. Escherichia Coli Labeled Propidium Iodide and Flow Cytometry
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- United States Department of Agriculture. United States Department of Agriculture Antimicrobial Resistance Action Plan; United States Department of Agriculture: Wasnington, DC, USA, 2014; pp. 1–28.
- Ruegg, P.L. Making antibiotic treatment decisions for clinical mastitis mastitis antibiotics udder health treatment milk quality. Vet. Clin. NA Food Anim. Pract. 2018, 34, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Tenhagen, B.A.; Köster, G.; Wallmann, J.; Heuwieser, W. Prevalence of mastitis pathogens and their resistance against antimicrobial agents in dairy cows in brandenburg, germany. J. Dairy Sci. 2006, 89, 2542–2551. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, L.; Ruegg, P.L. Treatments of clinical mastitis occurring in cows on 51 large dairy herds in wisconsin. J. Dairy Sci. 2014, 97, 5426–5436. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Barkema, H.W.; Zhang, L.; Liu, G.; Deng, Z.; Cai, L.; Shan, R.; Zhang, S.; Zou, J.; Kastelic, J.P. Incidence of clinical mastitis and distribution of pathogens on large chinese dairy farms. J. Dairy Sci. 2017, 100, 4797–4806. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, M.J.; Fricke, P.M.; Rueg, R.L. The association between occurrence and severity of subclinical and clinical mastitis on pregnancies per artificial insemination at first service of Holstein cows. J. Dairy Sci. 2015, 98, 3791–3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-level mastitis-associated costs on canadian dairy farms. Front. Vet. Sci. 2018, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Forsbäck, L.; Lindmark-Månsson, H.; Åndrén, A.; Kerstedt, M.; Svennersten-Sjaunja, K. Udder quarter milk composition at different levels of somatic cell count in cow composite milk. Animal 2009, 3, 710–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dürr, J.W.; Cue, R.I.; Monardes, H.G.; Moro-Méndez, J.; Wade, K.M. Milk losses associated with somatic cell counts per breed, parity and stage of lactation in canadian dairy cattle. Livest. Sci. 2008, 117, 225–232. [Google Scholar] [CrossRef]
- Beaudeau, F.; Seegers, H.; Ducrocq, V.; Fourichon, C.; Bareille, N. Effect of Health disorders on culling in dairy cows: A review and a critical discussion. Anim. Res. 2000, 49, 293–311. [Google Scholar] [CrossRef]
- Ruegg, P.L.; Pantoja, J.C.F. Understanding and using somatic cell counts to improve milk quality. Irish J. Agric. Food Res. 2013, 52, 101–117. [Google Scholar]
- Martins, L.; Barcelos, M.M.; Cue, R.I.; Anderson, K.L.; Dos Santos, M.V.; Gonçalves, J.L. Chronic subclinical mastitis reduces milk and components yield at the cow level. J. Dairy Res. 2020, 87, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Peton, V.; Bouchard, D.S.; Almeida, S.; Rault, L.; Falentin, H.; Jardin, J.; Jan, G.; Hernandez, D.; François, P.; Schrenzel, J.; et al. Fine-tuned characterization of staphylococcus aureus newbould 305, a strain associated with mild and Chronic mastitis in bovines. Vet. Res. 2014, 45, 106. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.M. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin. Hematol. 1997, 34, 311–318. [Google Scholar] [PubMed]
- Baggiolini, M.; Loetscher, P.; Moser, B. Interleukin-8 and the chemokine family. Int. J. Immunopharmacol. 1995, 17, 103–108. [Google Scholar] [CrossRef]
- Mitchell, G.B.; Albright, B.N.; Caswell, J.L.; Al, M.E.T.; Mmun, I.N.I. Effect of interleukin-8 and granulocyte colony-stimulating factor on priming and activation of bovine neutrophils. Infec. Immun. 2003, 71, 1643–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, A.; Yagi, Y.; Shiono, H.; Yokomizo, Y.; Inumaru, S. Effects of intramammary infusions of interleukin-8 on milk protein composition and induction of acute-phase protein in cows during mammary involution. Can. J. Vet. Res. 2008, 72, 291–296. [Google Scholar]
- Bicalho, M.L.S.; Zinicola, M.; Machado, V.S.; Lima, F.S.; Teixeira, A.G.V.; Narbus, C.; Xavier, M.R.; Higgins, H.; Bicalho, R.C. Effects of recombinant bovine interleukin-8 (RbIL-8) treatment on health, metabolism, and lactation performance in holstein cattle i: Production and functional characterization of Rbil-8 in vitro and in vivo. J. Dairy Sci. 2019, 102, 10304–10315. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Hardwick, A.J.; Lindley, I. Central actions of interleukin-8 in the rat are independent of prostaglandins. Horm. Metab. Res. 1990, 22, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Zampronio, A.R.; Souza, G.E.; Silva, C.A.; Cunha, F.Q.; Ferreira, S.H. Interleukin-8 induces fever by a prostaglandinindependent mechanism. Am. J. Physiol. 1994, 266, 1670–1674. [Google Scholar] [CrossRef]
- Molinari, P.C.C.; Blagitz, M.G.; Libera, A.M.M.P.D.; Batista, C.F.; Souza, F.N. Intracellular reactive oxygen species production and phagocytosis of staphylococcus aureus by milk neutrophils as tool to diagnose mastitis and identify susceptible dairy cows 1. Pesq. Vet. Bras. 2018, 38, 659–664. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein hmgb1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Takahashi, A.; Kobayashi, S.; Yonehara, S.; Sawai, H.; Okazaki, T.; Yamamoto, K.; Sasada, M. Caspases are activated in a branched protease cascade and Control distinct downstream processes in fas-induced apoptosis. J. Exp. Med. 1998, 187, 587–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinicola, M.; Bicalho, M.L.S.; Santin, T.; Marques, E.C.; Bisinotto, R.S.; Bicalho, R.C. Effects of recombinant bovine interleukin-8 (rbil-8) treatment on health, metabolism, and lactation performance in Holstein cattle II: Postpartum uterine health, ketosis, and milk production. J. Dairy Sci. 2019, 102, 10316–10328. [Google Scholar] [CrossRef] [PubMed]
- Paape, J.; Schultze, W.D.; Miller, R.H. Plasma corticosteroid, circulating leukocyte and milk somatic cell responses to Escherichia coli endotoxin-induced mastitis. Proc. Soc. Exp. Biol. Med. 1974, 145, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C.; Paape, M.J.; Berning, L.; Salgar, S.K.; Worku, M. Functional competence and monoclonal antibody reactivity of neutrophils from cows injected with Escherichia coli endotoxin. Comp. Haematol. Int. 1991, 1, 10–20. [Google Scholar] [CrossRef]
- Miller, R.H.; Guidry, A.J.; Paape, M.J.; Dulin, A.M.; Fulton, L.A. relationship between immunoglobulin concentrations in milk and phagocytosis by bovine neutrophils. Am. J. Vet. Res. 1988, 49, 42–45. [Google Scholar] [PubMed]
- Eckersall, P.D.; Young, F.J.; McComb, C.; Hogarth, C.J.; Safi, S.; Weber, A.; McDonald, T.; Nolan, A.M.; Fitzpatrick, J.L. Acute phase proteins in serum and milk from dairy cows with clinical mastitis. Vet. Rec. 2001, 148, 35–41. [Google Scholar] [CrossRef]
- Lai, I.H.; Tsao, J.H.; Lu, Y.P.; Lee, J.W.; Zhao, X.; Chien, F.L.; Mao, S.J.T. Neutrophils as one of the major haptoglobin sources in mastitis affected milk. Vet. Res. 2009, 40, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, F.C.; Waterston, M.; Hastie, P.; Parkin, T.; Haining, H.; Eckersall, P.D. The major acute phase proteins of bovine milk in a commercial dairy herd. BMC Vet. Res. 2015, 11, 207. [Google Scholar] [CrossRef] [Green Version]
- Galitzer, S.J.; Oehme, F.W. Creatine kinase isoenzymes in bovine tissue. Am. J. Vet. Res. 1985, 46, 1427–1429. [Google Scholar]
- Sattler, T.; Fürll, M. Creatine kinase and aspartate aminotransferase in cows as indicators for endometritis. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2004, 51, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Mehrzad, J.; Dosogne, H.; Vangroenweghe, F.; Burvenich, C. A comparative study of bovine blood and milk neutrophil functions with luminol-dependent chemiluminescence. Luminescence 2001, 16, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Hanel, R.M.; Crawford, P.C.; Hernandez, J.; Benson, N.A.; Levy, J.K. Neutrophil function and plasma opsonic capacity in colostrum-fed and colostrum-deprived neonatal kittens. Am. J. Vet. Res. 2003, 64, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvão, K.; Santos, J.E.P. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Item | Hours Relative to Treatment | Effect 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC 1 Δ% | 0 h 2 | 0–4 h | 4–8 h | 8–24 h | 24–48 h | Mean Δ% | Group | Hour | Group*Hour | Infused | SCC level |
| Control | 9.6 ± 5.9 | 14.2 ± 13.6 | −1.2 ± 13.6 | −3.0 ± 13.6 | 6.1 ± 13.6 | 4.0 ± 6.1 a | p-value | ||||
| rbIL-8 | 7.0 ± 4.2 | −15.7 ± 9.4 | 19.2 ± 9.4 | 7.0 ± 9.4 | 17.3 ± 9.4 | 6.9 ± 4.3 a | 0.816 | 0.930 | 0.167 | 0.880 | 0.539 |
| Saline | 15. ± 4.2 | 15.5 ± 9.4 | −1.2 ± 9.4 | −0.04 ± 9.4 | −1.6 ± 9.4 | 3.1 ± 4.3 a | |||||
| Item | Hours Relative to Treatment | Effect 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LogSCC 1 | 0 h | 4 h | 8 h | 12 h | 24 h | 48 h | 144 h | Mean | Group | Hour | Group*Hour | Infused | SCC Level |
| Control | 3.92 ± 0.2 a | 5.31 ± 0.2 a | 4.79 ± 0.2 a | 5.52 ± 0.2 a | 4.03 ± 0.2 a | 4.55 ± 0.2 a | 4.79 ± 0.2 a | 4.71 ± 0.1 a | p-value | ||||
| rbIL-8 | 6.47 ± 0.1 b | 6.55 ± 0.1 b | 6.34 ± 0.1 b | 6.50 ± 0.1 a | 6.54 ± 0.1 b | 6.56 ± 0.1 b | 6.15 ± 0.1 b | 6.44 ± 0.1 b | 0.0002 | <0.0001 | <0.0001 | 0.369 | <0.0001 |
| Saline | 6.31 ± 0.1 b | 6.49 ± 0.1 b | 6.36 ± 0.1 b | 6.45 ± 0.1 a | 6.07± 0.1 b | 6.18 ± 0.1 b | 6.17 ± 0.1 b | 6.29 ± 0.1 b | |||||
| Hours Relative to Treatment | Effect 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | 0 h | 4 h | 24 h | Mean | Group | Hour | Group*Hour | Infused | SCC Level |
| Total protein, g/dL | p-value | ||||||||
| Control | 7.8 ± 0.25 | 7.8 ± 0.25 | 8.2 ± 0.25 | 7.9 ± 0.17 a | |||||
| rbIL-8 | 8.5 ± 0.17 | 8.3 ± 0.17 | 8.8 ± 0.17 | 8.5 ± 0.12 b | 0.054 | 0.016 | 0.430 | 0.161 | 0.037 |
| Saline | 8.4 ± 0.17 | 8.2 ± 0.17 | 8.2 ± 0.17 | 8.3 ± 0.12 ab | |||||
| Albumin, g/dL | |||||||||
| Control | 3.9 ± 0.17 | 3.8 ± 0.17 | 3.4 ± 0.17 | 3.7 ± 0.11 a | |||||
| rbIL-8 | 3.4 ± 4.2 | 3.3 ± 0.12 | 3.2 ± 0.12 | 3.3 ± 0.07 b | 0.062 | 0.433 | 0.278 | 0.130 | 0.056 |
| Saline | 3.4 ± 0.12 | 3.5 ± 0.12 | 3.6 ± 0.12 | 3.5 ± 0.07 ab | |||||
| Globulin, g/dL | |||||||||
| Control | 15.3 ± 3.4 | 16.1 ± 3.4 | 23.6 ± 3.4 | 18.3 ± 2.3 a | |||||
| rbIL-8 | 26.6 ± 2.4 | 25.3 ± 2.4 | 30.8 ± 2.4 | 27.6 ± 1.6 b | 0.032 | 0.202 | 0.275 | 0.106 | 0.025 |
| Saline | 25.9 ± 2.4 | 22.5 ± 2.4 | 21.6 ± 2.4 | 23.3 ± 1.6 ab | |||||
| GGT, U/L | |||||||||
| Control | 18.1 ± 4.0 | 20.0 ± 4.9 | 16.6 ± 3.4 | 18.1 ± 2.8 a | |||||
| rbIL-8 | 26.6 ± 6.1 | 26.6 ± 6.1 | 33.3 ± 9.6 | 28.5 ± 5.0 a | 0.196 | 0.946 | 0.856 | 0.926 | 0.079 |
| Saline | 30.7 ± 8.2 | 28.5 ± 7.1 | 25 ± 5.4 | 27.9 ± 4.8 a | |||||
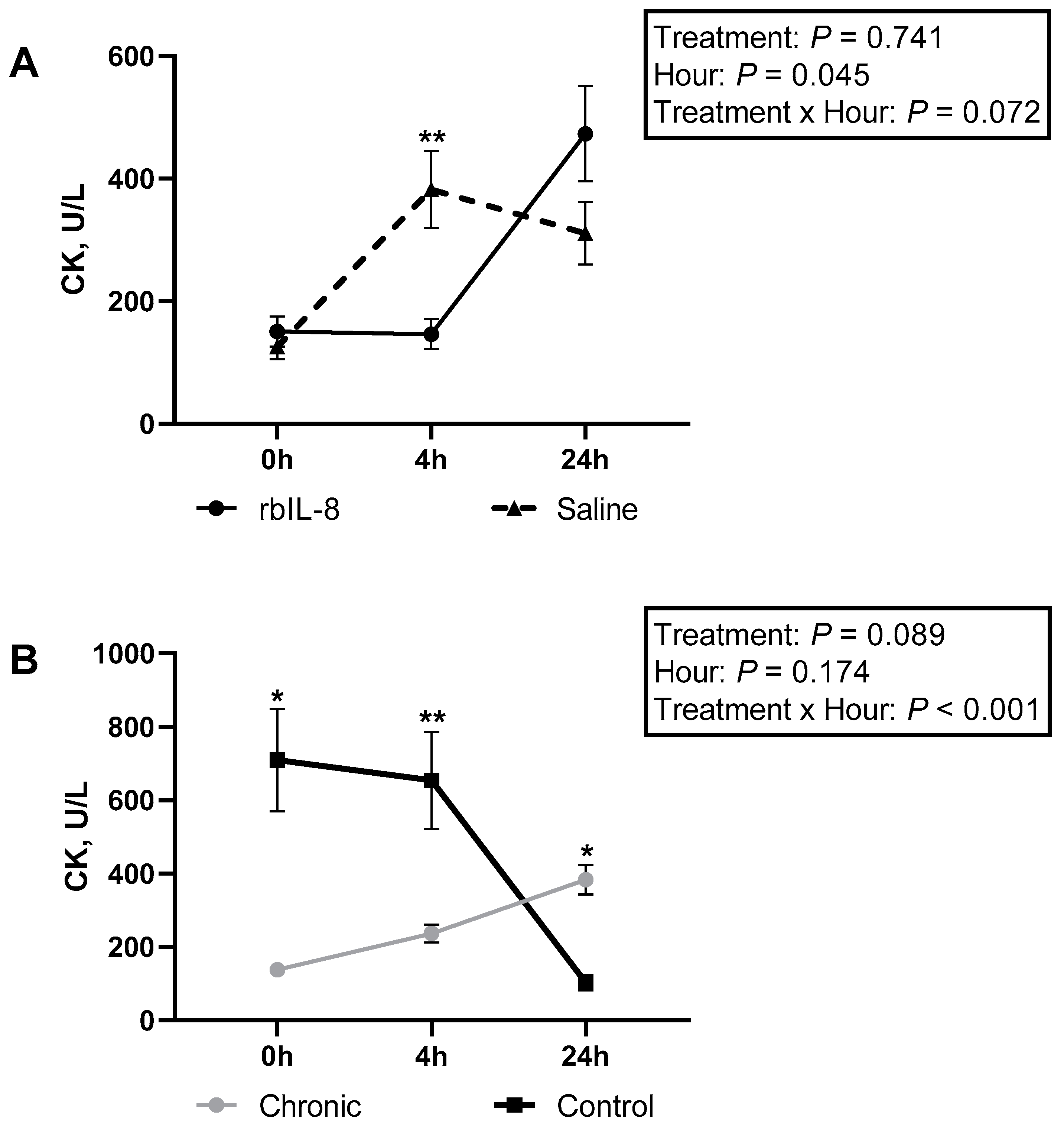
| CK, U/L | |||||||||
| Control | 709 ± 183 | 654 ± 183 | 103 ± 183 | 488 ± 129 a | |||||
| rbIL-8 | 156 ± 129 | 153 ± 129 | 691 ± 129 | 333 ± 91 a | 0.551 | 0.518 | 0.013 | 0.294 | 0.897 |
| Saline | 131 ± 129 | 428 ± 129 | 389 ± 129 | 316 ± 91 a | |||||
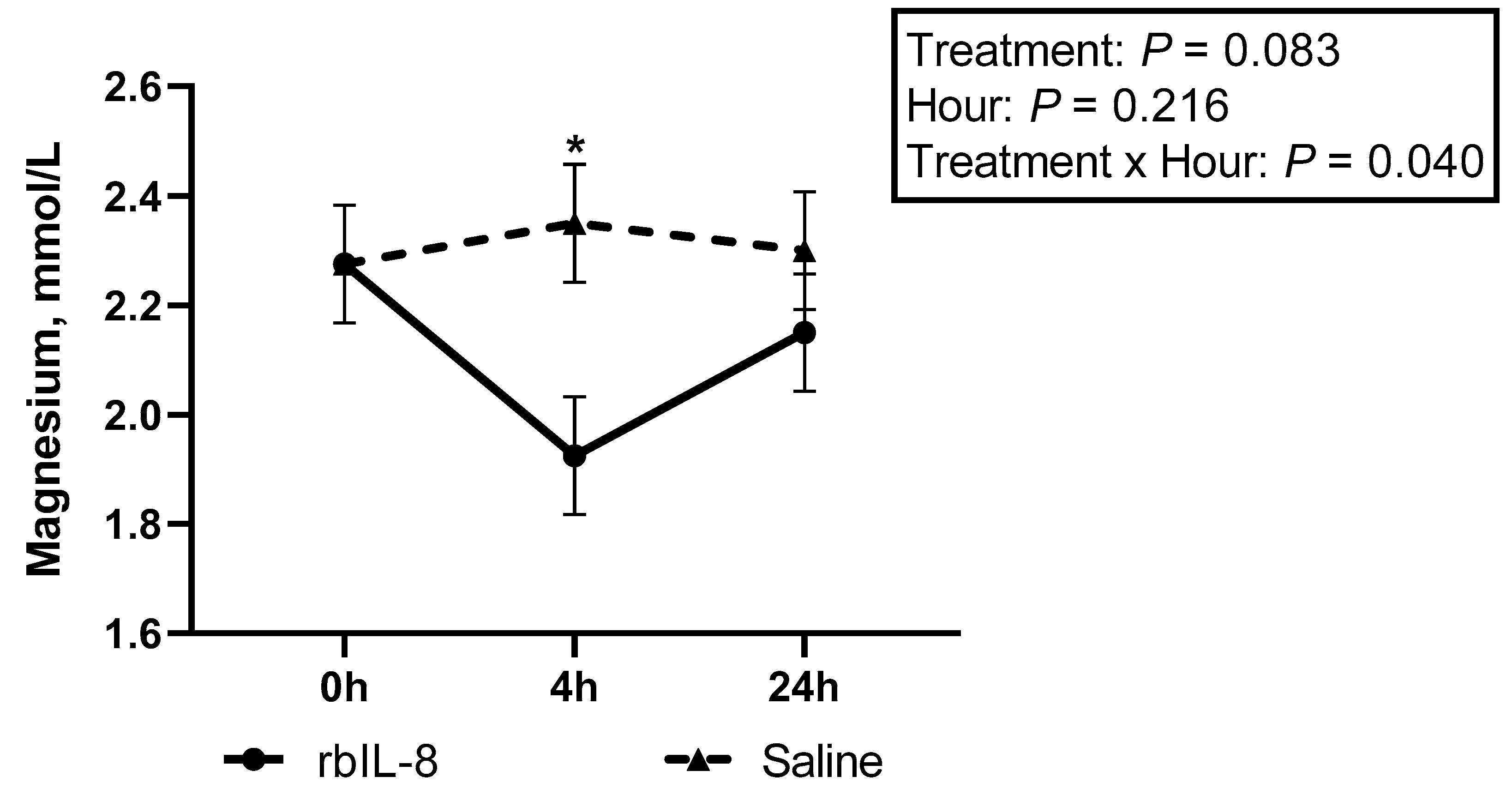
| Magnesium, mmol/L | |||||||||
| Control | 2.7 ± 0.15 | 2.6 ± 0.15 | 2.2 ± 0.15 | 2.5 ± 0.10 a | |||||
| rbIL-8 | 2.2 ± 0.10 | 1.9 ± 0.10 | 2.1 ± 0.10 | 2.1 ± 0.07 b | 0.028 | 0.096 | 0.036 | 0.085 | 0.027 |
| Saline | 2.2 ± 0.10 | 2.3 ± 0.10 | 2.3 ± 0.10 | 2.3 ± 0.07 ab | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, P.M.G.; Cunha, L.L.; Barbosa, L.; Coelho, W., Jr.; Podico, G.; Bicalho, R.C.; Canisso, I.F.; Lima, F.S. Evaluation of Recombinant Bovine Interleukin-8 (rbIL-8) as a Treatment for Chronic Intramammary Infection in Dairy Cows. Antibiotics 2022, 11, 1029. https://doi.org/10.3390/antibiotics11081029
Peixoto PMG, Cunha LL, Barbosa L, Coelho W Jr., Podico G, Bicalho RC, Canisso IF, Lima FS. Evaluation of Recombinant Bovine Interleukin-8 (rbIL-8) as a Treatment for Chronic Intramammary Infection in Dairy Cows. Antibiotics. 2022; 11(8):1029. https://doi.org/10.3390/antibiotics11081029
Chicago/Turabian StylePeixoto, Phillip M. G., Lais L. Cunha, Leonardo Barbosa, Wilson Coelho, Jr., Giorgia Podico, Rodrigo C. Bicalho, Igor F. Canisso, and Fabio S. Lima. 2022. "Evaluation of Recombinant Bovine Interleukin-8 (rbIL-8) as a Treatment for Chronic Intramammary Infection in Dairy Cows" Antibiotics 11, no. 8: 1029. https://doi.org/10.3390/antibiotics11081029
APA StylePeixoto, P. M. G., Cunha, L. L., Barbosa, L., Coelho, W., Jr., Podico, G., Bicalho, R. C., Canisso, I. F., & Lima, F. S. (2022). Evaluation of Recombinant Bovine Interleukin-8 (rbIL-8) as a Treatment for Chronic Intramammary Infection in Dairy Cows. Antibiotics, 11(8), 1029. https://doi.org/10.3390/antibiotics11081029







